Defense Responses Induced by Viral Movement Protein and Its Nuclear Localization Modulate Virus Cell-to-Cell Transport
Abstract
:1. Introduction
2. Results
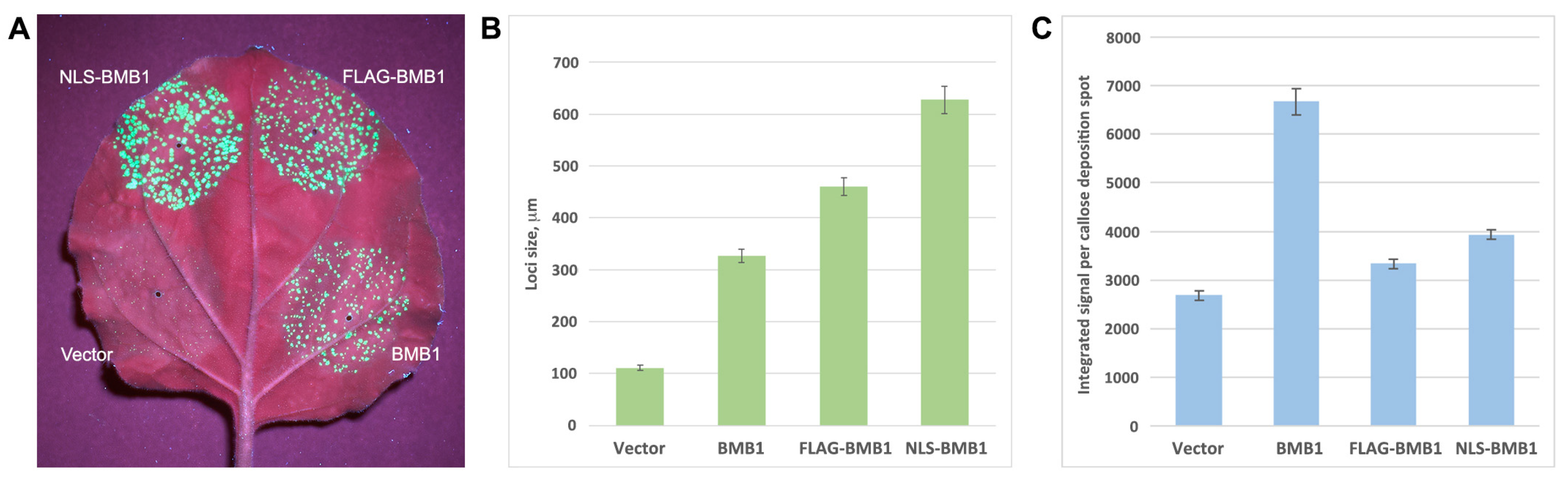
2.1. BMB1 Expression Induces Defense Response in N. benthamiana
2.2. BMB2 Suppresses Defense Response Induced by BMB1
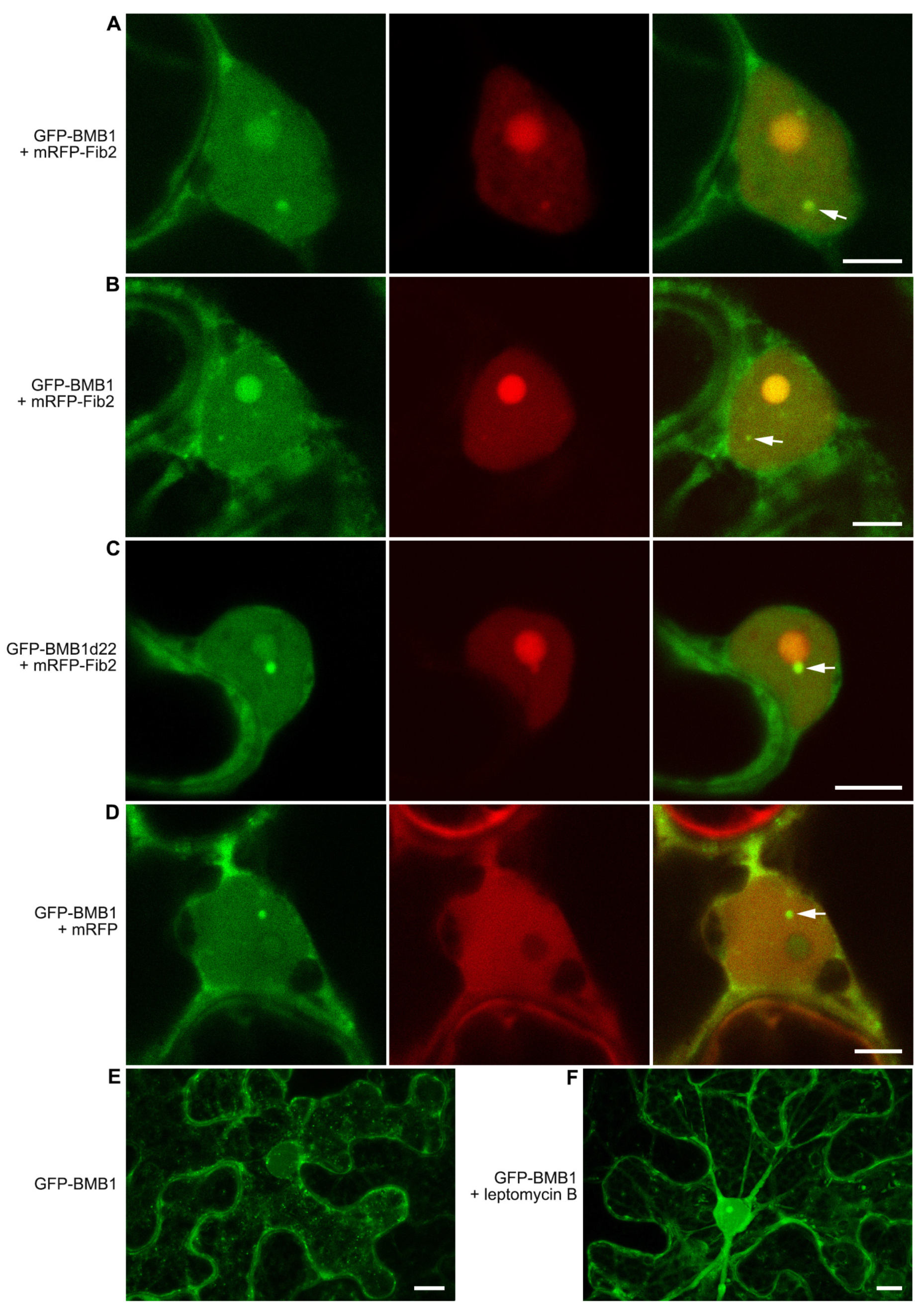
2.3. BMB1 Is Partially Localized to Nuclear Substructures
2.4. Search for Signals Directing BMB1 to Nuclear Substructures
2.5. Analysis of BMB1 with Artificially Added NES or NLS
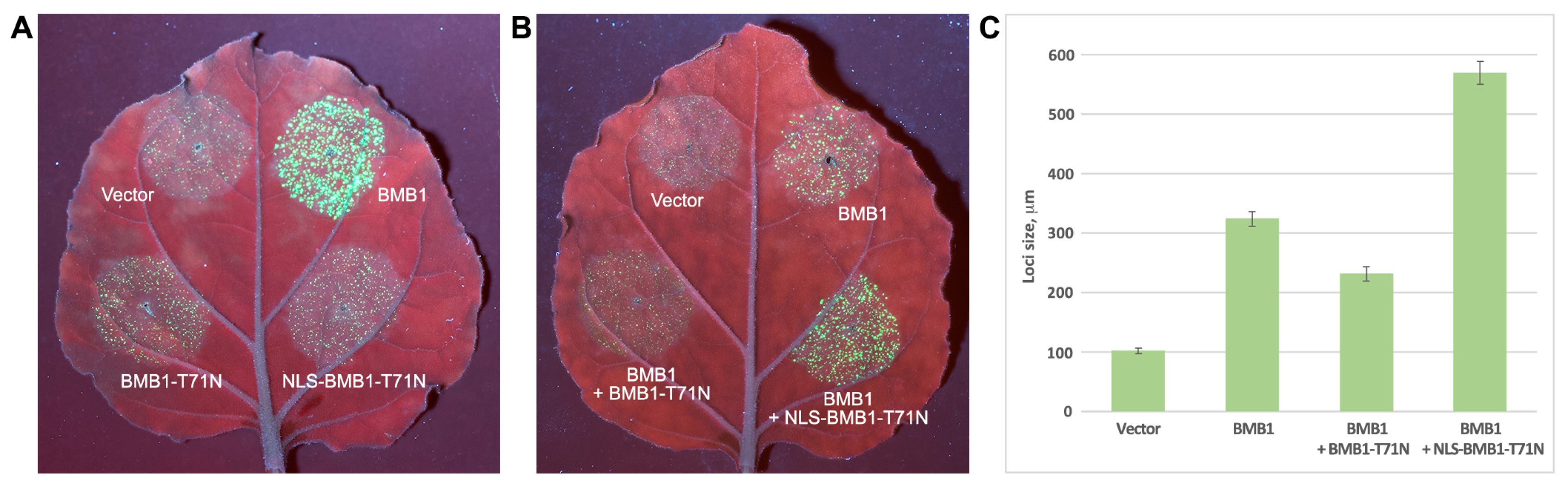
2.6. Dysfunctional BMB1 Localized to the Nucleus Enhances Virus Transport
3. Discussion
4. Materials and Methods
4.1. Molecular Cloning and Recombinant Constructs
4.2. Protein Sequence Analysis by Bioinformatics Tools
4.3. Plant Material
4.4. Plant Agroinfiltration
4.5. Aniline Blue Staining
4.6. DAB Staining
4.7. Leptomycin B Treatment
4.8. Confocal Microscopy and Virus Movement Visualization
4.9. Quantitative PCR
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucas, W.J. Plant Viral Movement Proteins: Agents for Cell-to-Cell Trafficking of Viral Genomes. Virology 2006, 344, 169–184. [Google Scholar] [CrossRef]
- Heinlein, M. Plant Virus Replication and Movement. Virology 2015, 479–480, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Tee, E.E.; Faulkner, C. Plasmodesmata and Intercellular Molecular Traffic Control. New Phytol. 2024, 243, 32–47. [Google Scholar] [CrossRef]
- Lanfermeijer, F.C.; Dijkhuis, J.; Sturre, M.J.G.; de Haan, P.; Hille, J. Cloning and Characterization of the Durable Tomato Mosaic Virus Resistance Gene Tm-2(2) from Lycopersicon Esculentum. Plant Mol. Biol. 2003, 52, 1037–1049. [Google Scholar] [CrossRef]
- Aguilar, E.; Almendral, D.; Allende, L.; Pacheco, R.; Chung, B.N.; Canto, T.; Tenllado, F. The P25 Protein of Potato Virus X (PVX) Is the Main Pathogenicity Determinant Responsible for Systemic Necrosis in PVX-Associated Synergisms. J. Virol. 2015, 89, 2090–2103. [Google Scholar] [CrossRef]
- Atabekova, A.K.; Solovieva, A.D.; Chergintsev, D.A.; Solovyev, A.G.; Morozov, S.Y. Role of Plant Virus Movement Proteins in Suppression of Host RNAi Defense. Int. J. Mol. Sci. 2023, 24, 9049. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant Immunity: Towards an Integrated View of Plant–Pathogen Interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-Triggered Immunity: From Pathogen Perception to Robust Defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef]
- Meshi, T.; Motoyoshi, F.; Maeda, T.; Yoshiwoka, S.; Watanabe, H.; Okada, Y. Mutations in the Tobacco Mosaic Virus 30-kD Protein Gene Overcome Tm-2 Resistance in Tomato. Plant Cell 1989, 1, 515–522. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Liu, S.; Zhang, D.-P.; Liu, Y. Tm-22 Confers Different Resistance Responses against Tobacco Mosaic Virus Dependent on Its Expression Level. Mol. Plant 2013, 6, 971–974. [Google Scholar] [CrossRef]
- Motoyoshi, F.; Oshima, N. Expression of Genetically Controlled Resistance to Tobacco Mosaic Virus Infection in Isolated Tomato Leaf Mesophyll Protoplasts. J. Gen. Virol. 1977, 34, 499–506. [Google Scholar] [CrossRef]
- Chen, T.; Liu, D.; Niu, X.; Wang, J.; Qian, L.; Han, L.; Liu, N.; Zhao, J.; Hong, Y.; Liu, Y. Antiviral Resistance Protein Tm-2 2 Functions on the Plasma Membrane. Plant Physiol. 2017, 173, 2399–2410. [Google Scholar] [CrossRef]
- Morozov, S.Y.; Solovyev, A.G. Triple Gene Block: Modular Design of a Multifunctional Machine for Plant Virus Movement. J. Gen. Virol. 2003, 84, 1351–1366. [Google Scholar] [CrossRef]
- Wright, K.M.; Cowan, G.H.; Lukhovitskaya, N.I.; Tilsner, J.; Roberts, A.G.; Savenkov, E.I.; Torrance, L. The N-Terminal Domain of PMTV TGB1 Movement Protein Is Required for Nucleolar Localization, Microtubule Association, and Long-Distance Movement. Mol. Plant Microbe Interact. 2010, 23, 1486–1497. [Google Scholar] [CrossRef]
- Lukhovitskaya, N.I.; Cowan, G.H.; Vetukuri, R.R.; Tilsner, J.; Torrance, L.; Savenkov, E.I. Importin-α-Mediated Nucleolar Localization of Potato Mop-Top Virus TRIPLE GENE BLOCK1 (TGB1) Protein Facilitates Virus Systemic Movement, Whereas TGB1 Self-Interaction Is Required for Cell-to-Cell Movement in Nicotiana benthamiana. Plant Physiol. 2015, 167, 738–752. [Google Scholar] [CrossRef]
- Semashko, M.A.; González, I.; Shaw, J.; Leonova, O.G.; Popenko, V.I.; Taliansky, M.E.; Canto, T.; Kalinina, N.O. The Extreme N-Terminal Domain of a Hordeivirus TGB1 Movement Protein Mediates Its Localization to the Nucleolus and Interaction with Fibrillarin. Biochimie 2012, 94, 1180–1188. [Google Scholar] [CrossRef]
- Semashko, M.A.; Rakitina, D.V.; González, I.; Canto, T.; Kalinina, N.O.; Taliansky, M.E. Movement Protein of Hordeivirus Interacts in Vitro and in Vivo with Coilin, a Major Structural Protein of Cajal Bodies. Dokl. Biochem. Biophys. 2012, 442, 57–60. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Jiang, Z.; Jin, X.; Zhang, K.; Wang, X.; Han, C.; Yu, J.; Li, D. Hijacking of the Nucleolar Protein Fibrillarin by TGB1 Is Required for Cell-to-Cell Movement of Barley Stripe Mosaic Virus. Mol. Plant Pathol. 2018, 19, 1222–1237. [Google Scholar] [CrossRef]
- Ryabov, E.V.; Robinson, D.J.; Taliansky, M.E. A Plant Virus-Encoded Protein Facilitates Long-Distance Movement of Heterologous Viral RNA. Proc. Natl. Acad. Sci. USA 1999, 96, 1212–1217. [Google Scholar] [CrossRef]
- Kim, S.H.; Ryabov, E.V.; Kalinina, N.O.; Rakitina, D.V.; Gillespie, T.; MacFarlane, S.; Haupt, S.; Brown, J.W.S.; Taliansky, M. Cajal Bodies and the Nucleolus Are Required for a Plant Virus Systemic Infection. EMBO J. 2007, 26, 2169–2179. [Google Scholar] [CrossRef]
- Kim, S.H.; Macfarlane, S.; Kalinina, N.O.; Rakitina, D.V.; Ryabov, E.V.; Gillespie, T.; Haupt, S.; Brown, J.W.S.; Taliansky, M. Interaction of a Plant Virus-Encoded Protein with the Major Nucleolar Protein Fibrillarin Is Required for Systemic Virus Infection. Proc. Natl. Acad. Sci. USA 2007, 104, 11115–11120. [Google Scholar] [CrossRef]
- Levy, A.; Zheng, J.Y.; Lazarowitz, S.G. The Tobamovirus Turnip Vein Clearing Virus 30-Kilodalton Movement Protein Localizes to Novel Nuclear Filaments To Enhance Virus Infection. J. Virol. 2013, 87, 6428–6440. [Google Scholar] [CrossRef]
- Cohen, Y.; Qu, F.; Gisel, A.; Morris, T.J.; Zambryski, P.C. Nuclear Localization of Turnip Crinkle Virus Movement Protein P8. Virology 2000, 273, 276–285. [Google Scholar] [CrossRef]
- Melzer, M.J.; Sether, D.M.; Borth, W.B.; Hu, J.S. Characterization of a Virus Infecting Citrus Volkameriana with Citrus Leprosis-like Symptoms. Phytopathology 2012, 102, 122–127. [Google Scholar] [CrossRef]
- Lazareva, E.A.; Lezzhov, A.A.; Komarova, T.V.; Morozov, S.Y.; Heinlein, M.; Solovyev, A.G. A Novel Block of Plant Virus Movement Genes. Mol. Plant Pathol. 2017, 18, 611–624. [Google Scholar] [CrossRef]
- Morozov, S.Y.; Solovyev, A.G. Did Silencing Suppression Counter-Defensive Strategy Contribute to Origin and Evolution of the Triple Gene Block Coding for Plant Virus Movement Proteins? Front. Plant Sci. 2012, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Morozov, S.Y.; Solovyev, A.G. Phylogenetic Relationship of Some “Accessory” Helicases of Plant Positive-Stranded RNA Viruses: Toward Understanding the Evolution of Triple Gene Block. Front. Microbiol. 2015, 6, 508. [Google Scholar] [CrossRef]
- Lazareva, E.A.; Lezzhov, A.A.; Chergintsev, D.A.; Golyshev, S.A.; Dolja, V.V.; Morozov, S.Y.; Heinlein, M.; Solovyev, A.G. Reticulon-like Properties of a Plant Virus-Encoded Movement Protein. New Phytol. 2021, 229, 1052–1066. [Google Scholar] [CrossRef]
- Lazareva, E.A.; Lezzhov, A.A.; Dolja, V.V.; Morozov, S.Y.; Heinlein, M.; Solovyev, A.G. Constriction of Endoplasmic Reticulum Tubules by the Viral Movement Protein BMB2 Is Associated with Local BMB2 Anchorage at Constriction Sites. Plant Signal. Behav. 2020, 00, 1856547. [Google Scholar] [CrossRef]
- Atabekova, A.K.; Golyshev, S.A.; Lezzhov, A.A.; Skulachev, B.I.; Moiseenko, A.V.; Yastrebova, D.M.; Andrianova, N.V.; Solovyev, I.D.; Savitsky, A.P.; Morozov, S.Y.; et al. Fine Structure of Plasmodesmata-Associated Membrane Bodies Formed by Viral Movement Protein. Plants 2023, 12, 4100. [Google Scholar] [CrossRef] [PubMed]
- Atabekova, A.K.; Lazareva, E.A.; Lezzhov, A.A.; Solovieva, A.D.; Golyshev, S.A.; Skulachev, B.I.; Solovyev, I.D.; Savitsky, A.P.; Heinlein, M.; Morozov, S.Y.; et al. Interaction between Movement Proteins of Hibiscus Green Spot Virus. Viruses 2022, 14, 2742. [Google Scholar] [CrossRef]
- MacFarlane, S.A.; Popovich, A.H. Efficient Expression of Foreign Proteins in Roots from Tobravirus Vectors. Virology 2000, 267, 29–35. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. Regulation and Function of Defense-Related Callose Deposition in Plants. Int. J. Mol. Sci. 2021, 22, 2393. [Google Scholar] [CrossRef]
- García-Marcos, A.; Pacheco, R.; Manzano, A.; Aguilar, E.; Tenllado, F. Oxylipin Biosynthesis Genes Positively Regulate Programmed Cell Death during Compatible Infections with the Synergistic Pair Potato Virus X-Potato Virus Y and Tomato Spotted Wilt Virus. J. Virol. 2013, 87, 5769–5783. [Google Scholar] [CrossRef]
- Lazareva, E.A.; Atabekova, A.K.; Lezzhov, A.A.; Morozov, S.Y.; Heinlein, M.; Solovyev, A.G. Virus Genome-Based Reporter for Analyzing Viral Movement Proteins and Plasmodesmata Permeability. Methods Mol. Biol. 2022, 2457, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.J.; Brown, J.W. Plant Nuclear Bodies. Curr. Opin. Plant Biol. 2004, 7, 614–620. [Google Scholar] [CrossRef]
- Nishi, K.; Yoshida, M.; Fujiwara, D.; Nishikawa, M.; Horinouchi, S.; Beppu, T. Leptomycin B Targets a Regulatory Cascade of Crm1, a Fission Yeast Nuclear Protein, Involved in Control of Higher Order Chromosome Structure and Gene Expression. J. Biol. Chem. 1994, 269, 6320–6324. [Google Scholar] [CrossRef]
- Kalderon, D.; Roberts, B.L.; Richardson, W.D.; Smith, A.E. A Short Amino Acid Sequence Able to Specify Nuclear Location. Cell 1984, 39, 499–509. [Google Scholar] [CrossRef]
- Wen, W.; Meinkotht, J.L.; Tsien, R.Y.; Taylor, S.S. Identification of a Signal for Rapid Export of Proteins from the Nucleus. Cell 1995, 82, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Hopp, T.P.; Prickett, K.S.; Price, V.L.; Libby, R.T.; March, C.J.; Pat Cerretti, D.; Urdal, D.L.; Conlon, P.J. A Short Polypeptide Marker Sequence Useful for Recombinant Protein Identification and Purification. Nat. Biotechnol. 1988, 6, 1204–1210. [Google Scholar] [CrossRef]
- Behrens, R.T.; Aligeti, M.; Pocock, G.M.; Higgins, C.A.; Sherer, N.M. Nuclear Export Signal Masking Regulates HIV-1 Rev Trafficking and Viral RNA Nuclear Export. J. Virol. 2017, 91, e02107-16. [Google Scholar] [CrossRef]
- Solovieva, A.D.; Frolova, O.Y.; Solovyev, A.G.; Morozov, S.Y.; Zamyatnin, A.A., Jr. Effect of Mitochondria-Targeted Antioxidant SkQ1 on Programmed Cell Death Induced by Viral Proteins in Tobacco Plants. Biochemistry 2013, 78, 1006–1012. [Google Scholar] [CrossRef]
- Vicente, J.; Cascón, T.; Vicedo, B.; García-Agustín, P.; Hamberg, M.; Castresana, C. Role of 9-Lipoxygenase and α-Dioxygenase Oxylipin Pathways as Modulators of Local and Systemic Defense. Mol. Plant 2012, 5, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Marcos, R.; Izquierdo, Y.; Vellosillo, T.; Kulasekaran, S.; Cascón, T.; Hamberg, M.; Castresana, C. 9-Lipoxygenase-Derived Oxylipins Activate Brassinosteroid Signaling to Promote Cell Wall-Based Defense and Limit Pathogen Infection. Plant Physiol. 2015, 169, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Epel, B.L. Plant Viruses Spread by Diffusion on ER-Associated Movement-Protein-Rafts through Plasmodesmata Gated by Viral Induced Host Beta-1,3-Glucanases. Semin. Cell Bev. Biol. 2009, 20, 1074–1081. [Google Scholar] [CrossRef]
- Huang, C.; Sede, A.R.; Elvira-González, L.; Yan, Y.; Rodriguez, M.; Mutterer, J.; Boutant, E.; Shan, L.; Heinlein, M. DsRNA-Induced Immunity Targets Plasmodesmata and Is Suppressed by Viral Movement Proteins. Plant Cell 2023, 35, 3845–3869. [Google Scholar] [CrossRef]
- Gushchin, V.A.; Lukhovitskaya, N.I.; Andreev, D.E.; Wright, K.M.; Taliansky, M.E.; Solovyev, A.G.; Morozov, S.Y.; MacFarlane, S.A. Dynamic Localization of Two Tobamovirus ORF6 Proteins Involves Distinct Organellar Compartments. J. Gen. Virol. 2013, 94, 230–240. [Google Scholar] [CrossRef]
- Lazareva, E.A.; Lezzhov, A.A.; Golyshev, S.A.; Morozov, S.Y.; Heinlein, M.; Solovyev, A.G. Similarities in Intracellular Transport of Plant Viral Movement Proteins BMB2 and TGB3. J. Gen. Virol. 2017, 98, 2379–2391. [Google Scholar] [CrossRef]
- Solovyev, A.G.; Minina, E.A.; Makarova, S.S.; Erokhina, T.N.; Makarov, V.V.; Kaplan, I.B.; Kopertekh, L.; Schiemann, J.; Richert-Pöggeler, K.R.; Morozov, S.Y. Subcellular Localization and Self-Interaction of Plant-Specific Nt-4/1 Protein. Biochimie 2013, 95, 1360–1370. [Google Scholar] [CrossRef]
- Høie, M.H.; Kiehl, E.N.; Petersen, B.; Nielsen, M.; Winther, O.; Nielsen, H.; Hallgren, J.; Marcatili, P. NetSurfP-3.0: Accurate and Fast Prediction of Protein Structural Features by Protein Language Models and Deep Learning. Nucleic Acids Res. 2022, 50, W510–W515. [Google Scholar] [CrossRef] [PubMed]
- Bernhofer, M.; Dallago, C.; Karl, T.; Satagopam, V.; Heinzinger, M.; Littmann, M.; Olenyi, T.; Qiu, J.; Schütze, K.; Yachdav, G.; et al. PredictProtein—Predicting Protein Structure and Function for 29 Years. Nucleic Acids Res. 2021, 49, W535–W540. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Prediction of Solvent Accessibility and Sites of Deleterious Mutations from Protein Sequence. Nucleic Acids Res. 2005, 33, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.; Savojardo, C.; Martelli, P.L.; Casadio, R. DeepREx-WS: A Web Server for Characterising Protein–Solvent Interaction Starting from Sequence. Comput. Struct. Biotechnol. J. 2021, 19, 5791–5799. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, L.; Liang, S.; Zhang, C. SVMTriP: A Method to Predict Antigenic Epitopes Using Support Vector Machine to Integrate Tri-Peptide Similarity and Propensity. PLoS ONE 2012, 7, e45152. [Google Scholar] [CrossRef]
- Amari, K.; Di Donato, M.; Dolja, V.V.; Heinlein, M. Myosins VIII and XI Play Distinct Roles in Reproduction and Transport of Tobacco Mosaic Virus. PLoS Pathog. 2014, 10, e1004448. [Google Scholar] [CrossRef]
- Huang, C.; Mutterer, J.; Heinlein, M. In Vivo Aniline Blue Staining and Semiautomated Quantification of Callose Deposition at Plasmodesmata. Methods Mol. Biol. 2022, 2457, 151–165. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atabekova, A.K.; Lazareva, E.A.; Lezzhov, A.A.; Golyshev, S.A.; Skulachev, B.I.; Morozov, S.Y.; Solovyev, A.G. Defense Responses Induced by Viral Movement Protein and Its Nuclear Localization Modulate Virus Cell-to-Cell Transport. Plants 2024, 13, 2550. https://doi.org/10.3390/plants13182550
Atabekova AK, Lazareva EA, Lezzhov AA, Golyshev SA, Skulachev BI, Morozov SY, Solovyev AG. Defense Responses Induced by Viral Movement Protein and Its Nuclear Localization Modulate Virus Cell-to-Cell Transport. Plants. 2024; 13(18):2550. https://doi.org/10.3390/plants13182550
Chicago/Turabian StyleAtabekova, Anastasia K., Ekaterina A. Lazareva, Alexander A. Lezzhov, Sergei A. Golyshev, Boris I. Skulachev, Sergey Y. Morozov, and Andrey G. Solovyev. 2024. "Defense Responses Induced by Viral Movement Protein and Its Nuclear Localization Modulate Virus Cell-to-Cell Transport" Plants 13, no. 18: 2550. https://doi.org/10.3390/plants13182550




