Comparative Metabolomic Responses of Three Rhododendron Cultivars to the Azalea Lace Bug (Stephanitis pyrioides)
Abstract
1. Introduction
2. Results
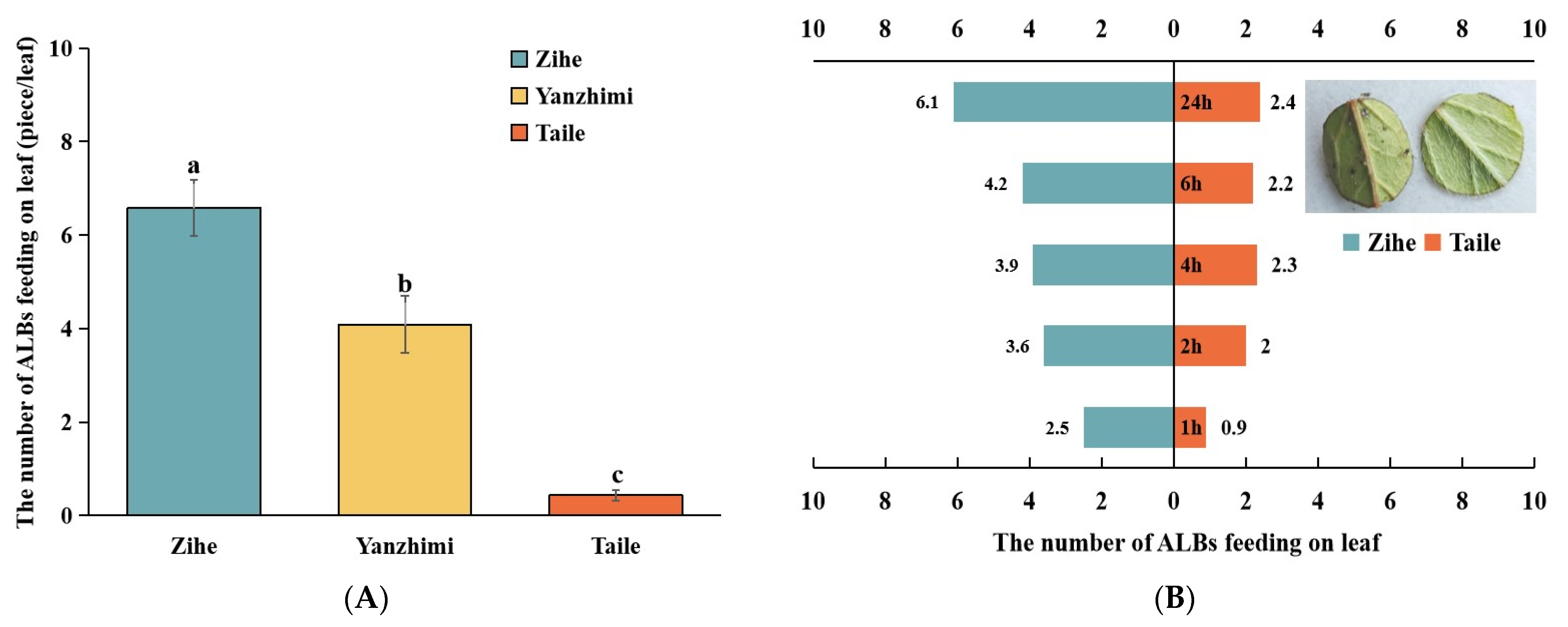
2.1. Comparison of ALB Susceptibility among Different Rhododendron Cultivars
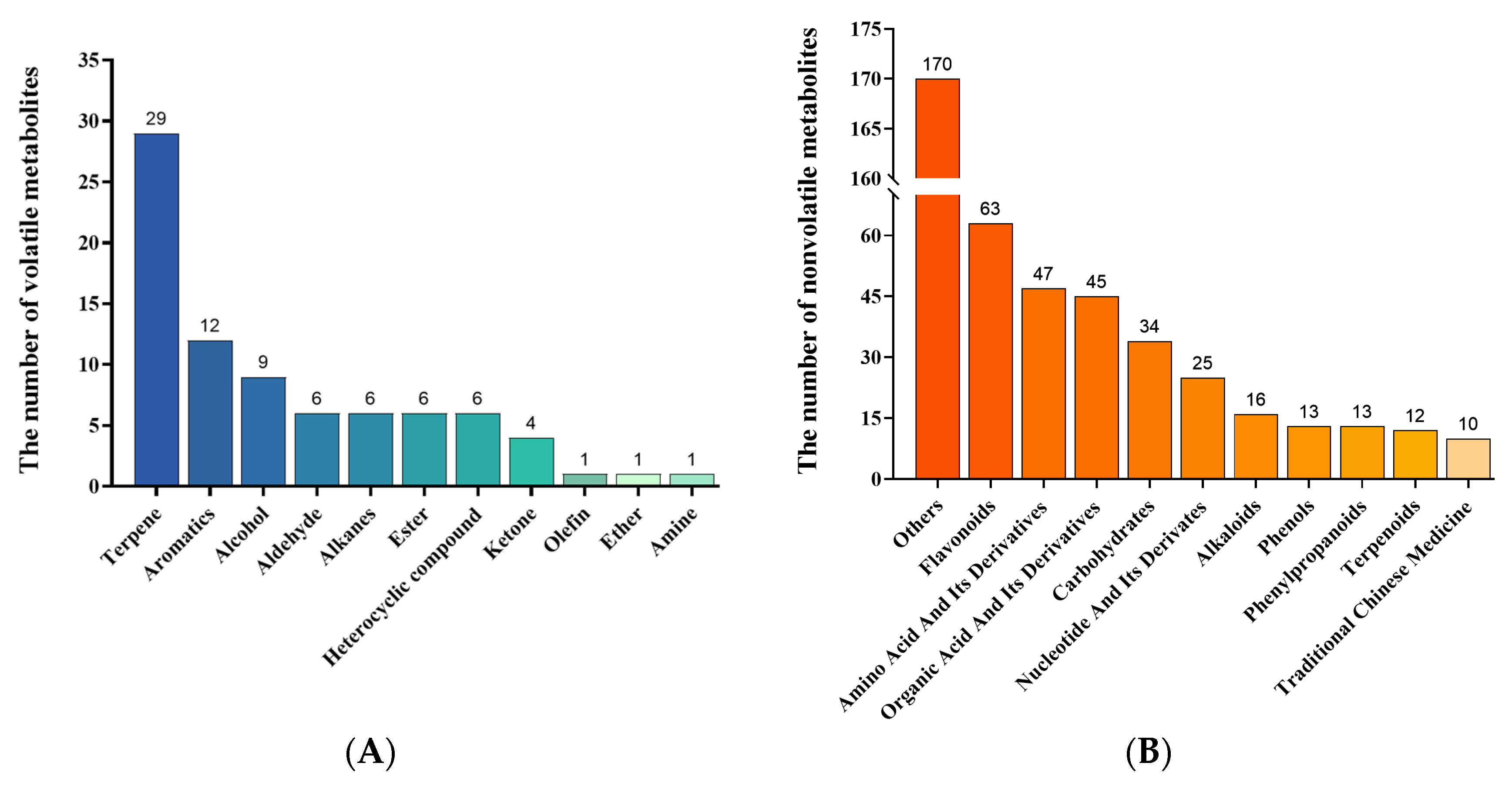
2.2. Identification of Metabolites in Different Rhododendron Cultivars
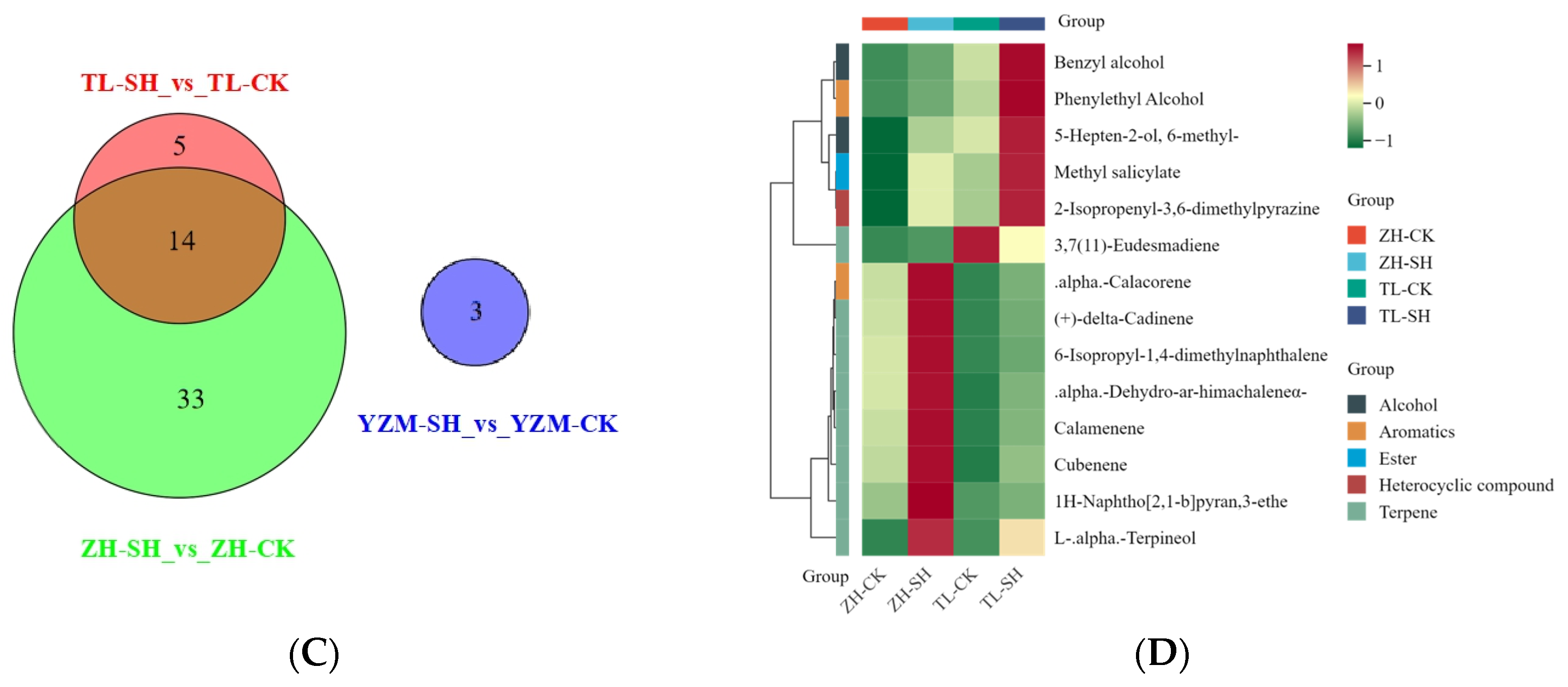
2.3. Differential Accumulated Metabolites after ALB Infestation
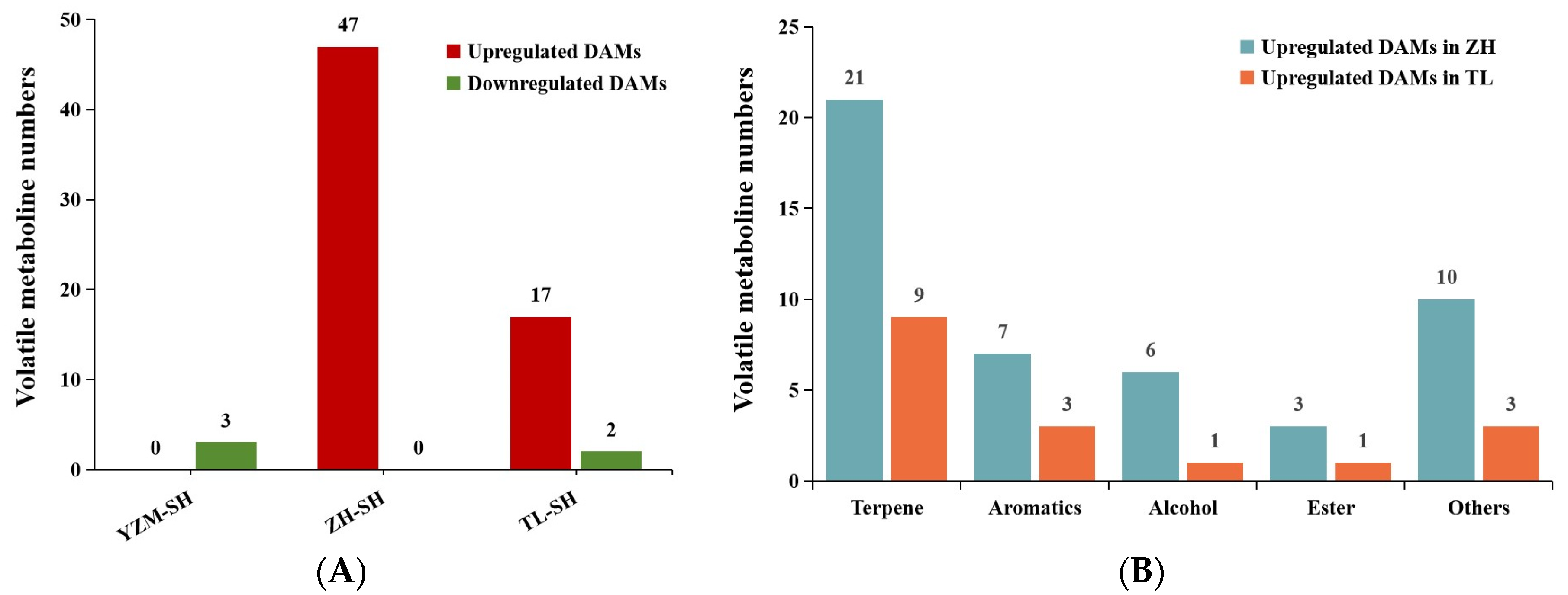
2.3.1. Volatile Differential Accumulated Metabolites in GCMS
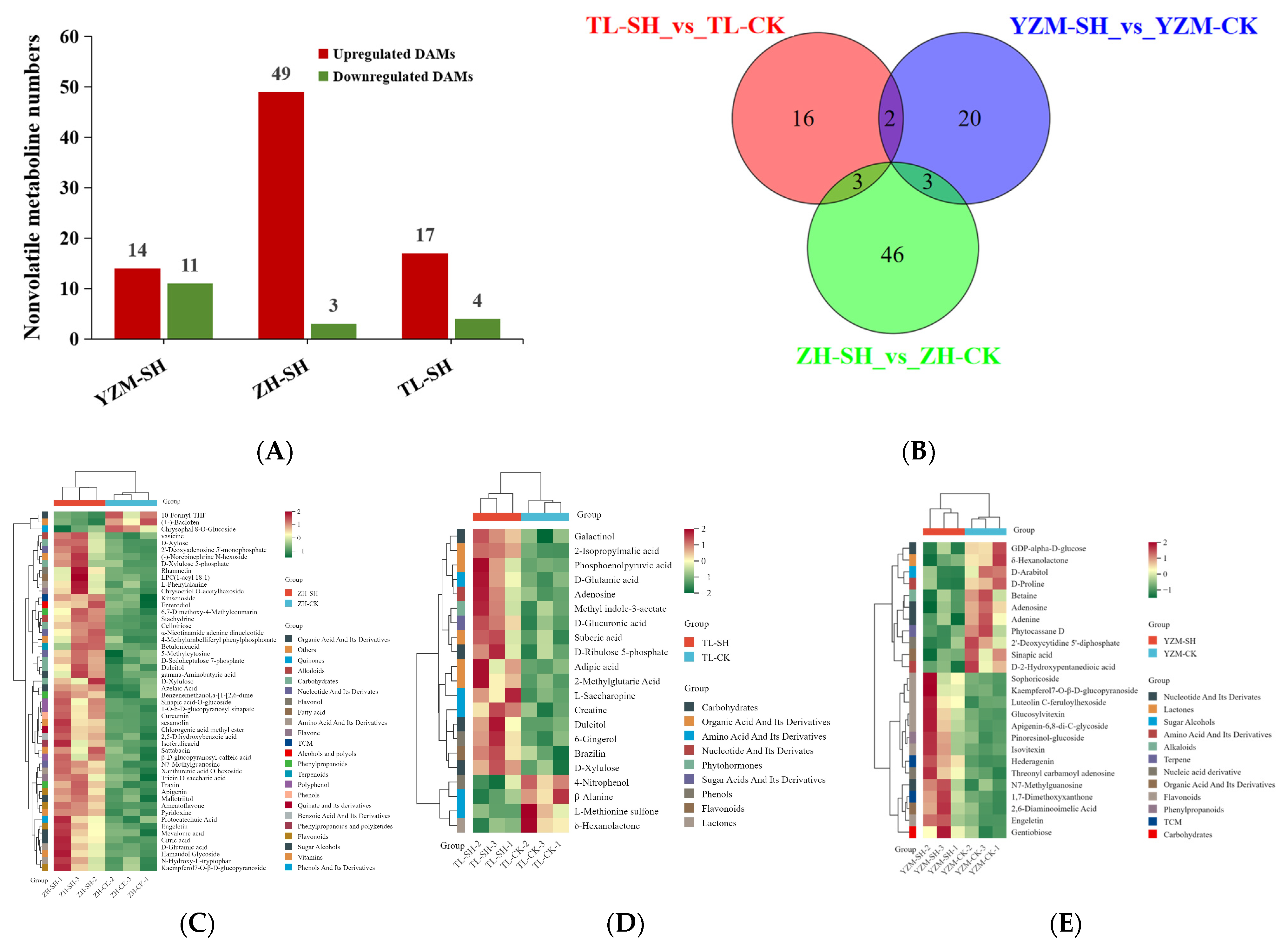
2.3.2. Nonvolatile DAMs in LCMS
2.4. The KEGG (Kyoto Encyclopedia of Genes and Genomes) Annotation Analysis of DAMs
2.4.1. The KEGG Annotation of Volatile DAMs
2.4.2. The KEGG Annotation of Nonvolatile DAMs
3. Discussion
3.1. Metabolic Profiles of Different Rhododendron Cultivars in Response to Insect Herbivory
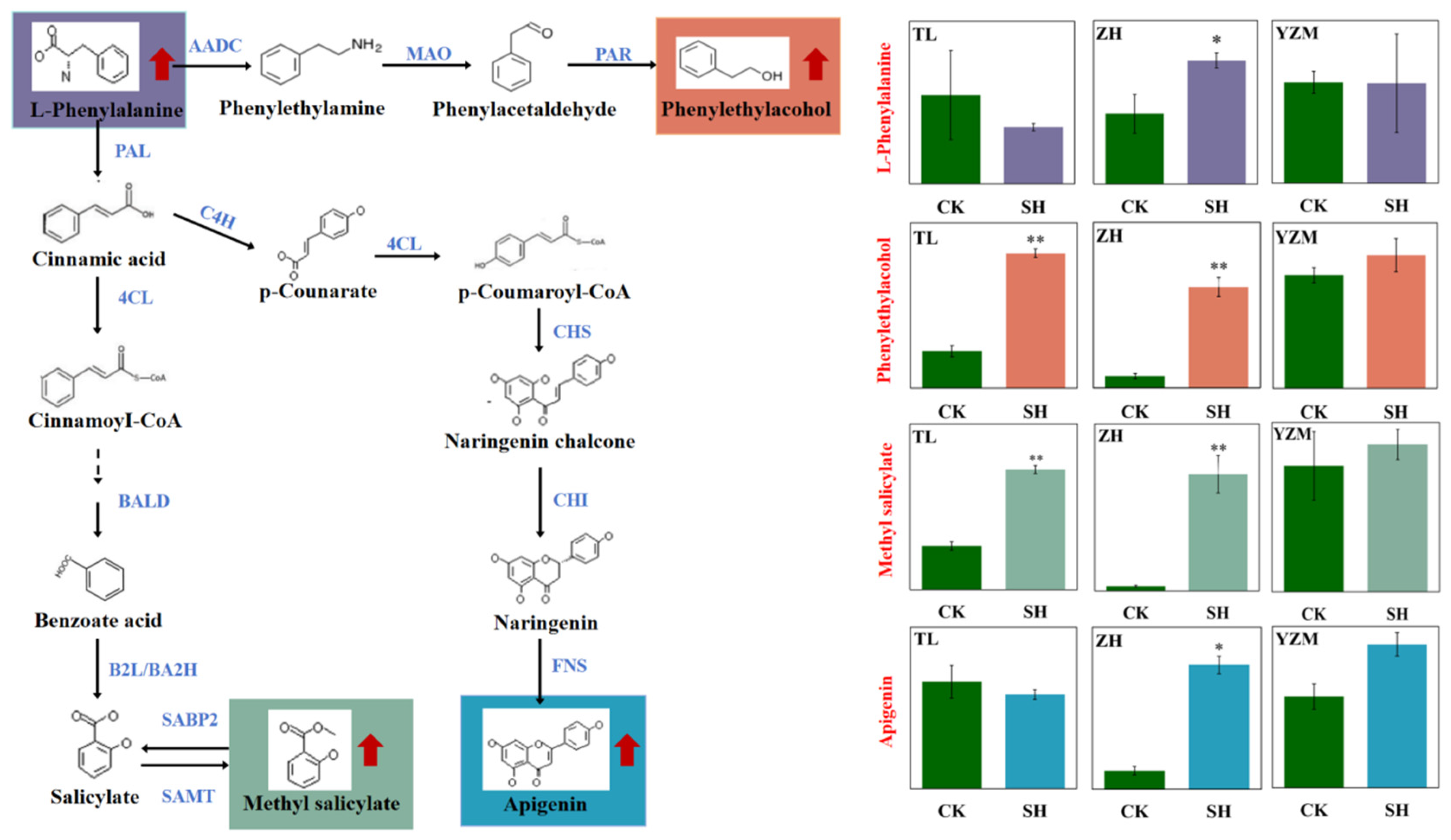
3.2. The Pivotal Role of the Phenylalanine Metabolism in Rhododendron Insect Resistance
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Field Investigation of Insect Resistance among the Three Cultivars
4.2.2. Comparison of Feeding Preference among ALBs to the Three Cultivars
4.2.3. ALB Inoculation and Sample Collection
4.2.4. Headspace Solid-Phase Microextraction (HS-SPEM) and GCMS Analysis
4.2.5. Sample Extraction and HPLC-MS/MS Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Graham, K.V.; Choi, M.Y.; Lee, J.C.; Godfrey, K. Attracting Chrysopidae with Plant Volatiles for Lace Bug (Hemiptera: Tingidae) Control in Rhododendrons and Azaleas. J. Insect Sci. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Garrison, R.R.; Tobin, P.C. Development of Azalea Lace Bug, Stephanitis pyrioides, on Susceptible and Resistant Rhododendron species in Western Washington. J. Econ. Entomol. 2022, 115, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Joseph, V.S. Influence of Insect Growth Regulators on Stephanitis pyrioides (Hemiptera: Tingidae) Eggs and Nymphs. Insects 2019, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Joseph, V.S. Repellent effects of insecticides on Stephanitis pyrioides Scott (Hemiptera: Tingidae) under laboratory conditions. Crop Prot. 2020, 127, 104985. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Lou, Y.R.; Tzin, V.; Jander, G. Alteration of Plant Primary Metabolism in Response to Insect Herbivory. Plant Physiol. 2015, 169, 1488–1498. [Google Scholar] [CrossRef]
- Shavit, R.; Batyrshina, Z.S.; Yaakov, B.; Florean, M.; Köllner, T.G.; Tzin, V. The wheat dioxygenase BX6 is involved in the formation of benzoxazinoids in planta and contributes to plant defense against insect herbivores. Plant Sci. 2021, 316, 111171. [Google Scholar] [CrossRef]
- Uzma, S.; Shakir, U.; Tang, Z.H.; Elateeq, A.A.; Yaseen, K.; Jafar, K.; Asif, K.; Sajid, A. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Francesco, L.; Sabato, D. How do plants sense volatiles sent by other plants? Trends Plant Sci. 2021, 27, 29–38. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.J.; He, L.F.; Huang, F.; Zhang, D.F.; Wang, Y.; Wei, X.; Han, M.; Deng, H.T.; Luo, L.; et al. Molecular basis of methyl salicylate-mediated plant airborne defence. Nature 2023, 622, 139–148. [Google Scholar] [CrossRef]
- Wang, F.; Park, Y.L.; Gutensohn, M. Glandular trichome-derived sesquiterpenes of wild tomato accessions (Solanum habrochaites) affect aphid performance and feeding behavior. Phytochemistry 2020, 180, 112532. [Google Scholar] [CrossRef] [PubMed]
- Aljbory, Z.; Chen, M.S. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Kim, N.S.; Park, Y.E.; Park, S.Y.; Kim, J.K.; Park, S.U. Metabolomic Profiling of the White, Violet, and Red Flowers of Rhododendron schlippenbachii Maxim. Molecules 2018, 23, 827. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.X.; Qin, Y.; Jia, Y.H.; Xie, X.H.; Li, D.B.; Jiang, B.X.; Wang, Q.; Feng, S.Y.; Wu, Y.Y. Transcriptomic and metabolomic data reveal key genes that are involved in the phenylpropanoid pathway and regulate the floral fragrance of Rhododendron fortunei. BMC Plant Biol. 2023, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Duan, S.G.; Xia, Y.; Li, J.T.; Liu, L.X.; Tang, M.; Tang, J.; Sun, W.; Yi, Y. Transcriptomic, Physiological, and Metabolomic Response of an Alpine Plant, Rhododendron delavayi, to Waterlogging Stress and Post-Waterlogging Recovery. Int. J. Mol. Sci. 2023, 24, 10509. [Google Scholar] [CrossRef]
- Williams, M.; Eveleigh, E.; Forbes, G.; Lamb, R.; Roscoe, L.; Silk, P. Evidence of a direct chemical plant defense role for maltol against spruce budworm. Entomol. Exp. Et Appl. 2019, 167, 755–762. [Google Scholar] [CrossRef]
- Rodrigues, R.M.B.d.A.; Fontes, L.d.S.; Brito, R.d.C.; Barbosa, D.R.e.S.; Citó, A.M.d.G.L.; Carmo, I.S.D.; Sousa, E.M.d.J.; Silva, G.N. A sustainable approach in the management of Callosobruchus maculatus: Essential oil of Protium heptaphyllum and its major compound D-limonene as biopesticides. J. Plant Dis. Prot. 2022, 129, 831–841. [Google Scholar] [CrossRef]
- Lin, H.R.; Li, Z.Y.; Sun, Y.; Zhang, Y.Y.; Wang, S.; Zhang, Q.; Cai, T.; Xiang, W.L.; Zeng, C.Y.; Tang, J. D-Limonene: Promising and Sustainable Natural Bioactive Compound. Appl. Sci. 2024, 14, 4605. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, Y.R.; Han, J.Y.; Choi, Y.E. Nematicidal Properties and Chemical Composition of Pinus rigida Mill. Resin against Pinewood Nematodes. Forests 2022, 13, 1131. [Google Scholar] [CrossRef]
- Victoria, F.O.; Susana, S.M.; José, Z.V.; Georg, J.; José, L.C. Changes in the free amino acid composition of Capsicum annuum (pepper) leaves in response to Myzus persicae (green peach aphid) infestation. A comparison with water stress. PLoS ONE 2018, 13, 0198093. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Zhao, X.; Zhao, S.; Gu, X.; Du, W.; Wei, H.; Ji, R.; Zhao, L. Metabolomics Reveals the "Invisible" Responses of Spinach Plants Exposed to CeO2 Nanoparticles. Environ. Sci. Technol. 2019, 53, 6007–6017. [Google Scholar] [CrossRef] [PubMed]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of Drought on Soluble Sugars and Free Proline Content in Selected Arabidopsis Mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, K.J.; Yang, S.Y.; Straube, H.; Medina, E.N.; Varbanova, H.M.; Herde, M.; Rhee, S.; Witte, C.P. Initiation of cytosolic plant purine nucleotide catabolism involves a monospecific xanthosine monophosphate phosphatase. Nat. Commun. 2021, 12, 6846. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Q.; Du, K.; Kang, X.Y.; Wei, H.R. The diverse roles of cytokinins in regulating leaf development. Hortic. Res. 2021, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Yue, L.; Xia, X.; Liu, K.; Zhang, W.Q. Comparative metabolomics analysis of different resistant rice varieties in response to the brown planthopper Nilaparvata lugens Hemiptera: Delphacidae. Metabolomics 2019, 15, 62. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Y.; Wang, H.; Zhang, J.J.; Song, C.P.; Shangguan, X.X.; Zhu, L.L.; He, G.C. Comparative metabolomics of the interaction between rice and the brown planthopper. Metabolomics 2016, 12, 132. [Google Scholar] [CrossRef]
- Seong, G.; Yun, D.Y.; Shin, D.H.; Cho, J.S.; Lee, G.; Choi, J.H.; Park, K.J.; Ku, K.H.; Lim, J.H. Comparative 1H NMR-Based Metabolomics of Traditional Landrace and Disease-Resistant Chili Peppers (Capsicum annuum L.). Foods 2024, 13, 1966. [Google Scholar] [CrossRef]
- Oliveira, D.C.; Isaias, R.M.S.; Fernandes, G.W.; Ferreira, B.G.; Carneiro, R.G.; Fuzaro, L. Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. J. Insect Physiol. 2016, 84, 103–113. [Google Scholar] [CrossRef]
- Sheng, H.L.; Hsin, Y.C.; Hsiun, M.H. Glutamate: A multifunctional amino acid in plants. Plant Sci. 2022, 318, 111238. [Google Scholar] [CrossRef]
- Jin, Y.; Zhi, L.L.; Tang, X.; Chen, Y.L.; Hancock, J.T.; Hu, X.Y. The Function of GABA in Plant Cell Growth, Development and Stress Response. Phyton-Int. J. Exp. Bot. 2023, 92, 2211–2225. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.R.; Wei, Y.Y.; Jiang, S.; Cao, Z.D.; Xu, F.; Wang, H.F.; Zhan, P.P.; Shao, X.F. Volatile organic compounds and rapid proliferation of Candida pseudolambica W16 are modes of action against gray mold in peach fruit. Postharvest Biol. Technol. 2022, 183, 111751. [Google Scholar] [CrossRef]
- Lu, J.; Li, J.; Li, L.; Qi, L.L.; Wang, Y.X.; Yang, S.; Xu, G.Y.; Dou, D.L.; Wang, X.D.; Liu, J. Natural product 2-Phenylethanol inhibits ATP synthesis of P. infestans by blocking the oxidative phosphorylation pathway to prevent potato late blight. Postharvest Biol. Technol. 2023, 199, 112310. [Google Scholar] [CrossRef]
- Çevikbaş, H.; Ulusoy, S.; Kinaytürk, K.N. Exploring rose absolute and phenylethyl alcohol as novel quorum sensing inhibitors in Pseudomonas aeruginosa and Chromobacterium violaceum. Sci. Rep. 2024, 14, 15666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Wang, X.; Zhang, F.; Dong, L.D.; Wu, J.J.; Cheng, Q.; Qi, D.Y.; Yan, X.F.; Jiang, L.Y.; Fan, S.J.; et al. Phenylalanine ammonia-lyase2.1 contributes to the soybean response towards Phytophthora sojae infection. Sci. Rep. 2017, 7, 7242. [Google Scholar] [CrossRef] [PubMed]
- Xoaquín, M.; Collen, S.N.; Angelos, K.; Sergio, R.; Kailen, A.M. Herbivore specificity and the chemical basis of plant-plant communication in Baccharis salicifolia (Asteraceae). New Phytol. 2018, 220, 703–713. [Google Scholar] [CrossRef]
- Velemir, N.; Robert, G.; Ayse, G.Ü.; Suresh, G.J.; Unelius, R.C. Effects of Methyl Salicylate on Host Plant Acceptance and Feeding by the Aphid Rhopalosiphum padi. Front. Plant Sci. 2021, 12, 710268. [Google Scholar] [CrossRef]
- Gatica, H.I.; Florencia, P.; Ivana, M.; Rómulo, G.C.; Marcelo, F.W. Appetitive behavior of the honey bee Apis mellifera in response to phenolic compounds naturally found in nectars. J. Exp. Biol. 2019, 222, 189910. [Google Scholar] [CrossRef]
- Stec, K.; Kozłowska, J.; Anna, W.K.; Kordan, B.; Anioł, M.; Gabryś, B. Effect of Naringenin and Its Derivatives on the Probing Behavior of Myzus persicae (Sulz.). Molecules 2020, 25, 3185. [Google Scholar] [CrossRef]
- Su, P.S.; Zhao, L.F.; Li, W.; Zhao, J.X.; Yan, J.; Ma, X.; Li, A.F.; Wang, H.W.; Kong, L.R. Integrated metabolo-transcriptomics and functional characterization reveals that the wheat auxin receptor TIR1 negatively regulates defense against Fusarium graminearum. J. Integr. Plant Biol. 2021, 63, 340–352. [Google Scholar] [CrossRef]
- Ramaroson, M.L.; Koutouan, C.; Helesbeux, J.J.; Le, C.V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef] [PubMed]

| Group | Metabolic Pathway | Annotated Differential Metabolites |
|---|---|---|
| TL-SH vs. TL-CK | Phenylalanine metabolism | Phenylethyl alcohol ↑ |
| Sesquiterpenoid and triterpenoid biosynthesis | (+)-delta-Cadinene ↑ | |
| Metabolic pathways | Benzyl alcohol ↑; L-.alpha.-Terpineol ↑; (+)-delta-Cadinen ↑ | |
| Biosynthesis of secondary metabolites | L-.alpha.-Terpineol ↑; (+)-delta-Cadinen ↑ | |
| Monoterpenoid | L-.alpha.-Terpineol ↑ | |
| ZH-SH vs. ZH-CK | Polyketide sugar unit biosynthesis | Maltol ↑ |
| Glycine, serine and threonine metabolism | 1,3-Propanediamine ↑ | |
| beta-Alanine metabolism | 1,3-Propanediamine ↑ | |
| Monoterpenoid biosynthesis | D-Limonene ↑; L-.alpha.-Terpineol ↑; (1S,5S)-6,6-Dimethyl-2-methylenebicyclo[3.1.1]heptan ↑ | |
| Biosynthesis of secondary metabolites | Maltol ↑; (+)-delta-Cadinen ↑; D-Limonene ↑, etc. | |
| Limonene and pinene degradation | D-Limonene ↑ | |
| Arginine and proline metabolism | 1,3-Propanediamine ↑ | |
| Sesquiterpenoid and triterpenoid biosynthesis | (+)-delta-Cadinen ↑ | |
| Metabolic pathways | D-Limonene ↑; Benzyl alcohol ↑; 1,3-Propanediamine ↑, etc. | |
| Phenylalanine metabolism | Phenylethyl alcohol ↑ | |
| Diterpenoid biosynthesis | Neoabietadiene ↑ | |
| YZM-SH vs. YZM-CK | Nothing | Nothing |
| Group | Metabolic Pathway | Annotated Differential Metabolites |
|---|---|---|
| YZM-SH vs. YZM-CK | Purine metabolism | Adenosine ↓; Adenine ↓ |
| Zeatin biosynthesis | Adenine ↓ | |
| Metabolic pathways | GDP-alpha-D-glucose ↓; Sinapic acid ↓; Betaine ↓; Adenosine ↓; Adenine ↓; D-Arabitol ↓; 2′-Deoxycytidine 5′-diphosphate ↓; D-Proline ↓ | |
| TL-SH vs. TL-CK | Biosynthesis of amino acids | Phosphoenolpyruvic acid ↑; D-Ribulose 5-phosphate ↑; 2-Isopropylmalic acid ↑; L-Saccharopine ↑ |
| Pyruvate metabolism | Phosphoenolpyruvic acid ↑; 2-Isopropylmalic acid ↑ | |
| Pentose and glucuronate interconversions | D-Ribulose 5-phosphate ↑; D-Xylulose ↑ | |
| Carbon fixation in photosynthetic organisms | Phosphoenolpyruvic acid ↑; D-Ribulose 5-phosphate ↑ | |
| Phosphonate and phosphinate metabolism | Phosphoenolpyruvic acid ↑ | |
| Riboflavin metabolism | D-Ribulose 5-phosphate ↑ | |
| Galactose metabolism | Galactinol ↑; Dulcitol ↑ | |
| Lysine biosynthesis | L-Saccharopine ↑ | |
| Carbon metabolism | Phosphoenolpyruvic acid ↑; D-Ribulose 5-phosphate ↑ | |
| Metabolic pathways | Phosphoenolpyruvic acid ↑; D-Ribulose 5-phosphate ↑; Creatine ↑; 2-Isopropylmalic acid ↑; Adenosine ↑; L-Saccharopine ↑; Dulcitol ↑; D-Xylulose ↑; D-Glutamic acid ↑, etc. | |
| ZH-SH vs. ZH-CK | Carbon metabolism | D-Xylulose 5-phosphate ↑; 10-Formyl-THF ↓; Citric acid ↑; D-Sedoheptulose 7-phosphate ↑ |
| Pentose and glucuronate interconversions | D-Xylulose 5-phosphate ↑; D-Xylose ↑; D-Xylulose ↑ | |
| Metabolic pathways | 2,5-Dihydroxybenzoic acid ↑; Apigenin-6,8-di-C-glycoside ↑; Mevalonic acid ↑; Pyridoxine ↑; Dulcitol ↑; D-Glutamic acid ↑; 2′-Deoxyadenosine 5′-monophosphate ↑, etc. | |
| One carbon pool by folate | 10-Formyl-THF ↓ | |
| Terpenoid backbone biosynthesis | Mevalonic acid ↑ | |
| Pentose phosphate pathway | D-Xylulose 5-phosphate ↑; D-Sedoheptulose 7-phosphate ↑ | |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | Protocatechuic Acid ↑; L-Phenylalanine ↑ | |
| Isoflavonoid biosynthesis | Apigenin ↑ | |
| Biosynthesis of amino acids | D-Xylulose 5-phosphate ↑; L-Phenylalanine ↑; Citric acid ↑; D-Sedoheptulose 7-phosphate ↑ | |
| Alanine, aspartate and glutamate metabolism | γ-Aminobutyric acid ↑; Citric acid ↑ | |
| Carbon fixation in photosynthetic organisms | D-Xylulose 5-phosphate ↑; D-Sedoheptulose 7-phosphate ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Zhou, Y.; Peng, Y.; Xu, D.; Tong, J.; Dong, Y.; Fang, L.; Mao, J. Comparative Metabolomic Responses of Three Rhododendron Cultivars to the Azalea Lace Bug (Stephanitis pyrioides). Plants 2024, 13, 2569. https://doi.org/10.3390/plants13182569
He B, Zhou Y, Peng Y, Xu D, Tong J, Dong Y, Fang L, Mao J. Comparative Metabolomic Responses of Three Rhododendron Cultivars to the Azalea Lace Bug (Stephanitis pyrioides). Plants. 2024; 13(18):2569. https://doi.org/10.3390/plants13182569
Chicago/Turabian StyleHe, Bei, Yuan Zhou, Yu Peng, Dongyun Xu, Jun Tong, Yanfang Dong, Linchuan Fang, and Jing Mao. 2024. "Comparative Metabolomic Responses of Three Rhododendron Cultivars to the Azalea Lace Bug (Stephanitis pyrioides)" Plants 13, no. 18: 2569. https://doi.org/10.3390/plants13182569
APA StyleHe, B., Zhou, Y., Peng, Y., Xu, D., Tong, J., Dong, Y., Fang, L., & Mao, J. (2024). Comparative Metabolomic Responses of Three Rhododendron Cultivars to the Azalea Lace Bug (Stephanitis pyrioides). Plants, 13(18), 2569. https://doi.org/10.3390/plants13182569






