Whole-Genome Resequencing Identifies SNPs in Sucrose Synthase and Sugar Transporter Genes Associated with Sweetness in Coconut
Abstract
:1. Introduction
2. Results
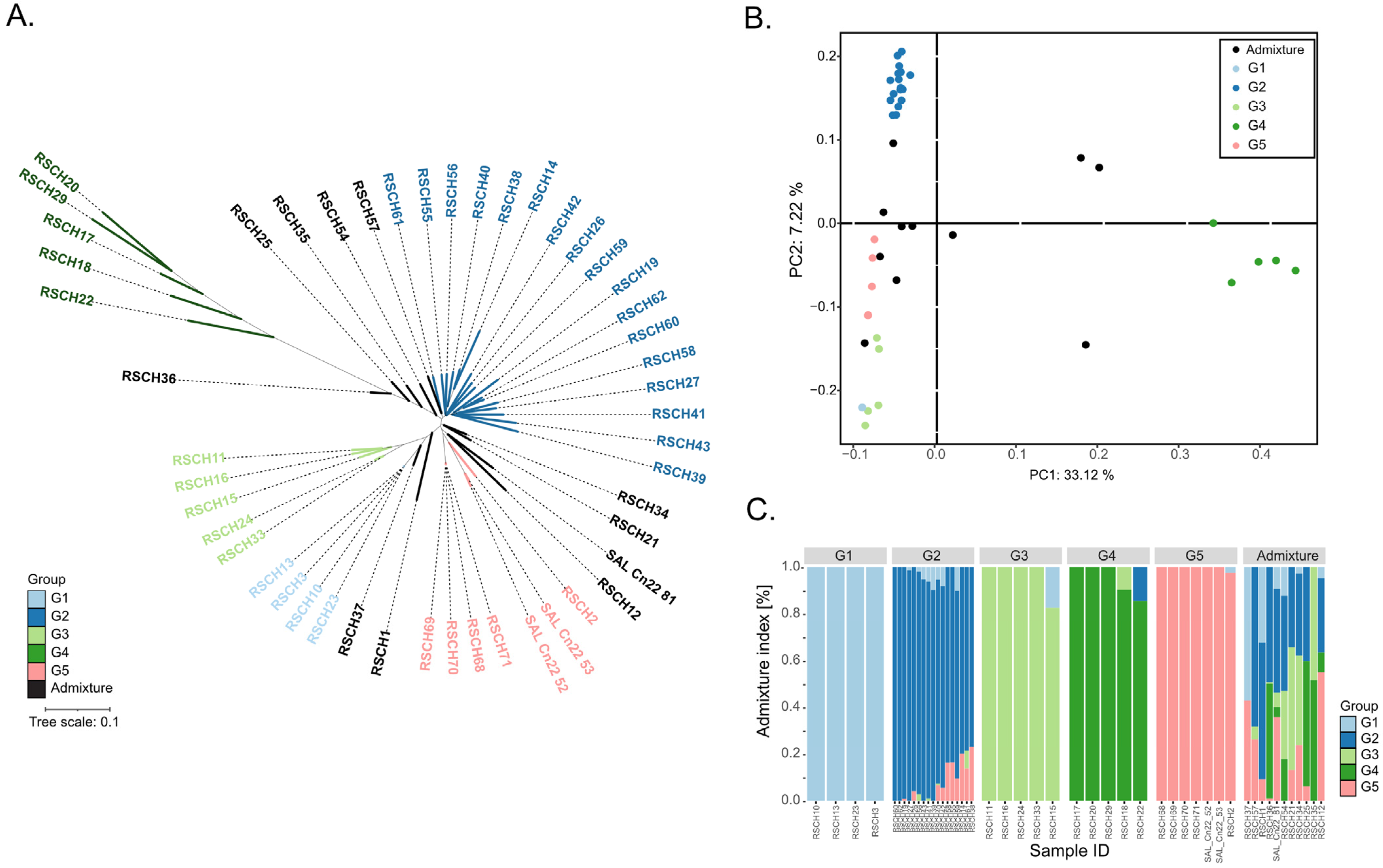
2.1. Whole-Genome Resequencing, Variant Calling and Population Study of Coconut Populations
2.2. Analysis of Sweetness and Sugar Profile
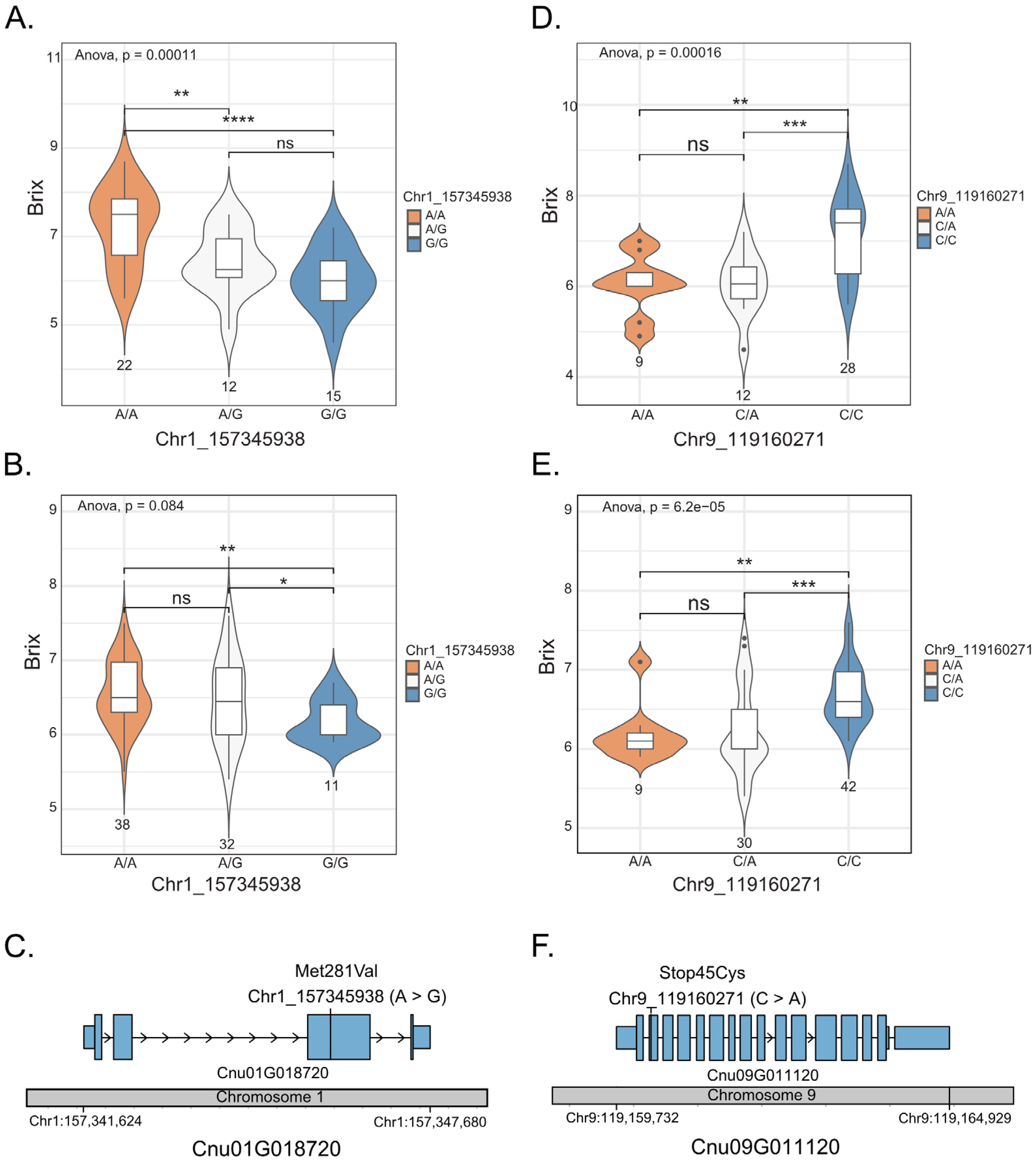
2.3. Identification of Genes Associated with Sweetness in Coconut
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. DNA Extraction, Whole-Genome Sequencing and Variant Calling
4.3. Population Structure Analysis
4.4. Sweetness Evaluation and Sugar Profile Analysis
4.5. Allele Mining Genes in Sugar Metabolism and Transport and Genotype–Phenotype Association Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muralidharan, K.; Jayashree, A. Value addition, product diversification and by-product utilization in coconut. Indian Coconut J. 2011, 7, 4–10. [Google Scholar]
- Lédo, A.d.S.; Passos, E.E.M.; Fontes, H.R.; Ferreira, J.M.S.; Talamini, V.; Vendrame, W.A. Advances in Coconut palm propagation. Rev. Bras. Frutic. 2019, 41, e-159. [Google Scholar] [CrossRef]
- Ignacio, I.-F.; Miguel, T.-S. Research opportunities on the coconut (Cocos nucifera L.) using new technologies. S. Afr. J. Bot. 2021, 141, 414–420. [Google Scholar] [CrossRef]
- Gunn, B.F.; Baudouin, L.; Olsen, K.M. Independent origins of cultivated coconut (Cocos nucifera L.) in the old world tropics. PLoS ONE 2011, 6, e21143. [Google Scholar] [CrossRef]
- Perera, L.; Perera, S.A.; Bandaranayake, C.K.; Harries, H.C. Coconut. In Oil Crops; Springer: New York, NY, USA, 2010; pp. 369–396. [Google Scholar]
- Riangwong, K.; Wanchana, S.; Aesomnuk, W.; Saensuk, C.; Nubankoh, P.; Ruanjaichon, V.; Kraithong, T.; Toojinda, T.; Vanavichit, A.; Arikit, S. Mining and validation of novel genotyping-by-sequencing (GBS)-based simple sequence repeats (SSRs) and their application for the estimation of the genetic diversity and population structure of coconuts (Cocos nucifera L.) in Thailand. Hortic. Res. 2020, 7, 156. [Google Scholar] [CrossRef]
- Kalaipandian, S.; Mu, Z.; Kong, E.Y.Y.; Biddle, J.; Cave, R.; Bazrafshan, A.; Wijayabandara, K.; Beveridge, F.C.; Nguyen, Q.; Adkins, S.W. Cloning Coconut via Somatic Embryogenesis: A Review of the Current Status and Future Prospects. Plants 2021, 10, 2050. [Google Scholar] [CrossRef]
- Dumhai, R.; Wanchana, S.; Saensuk, C.; Choowongkomon, K.; Mahatheeranont, S.; Kraithong, T.; Toojinda, T.; Vanavichit, A.; Arikit, S. Discovery of a novel CnAMADH2 allele associated with higher levels of 2-acetyl-1-pyrroline (2AP) in yellow dwarf coconut (Cocos nucifera L.). Sci. Hortic. 2019, 243, 490–497. [Google Scholar] [CrossRef]
- Burns, D.T.; Johnston, E.L.; Walker, M.J. Authenticity and the potability of coconut water-a critical review. J. AOAC Int. 2020, 103, 800–806. [Google Scholar] [CrossRef]
- Yong, J.W.H.; Ge, L.; Ng, Y.F.; Tan, S.N. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef]
- Ab Mutalib, S.R.; Jailani, F. Physicochemical properties and sensory acceptability of different varieties of coconut water and flesh. Sci. Res. J. 2022, 19, 75. [Google Scholar] [CrossRef]
- Assa, R.R.; Prades, A.; Konan, A.G.; Nemlin, J.; Konan, J.-L. Sensory evaluation and sugars contents of coconut (Cocos nucifera L.) water during nuts ripening. Afr. J. Food Sci. 2013, 7, 186–192. [Google Scholar]
- Li, P.; Wang, L.; Liu, H.; Yuan, M. Impaired SWEET-mediated sugar transportation impacts starch metabolism in developing rice seeds. Crop J. 2022, 10, 98–108. [Google Scholar] [CrossRef]
- Wu, Y.; Lee, S.-K.; Yoo, Y.; Wei, J.; Kwon, S.-Y.; Lee, S.-W.; Jeon, J.-S.; An, G. Rice transcription factor osdof11 modulates sugar transport by promoting expression of sucrose transporter and SWEET genes. Mol. Plant 2018, 11, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Moore, B.; Sheen, J. Sugar sensing and signaling in plants. Plant Cell 2002, 14, S185–S205. [Google Scholar] [CrossRef]
- Shangguan, L.; Song, C.; Leng, X.; Kayesh, E.; Sun, X.; Fang, J. Mining and comparison of the genes encoding the key enzymes involved in sugar biosynthesis in apple, grape, and sweet orange. Sci. Hortic. 2014, 165, 311–318. [Google Scholar] [CrossRef]
- Nguyen-Quoc, B.; Foyer, C.H. A role for “futile cycles” involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. J. Exp. Bot. 2001, 52, 881–889. [Google Scholar] [CrossRef]
- Aba, R.P.M.; Garcia, P.Y.Q.; Juan, J.K.B.; Linsangan, A.T. Influence of food safety knowledge, attitudes, and practices (KAP) of vendors in the City of Manila on microbiological quality of ready-to-drink coconut water. Food Humanit. 2023, 1, 119–127. [Google Scholar] [CrossRef]
- Badenes, M.L.; Fernández I Martí, A.; Ríos, G.; Rubio-Cabetas, M.J. Application of genomic technologies to the breeding of trees. Front. Genet. 2016, 7, 198. [Google Scholar] [CrossRef]
- Lokeshkumar, B.M.; Katral, A.; Sunitha, N.C.; Sah, R.P.; Krishnamurthy, S.L.; Molla, K.A.; Anilkumar, C. Allele mining in rice. In Allele Mining for Genomic Designing of Cereal Crops; CRC Press: Boca Raton, FL, USA, 2024; pp. 39–59. ISBN 9781003385004. [Google Scholar]
- Huang, Y.-C.; Hsiang, E.-C.; Yang, C.-C.; Wang, A.-Y. New insight into the catalytic properties of rice sucrose synthase. Plant Mol. Biol. 2016, 90, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.D.; Yan, J.; Mansfield, S.D. Sucrose synthase affects carbon partitioning to increase cellulose production and altered cell wall ultrastructure. Proc. Natl. Acad. Sci. USA 2009, 106, 13118–13123. [Google Scholar] [CrossRef]
- Geiger, D. Plant glucose transporter structure and function. Pflügers Arch. 2020, 472, 1111–1128. [Google Scholar] [CrossRef]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 11, 11.10.1–11.10.33. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Perrier, X. DARwin Software. 2006. Available online: http://darwin.cirad.fr (accessed on 1 June 2024).
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant Graphics for Data Analysis (2nd ed.). Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]

| Accession ID | Name | Group | Type | Brix | Raw Read (Million) | Mapped Reads (%) | Average Depth | No. of SNPs | No. of InDels |
|---|---|---|---|---|---|---|---|---|---|
| RSCH1 | Mu Si Luang | Admixture | Tall | 6.8 | 360.14 | 98.96 | 15.85 | 620,252 | 59,543 |
| RSCH2 | Nam Wan | G5 | Dwarf | 8.0 | 361.69 | 98.96 | 15.90 | 770,210 | 72,936 |
| RSCH3 | Pathiw | G1 | Dwarf | 6.0 | 350.02 | 98.85 | 15.40 | 605,095 | 59,835 |
| RSCH10 | Tung Kret | G1 | Dwarf | 7.7 | 342.66 | 98.89 | 15.05 | 600,709 | 58,792 |
| RSCH11 | MuSi Som (orange) | G3 | Dwarf | 7.5 | 349.96 | 98.74 | 15.36 | 646,291 | 61,172 |
| RSCH12 | Puang Roi | Admixture | Tall | 8.1 | 290.36 | 98.92 | 12.80 | 673,944 | 59,142 |
| RSCH13 | Nam Hom #1 | G1 | Dwarf | 7.5 | 350.05 | 98.94 | 15.41 | 607,824 | 59,930 |
| RSCH14 | Pak Chok #1 | G2 | Tall | 5.6 | 349.98 | 98.88 | 15.38 | 1,293,375 | 117,102 |
| RSCH15 | Papua Nugini brown dwarf | G3 | Dwarf | 7.4 | 364.15 | 98.97 | 16.03 | 636,506 | 60,933 |
| RSCH16 | Cameroon yellow dwarf | G3 | Dwarf | 5.6 | 309.68 | 98.80 | 13.64 | 615,516 | 59,726 |
| RSCH17 | West African Tall #1 | G4 | Tall | 6.0 | 350.02 | 98.96 | 15.38 | 5,078,243 | 280,368 |
| RSCH18 | West African Tall #2 | G4 | Tall | 6.2 | 321.10 | 98.72 | 14.05 | 4,091,019 | 228,083 |
| RSCH19 | Kalok #1 | G2 | Tall | 6.0 | 306.81 | 98.77 | 13.43 | 3,817,502 | 227,197 |
| RSCH20 | Talai Roi | G4 | Tall | 6.8 | 323.94 | 98.79 | 14.24 | 3,075,937 | 180,670 |
| RSCH21 | Tahiti | Admixture | Tall | 7.1 | 330.79 | 98.76 | 14.46 | 4,704,771 | 270,072 |
| RSCH22 | Pak Chok #2 | G4 | Tall | 7.0 | 322.17 | 98.73 | 14.13 | 4,435,916 | 251,018 |
| RSCH23 | Nok Khum | G1 | Dwarf | 8.7 | 355.14 | 98.85 | 15.61 | 613,071 | 60,224 |
| RSCH24 | Nalike (yellow) | G3 | Dwarf | 6.2 | 366.09 | 98.82 | 16.11 | 644,087 | 62,993 |
| RSCH25 | Chumphon 60 | Admixture | Tall | 6.0 | 344.14 | 97.05 | 14.75 | 9,243,464 | 469,432 |
| RSCH26 | Thai Tall #6 | G2 | Tall | 5.2 | 307.77 | 98.59 | 13.42 | 3,881,637 | 216,543 |
| RSCH27 | Tapsakae | G2 | Tall | 5.9 | 371.28 | 98.62 | 16.21 | 4,695,620 | 253,492 |
| RSCH29 | West African Tall #3 | G4 | Tall | 6.3 | 353.19 | 98.57 | 15.48 | 3,595,949 | 206,013 |
| RSCH33 | Malayan yellow dwarf | G3 | Dwarf | 7.2 | 378.57 | 98.31 | 16.56 | 617,018 | 60,060 |
| RSCH34 | Chumphon 2 | Admixture | Tall | 6.2 | 325.48 | 98.74 | 14.28 | 4,229,504 | 241,368 |
| RSCH35 | MaWa | Admixture | Tall | 6.0 | 321.83 | 98.56 | 14.05 | 9,608,775 | 482,085 |
| RSCH36 | Khom #1 | Admixture | Dwarf | 5.5 | 304.23 | 98.38 | 13.27 | 8,237,781 | 414,489 |
| RSCH37 | Maphraw Fai | Admixture | Tall | 6.5 | 378.46 | 98.56 | 16.60 | 619,315 | 60,671 |
| RSCH38 | Pak Chok #3 | G2 | Tall | 6.2 | 330.42 | 98.50 | 14.46 | 3,587,634 | 222,083 |
| RSCH39 | Pak Chok #4 | G2 | Tall | 6.3 | 268.22 | 98.56 | 11.78 | 2,428,643 | 144,064 |
| RSCH40 | Pak Chok #5 | G2 | Tall | 6.9 | 283.06 | 98.48 | 12.39 | 3,694,255 | 215,166 |
| RSCH41 | Pleuk Wan | G2 | Tall | 7.2 | 334.88 | 98.64 | 14.67 | 3,943,537 | 234,280 |
| RSCH42 | Saw | G2 | Tall | 7.4 | 299.19 | 98.62 | 13.08 | 4,195,785 | 232,817 |
| RSCH43 | Khom #2 | G2 | Tall | 6.8 | 356.35 | 98.61 | 15.63 | 2,418,467 | 148,546 |
| RSCH54 | Thai Tall #1 | Admixture | Tall | 5.7 | 324.68 | 98.75 | 14.23 | 5,883,454 | 314,838 |
| RSCH55 | Thai Tall #2 | G2 | Tall | 6.1 | 323.13 | 98.65 | 14.16 | 4,201,893 | 250,662 |
| RSCH56 | Thai Tall #3 | G2 | Tall | 5.8 | 356.15 | 98.58 | 15.60 | 4,116,409 | 249,872 |
| RSCH57 | Thai Tall #4 | Admixture | Tall | 6.4 | 349.92 | 98.70 | 15.33 | 4,026,836 | 224,703 |
| RSCH58 | Thai Tall #4 | G2 | Tall | 6.4 | 325.44 | 98.69 | 14.24 | 4,316,205 | 232,021 |
| RSCH59 | Thai Tall #5 | G2 | Tall | 6.5 | 334.18 | 98.69 | 14.63 | 3,917,338 | 223,471 |
| RSCH60 | Kalok #2 | G2 | Tall | 5.5 | 352.21 | 98.75 | 15.43 | 4,189,965 | 242,214 |
| RSCH61 | Kalok #3 | G2 | Tall | 4.9 | 324.38 | 98.80 | 14.23 | 4,053,617 | 234,133 |
| RSCH62 | Kalok #4 | G2 | Tall | 4.6 | 382.47 | 98.70 | 16.75 | 4,308,424 | 245,350 |
| RSCH68 | Nam Hom #2 | G5 | Dwarf | 7.7 | 350.06 | 98.73 | 15.33 | 2,074,451 | 118,256 |
| RSCH69 | Nam Hom #3 | G5 | Dwarf | 7.9 | 382.92 | 98.74 | 16.78 | 2,112,589 | 121,664 |
| RSCH70 | Nam Hom #4 | G5 | Dwarf | 8.3 | 313.20 | 98.74 | 13.74 | 2,041,130 | 115,977 |
| RSCH71 | Nam Hom #5 | G5 | Dwarf | 8.2 | 289.44 | 98.76 | 12.71 | 1,997,857 | 113,526 |
| SAL_Cn22_52 | Brown sweet #1 | G5 | Dwarf | 7.5 | 290.16 | 96.61 | 12.25 | 1,012,175 | 53,552 |
| SAL_Cn22_53 | Brown sweet #2 | G5 | Dwarf | 7.5 | 312.45 | 96.23 | 13.12 | 1,105,773 | 55,419 |
| SAL_Cn22_81 | Maphreaw | Admixture | Tall | 7.5 | 89.47 | 94.63 | 5.58 | 1,342,349 | 57,232 |
| SNP | Significant Codes | Adjusted R2 | Ref | ATL | SNP Eff | Gene ID | Annotation |
|---|---|---|---|---|---|---|---|
| 1_135984088 | ** | 0.1264 | T | C | missense_variant | Cnu01G011700 | Sugar (and other) transporter |
| 1_145882242 | ** | 0.1895 | C | T | missense_variant | Cnu01G014570 | Fructokinase |
| 1_157345938 | *** | 0.3016 | A | G | missense_variant | Cnu01G018720 | Sugar (and other) transporter |
| 1_157347379 | ** | 0.1333 | T | G | missense_variant | Cnu01G018720 | Sugar (and other) transporter |
| 1_157352010 | ** | 0.1184 | A | G | missense_variant | Cnu01G018730 | Sugar (and other) transporter |
| 1_163329328 | *** | 0.2951 | A | G | missense_variant | Cnu01G021210 | alpha-1,3/1,6-mannosyltransferase ALG2-like |
| 1_163346328 | *** | 0.2881 | A | G | missense_variant | Cnu01G021210 | alpha-1,3/1,6-mannosyltransferase ALG2-like |
| 1_168918498 | ** | 0.1522 | G | C | missense_variant | Cnu01G023700 | Sugar (and other) transporter |
| 2_21952206 | ** | 0.1848 | G | A | missense_variant | Cnu02G009280 | Sucrose synthase |
| 2_28930455 | ** | 0.1294 | A | G | missense_variant | Cnu02G010820 | Sucrose nonfermenting 4-like protein |
| 2_28931901 | ** | 0.1823 | C | G | missense_variant&splice_region_variant | Cnu02G010820 | Sucrose nonfermenting 4-like protein |
| 2_36533852 | ** | 0.1568 | G | C | missense_variant | Cnu02G012160 | Hexokinase |
| 3_13921395 | ** | 0.1513 | T | A | missense_variant | Cnu03G006580 | Phosphofructokinase |
| 3_13936725 | ** | 0.1305 | T | C | missense_variant | Cnu03G006580 | Phosphofructokinase |
| 3_29827288 | ** | 0.1375 | G | C,A | missense_variant | Cnu03G011100 | Fructokinase |
| 3_35378092 | ** | 0.1374 | T | C | missense_variant | Cnu03G012010 | Sugar efflux transporter for intercellular exchange |
| 4_120857213 | ** | 0.1501 | A | C | missense_variant | Cnu04G012550 | Sugar (and other) transporter |
| 4_13014850 | ** | 0.1725 | C | T | missense_variant | Cnu04G004950 | Hexokinase |
| 4_13016579 | ** | 0.1464 | C | T | stop_gained | Cnu04G004950 | Hexokinase |
| 4_13016642 | ** | 0.1464 | C | A | missense_variant | Cnu04G004950 | Hexokinase |
| 4_13016651 | ** | 0.1464 | G | A | missense_variant | Cnu04G004950 | Hexokinase |
| 4_13017837 | ** | 0.1464 | G | C | missense_variant | Cnu04G004950 | Hexokinase |
| 4_13017876 | *** | 0.2613 | T | C | missense_variant | Cnu04G004950 | Hexokinase |
| 4_133364483 | ** | 0.1327 | A | T | missense_variant | Cnu04G017640 | Hexokinase |
| 4_133364586 | *** | 0.2052 | T | TA | frameshift_variant | Cnu04G017640 | Hexokinase |
| 5_9583913 | ** | 0.1344 | A | G | missense_variant | Cnu05G004150 | Sucrose synthase |
| 8_15530732 | ** | 0.1364 | C | T | stop_gained | Cnu08G006450 | Sugar efflux transporter for intercellular exchange |
| 8_15530840 | ** | 0.1364 | G | A | missense_variant | Cnu08G006450 | Sugar efflux transporter for intercellular exchange |
| 8_19928242 | ** | 0.1167 | C | T | missense_variant | Cnu08G008560 | Sugar efflux transporter for intercellular exchange |
| 8_19928244 | ** | 0.136 | A | T | missense_variant | Cnu08G008560 | Sugar efflux transporter for intercellular exchange |
| 8_19934962 | ** | 0.1222 | A | G | missense_variant | Cnu08G008560 | Sugar efflux transporter for intercellular exchange |
| 8_19935070 | ** | 0.128 | G | C | missense_variant | Cnu08G008560 | Sugar efflux transporter for intercellular exchange |
| 8_9181379 | ** | 0.1732 | G | T | missense_variant | Cnu08G003810 | Phosphofructokinase |
| 8_9182540 | *** | 0.2238 | A | T | missense_variant | Cnu08G003810 | Phosphofructokinase |
| 8_9913460 | ** | 0.1256 | A | G | missense_variant | Cnu08G004000 | Hexokinase |
| 9_119160271 | *** | 0.2473 | C | A | stop_gained | Cnu09G011120 | Sucrose synthase |
| 9_119162558 | ** | 0.1534 | A | G | missense_variant | Cnu09G011120 | Sucrose synthase |
| 9_121551153 | *** | 0.3985 | G | A | missense_variant | Cnu09G012520 | Major facilitator superfamily |
| 10_183086020 | *** | 0.3244 | T | C | missense_variant | Cnu10G024280 | Sugar (and other) transporter |
| 10_192176452 | *** | 0.3358 | G | C,A | missense_variant | Cnu10G028040 | Sugar efflux transporter for intercellular exchange |
| 10_192177601 | *** | 0.2312 | G | A | missense_variant | Cnu10G028040 | Sugar efflux transporter for intercellular exchange |
| 10_20363231 | ** | 0.1879 | T | C | missense_variant&splice_region_variant | Cnu10G005750 | Sucrose nonfermenting 4-like protein |
| 10_57193853 | ** | 0.1463 | G | T | missense_variant | Cnu10G013990 | beta-fructofuranosidase |
| 11_15951447 | *** | 0.2444 | C | T | missense_variant | Cnu11G006980 | Hexokinase |
| 11_43908723 | ** | 0.1306 | G | T | missense_variant | Cnu11G018990 | Sugar (and other) transporter |
| 12_89966688 | *** | 0.2144 | C | T | missense_variant | Cnu12G018320 | Sugar (and other) transporter |
| 12_89966974 | ** | 0.1693 | A | G | missense_variant | Cnu12G018320 | Sugar (and other) transporter |
| 12_89967123 | *** | 0.2076 | G | A | missense_variant | Cnu12G018320 | Sugar (and other) transporter |
| 12_89967124 | *** | 0.2076 | C | A | missense_variant | Cnu12G018320 | Sugar (and other) transporter |
| 13_65960883 | ** | 0.1461 | C | T | missense_variant&splice_region_variant | Cnu13G003960 | Sugar (and other) transporter |
| 14_124469111 | ** | 0.1566 | A | G | missense_variant | Cnu14G014760 | Raffinose synthase or seed imbibition protein Sip1 |
| 14_124469119 | ** | 0.1566 | A | G | missense_variant | Cnu14G014760 | Raffinose synthase or seed imbibition protein Sip1 |
| 14_144741125 | ** | 0.1605 | A | C | missense_variant | Cnu14G021780 | Phosphofructokinase |
| 14_149652548 | *** | 0.2045 | G | A | missense_variant | Cnu14G024290 | Sugar (and other) transporter |
| 14_149652623 | *** | 0.2593 | T | A | missense_variant | Cnu14G024290 | Sugar (and other) transporter |
| 14_161558915 | *** | 0.2522 | A | G | missense_variant | Cnu14G029310 | Sucrose nonfermenting 4-like protein |
| 14_7085864 | ** | 0.12 | T | G | missense_variant | Cnu14G002810 | Sugar efflux transporter for intercellular exchange |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khongmaluan, M.; Aesomnuk, W.; Dumhai, R.; Pitaloka, M.K.; Xiao, Y.; Xia, R.; Kraithong, T.; Phonsatta, N.; Panya, A.; Ruanjaichon, V.; et al. Whole-Genome Resequencing Identifies SNPs in Sucrose Synthase and Sugar Transporter Genes Associated with Sweetness in Coconut. Plants 2024, 13, 2548. https://doi.org/10.3390/plants13182548
Khongmaluan M, Aesomnuk W, Dumhai R, Pitaloka MK, Xiao Y, Xia R, Kraithong T, Phonsatta N, Panya A, Ruanjaichon V, et al. Whole-Genome Resequencing Identifies SNPs in Sucrose Synthase and Sugar Transporter Genes Associated with Sweetness in Coconut. Plants. 2024; 13(18):2548. https://doi.org/10.3390/plants13182548
Chicago/Turabian StyleKhongmaluan, Manlika, Wanchana Aesomnuk, Reajina Dumhai, Mutiara K. Pitaloka, Yong Xiao, Rui Xia, Tippaya Kraithong, Natthaporn Phonsatta, Atikorn Panya, Vinitchan Ruanjaichon, and et al. 2024. "Whole-Genome Resequencing Identifies SNPs in Sucrose Synthase and Sugar Transporter Genes Associated with Sweetness in Coconut" Plants 13, no. 18: 2548. https://doi.org/10.3390/plants13182548





