Abstract
Passion fruit is a valued tropical fruit crop that faces environment-related growth strains. TCP genes are important for both growth modulation and stress prevention in plants. Herein, we systematically analyzed the TCP gene family in passion fruit, recognizing 30 members. Genes exhibiting closer phylogenetic relationships exhibited similar protein and gene structures. Gene members of the TCP family showed developmental-stage- or tissue-specific expression profiles during the passion fruit life cycle. Transcriptome data also demonstrated that many PeTCPs showed induced expression in response to hormonal treatments and cold, heat, and salt stress. Based on transcriptomics data, eight candidate genes were chosen for preferential gene expression confirmation under cold stress conditions. The qRT-PCR assays suggested PeTCP15/16/17/19/23 upregulation, while PeTCP1/11/25 downregulation after cold stress. Additionally, TCP19/20/29/30 exhibited in silico binding with cold-stress-related miRNA319s. GFP subcellular localization assays exhibited PeTCP19/1 were localized at the nucleus. This study will aid in the establishment of novel germplasm, as well as the further investigation of the roles of PeTCPs and their cold stress resistance characteristics.
1. Introduction
Since passion fruit (Passiflora edulis) has significant edible, medicinal, and ornamental value, it is widely grown in tropical and subtropical regions worldwide. Many consumers relish its egg-shaped fruit due to its unique flavor, rich aroma, acid pulp, and yellow juice [1]. Owing to the abundance of alkaloids, flavonoids, and other physiologically active components found in passion fruit, extracts derived from its leaves, fruits, peels, and seeds have therapeutic properties that include anti-inflammatory, soothing, antioxidant, and anticancer characteristics [2]. Studies suggest that passion fruit is an effective resource for managing rocky desertification and fostering industrial growth [3]. Passion fruit peels (PFPs) are increasingly being recognized as valuable waste materials that can serve as feedstock for pectin extraction [4]. Because most passion fruit varieties have large floral organs, bright coronal filaments, a rich fragrance, and lush branches and leaves, they are used as ornamental plants for flower racks due to their ornamental value [5]. Passion fruit has a huge agricultural significance as it has a short growth period, can grow in a variety of soils, uses less soil surface due to vertical climbing habits, has 5–7-year semi-perennial vines, is easy to propagate from stem cuttings, and is adapted to subtropical and tropical regions [6]. Identifying and characterizing important gene families in passion fruit might help boost the growth of the world’s agricultural economy.
Transcription factors (TFs) are major elements of the genetic foundation for phenotypic evolution. TCP proteins (TCPs) are a class of plant-specific TFs, that was first identified and designated as TEOSINTE BRANCHED 1 (TB1) in Zea mays [7], PROLIFERATING CELL FACTORS 1 and 2 (PCF1 and PCF2) in rice [8], and CYCLOIDEA (CYC) in Antirrhinum majus [9]. TCP domains are defined by an atypical 59-amino acid basic helix–loop–helix motif structural feature and are unrelated to the DNA-binding bHLH domain. Based on TCP domains, this class of protein is partitioned into Class I (TCP-P or PCF type) and Class II (TCP-C type) [10]. Class I is distinguished by a four-amino-acid deletion which is a conserved feature. Class II is divided into the CIN and CYC clades [11]. Two duplication events in the CYC clade among the main eudicots led to three subgroups designated as CYC1, 2, and 3 [12]. The accumulated research evidence suggested that TCP genes were implicated in many growth-related mechanisms including axillary meristem development, flower and leaf morphology, circadian rhythm regulation, hormone signaling, seed germination, and defense [13,14].
Plants constantly face adverse environmental conditions, and researchers have identified a correlation between TCPs (TEOSINTE BRANCHED 1, CYCLOIDEA, and PCF) and plant responses to abiotic stresses [15]. Plant growth and development are significantly constrained by cold stress [16]. Plants cope with cold stress through modification such as promptly increasing the concentration of calcium ions, a saturation of cytosol affecting plant hormones such as gibberellin (GA), abscisic acid (ABA), and brassinolide (BR), arresting the growth, leading to lipid peroxidation and fatty acid unsaturation, modifications in phospholipid configurations, and accumulation of osmoprotectants like soluble sugars, proline, and betaine [16]. Knockdown of two rice TCP genes OsPCF5 and OsPCF8 resulted in enhanced tolerance to cold stress after chilling treatment [17]. Notably, two OsTCPs, OsPCF6 and OsTCP21, demonstrated significant cold-induced expression, with knockdown plants exhibiting enhanced cold tolerance compared to wild-type plants due to improved reactive oxygen species scavenging [18]. PCF6 expression in sugarcane seedlings exposed to cold stress for 24 h was reduced to 50 percent [19]. Similarly, the cassava TCP gene MeTCP3a and MeTCP4 expressions were reduced after their seedlings were treated with 4 °C cold stress [20]. In transgenic creeping bentgrass four members of the TCP gene family, namely AsPCF5/6/8/14, exhibited expressional depression in conjunction with increased salt and drought tolerance linked with enhanced water retention and leaf wax contents [21]. Contrarily, overexpression of the rice OsTCP19 resulted in abnormal development including reduced formation of lateral roots and enhanced abiotic stress tolerance [22]. ZmTCP14 overexpression under drought conditions led to a significant reduction of drought tolerance, while gene-edited lines of ZmTCP14 demonstrated enhanced drought tolerance, suggesting it acts as a negative regulator of drought stress [23]. TCP10 from Moso bamboo exhibited induced expressions under drought stress and its overexpression in rice and Arabidopsis enhanced drought tolerance in transgenic plants [24].
Owing to the development of genome sequencing technologies, TCP gene families have been studied in various modal organisms of agro-botanical importance like cultivated rye [25], switchgrass [26], potato [27], and Arabidopsis [28]. Recently, the genome sequence of passion fruit was publicly released [20,29,30], offering valuable genome resources for the identification of novel genes. Despite the agricultural importance of passion fruit, there is a significant lack of information regarding the comprehensive characterization and functional analysis of the TCP transcription factor gene family in this plant. This study aimed to address this gap by achieving the following objectives: (1) Systematic identification of passion fruit TCP genes. (2) Description of the conserved domains and cis-regulatory elements in their sequences. (3) Exploration of the distribution of TCP genes in the passion fruit genome. (4) Analysis of the evolutionary relationships among these genes to understand their origins and divergence between different subgroups. (5) Expression profiling of TCP genes in various sugarcane tissues, developmental stages, and under different stresses. (6) Expressional validation of a few promising candidate genes under cold stress. (7) Confirmation of TCP proteins’ subcellular localization through GFP assays.
This study provides valuable insights into the sequence characteristics, genomic distribution, evolutionary background, and functionality of TCP genes in passion fruit. This study will serve as raw materials for further molecular characterization and stress breeding of passion fruit employing TCP transcription factors.
2. Material and Methods
2.1. Identification and Sequence Analysis of Passion Fruit TCP Proteins
Firstly, TCP protein sequences were retrieved from the yellow passion fruit genome in the Passion Fruit Genomic Networks database (http://passionfruit.com.cn/cgi-bin/motif_expression.pl (accessed on 4 August 2023)) using the TCP PFAM accession ID (PF03634). Later on, two published genomic assemblies named GWHAZTM00000000 and GWHANWG00000000 of purple passion fruit types were downloaded from Genome Warehouse (https://ngdc.cncb.ac.cn/gwh/Genome/557/show (accessed on 4 August 2023)). Similarly, the latest whole-genome protein sequences of Arabidopsis from the Phytozome database (https://phytozome-next.jgi.doe.gov/ (accessed on 4 August 2023)) were downloaded and uploaded to Bioedit software 7.2 (accessed on 10 August 2023) to generate three local genome files. The yellow passion fruit TCP protein sequences were used as queries to perform a local BLASTP against the proteomes in Bioedit software with rigorous thresholds of E value < 1 × 10−5, query cover > 50%, and protein identity > 30%. The Online Conserved Domains search tool in the NCBI database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi (accessed on 19 August 2023)) was utilized to confirm the protein sequences with the characteristics of bHLH domains. The physicochemical properties of yellow passion fruit TCP proteins were computed using Expasy ProtParam (https://web.expasy.org/protparam/ (accessed on 22 September 2023)). The Euk-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/euk-multi/ (accessed on 25 September 2023)) tool was used to predict the subcellular localization of all putative TCPs.
2.2. Multiple Sequence Alignment and Phylogenetic Tree Construction
Using MEGA-11 software (accessed on 4 September 2023) [31], the full-length protein sequences of Arabidopsis (24) and PeTCPs (30) were aligned to construct a neighbor-joining phylogenetic tree. The criteria were adopted using 1000 bootstrap replications of the Poisson correction model and a pairwise deletion option. Gene structures of the identified PeTCPs were determined by the Gene Structure Display Server 2.0 tool (http://gsds.gao-lab.org/ (accessed on 9 October 2023)). Domain organizations of the likely PeTCPs were initially identified using the NCBI Batch CDD server (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi (accessed on 10 October 2023)) and, later on, depicted through TBtools software (version 2.019) accessed on 11 October 2023) [32]. Using the TBtools program, the arrangement of PebTCPs throughout the nine chromosomes of the P. edulis genome as well as the duplications was mapped, and Ka/Ks values were computed.
2.3. Structural Analysis of TCP Proteins
Homology modeling of PeTCP proteins was performed and a single representative member structure of three subfamilies was modeled based on the SWISS-MODEL database in user-specified template mode. The Arabidopsis TCP proteins representing each subfamily AlphaFold structure were downloaded from UniProt and used as a template. Then these templates were used in SWISS-MODEL (https://swissmodel.expasy.org/ (accessed on 19 January 2024) ) to model the characteristic DNA-binding 59-amino-acid bHLH domains of PeTCPs. Eventually, the modeled domains were visualized and superimposed in Chimera 1.15 software (accessed on 25 January 2024) to observe the structural differences [33].
2.4. Cis-Regulatory Element Analysis of the Promoter Regions of TCP Genes
The yellow passion fruit genome was used in this study, therefore the full genomic sequence of yellow passion fruit was downloaded from the Passionfruit Genomics Network website (http://passionfruit.com.cn/search_downloads.html (accessed on 6 February 2024)). The full genome was viewed in UltraEdit software (https://www.ultraedit.com/ (accessed on 6 February 2024)). The first 20 bp of the first starting codon of each PeTCP coding sequence was searched manually using Ctrl+F keyboard functions. Following matching, the upstream 2000 bp promoter sequences were collected. Using genome browser functions on the Passionfruit Genomics Network website, the upstream gene cds sequences were also examined to partition the upstream gene CDS and under-query gene promoters. The promoter sequences were then fed to the PlantCARE web tool [34]. Subsequently, overlapping sites were removed manually in MS Excel software (v2016) (accessed on 10 February 2024) and the final representations were created through Tbtools software [32].
2.5. In Silico Binding Prediction between TCP Genes and miRNA319
Rice microRNA319 positively regulates cold stress by repressing the expression of its target OsTCPs, and since microRNA sequences are conserved among plant species, we tested whether OsmiRNA319 could target PeTCPs. Rice miR319, miR319a, and miR319b sequences were downloaded from the plant microRNA database (PMRD) (http://bioinformatics.cau.edu.cn/PMRD (accessed on 14 March 2024)) and, later on, were analyzed with PeTCPs CDS sequences as target genes in psRNATarget (https://www.zhaolab.org/psRNATarget/ (accessed on 16 March 2024)).
2.6. Expression Profile Analysis Based on RNA-Seq Data
RNA-seq datasets of all the putative PeTCPs were downloaded from the Passionfruit Genomics Network database (http://passionfruit.com.cn/cgi-bin/gene_expression.pl?name=Pe2g01742 (accessed on 6 April 2024)) for eight tissue samples (leaf, stem, root, petal, stamen, pistil, mature and immature fruit), abiotic stresses (heat, cold, salt, and drought), and hormonal treatments (ABA, Eth, Auxin, GA, and MeJA) with three biological replicates. For cold and heat stress treatments, 4 °C and 42 °C temperatures were used, respectively, while 25 °C was used as a control. The fragments per kilobase of transcript per million fragments (FPKM) gene expression values were used to generate heatmaps using the Tbtools software.
2.7. GO Annotation and KEGG Analysis of PeTCP Genes
The GO annotation and KEGG analysis of identified PeTCPs were performed through the Passionfruit Genomic Networks database and graphical depictions were made through the https://bioinformatics.com.cn/ (accessed on 16 April 2024) web tool.
2.8. Functional Validation of the Candidate PeTCP Genes through qRT-PCR
qRT-PCR assays were used to study the PeTCP gene expression changes under cold stress. Green healthy stem cuttings having two buds of the same length were made from yellow passion fruit vines growing in the Guangxi University vineyard. The leaves attached to the cutting were cut in half. The stem cuttings were gently inserted into pods containing compost–vermiculite mix with one bud inside the soil mix and the other outside. The roots sproute, d from the bud underneath while leaves and shoots emerged from the aerial bud. Seedlings were grown at 30 °C in a light room using 16 h light and 8 h dark periods. Cold stress treatments were applied to the two-month-old healthy passion fruit seedlings with fully developed roots and shoots. For each cold stress treatment, 8–10 seedlings were used. To apply cold stress, healthy plants were put in a growth chamber with temperatures set at 6 °C. At least three leaves (top, middle, and bottom) from each treated plant were collected at 0, 6, 12, and 24 h intervals, respectively. After stress treatments, the leaves were frozen and the RNAs of all samples were extracted employing a Vazyme RNA isolation kit, Nanjing, Jiangsu, China. An Aidlab Truescript 1st Strand cDNA Synthesis Kit (Aidlab Biotechnologies, Ltd., Beijing, China) was used to synthesize cDNA from extracted RNA. Real-time PCR was performed using the PC59-2 × SYBR Green qPCR Mix (Aidlab Biotechnologies, Ltd., Beijing, China) in the DLAB accurate96 system and primers are listed in Table S7. The qRT-PCR conditions were 95 °C for 2 min; 40 cycles of 95 °C for 15 s, 60 °C for 30 s; and 72 °C for 30 s. Three technical replicates from three biological replicates were used for each analysis, and the 2–∆∆Ct method was used to determine the fold change of each gene. For normalization of mRNA levels, the passion fruit PeEF1a gene was used as an internal control [35].
2.9. Subcellular Localization of Representative TCP Proteins
The GFP subcellular localization of PeTCP19 and PeTCP1 proteins was performed according to the methods described in Xiaojin Huang et al., 2024 [36]. The primers used for cloning of coding sequences of PeTCP genes are listed in Table S8.
3. Results
3.1. Identification and Phylogeny of TCP Proteins in Passion Fruit
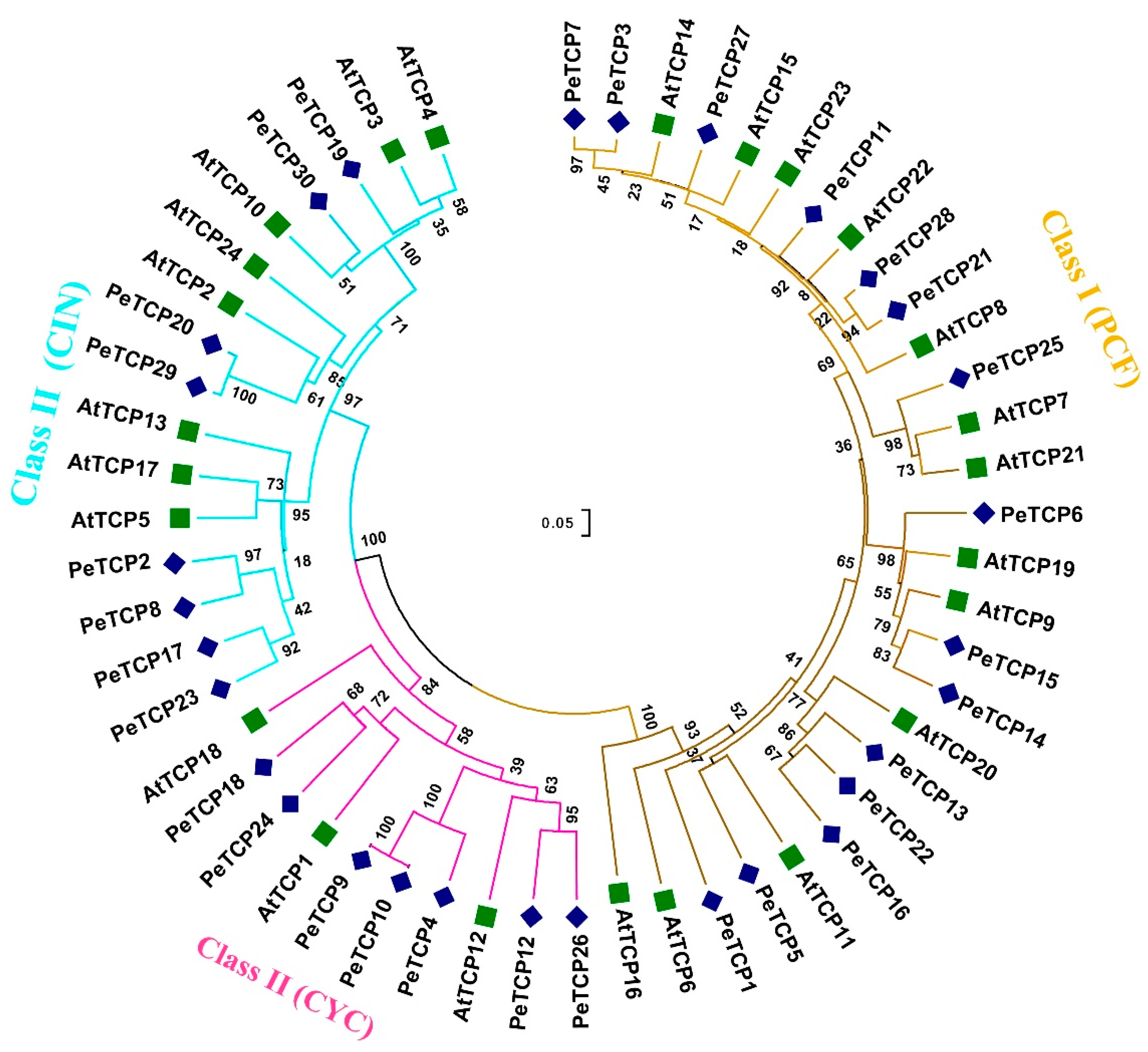
A total of 30 PeTCP genes that encode TCP proteins were identified in the yellow passion fruit genome. The genes were renamed, from PeTCP1 to PeTCP30, based on their ascending order of chromosomal locations (Table 1). Furthermore, 10 and 24 PeTCP genes were identified from GWHAZTM00000000 (purple type) and GWHANWG00000000 genome assemblies, respectively (Table S1). To study how all 30 identified TCP proteins are related, a neighbor-joining phylogenetic tree was constructed along with 24 TCPs of Arabidopsis (Figure 1). Passion fruit TCP members were classified into two classes and three subfamilies. Among passion fruit TCP proteins 15 were Class I members of the PCF subfamily. The rest of the fifteen PeTCPs of Class II were distributed into CIN and CYC subfamilies having eight and seven proteins, respectively.

Table 1.
Information of TCP family in passion fruit.
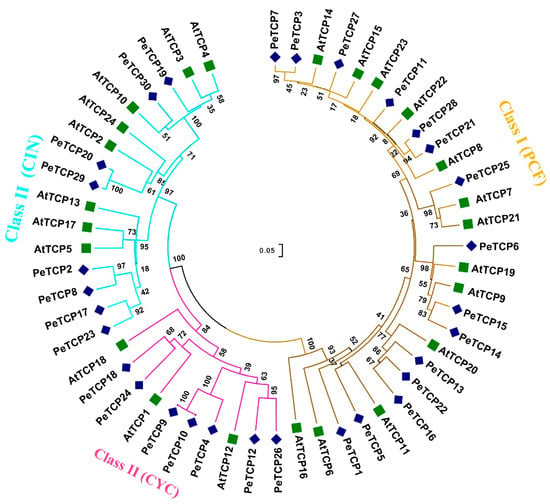
Figure 1.
Phylogenetic analysis of TCP gene family proteins among passion fruit and Arabidopsis. The phylogenetic tree was built using MEGA-11 software employing the neighbor-joining tree method with 1000 bootstrap replicates. Passion fruit TCP proteins are designated by “Pe” while Arabidopsis proteins are depicted with the “At” prefix.
Additionally, we calculated the physiochemical properties of 30 TCP proteins (Table 1). The amino acids in the proteins encoded by the 30 TCP genes ranged from 164 (PeTCP1) to 759 (PeTCP16), while their molecular weights (MWs) varied from 17,985.36 Da (PeTCP1) to 83,909.83 Da (PeTCP16). Protein isoelectric points (pI) were predicted, ranging from 5.61 (PeTCP6) to 8.86 (PeTCP10). Proteins’ thermal stability varied little among TCP proteins, with the aliphatic amino index (A.I.) revealing it ranged from 52.83 (PeTCP28) to 86.96 (PeTCP16). All TCP proteins exhibited a negative grand average of hydropathicity score (GRAVY), suggesting they are primarily hydrophilic. Finally, the subcellular localization prediction analysis revealed that except for membrane-localized PeTCP6/15/16 and cytoplasm-localized PeTCP3/13/23/26, the rest of the TCP proteins were predicted to be localized in the nucleus.
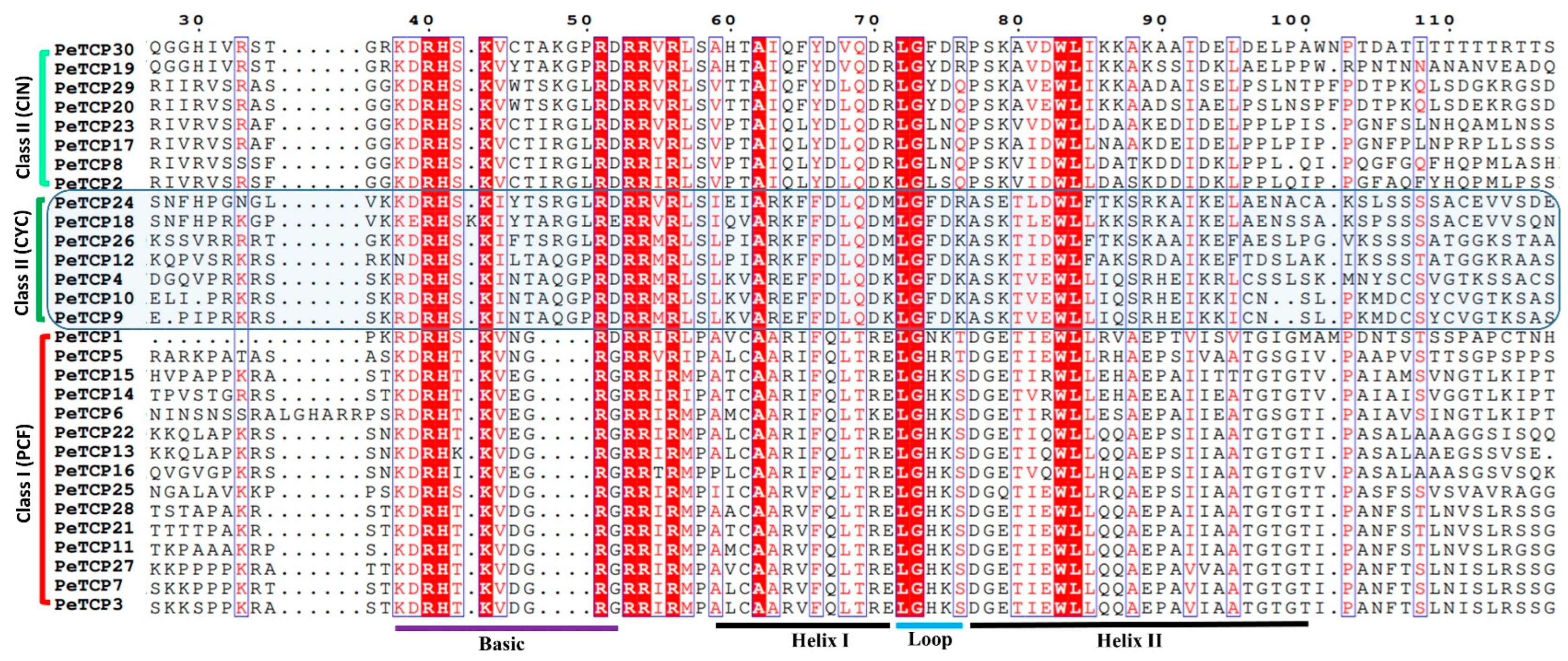
After ascertaining the phylogenetic classification and distribution of TCP proteins in the three abovementioned subfamilies we performed the multiple sequence alignment (MSA) of bHLH domains in MEGA-11 software and depicted it through the ESPript online server (Figure 2). The results indicated that the four characteristic amino acid deletions in the basic region led to the diversification of PeTCPs into two major phylogenetic classes.
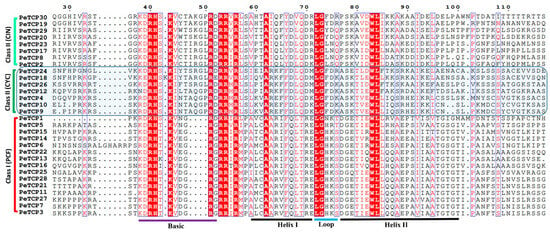
Figure 2.
Multiple sequence alignment of passion fruit TCP proteins. Sequences were aligned in the MEGA software and sequence alignment was visualized in the ESPript 3.0 web tool. The residues highlighted in red vertical lines indicate completely conserved while those inside blue verticle lines are highly conserved amino acids.
3.2. Gene Structural Analysis and Domain Organization of PeTCP Genes
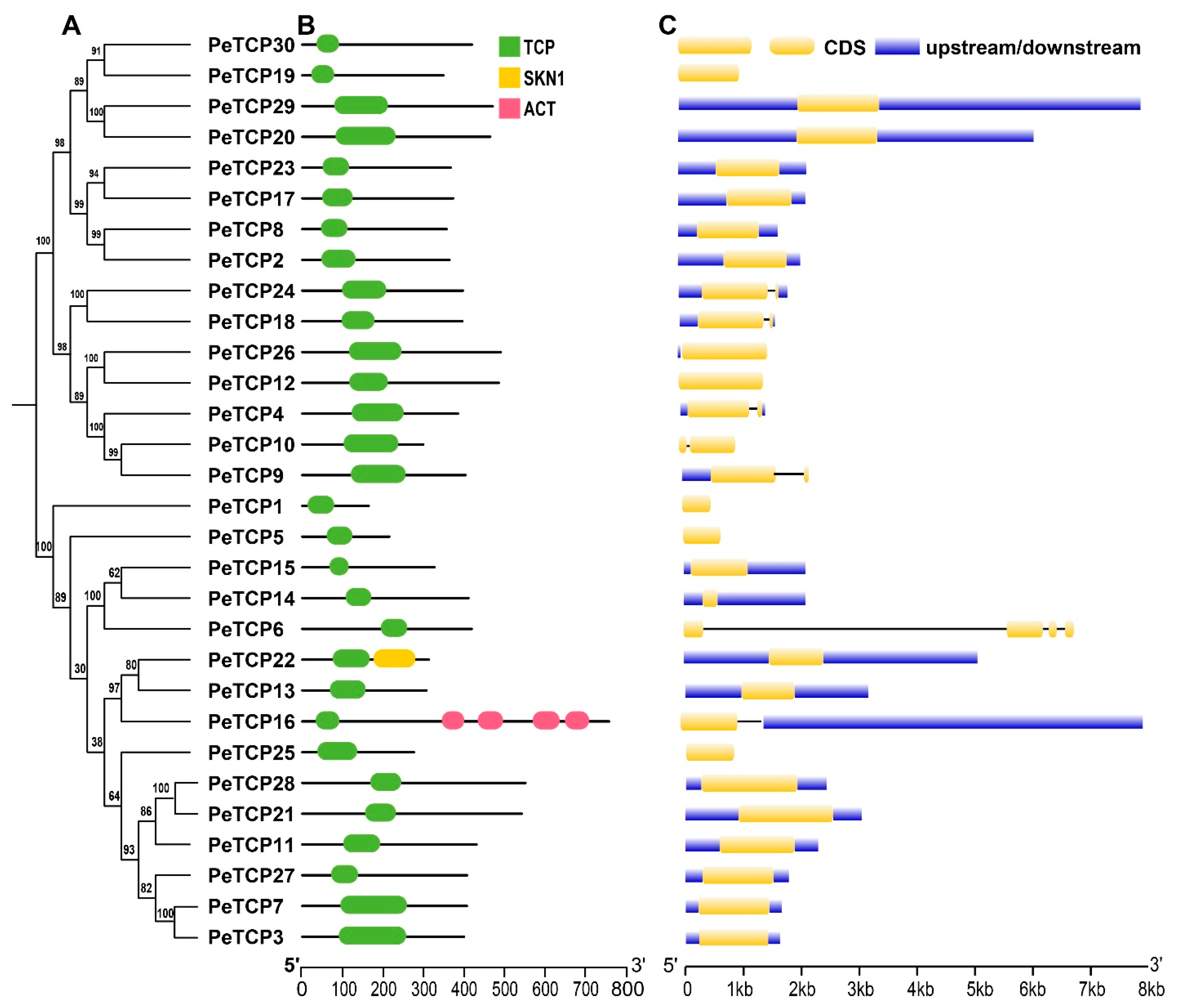
The TCP gene family of passion fruit was examined for its conserved domain composition and gene structure features, and their phylogenetic connections are exhibited (Figure 3A–C). The domain analysis revealed all the TCP proteins possess only TCP domains, however, two members of Class I, PeTCP22, and PeTCP16, contained additional domains called SKN1 and ACT, respectively (Figure 3A). Gene structure organization depicted that PeTCP6 had the highest number of exons (four), whereas five genes of the CYC subfamily PeTCP4/9/10/18/24 possessed two exons each (Figure 3B). The rest of the PeTCPs contained only a single exon. Gene structures and domain composition of PeTCP genes clustered in the same subclasses are comparable, suggesting a strong correlation between gene structures and evolutionary relationships among PeTCPs.
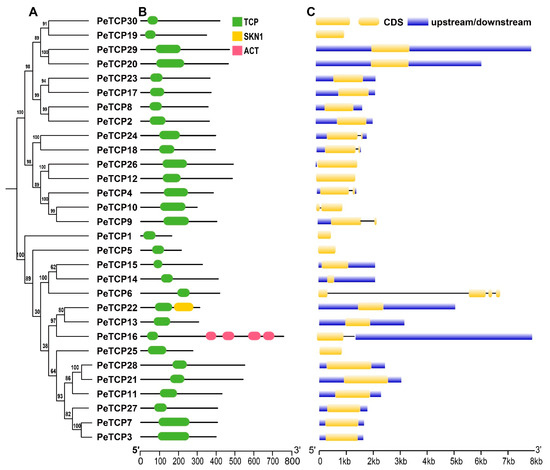
Figure 3.
TCP protein domain composition and gene structure organization. (A) The phylogenetic tree was generated in MEGA-X software. (B) Domain attributes were downloaded from the NCBI batch CD server. TCP, SKN1, and ACT domains are depicted in green, yellow, and pink, respectively. (C) The gene coordinate information was drawn through the TB tool. CDS, introns, and upstream/downstream regions of gene structure are shown in yellow, black, and blue, respectively.
3.3. Homology Modeling and 3D Structural Comparisons of PeTCP Proteins
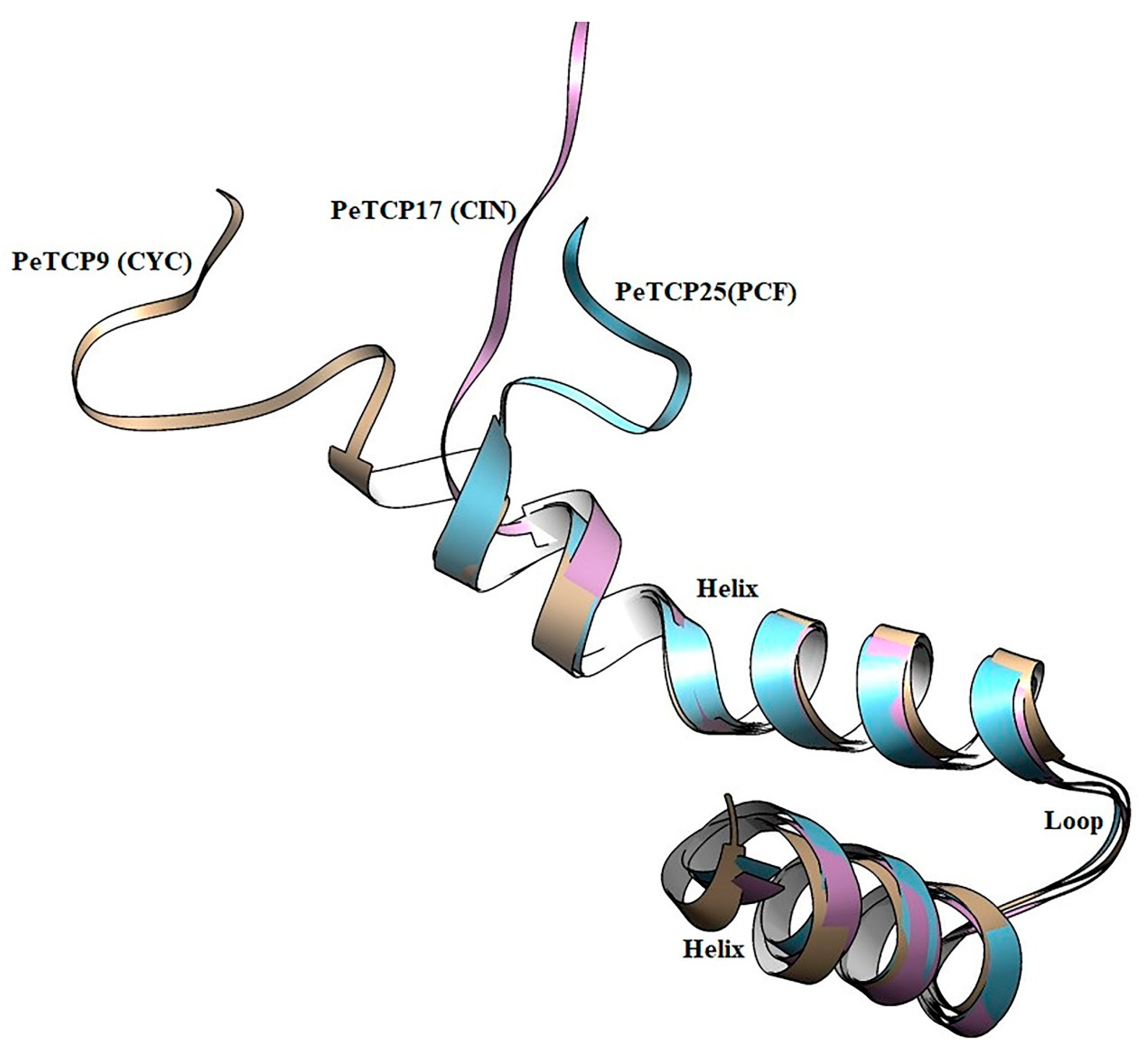
Structural examination of proteins has significant effects on comprehending their functions. We performed the homology modeling of PeTCP9 from CYC, PeTCP16 from CIN, and PeTCP25 from PCF subfamilies (Figure 4). Each subfamily protein was made up of single chains, consisting of a typical DNA-binding 59-amino-acid basic helix–loop–helix motif. The first helix was small with three turns and highly similar in all three representative proteins along with the loop region, suggesting this region is highly conserved. Contrarily, the 2nd helix exhibited structural differences such as PeTCP9 (CYC) having the longest alpha-helix with seven turns while PeTCP25 (PCF) and PeTCP17 (CIN) possessed six and five turns, respectively. These differences in 2nd helix length might be attributed to the functional variation of these proteins either through homodimerization with other TCP proteins or DNA binding. Taken together, these proteins’ homology models offer a foundational framework for delving deeper into the molecular roles of TCP proteins.
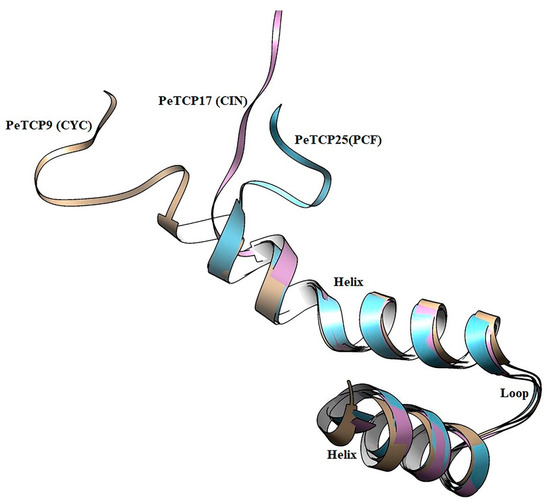
Figure 4.
Structural modeling and superimposition of bHLH domains of three representative proteins from each subfamily of PeTCP. PeTCP9 depicted in brown, PeTCP17 shown in magenta, and PeTCP25 exhibited in blue represent CYC, CIN, and PCF subfamilies, respectively. Protein 3D modeling was performed using the SWISS-MODEL employing orthologous Arabidopsis TCP proteins as templates. Modeled proteins were visualized and structurally aligned using the Chimera software.
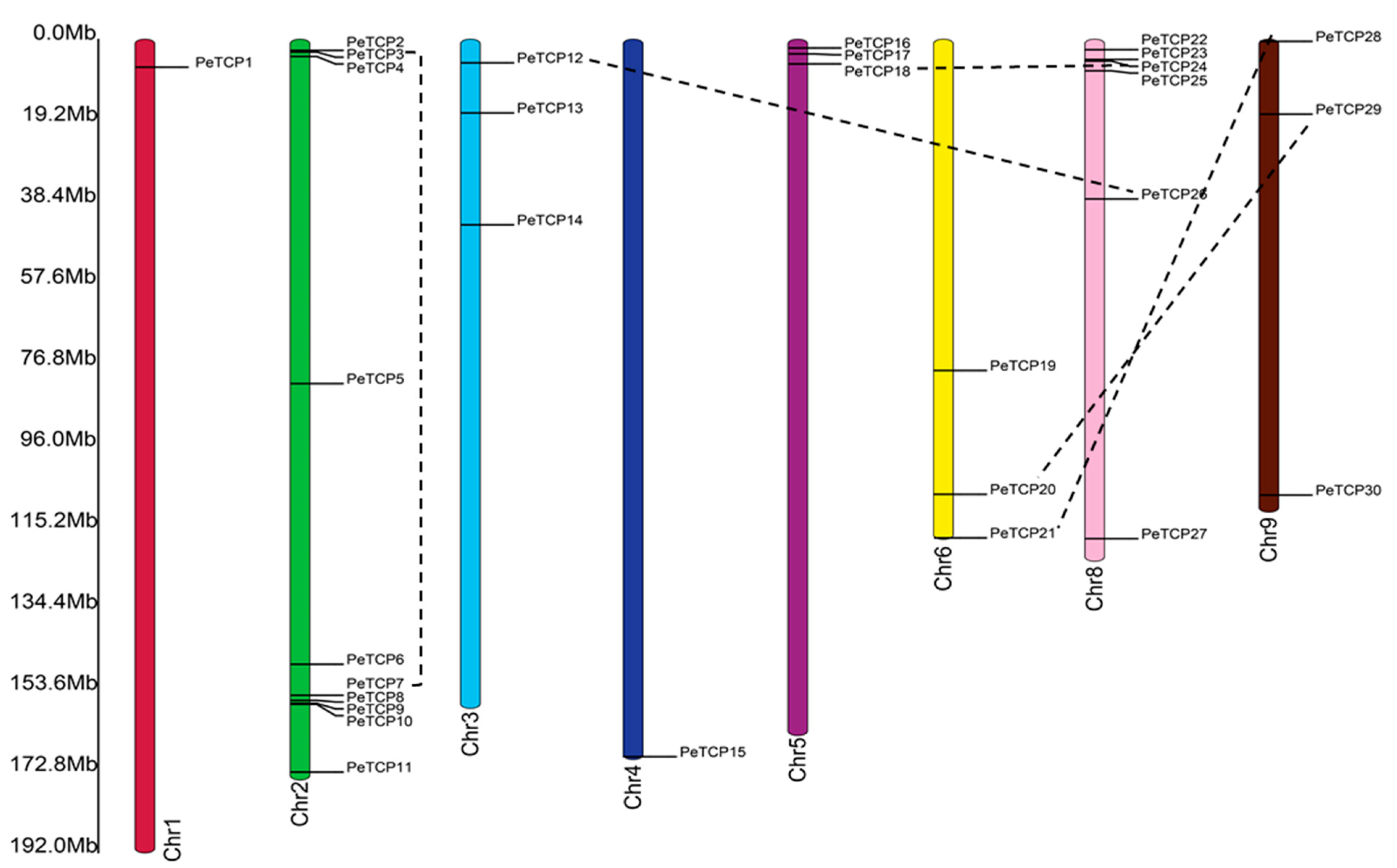
3.4. Chromosomal Distributions and Gene Duplication Analysis of PeTCPs
In principle, different gene duplication patterns are assumed to be the driving force behind gene family formation and the evolution of species. Among all nine passion fruit linkage groups, the PeTCPs were unevenly distributed (Figure 5). Chromosome 2 possessed the highest (10) number of PeTCPs, followed by chromosome 8 which contained 6 genes. Chromosomes 3, 5, 6, and 9 each had three PeTCPs, while chromosomes 1 and 4 each possessed a single gene. Duplicated genes were determined through reciprocal BLAST approaches. The location of duplicated genes on different chromosomes implied that PeTCPs might have arisen majorly through segmental gene duplications. Substitution ratio Ka/Ks has the functionality to describe evolutionary processes and the nature of selection or selection pressure, therefore we estimated the Ka/Ks ratios for all duplicated PeTCP gene pairs (Table S2). Ka/Ks estimates indicated that the values of all duplicated gene pairs were less than 1, indicating that the TCP gene family might have experienced purifying selection during evolution. It is possible that the purifying selection was crucial in preserving the TCP genes’ conserved structure over time.
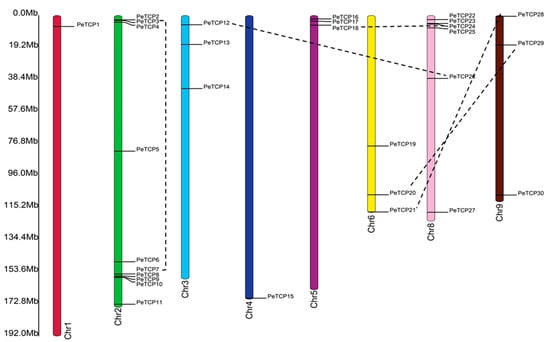
Figure 5.
Distribution of 30 TCP genes in passion fruit genome. The chromosome structures and gene positions were depicted in TBtools employing the genomic information from the Passionfruit Genomics Network database. The scale on the left indicates the chromosome size in Mbps. The colored bars indicate chromosomes, while black dotted lines show the duplicated genes.
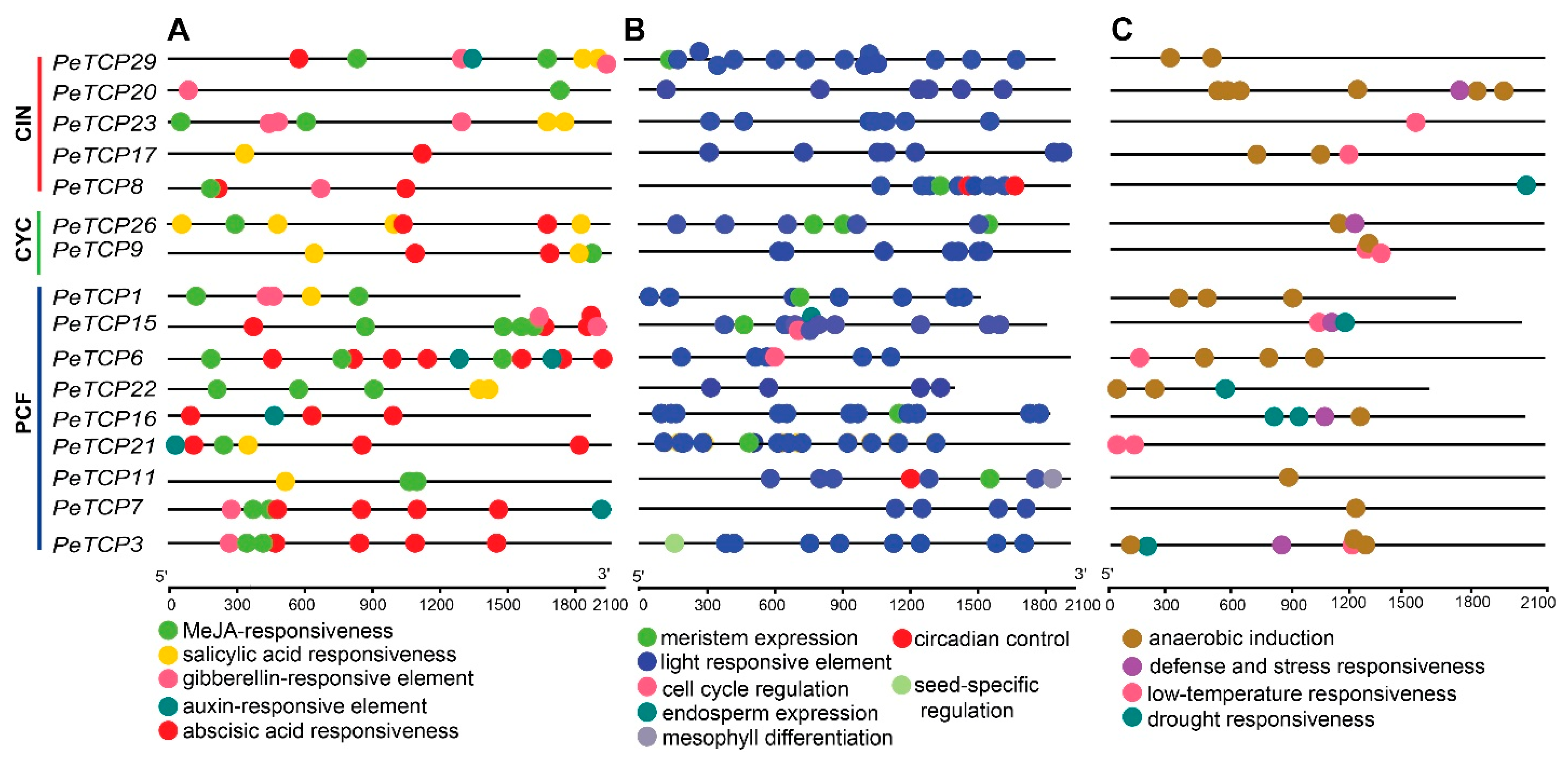
3.5. Prediction of Cis-Regulatory Elements in PeTCP Promoters
Probable roles of PeTCP genes in phytohormone responses, growth and development, and abiotic stresses were examined by analyzing the cis-regulatory elements (CREs) inside their promoter regions (Figure 6). A total of 291 CREs were estimated in 16 PeTCP genes. The predicted 291 CREs could be distributed into 134, 94, and 63 related to growth, hormones, and stress, respectively. Among all the genes, PeTCP15 had the most CREs (25), whereas PeTCP22 possessed the fewest with 13 CREs. Abscisic acid (ABA) responsiveness (ABRE, 33, 35.0%) and MeJA responsiveness (CGGTA-motif and TGACG-motif, 27, 28.0%) were relatively abundant among CREs related to hormonal responsiveness in the promoter regions of PeTCPs (Figure 6A). On the other hand, CREs associated with salicylic acid (SA) responsiveness (16, 17.0%), gibberellin (GA) responsiveness (12, 12.7%), and CREs linked to auxin (IAA) responsiveness (6, 6.8%) were sporadically distributed. CREs involved in regulating growth and development (Figure 6B), such as CAT-box for meristem expression (10, 7.4%), GCN4_motif for endosperm expression (1, 0.7%), RY-element for seed-specific regulation (1, 0.7%), and circadian for circadian control (3, 2.2%), were relatively insufficient compared to the light-responsive elements (116, 86.5%). Regarding CREs associated with stress responsiveness (Figure 6C), the anaerobic induction elements (AREs) (38, 60.3%) were the most prevalent across all genes. Conversely, low-temperature responsiveness (LTR) (9, 14.0%), defense, stress responsiveness (10, 15.0%), and drought responsiveness (6, 9.5%) CREs were unevenly distributed among different genes. These results reveal significant diversity in the composition and quantity of CREs in the promoter regions of PeTCPs, suggesting that different CREs regulate TCP gene expression in passion fruit.
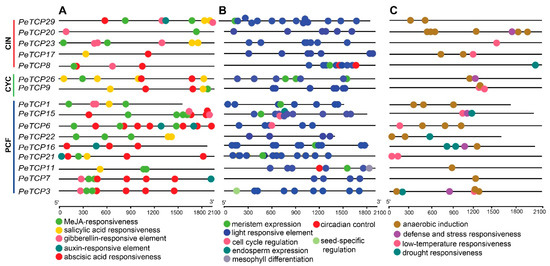
Figure 6.
Cis-regulatory element (CRE) distribution in the predicted promoter regions of PeTCPs. (A) Hormonal responsiveness. (B) Growth and development related. (C) Stress responsiveness.
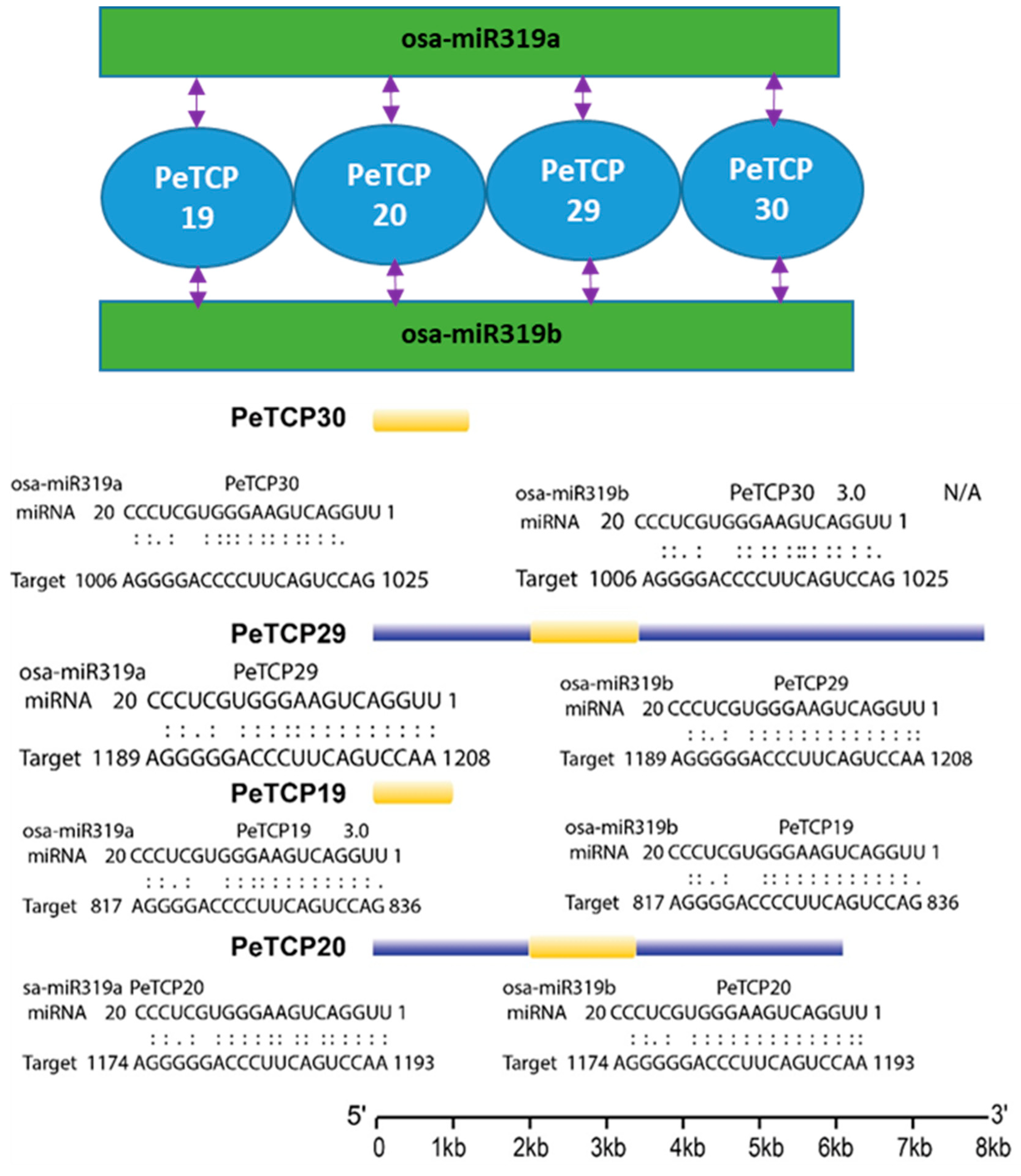
3.6. Prediction of Putative TCP Genes Targeted by miRNA319
We tested the in silico binding of miRNA319, a conserved class of plant cold-stress-related microRNAs with PeTCP genes. Our analysis indicated that miRNA319a and miRNA319b can bind with TCP19/20/29/30 genes (Figure 7). Interestingly all four genes were phylogenetically conserved, belonging to the CIN subfamily of Class II.
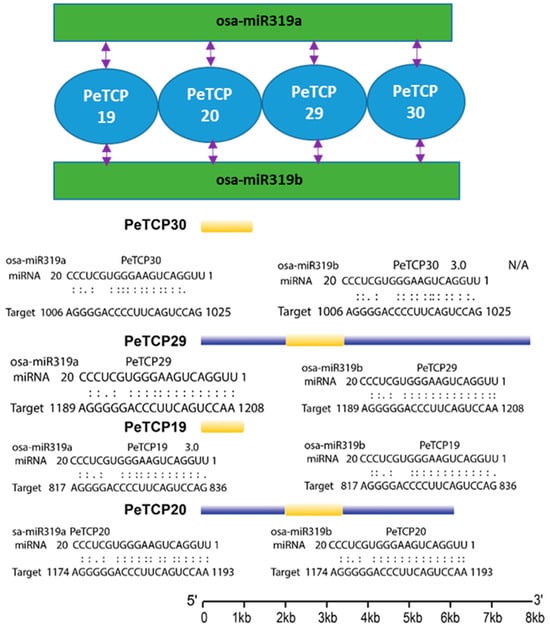
Figure 7.
Predicted miRNA319a/b–TCP-binding modules.
3.7. Expression and GO/KEGG Enrichment Analysis of TCP Genes
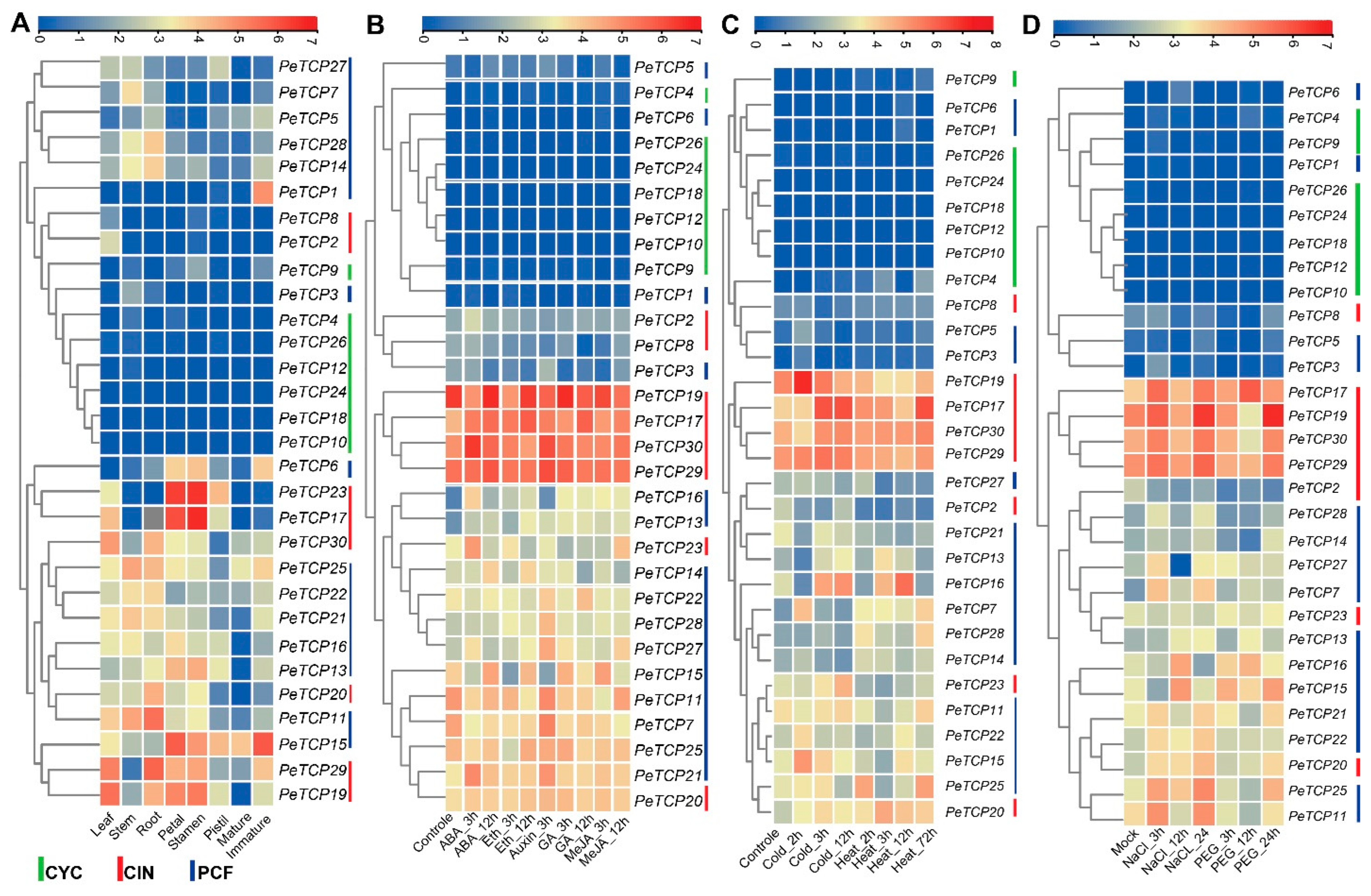
Transcriptomic data were used to characterize the expression profiles of the PeTCP genes at different developmental stages to investigate the potential functions of these genes (Figure 8A, Table S3). The hierarchical clustering of expression patterns allowed the PeTCP genes to be sorted into different groups. Different groups of PeTCP genes had unique patterns of temporal and spatial expressions. The heatmap clustering indicated that PeTCP17/23 in petals and stamens, PeTCP11/29 in root, PeTCP19/29 in leaf, and PeTCP1/15 in immature fruit tissue exhibited high preferential gene expressions.
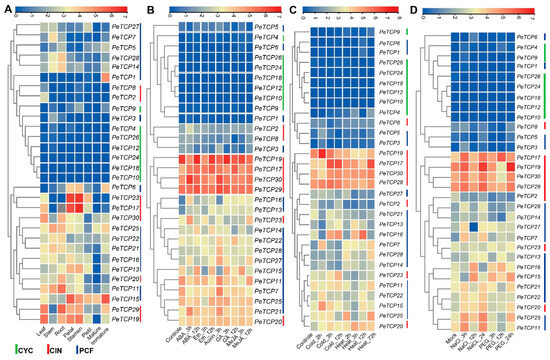
Figure 8.
Transcriptome profiling of TCP gene expression levels. (A) Different growth stages and tissues. (B) Hormonal treatments. (C) Cold and heat stress conditions. (D) Salt and drought stress. The scale above each heatmap indicates the levels of relative gene expression, red, yellow, and blue correspond to high, medium, and low expressions, respectively. The vertical bars at the extreme right of each panel and below 8A indicate a phylogenetic subclass and green, red, and blue represent CYC, CIN, and PCF subclasses, respectively.
To anticipate potential roles for TCP genes in hormonal regulation, we examined the expression profile of TCP genes in response to a range of phytohormones, such as ABA, ethylene, GA, auxin, and MeJA (Figure 8B, Table S4). In reaction to ABA’s hormonal treatments, PeTCP17/23/30/15/21 showed instantaneous induction. On ethylene treatments, PeTCP15/17 exhibited preferential upregulation. Treatments with auxin resulted in elevated PeTCP21/27/28/29/30 gene expression. In reaction to the GA treatment, PeTCP15/17/22 showed induced expressions, while PeTCP15/17/27 exhibited preferential upregulation in response to MeJA treatments.
TCP genes are crucial for protecting cells from stress-induced oxidative damage [37]. We investigated the expression profiles of PeTCPs under heat, cold (Figure 8C, Table S5), salt, and drought stress (Figure 8D, Table S6) conditions using transcriptome data. Many genes, including PeTCP15/16/17/19, showed increased gene expressions and responded immediately to the cold treatments. Similarly, PeTCP16/17/20/25 exhibited quick accumulation of mRNA transcripts in a short period after being rapidly induced under heat stress, indicating their speedy response to heat stress conditions. Furthermore, in response to salt stress, elevated gene expression was observed in CIN-type PeTCP17/19/29/30 and PCF-type PeTCP11/15/16/25. The gene expression of PeTCP15/16/17/19//25 increased in response to the drought stress.
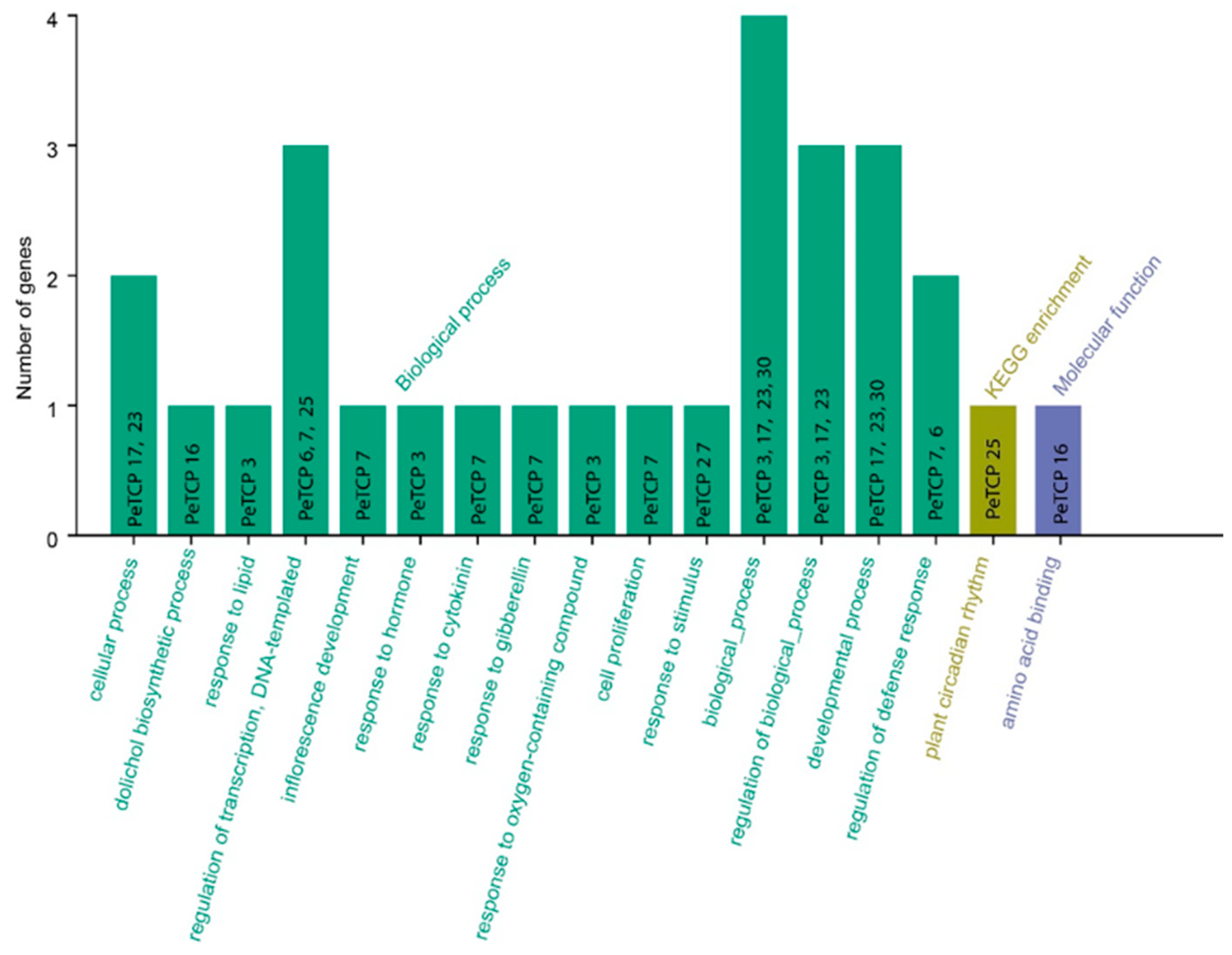
GO and KEGG enrichment analyses of passion fruit were carried out for 30 TCP genes (Figure 9). The PeTCPs were majorly enriched with GO terms such as regulation of transcription (PeTCP7/25), response to lipids (PeTCP3) and hormones (PeTCP7), inflorescence development (PeTCP7), defense responses (PeTCP6/7), response to stimulus (PeTCP27), and amino acid binding (PeTCP16), while only a single gene, PeTCP25, was significantly enriched for KEGG pathways for plant circadian rhythms.
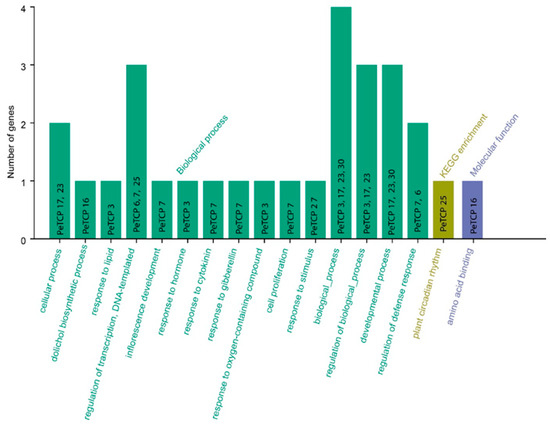
Figure 9.
GO annotations and KEGG analysis of TCP gene family. The GO enrichment terms’ names are on the X axis while the number of genes belonging to each category is along the Y axis.
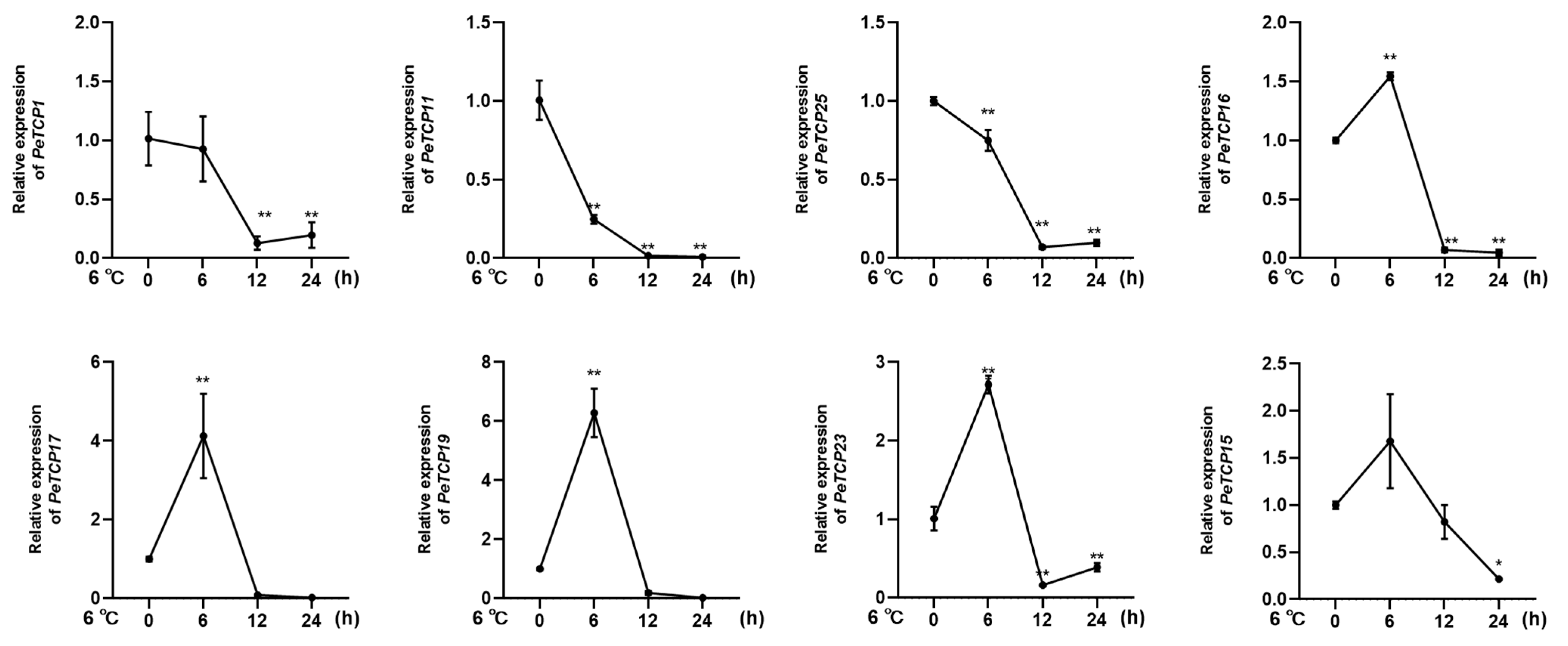
Given the considerable impact of low temperatures on passion fruit cultivation [38], we selected eight notable PeTCPs for qRT-PCR analysis based on their significantly varied expression in response to cold stress at 6 °C imitating the local winter climatic conditions of Guangxi Province, China [16]. In conformation to the transcriptomics data, CIN-type PeTCP19/17/23 and PCF-type PeTCP16/15 expressions were significantly increased by application of cold stress treatment at 6h intervals, declined sharply at 12 h intervals, and steadied at 24 h (Figure 10), suggesting expressional induction of these genes. Contrarily, PCF-type PeTCP25/1 and CYC-type PeTCP11 exhibited decreased expressions with subsequent cold treatments compared to control (Figure 10). Interestingly, among treatment intervals, the 6 h interval was the most influential. These results indicate that passion fruit’s TCP genes are positively or negatively induced by cold stress.
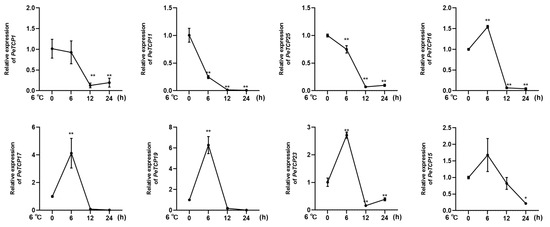
Figure 10.
Expression profiles of 8 TCP genes (PeTCP1, 11, 25, 16, 17, 19, 23, 15) in response to the cold stress treatments. Three independent biological replicates’ standard deviations of means are represented by error bars. Significant variations of the transcript levels between treatments and blank control (0 h) are indicated by asterisks (* p < 0.05, ** p < 0.01).
3.8. Subcellular Localization of PeTCP Proteins
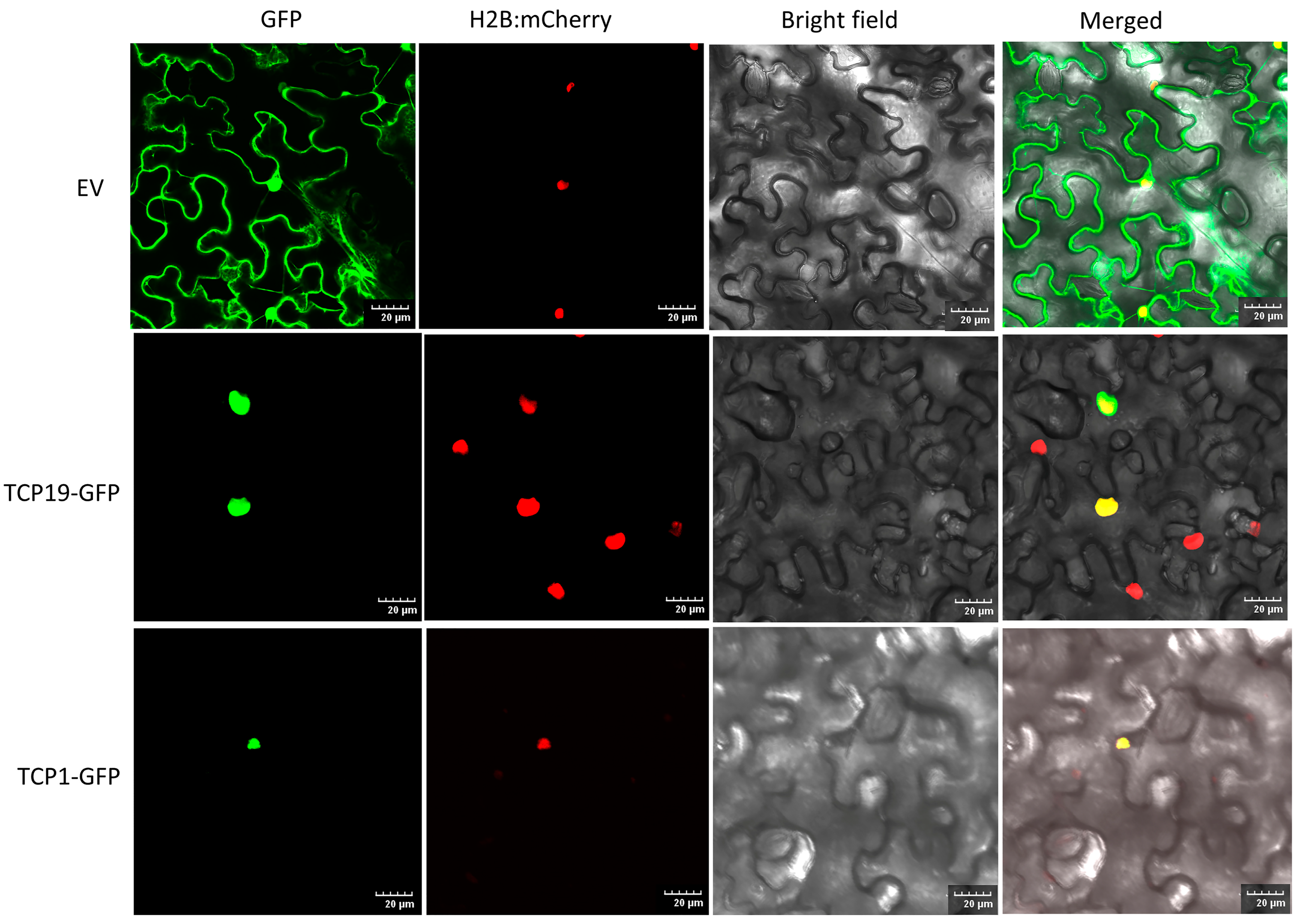
The nuclear localization of transcription factors is widely recognized as crucial for regulating the transcription of target genes by binding to specific cis-regulatory elements in their promoters [39]. Earlier studies in grapevines [39] and strawberries [40] suggested TCP proteins are majorly localized to the nucleus. In this research, most PeTCP proteins were forecasted to be localized in the nucleus using Euk-mPLoc (Table 1). To study PeTCP locations within cells, two cloned PeTCP genes (PeTCP1-GFP and PeTCP19-GFP) were inserted into the pCAMBIA1300-GFP vector harboring the CaMV 35S promoter. The fusion constructs were then introduced into the epidermal cells of Nicotiana benthamiana, and the green fluorescence emitted by the PeTCP1-GFP and PeTCP19-GFP fusion proteins was specifically found within the nuclei, as validated by a mCherry-labeled nuclear marker (H2B-mCherry) (Figure 11). Our findings suggest that PeTCP1 and PeTCP19 proteins localize within the cell nucleus, aligning with predicted outcomes.
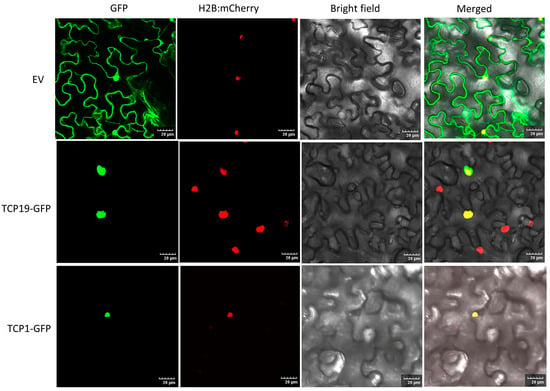
Figure 11.
Subcellular localization of two representative TCP proteins. The top panels represent an empty vector (EV). The PeTCP19-GFP and PeTCP1-GFP fusion proteins were introduced into tobacco leaves for transient expression. After 60 h, confocal microscopy was used to observe the localization of these proteins. Nuclei were visualized using a co-transformed mCherry-labeled nuclear marker (H2B-mCherry). Scale bar, 20 μm.
4. Discussion
Tropical fruit crops like passion fruit have significant agricultural, commercial, and ornamental value, however, environmental conditions have a big impact on the fruit’s growth and development. TCP transcription factors play pivotal roles in growth and development and coping with biotic and abiotic stresses.
In the current study, a total of 30 members of the TCP transcription factor gene family in the passion fruit genome were identified. Using homologous genes from Arabidopsis, phylogenetic analysis exhibited TCP gene family divergence into two classes and three subfamilies (Figure 1 and Figure 2). The results of the phylogenetic analysis and classification were independently supported by domain architecture, and gene exon/intron structure analysis indicated that closely related gene members typically exhibit similar structural characteristics (Figure 3), as observed in other plants like rice [41] and rye [25]. We predicted that most of the TCP proteins were located in the nucleus except a few in the cytoplasm (Table 1). There is a chance that some TCP proteins might be transiently located in the cytoplasm, as suggested by subcellular localization assays of strawberry FvTCP7 and FvTCP17. These proteins exhibited a punctated pattern of GFP signals in Arabidopsis mesophyll protoplasts [40]. Furthermore, the results of homologous protein modeling of the representative proteins of each gene subfamily exhibited divergent 3D structures and distinctive features associated with their varied functions (Figure 4). The structural diversity of these proteins may contribute to the functional diversity of TCP gene subfamilies.
Passion fruit had a slightly higher number of TCP proteins than Arabidopsis with 24 protein members. Among the 30 total TCP genes in passion fruit, five pairs of segmental duplication were identified, largely responsible for the expansion of the TCP family. The Ka/Ks ratios of all the duplicated gene pairs were less than 1 (Table S2), suggesting that these pairs have undergone negative or purifying selection over their evolutionary history. Except for the lowly expressed duplicated pairs PeTCP18/24 and 12/26, the other members of duplicated gene pairs exhibited opposite or varied gene expressions, suggesting these genes following duplication might have experienced subfunctionalization or neofunctionalization. In contrast to model laboratory species such as Arabidopsis, passion fruit had to adapt to a greater variety of abiotic stressors to flourish during its growth and development. The expansion of PeTCPs and their diverse roles may enhance plants’ resilience to various adverse conditions, thereby improving passion fruit’s adaptability to changing environments.
In the meantime, four genes (PeTCP19/20/29/30) potentially targeting miRNA319 in passion fruit were identified through bioinformatics analysis (Figure 7). Interestingly, all four genes belonged to the CIN subfamily and showed similar expression profiles (Figure 8C). miR319 is one of the primitive and the most evolutionarily conserved miRNAs in plants [42]. Growing data indicate that miR319-regulated TCP (MRTCP) genes play a major role in the development of plants and stress responses [15]. Interestingly, miR319 appears to have conserved roles in modulating stress responses in passion fruit, as evidenced by differential expression of all three targeted genes (PeTCP19/29/30) under stress conditions (Figure 8C and Figure 9). These findings predict that miR319 may influence the transcriptional levels of TCP genes in passion fruit, affecting growth, development, and stress responses. However, further experimental validation of the predicted miRNA319-PeTCP module is required relating to the cold stress tolerance.
Although TCP genes’ involvement in growth/development and stress management has been reported in other plant species, studies of the TCP gene family in passion fruit are missing. The functional variety of the TCP genes was identified by analysis of the gene expression of the passion fruit. Some genes showed tissue-specific expression. For instance, PeTCP17/23 exhibited obvious high expressions in petals and stamen tissues, whereas PeTCP29 showed preferential upregulation in leaf and root tissues (Figure 8A). Additionally, PeTCP15 exhibited obvious high expressions in petals and immature fruit tissues. Transgenic expression of CmTPC14 in Arabidopsis, which is a transcription factor of the chrysanthemum TCP-P gene family, resulted in postponing senescence and reduction of organ size [43]. TCP TF RETARDED PALEA1 (REP1) of Oryza sativa has interactions with protein AT-hook DEPRESSED PALEA1 (DP1), similar to maize, suggesting an evolutionarily preserved pathway of inflorescence and flower development in the gramineae family [44].
Members of the TCP gene family are known to be crucial components of hormonal signaling networks in various plant species [45]. In response to ABA treatments, PeTCP15, PeTCP17, PeTCP21, and PeTCP30 exhibited immediate induction. PeTCP15/17 were upregulated upon ethylene treatments as well. Auxin treatments led to increased gene expression of PeTCP21/27/28/29/30 (Figure 8B). Furthermore, the snapdragon CIN gene is linked to the regulation of genes involved in cytokinin and auxin signaling pathways along with lateral organ formation [46]. PeTCP15/22/17 exhibited expressional induction responding to the GA treatment, meanwhile, PeTCP15/17/27 showed preferential upregulation in response to methyl jasmonate (MeJA) treatments (Figure 8B). The altered gene expression levels measured during MeJA treatment in Senna tora suggested that StTCP11 and StTCP4.1 may be implicated in jasmonic acid (JA) response signaling [47].
Passion fruit’s growth and development are extremely susceptible to fluctuations in the climate. The expression of some gene members, such as PeTCP15/16/17/19, increased rapidly with the cold treatments (Figure 8C). It is well documented that the miR319-TCP module regulates cold stress in rice [17,18,41], sugarcane [19], and cassava [20]. Similarly, PeTCP16/17/20/25 were induced quickly under heat stress and showed high transcript accumulation in a short period (Figure 8C). Additionally, CIN-type genes PeTCP17/19/29/30 and PCF-type PeTCP11/15/16/25 exhibited enhanced mRNA transcripts in response to salt stress (Figure 8D). A comparative transcriptome investigation between salt-sensitive and salt-tolerant genotypes of common beans demonstrated salt-responsive expression patterns for Pvul-TCP1/11/13/22/27, which are targets of miR319 [48]. Furthermore, a recent study of the pecan (Carya illinoensis) TCP gene family reported that transgenic Arabidopsis CIN-type CiTCP8 enhanced salt stress tolerance [49]. In response to the drought stress, PeTCP17/19/15/16/25 showed increased gene expressions (Figure 8D). Drought treatments induced ZmTCP42 and ZmTCP32 expressions, and overexpression of ZmTCP42 in Arabidopsis resulted in enhanced drought tolerance [50], however, in another study overexpression of ZmTCP14 showed the opposite results [23], confirming TCP roles in drought resistance. In Allium senescens during drought stress, eight members of the TCP gene family exhibited induced gene expressions, including the AsTCP17 whose heterologous overexpression in Arabidopsis helped cope with drought stress [51]. Interestingly, all the PeTCPs belonging to the CYC subfamily exhibited low expression across all the samples, while four CIN subfamily genes, PeTCP17/19/29/30, exhibited strong differential expressions in reaction to the stress conditions (Figure 8). Furthermore, qRT-PCR expression validations of eight selective PeTCPs (Figure 10) suggested highly consistent results with RNA-seq data (Figure 8B). Finally, two representative proteins from each phylogenetic class (PeTCP1 from Class I and PeTCP19 from Class II) were confirmed to be localized to the nucleus (Figure 11), in conformation to the universal localization of transcription factors to the nucleus [38] and previous studies [39,40,49].
Some of these promising genes such as PeTCP15/17/19/29/30 may have significant application potential for the genetic improvement of passion fruit with improved tolerance to abiotic stress because they respond to various stimuli. In their promoter regions, they had a variety of cis-regulatory elements (CREs) linked to hormonal responses (ABA responsiveness, MeJA responsiveness), growth (light-responsive element, meristem expression), and stress responses (anaerobic induction, low-temperature responsiveness) (Figure 6), which play a significant role in the transduction of biological information [52]. In rice, TCP19 modulates drought-induced ABA signalling through its interaction with OsABI4, which encodes a TF implicated in the transmission of the ABA signaling [22]. AtTCP14 inhibits ABA signaling by interacting with the DNA binding with one finger (DOF6) TF, preventing the induction of additional ABA-responsive genes in Arabidopsis as well as the downstream ABA biosynthesis gene ABA deficient 1 (ABA1) [53]. In addition, the co-occurrence of several CREs in the promoter regions of PeTCPs may be intimately associated with the functions of these genes in the growth and development of passion fruit in response to various environmental modifications [54].
Recent developments in genetic modification, somatic hybridization, micropropagation, somatic embryogenesis, and cryopreservation have enabled transgenic improvements in the Passiflora genus [55,56]. Additionally, candidate passion fruit genes can be overexpressed in other organisms like Arabidopsis, tobacco, and yeast to study their role in plant development and stress tolerance/avoidance [38]. Therefore, further studies are required to help establish links between candidate PeTCP genes identified in this research and their phenotypic or stress responses.
5. Conclusions
This study is the first deciphering the phylogeny, gene structure, cis-regulatory elements, and expression of the TCP family, one of the important regulatory factors, in passion fruits. A total of 30 PeTCPs were identified distributed on nine chromosomes and can be classified into three subgroups. The motif composition and the 3D models of the TCP proteins further exhibited similarity at the basic helix I and loop regions and the variations at helix II. The differential expression patterns of PeTCPs across the tissues and organs were revealed, some of which were tissue-specific. In addition, transcriptome expression analysis of PeTCPs under cold, heat, salt, and PEG treatments highlighted their predominant roles under stress conditions. Finally, the subcellular localization of two PeTCP proteins was confirmed in the nucleus. Our results provide insights into functional analysis of TCP genes and a few of them (PeTCP17/19/15/16/23 and PeTCP1/11/25) would be promising targets for the genetic improvement of cold stress tolerance of passion fruits.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13182568/s1, Table S1. Number of TCP genes identified in this study and their sequence similarity with two gene-level genomic assemblies of passion fruit; Table S2. Ka/Ks values and estimated time of evolution among duplicated genes of TCP gene family in passion fruit; Table S3. Transcriptome data of PeTCPs in different tissues of passion fruit; Table S4. Transcriptome data of PeTCPs under hormonal treatments; Table S5. Transcriptome data of PeTCPs under cold and heat treatments; Table S6. Transcriptome data of PeTCPs under salt and drought stresses; Table S7. Primer names and sequences of PeTCPs used for qRT-PCR; Table S8. Primer sequences for cloning of TCP-GFP fusion constructs.
Author Contributions
M.A.S., C.M., L.H., and L.W. conceived the project and designed the experiments; X.H., Y.W., S.W., and M.A.S. conducted the gene family analysis; M.A.S. drafted the manuscript; M.A.S. and S.W. collected the leaf samples and conducted cold stress treatments; M.A.S., S.W., and X.L. performed the qRT-PCR experiments; M.A.S., Y.Z., S.W., and L.H. performed subcellular localization experiments; the manuscript was reviewed and edited by L.H., M.J.R., and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Open Research Project (FMR2023004Z) from Henry Fok School of Biology and Agriculture, Shaoguan University; the Open Fund (FMR2023004Z) of Guangdong Provincial Key Laboratory of Utilization and Conservation of Food and Medicinal Resources in Northern Region, and Natural Science Foundation of Guangxi (2020GXNSFDA238027).
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Teixeira, N.; Melo, J.C.S.; Batista, L.F.; Paula-Souza, J.; Fronza, P.; Brandão, M.G.L. Edible fruits from Brazilian biodiversity: A review on their sensorial characteristics versus bioactivity as tool to select research. Food Res. Int. 2019, 119, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, M.M.; Lima, G.C.; Batista, Â.G.; Carazin, C.B.B.; Cintra, D.E.; Prado, M.A.; Júnior, M.R.M. Passion fruit peel intake decreases inflammatory response and reverts lipid peroxidation and adiposity in diet-induced obese rats. Nutr. Res. 2020, 76, 106–117. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, F.; Cai, G.; Xi, P.; Guo, Y.; Li, A. Physiological Response Mechanism and Drought Resistance Evaluation of Passiflora edulis Sims under Drought Stress. Phyton Int. J. Exp. Bot. 2024, 93, 1345–1363. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Zapata Zapata, A.D. Enzymatic extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) at laboratory and bench scale. LWT 2017, 80, 280–285. [Google Scholar] [CrossRef]
- da Silva Nóbrega, D.; Peixoto, J.R.; Vilela, M.S.; Faleiro, F.G.; de Paula Silva Gomes, K.; de Deus Sousa, R.M.; Nogueira, I. Agronomic descriptors and ornamental potential of passion fruit species. Ornam. Hortic. 2017, 23, 357–362. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Xing, W.; Wu, B.; Huang, D.; Ma, F.; Zhan, R.; Sun, P.; Xu, Y.; Song, S. Identification of the passion fruit (Passiflora edulis Sims) MYB family in fruit development and abiotic stress, and functional analysis of PeMYB87 in abiotic stresses. Front. Plant Sci. 2023, 14, 1124351. [Google Scholar] [CrossRef]
- Doebley, J.; Stec, A.; Hubbard, L. The evolution of apical dominance in maize. Nature 1997, 386, 485–488. [Google Scholar] [CrossRef]
- Kosugi, S.; Ohashi, Y. DNA binding and dimerization specificity and potential targets for the TCP protein family. Plant J. 2002, 30, 337–348. [Google Scholar] [CrossRef]
- Luo, D.; Carpenter, R.; Vincent, C.; Copsey, L.; Coen, E. Origin of floral asymmetry in Antirrhinum. Nature 1996, 383, 794–799. [Google Scholar] [CrossRef]
- Navaud, O.; Dabos, P.; Carnus, E.; Tremousaygue, D.; Hervé, C. TCP transcription factors predate the emergence of land plants. J. Mol. Evol. 2007, 65, 23–33. [Google Scholar] [CrossRef]
- Liu, M.-M.; Wang, M.-M.; Yang, J.; Wen, J.; Guo, P.-C.; Wu, Y.-W.; Ke, Y.-Z.; Li, P.-F.; Li, J.-N.; Du, H. Evolutionary and Comparative Expression Analyses of TCP Transcription Factor Gene Family in Land Plants. Int. J. Mol. Sci. 2019, 20, 3591. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Leebens-Mack, J.H.; Burke, J.M. Positive selection and expression divergence following gene duplication in the sunflower CYCLOIDEA gene family. Mol. Biol. Evol. 2008, 25, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Lucero, L.E.; Manavella, P.A.; Gras, D.E.; Ariel, F.D.; Gonzalez, D.H. Class I and Class II TCP Transcription Factors Modulate SOC1-Dependent Flowering at Multiple Levels. Mol. Plant 2017, 10, 1571–1574. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Yuan, L.; Di, Z.; Jia, Y.; Zhang, Z.; Li, S.; Xing, L.; Qi, Z.; Wang, X.; Zhu, J.; et al. A CYC/TB1-type TCP transcription factor controls spikelet meristem identity in barley. J. Exp. Bot. 2020, 71, 7118–7131. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zheng, Y.; Lu, W.; Li, J.; Duan, Y.; Zhang, S.; Wang, Y. Roles of miR319-regulated TCPs in plant development and response to abiotic stress. Crop J. 2021, 9, 17–28. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, W.; Tian, Q.; Liu, J.; Xia, X.; Yang, X.; Mou, H. Comparative transcriptomic analysis reveals the cold acclimation during chilling stress in sensitive and resistant passion fruit (Passiflora edulis) cultivars. PeerJ 2021, 9, e10977. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, D.; Mao, D.; Liu, X.; Ji, C.; Li, X.; Zhao, X.; Cheng, Z.; Chen, C.; Zhu, L. Overexpression of microRNA319 impacts leaf morphogenesis and leads to enhanced cold tolerance in rice (Oryza sativa L.). Plant. Cell Environ. 2013, 36, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, X.; Hoshino, Y.; Yu, Y.; Jia, B.; Sun, Z.; Sun, M.; Duan, X.; Zhu, Y. MicroRNA319 positively regulates cold tolerance by targeting OsPCF6 and OsTCP21 in rice (Oryza sativa L.). PLoS ONE 2014, 9, e91357. [Google Scholar] [CrossRef]
- Thiebaut, F.; Rojas, C.A.; Almeida, K.L.; Grativol, C.; Domiciano, G.C.; Lamb, C.R.C.; Engler, J.D.A.; Hemerly, A.S.; Ferreira, P.C.G. Regulation of miR319 during cold stress in sugarcane. Plant Cell Environ. 2012, 35, 502–512. [Google Scholar] [CrossRef]
- Lei, N.; Yu, X.; Li, S.; Zeng, C.; Zou, L.; Liao, W.; Peng, M. Phylogeny and expression pattern analysis of TCP transcription factors in cassava seedlings exposed to cold and/or drought stress. Sci. Rep. 2017, 7, 10016. [Google Scholar] [CrossRef]
- Zhou, M.; Li, D.; Li, Z.; Hu, Q.; Yang, C.; Zhu, L.; Luo, H. Constitutive expression of a miR319 gene alters plant development and enhances salt and drought tolerance in transgenic creeping bentgrass. Plant Physiol. 2013, 161, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Tyagi, A.K. OsTCP19 influences developmental and abiotic stress signaling by modulating ABI4-mediated pathways. Sci. Rep. 2015, 5, 9998. [Google Scholar] [CrossRef]
- Jiao, P.; Liu, T.; Zhao, C.; Fei, J.; Guan, S.; Ma, Y. ZmTCP14, a TCP transcription factor, modulates drought stress response in Zea mays L. Environ. Exp. Bot. 2023, 208, 105232. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Wu, M.; Shi, Y.; Wang, H.; Wu, L.; Xiang, Y. TCP10, a TCP transcription factor in moso bamboo (Phyllostachys edulis), confers drought tolerance to transgenic plants. Environ. Exp. Bot. 2020, 172, 104002. [Google Scholar] [CrossRef]
- Zhan, W.; Cui, L.; Guo, G.; Zhang, Y. Genome-wide identification and functional analysis of the TCP gene family in rye (Secale cereale L.). Gene 2023, 854, 147104. [Google Scholar] [CrossRef]
- Zheng, A.; Sun, F.; Cheng, T.; Wang, Y.; Xie, K.; Zhang, C.; Xi, Y. Genome-wide identification of members of the TCP gene family in switchgrass (Panicum virgatum L.) and analysis of their expression. Gene 2019, 702, 89–98. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Li, T.; Yang, J.; Zhu, X.; Fang, C.; Li, S.; Si, H. Genome-wide identification and expression analysis of StTCP transcription factors of potato (Solanum tuberosum L.). Comput. Biol. Chem. 2019, 78, 53–63. [Google Scholar] [CrossRef]
- Xu, R.; Gao, H.; Zhang, S.; Liu, P.; Wang, X.; Hao, Y. Genome-wide identification and phylogenetic, comparative genomic, alternative splicing, and expression analyses of TCP genes in plants. Plant Gene 2017, 12, 23–32. [Google Scholar] [CrossRef]
- Ma, D.; Dong, S.; Zhang, S.; Wei, X.; Xie, Q.; Ding, Q.; Xia, R.; Zhang, X. Chromosome-level reference genome assembly provides insights into aroma biosynthesis in passion fruit (Passiflora edulis). Mol. Ecol. Resour. 2021, 21, 955–968. [Google Scholar] [CrossRef]
- Xia, Z.; Huang, D.; Zhang, S.; Wang, W.; Ma, F.; Wu, B.; Xu, Y.; Xu, B.; Chen, D.; Zou, M.; et al. Chromosome-scale genome assembly provides insights into the evolution and flavor synthesis of passion fruit (Passiflora edulis Sims). Hortic. Res. 2021, 8, 14. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tian, Q.; Huang, W.; Liu, J.; Xia, X.; Yang, X.; Mou, H. Identification and evaluation of reference genes for quantitative real-time PCR analysis in Passiflora edulis under stem rot condition. Mol. Biol. Rep. 2020, 47, 2951–2962. [Google Scholar] [CrossRef]
- Huang, X.; Shad, M.A.; Shu, Y.; Nong, S.; Li, X.; Wu, S.; Yang, J.; Rao, M.J.; Aslam, M.Z.; Huang, X.; et al. Genome-Wide Analysis of the Auxin/Indoleacetic Acid (Aux/IAA) Gene Family in Autopolyploid Sugarcane (Saccharum spontaneum). Int. J. Mol. Sci. 2024, 25, 7473. [Google Scholar] [CrossRef]
- Willig, J.-J.; Guarneri, N.; van Steenbrugge, J.J.M.; de Jong, W.; Chen, J.; Goverse, A.; Lozano Torres, J.L.; Sterken, M.G.; Bakker, J.; Smant, G. The Arabidopsis transcription factor TCP9 modulates root architectural plasticity, reactive oxygen species-mediated processes, and tolerance to cyst nematode infections. Plant J. 2022, 112, 1070–1083. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Ma, F.; Huang, D.; Xing, W.; Wu, B.; Sun, P.; Xu, B.; Song, S. Characterization of the NAC Transcription Factor in Passion Fruit (Passiflora edulis) and Functional Identification of PeNAC-19 in Cold Stress. Plants 2023, 12, 1393. [Google Scholar] [CrossRef]
- Leng, X.; Wei, H.; Xu, X.; Ghuge, S.A.; Jia, D.; Liu, G.; Wang, Y.; Yuan, Y. Genome-wide identification and transcript analysis of TCP transcription factors in grapevine. BMC Genom. 2019, 20, 786. [Google Scholar] [CrossRef]
- Wei, W.; Hu, Y.; Cui, M.-Y.; Han, Y.-T.; Gao, K.; Feng, J.-Y. Identification and Transcript Analysis of the TCP Transcription Factors in the Diploid Woodland Strawberry Fragaria vesca. Front. Plant Sci. 2016, 7, 1937. [Google Scholar] [CrossRef]
- Gull, S.; Uddin, S.; Hussain, H.A.; Wang, S.; Bayar, J.; Liu, J. Genome-wide analysis reveals the TCP-miR159-miR319 module is crucial for rice (Oryza sativa L.) growth and response to drought and salinity. Plant Stress 2023, 10, 100215. [Google Scholar] [CrossRef]
- Koyama, T.; Sato, F.; Ohme-Takagi, M. Roles of miR319 and TCP Transcription Factors in Leaf Development. Plant Physiol. 2017, 175, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qu, Y.; Wang, H.; Wang, J.; Song, A.; Hu, Y.; Chen, S.; Jiang, J.; Chen, F. The heterologous expression of a chrysanthemum TCP-P transcription factor CmTCP14 suppresses organ size and delays senescence in Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 115, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Liu, X.; Shi, Z.; Li, D.; Zhu, L. An AT-hook protein DEPRESSED PALEA1 physically interacts with the TCP Family transcription factor RETARDED PALEA1 in rice. Biochem. Biophys. Res. Commun. 2018, 495, 487–492. [Google Scholar] [CrossRef]
- Bao, S.; Zhang, Z.; Lian, Q.; Sun, Q.; Zhang, R. Evolution and expression of genes encoding TCP transcription factors in Solanum tuberosum reveal the involvement of StTCP23 in plant defence. BMC Genet. 2019, 20, 91. [Google Scholar] [CrossRef]
- Das Gupta, M.; Aggarwal, P.; Nath, U. CINCINNATA in Antirrhinum majus directly modulates genes involved in cytokinin and auxin signaling. New Phytol. 2014, 204, 901–912. [Google Scholar] [CrossRef]
- Liu, S.; Yin, X.; Feng, T.; Kang, Z.; Zhang, X.; Dong, J.; Liang, Z. Genome-wide identification and expression analysis of the TCP genes in Senna tora reveal the regulatory mechanism of their response to MeJA. Ind. Crops Prod. 2022, 187, 115511. [Google Scholar] [CrossRef]
- İlhan, E.; Büyük, İ.; İnal, B. Transcriptome—Scale characterization of salt responsive bean TCP transcription factors. Gene 2018, 642, 64–73. [Google Scholar] [CrossRef]
- Wang, Y.; He, W.; Wang, L.; Lan, Y.; Wu, M. TCP transcription factor identification in pecan (carya illinoensis) and salt tolerance function analysis of CiTCP8. Sci. Hortic. 2024, 330, 113051. [Google Scholar] [CrossRef]
- Ding, S.; Cai, Z.; Du, H.; Wang, H. Genome-Wide Analysis of TCP Family Genes in Zea mays L. Identified a Role for ZmTCP42 in Drought Tolerance. Int. J. Mol. Sci. 2019, 20, 2762. [Google Scholar] [CrossRef]
- FU, X.; ZHAO, J.I.E.; CAO, D.; HE, C.; WANG, Z.; JIANG, Y.; LIU, J.; LIU, G. Characteristics and expression of the TCP transcription factors family in Allium senescens reveal its potential roles in drought stress responses. Biocell 2023, 47, 905–917. [Google Scholar] [CrossRef]
- Inukai, S.; Kock, K.H.; Bulyk, M.L. Transcription factor-DNA binding: Beyond binding site motifs. Curr. Opin. Genet. Dev. 2017, 43, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Romero, P.; Barrero-Sicilia, C.; Gómez-Cadenas, A.; Carbonero, P.; Oñate-Sánchez, L. Arabidopsis thaliana DOF6 negatively affects germination in non-after-ripened seeds and interacts with TCP14. J. Exp. Bot. 2012, 63, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Lu, L.; Zhang, W.; Chi, M.; Shen, M.; An, C.; Chen, S.; Wang, X.; Liu, R.; Qin, Y.; et al. Comprehensive characterization and expression analysis of enzymatic antioxidant gene families in passion fruit (Passiflora edulis). iScience 2023, 26, 108329. [Google Scholar] [CrossRef]
- da Silva, M.L.; Paim Pinto, D.L.; Passos, A.B.; Marcelino-Guimarães, F.C.; Rossi, A.A.B.; Krause, W.; de Carvalho, I.F.; Batista, D.S.; Rocha, D.I.; Otoni, W.C. Novel and efficient transformation of wild passion fruit (Passiflora cincinnata Mast.) using sonication-assisted Agrobacterium-mediated transformation. Vitr. Cell. Dev. Biol. Plant 2021, 57, 380–386. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Wai, M.H.; Rizwan, H.M.; Qarluq, A.Q.; Xu, M.; Wang, L.; Cheng, Y.; Aslam, M.; Zheng, P.; Wang, X.; et al. Advances in micropropagation, somatic embryogenesis, somatic hybridizations, genetic transformation and cryopreservation for Passiflora improvement. Plant Methods 2023, 19, 50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).