Spatial Analysis of the Ecogeographic Diversity of Wild Creeping Cucumber (Melothria pendula L.) for In Situ and Ex Situ Conservation in Mexico
Abstract
1. Introduction
2. Results
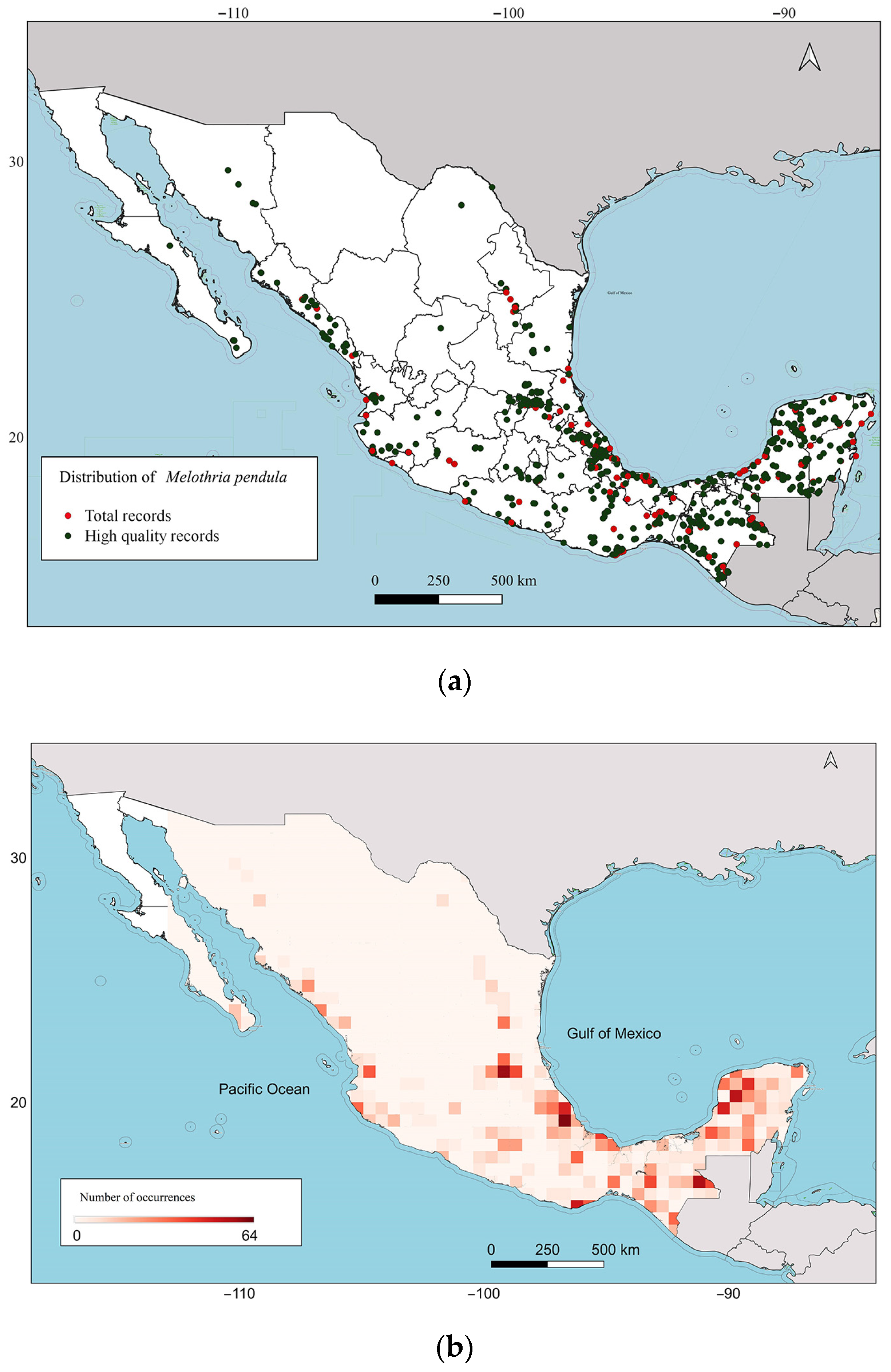
2.1. Distribution of M. pendula
2.2. Ecogeographic Variables of Importance
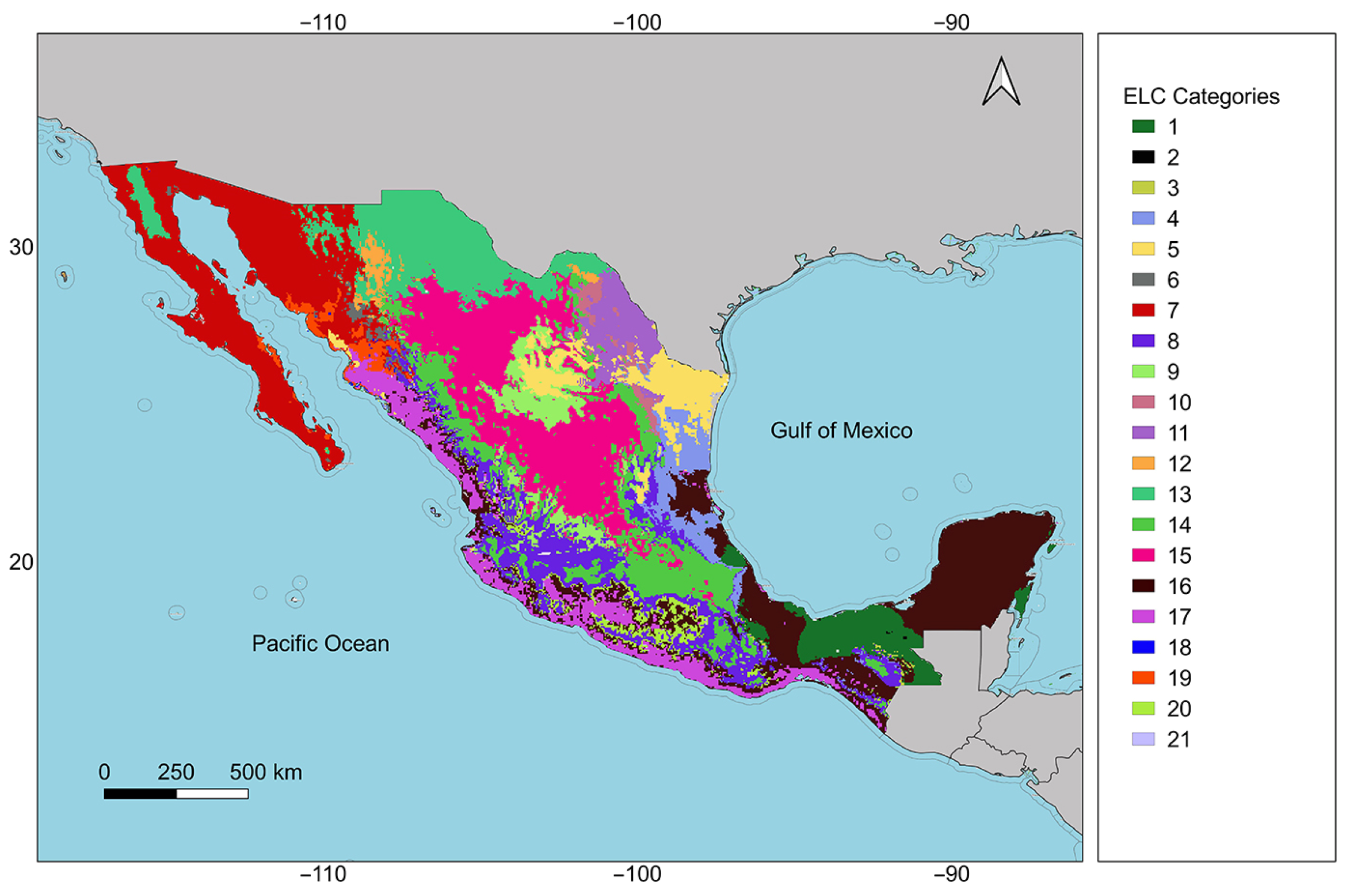
2.3. Ecogeographic Adaptive Scenarios
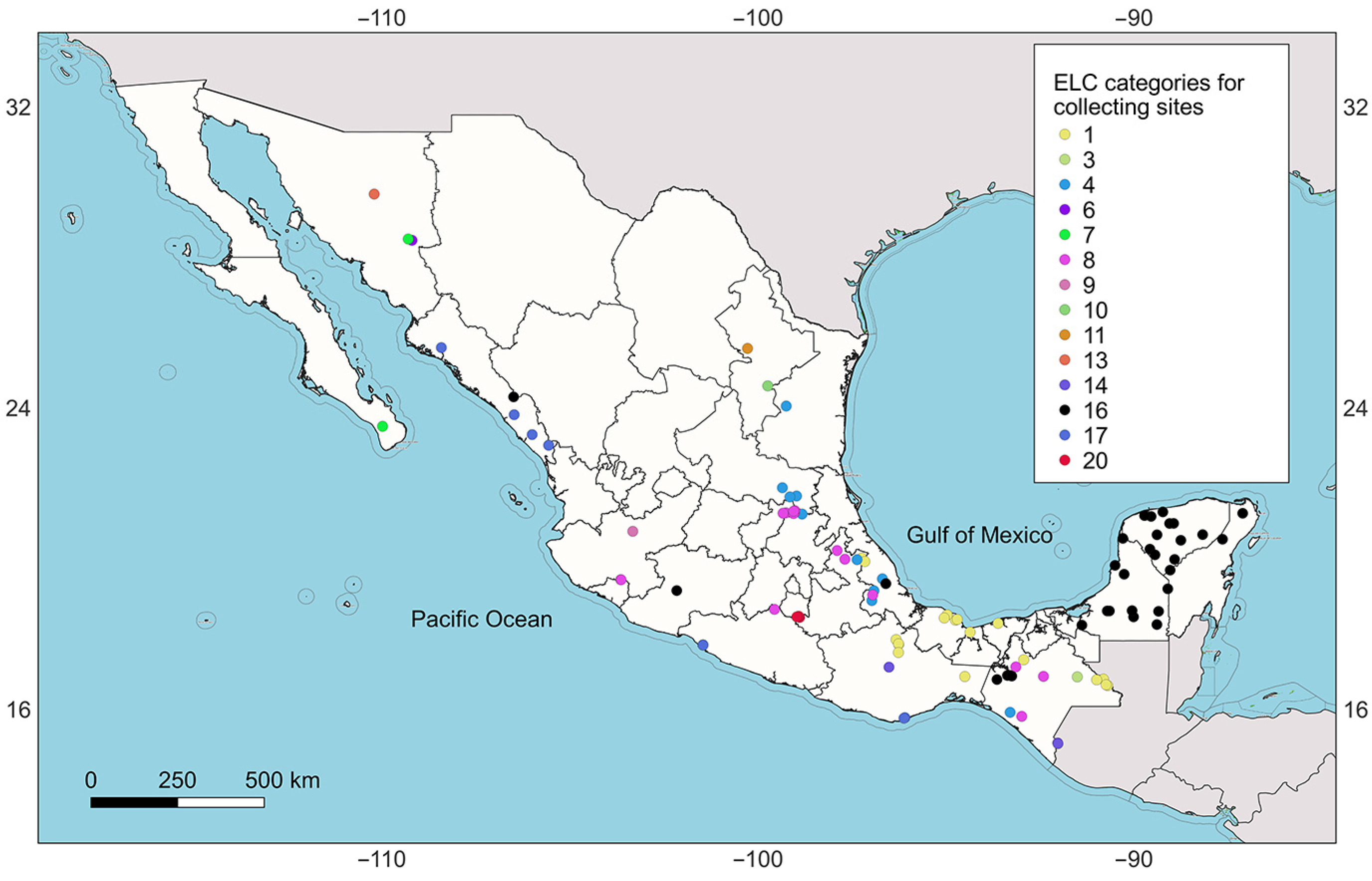
2.4. A Design for a Representative Germaplsm Collection
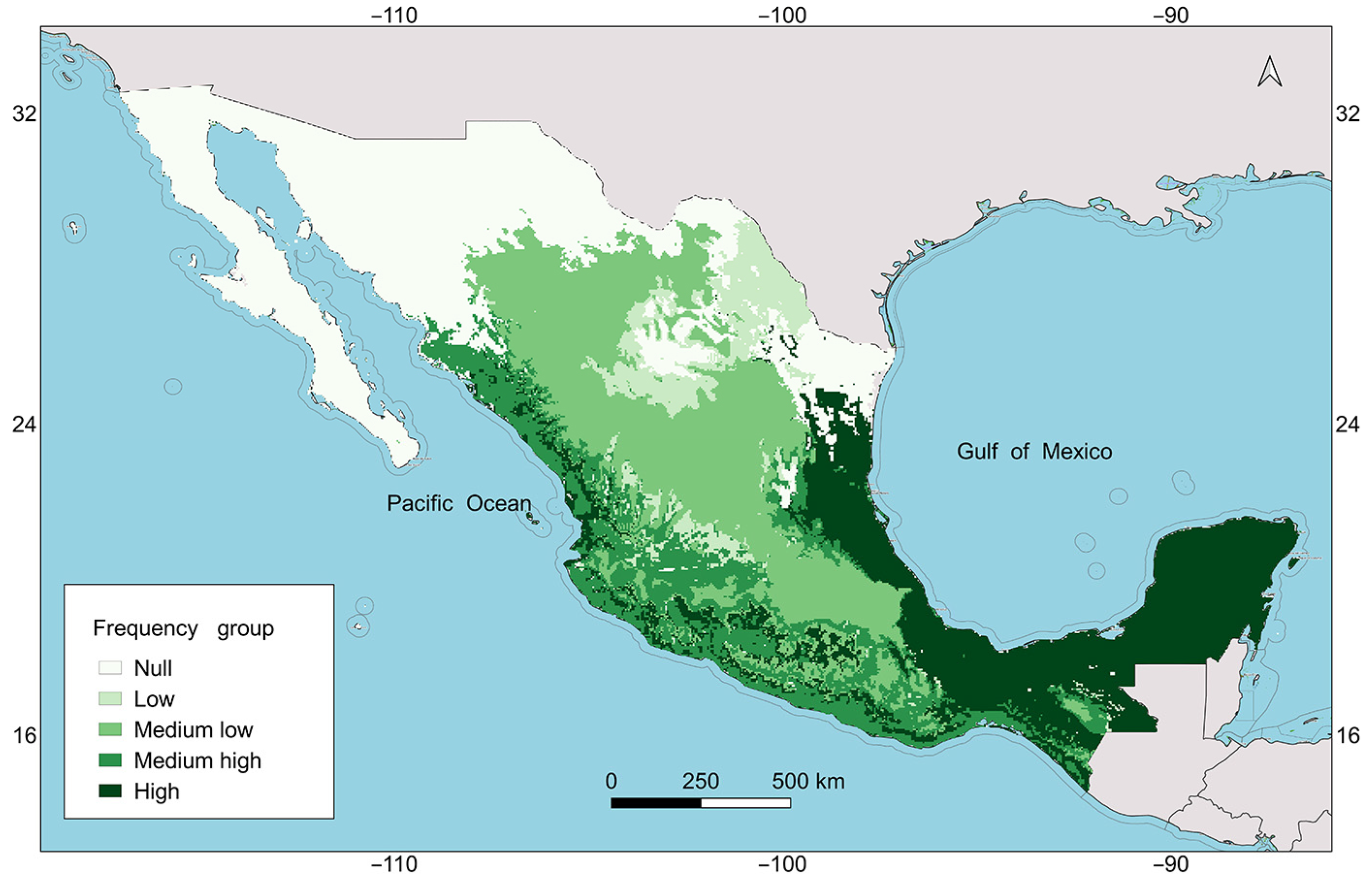
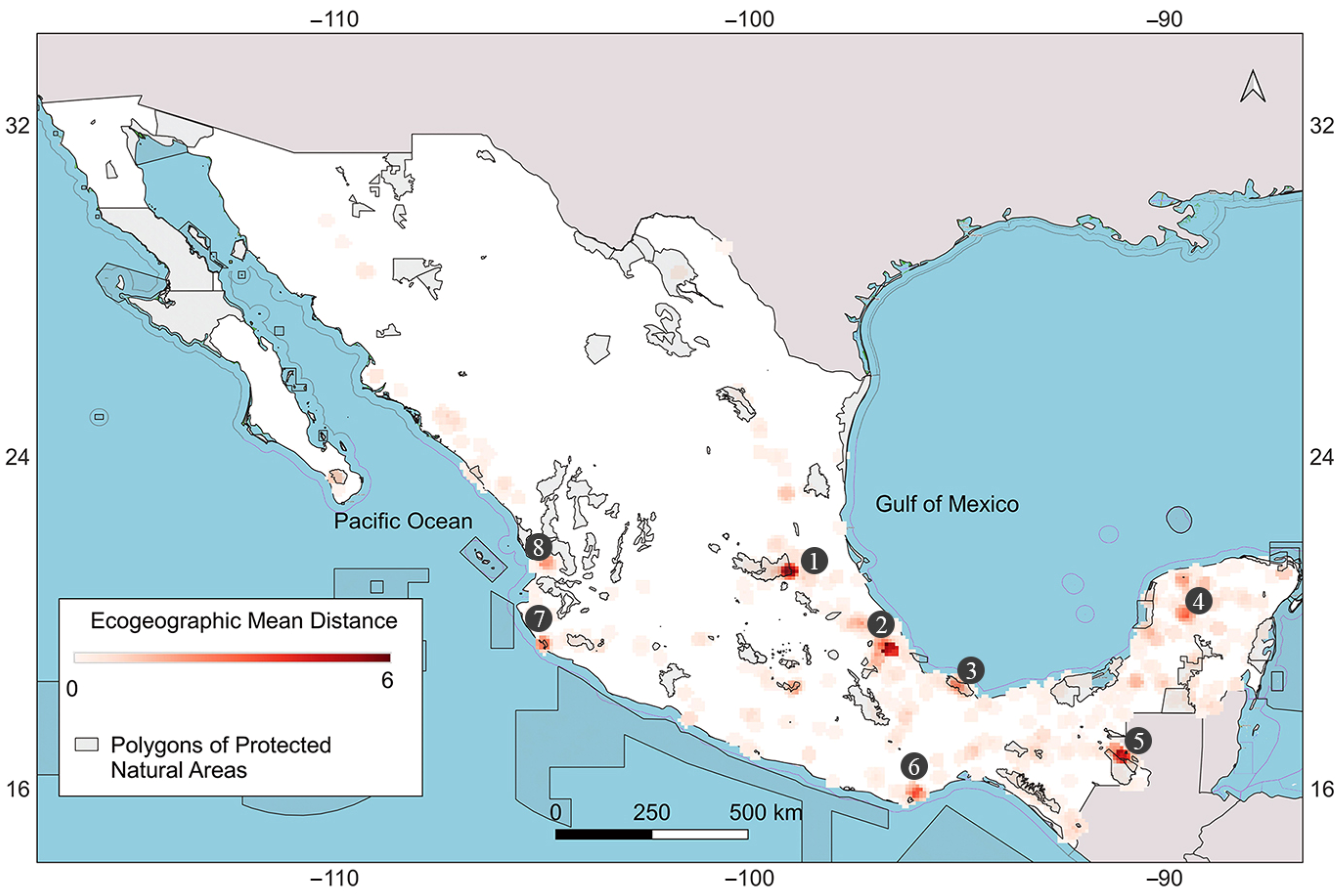
2.5. Map of Ecogeographic Diversity
3. Discussion
4. Materials and Methods
4.1. Occurrence and Distribution Data
4.2. Selection of Ecogeographic Variables of Importance
4.3. Ecogeographic Land Characterization
4.4. Optimized Germplasm Collecting Design
4.5. Map of Ecogeographic Diversity and Hotspot Coverage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Bioclimatic Variables | Edaphic Variables | Geophysical Variables |
|---|---|---|
| Mean temp warmest quarter | Avail soil water cap h1 top | Elevation |
| Mean temp coldest quarter | Avail soil water cap h2 top | Aspect |
| Prec wettest month | Avail soil water cap h3 top | Eastness |
| Prec driest month | edaphv: Sat water cont top | Northness |
| Prec wettest quarter | Depth to bedrock | Slope |
| Prec driest quarter | R horizon | solar radiant 1 |
| Prec warmest quarter | Bulk density top | solar radiant 2 |
| Prec coldest quarter | Cation exchange cap top | solar radiant 3 |
| Max temp warmest month | Clay content top | solar radiant 4 |
| Min temp coldest month | Coarse fragments top | solar radiant 5 |
| Mean temp wettest quarter | Organic carbon dens top | solar radiant 6 |
| Mean temp driest quarter | Organic carbon stock top | solar radiant 7 |
| Organic carbon content top | solar radiant 8 | |
| Soil pH H2O top | solar radiant9 | |
| Soil pH KCl top | solar radiant 10 | |
| Sodic soil grade | solar radiant 11 | |
| Silt content top | solar radiant 12 | |
| Sand content top | ||
| Avail soil water cap top |
| Parameter | |
|---|---|
| pais | Mexico |
| distdup | 1 |
| geoqual | TRUE |
| totalqual | 70 |
| buffy | FALSE |
| tamp | 0 |
| resol1 bioclimv edaphv geophysv latitude longitud | “Celdas 5 × 5 km aprox (2.5 arc-min)” The list of bioclimatic variables is shown in Table A1 The list of edaphic variables is shown in Table A1 The list of geophysical variables is shown in Table A1 TRUE TRUE |
| percenRF | 0.66 |
| percenCorr | 0.33 |
| CorrValue | 0.5 |
| p Value | 0.05 |
| nminvar | 3 |
| ecogeopcaxe | 5 |
| Parameter | |
|---|---|
| pais resol1 bioclimv geophysv latitude longitud edaphv | Mexico “Celdas 5 × 5 km aprox (2.5 arc-min)” The list of bioclimatic variables is shown in Table A1 The list of geophysical variables is shown in Table A1 TRUE TRUE The list of edaphic variables is shown in Table A1 |
| maxg | 8 |
| method | “calinski” |
| iterat | 10 |
| Parameter | |
|---|---|
| geoqual | TRUE |
| totalqual | 70 |
| fext | FALSE |
| geoqualfe | FALSE |
| duplibg | FALSE |
| gbifFE | FALSE |
| mapaelc | mapa_elc_Mexico.grd |
| statelc | Estadist_ELC_Mexico.xls |
| distdup | 1 |
| Parameter | |
|---|---|
| pais geoqual | Mexico TRUE |
| totalqual | 70 |
| buffy | FALSE |
| tamp | 0 |
| ecogeo | TRUE |
| resol1 | “Celdas 5 × 5 km aprox (2.5 arc-min)” |
| bioclimsn bioclimv | TRUE The list of bioclimatic variables is shown in Table A1 |
| edaphsn edaphv | TRUE The list of edaphic variables is shown in Table A1 |
| geophyssn geophysv latitud longitud | TRUE The list of geophysical variables is shown in Table A1 FALSE FALSE |
| phenotip | FALSE |
| genotip | FALSE |
| neigd | TRUE |
| csimilar | 5 |
| rgrid | 5 |
| buffer | 10 |
| ecogeoclus | TRUE |
| ecogeoclustype | ward |
| ecogeopca | TRUE |
| ecogeopcaxe | 5 |
| phenoclus | FALSE |
| genoclus | FALSE |
| mantelt | FALSE |
| mantelmeth mantelper | Pearson 999 |
References
- Hsu, T.W.; Peng, J.J.; Liu, H.Y. Melothria pendula L. (Cucurbitaceae), a newly naturalized plant in Taiwan. Taiwania 2001, 46, 193–198. [Google Scholar]
- Houdegbe, C.A.; Sogbohossou, O.E.D.; Achigan-Dako, E.G. Utilization and breeding perspective in the egusi gourd Melothria sphaerocarpa (Cogn.) H. Schaef. et S.S. Renner (syn: Cucumeropsis mannii Naudin). Genet. Resour. Crop Evol. 2016, 63, 545–559. [Google Scholar] [CrossRef]
- Arseniuk, A.F. Herbs from Distant Lands: Melothria pendula—Melonette. Available online: https://herbsfromdistantlands.blogspot.com/2018/04/melothria-pendula-melonette.html (accessed on 8 April 2024).
- Arzate-Fernández, A.M.; Grenón-Cascales, G.M. Contribución al conocimiento del pepinillo silvestre (Melothria pendula L.). CIENCIA Ergo-Sum 2002, 9, 78–86. [Google Scholar]
- Lira, R.; Caballero, J. Ethnobotany of the wild Mexican Cucurbitaceae. Econ. Bot. 2002, 56, 380–398. [Google Scholar] [CrossRef]
- Lira, R.; Casas, A. Uso y manejo de Ibervillea millspaughii (Cogn.) C. Jeffrey, Melothria pendula L. y otras especies silvestres de la familia Cucurbitaceae: Posibles procesos de domesticación incipiente. Bol. Soc. Bot. México 1998, 62, 77–89. [Google Scholar]
- Idris, A.; Linatoc, A.C.; Bin Abu Bakar, M.F. Effect of light intensity on the gas exchange characteristics of Melothria pendula. IOP Conf. Ser. Earth Environ. Sci. 2019, 269, 01202. [Google Scholar] [CrossRef]
- Schaefer, H.; Heibl, C.; Renner, S.S. Gourds afloat: A dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous oversea dispersal events. Proc. R. Soc. B: Biol. Sci. 2009, 276, 843–851. [Google Scholar] [CrossRef]
- Thormann, I.; Parra-Quijano, M.; Endresen, D.T.F.; Rubio-Teso, M.L.; Iriondo, M.J.; Maxted, N. Predictive Characterization of Crop Wild Relatives and Landraces: Technical Guidelines Version 1; Bioversity International: Rome, Italy, 2014; ISBN 978-92-9255-004-2. [Google Scholar]
- Castañeda-Álvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16022. [Google Scholar] [CrossRef]
- Ceballos-Silva, A.; López-Blanco, J. Evaluating biophysical variables to identify suitable areas for oat in Central Mexico: A multi-criteria and GIS approach. Agric. Ecosyst. Environ. 2003, 95, 371–377. [Google Scholar] [CrossRef]
- Williams, C.L.; Hargrove, W.W.; Liebman, M.; James, D.E. Agro-ecoregionalization of Iowa using multivariate geographical clustering. Agric. Ecosyst. Environ. 2008, 123, 161–174. [Google Scholar] [CrossRef]
- Parra-Quijano, M.; Iriondo, J.M.; Torres, E. Ecogeographical land characterization maps as a tool for assessing plant adaptation and their implications in agrobiodiversity studies. Genet. Resour. Crop Evol. 2012, 59, 205–217. [Google Scholar] [CrossRef]
- Parra-Quijano, M.; Iriondo, J.M.; Torres, E. Improving representativeness of genebank collections through species distribution models, gap analysis and ecogeographical maps. Biodivers. Conserv. 2012, 21, 79–96. [Google Scholar] [CrossRef]
- García, R.M.; Parra-Quijano, M.; Iriondo, J.M. Identification of ecogeographical gaps in the Spanish Aegilops collections with potential tolerance to drought and salinity. PeerJ 2017, 5, e3494. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.O.; Rhodes, J.R.; Riginos, C.; Fuller, R.A. Environmental and geographic variables are effective surrogates for genetic variation in conservation planning. Proc. Natl. Acad. Sci. USA 2017, 114, 12755–12760. [Google Scholar] [CrossRef]
- Di Santo, L.N.; Hamilton, J.A. Using environmental and geographic data to optimize ex situ collections and preserve evolutionary potential. Conserv. Biol. 2021, 35, 733–744. [Google Scholar] [CrossRef]
- Parra-Quijano, M.; Draper, D.; Torres, E.; Iriondo, J.M. Ecogeographical representativeness in crop wild relative ex situ collections. In Crop Wild Relative Conservation and Use; Maxted, N., Ford-Lloyd, B.V., Kell, S.P., Iriondo, J.M., Dulloo, M.E., Turok, J., Eds.; CAB International: Wallingford, UK, 2008; pp. 249–273. ISBN 978-1-84593-099-8. [Google Scholar]
- Marinoni, L.; Bortoluzzi, A.; Parra-Quijano, M.; Zabala, J.M.; Pensiero, J.F. Evaluation and improvement of the ecogeographical representativeness of a collection of genus Trichloris in Argentina. Genet. Resour. Crop Evol. 2015, 62, 593–604. [Google Scholar] [CrossRef]
- Van Zonneveld, M.; Scheldeman, X.; Escribano, P.; Viruel, M.A.; van Damme, P.; Garcia, W.; Tapia, C.; Romero, J.; Sigueñas, M.; Hormanza, J.I. Mapping genetic diversity of cherimoya (Annona cherimola Mill.): Application of spatial analysis for conservation and use of plant genetic resources. PLoS ONE 2012, 7, e29845. [Google Scholar] [CrossRef]
- Fitzgerald, H.; Palmé, A.; Asdal, A.; Endresen, D.; Kiviharju, E.; Lund, B.; Rasmussen, M.; Thorbjörnsson, H.; Weibull, J. A regional approach to Nordic crop wild relative in situ conservation planning. Plant Genet. Resour. Charact. Util. 2019, 17, 196–207. [Google Scholar] [CrossRef]
- Rao, M.N.; Ganeshaiah, K.N.; Shaanker, R.U. Assessing threats and mapping sandal resources to identify genetic ‘hot-spot’ for in situ conservation in peninsular India. Conserv. Genet. 2007, 8, 925–935. [Google Scholar]
- Parra-Quijano, M.; Iriondo, J.M.; Torres, M.E.; López, F.; Phillips, J.; Kell, S. CAPFITOGEN 3: A Toolbox for the Conservation and Promotion of the Use of Agricultural Biodiversity; Universidad Nacional de Colombia: Bogotá, Colombia, 2021; ISBN 978-958-505-038-9. [Google Scholar]
- Jarvis, A.; Yeaman, S.; Guarino, L.; Tohme, J. The role of geographic analysis in locating, understanding, and using plant genetic diversity. In Methods in Enzymology; Zimmer, E.A., Roalson, E.H., Eds.; Academic Press: San Diego, CA, USA, 2005; Volume 395, pp. 279–298. ISBN 978-012-182-800-4. [Google Scholar]
- Melothria pendula Accessions. Available online: https://www.genesys-pgr.org/a/v2zw59KWZq3 (accessed on 12 April 2024).
- SEMARNAT-CONANP. Información Especial de las Áreas Naturales Protegidas. Available online: http://sig.conanp.gob.mx/website/pagsig/info_shape.htm (accessed on 8 April 2024).
- García, R.M.; Parra-Quijano, M.; Iriondo, J.M. A multispecies collecting strategy for crop wild relatives based on complementary areas with a high density of ecogeographical gaps. Crop Sci. 2017, 57, 1059–1069. [Google Scholar] [CrossRef]
- Parra-Quijano, M.; Iriondo, J.M.; Torres, E. Applications of ecogeography and geographic information systems in conservation and utilization of plant genetic resources. Span. J. Agric. Res. 2012, 10, 419–429. [Google Scholar] [CrossRef]
- Hyman, G.; Hodson, D.; Jones, P. Spatial analysis to support geographic targeting of genotypes to environments. Front. Physiol. 2013, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ojeda, G.; Peralta, I.E.; Rodríguez-Guzmán, E.; Sahagún-Castellanos, J.; Chávez-Servia, J.L.; Medina-Hinostroza, T.C.; Rijalba-Vela, J.R.; Vásquez-Núñez, L.P.; Rodríguez-Pérez, J.E. Edaphoclimatic descriptors of wild tomato species (Solanum sect. Lycopersicon) and closely related species (Solanum sect. Juglandifolia and Sect. Lycopersicoides) in South America. Front. Genet. 2021, 12, 748979. [Google Scholar] [CrossRef] [PubMed]
- Tapia, C.; Paredes, N.; Lima, L. Representatividad de la diversidad del género Musa en el Ecuador. Rev. Científica Ecuat. 2019, 6, 53–58. [Google Scholar] [CrossRef]
- Tapia, C.; Torres, E.; Paredes, N.; Parra-Quijano, M. Morphological and ecogeographical diversity analysis of maize germplasm in the high altitude Andes region of Ecuador. Plant Genet. Resour. Charact. Util. 2021, 19, 183–194. [Google Scholar] [CrossRef]
- Hernández-Salinas, G.; Luna-Cavazos, M.; Soto-Estrada, A.; García-Pérez, E.; Pérez-Vázquez, A.; Córdova-Téllez, L. Distribution and eco-graphic characterization of Carica papaya L. native to Mexico. Genet. Resour. Crop Evol. 2022, 69, 99–116. [Google Scholar] [CrossRef]
- Meyer, C.; Weigelt, P.; Kreft, H. Multidimensional biases, gaps and uncertainties in global plant occurrence information. Ecol. Lett. 2016, 19, 992–1006. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Garrett, K.A.; Huamán, Z.; Zhang, D.P.; Schreuder, M.; Bonierbale, M. Assessing the geographic representativeness of genebank collections: The case of Bolivian wild potatoes. Conserv. Biol. 2000, 14, 1755–1765. [Google Scholar] [CrossRef]
- United Nations. Convention on Biological Diversity; United Nations: New York, NY, USA, 1992. [Google Scholar]
- FAO. Segundo Plan de Acción Mundial para los Recursos Fitogenéticos para la Alimentación y la Agricultura; FAO: Rome, Italy, 2011. [Google Scholar]
- Nduche, M.U.; Magos Brehm, J.; Parra-Quijano, M.; Maxted, N. In situ and ex situ conservation gap analyses of West African priority crop wild relatives. Genet. Resour. Crop Evol. 2023, 70, 333–351. [Google Scholar] [CrossRef]
- Khaki Mponya, N.; Chanyenga, T.; Magos Brehm, J.; Maxted, N. In situ and ex situ conservation gap analyses of crop wild relatives from Malawi. Genet. Resour. Crop Evol. 2021, 68, 759–771. [Google Scholar] [CrossRef]
- GBIF Global Biodiversity Information Facility. Melothria pendula. Available online: https://www.gbif.org/es/ (accessed on 8 April 2024).
- MEXU. Herbario Nacional de México. Bases de Datos de Herbario. Available online: https://herbanwmex.net/portal/collections/misc/collprofiles.php?collid=501 (accessed on 14 May 2024).
- UAQ. Herbario de Querétaro “Dr. Jerzy Rzedowski” (QMEX). Available online: https://fcn.uaq.mx/index.php/extension/herbario/presentacion-herbario (accessed on 14 May 2024).
- Ecogeographic Data Sets. Available online: https://www.capfitogen.net/en/access/ecogeodatasets/ (accessed on 8 April 2024).
- Cutler, D.R.; Edwards, T.C., Jr.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Calinski, T.; Harabasz, J. A dendrite method for cluster analysis. Commun. Stat. 1974, 3, 1–27. [Google Scholar] [CrossRef]
- Brown, A.H.D. Core collections: A practical approach to genetic resources management. Genome 1989, 31, 818–824. [Google Scholar] [CrossRef]
- R Core Team. The R Project for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 14 May 2024).
| Abiotic Component | Variable Names | CAPFITOGEN3 Variable Code |
|---|---|---|
| Climatic | Mean temperature of the coldest quarter | BIO11 |
| Mean temperature of the driest quarter | BIO9 | |
| Precipitation of the coldest quarter | BIO19 | |
| Edaphic | Bulk density top | t_bulk_dens |
| Cation exchange capacity top | t_cecsol | |
| Organic carbon density top | t_oc_dens | |
| Soil pH KCl top | t_ph_kcl | |
| Geophysical | Elevation | alt |
| Solar monthly radiation February | srad_2 | |
| Solar monthly radiation April | srad_4 | |
| Solar monthly radiation July | srad_7 |
| Ecogeographic Category of ELC Map of Mexico | Quartile Group by Frequency | Number of Occurrences and Percentage of Total Distribution | Initial Quotas by P Strategy and 10% Sampling Intensity | Final Quotas and Percentage of Total Future Collections |
|---|---|---|---|---|
| 1 | High | 183 (17.2%) | 18.3 | 18 (16.2%) |
| 2 | Null | 0 (0%) | 0 | 0 (0%) |
| 3 | Low | 1 (0.1%) | 0.1 | 1 (0.9%) |
| 4 | High | 108 (10.2%) | 10.8 | 11 (9.9%) |
| 5 | Null | 0 (0%) | 0 | 0 (0%) |
| 6 | Low | 1 (0.1%) | 0.1 | 1 (0.9%) |
| 7 | Medium-low | 15 (1.4%) | 1.5 | 2 (1.8%) |
| 8 | High | 154 (14.5%) | 15.4 | 15 (13.5%) |
| 9 | Low | 1 (0.1%) | 0.1 | 1 (0.9%) |
| 10 | Medium-low | 8 (0.8%) | 0.8 | 1 (0.9%) |
| 11 | Medium-low | 3 (0.3%) | 0.3 | 1 (0.9%) |
| 12 | Null | 0 (0%) | 0 | 0 (0%) |
| 13 | Low | 1 (0.1%) | 0.1 | 1 (0.9%) |
| 14 | Medium-high | 28 (2.6%) | 2.7 | 3 (2.7%) |
| 15 | Null | 0 (0%) | 0 | 0 (0%) |
| 16 | High | 418 (39.3%) | 41.8 | 42 (37.8%) |
| 17 | Medium-high | 108 (10.2%) | 10.8 | 11 (9.9%) |
| 18 | Null | 0 (0%) | 0 | 0 (0%) |
| 19 | Null | 0 (0%) | 0 | 0 (0%) |
| 20 | Medium-high | 34 (3.2%) | 3.4 | 3 (2.7%) |
| 21 | Null | 0 (0%) | 0 | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Santos, R.; Hernández-Sandoval, L.; Parra-Quijano, M. Spatial Analysis of the Ecogeographic Diversity of Wild Creeping Cucumber (Melothria pendula L.) for In Situ and Ex Situ Conservation in Mexico. Plants 2024, 13, 2572. https://doi.org/10.3390/plants13182572
González-Santos R, Hernández-Sandoval L, Parra-Quijano M. Spatial Analysis of the Ecogeographic Diversity of Wild Creeping Cucumber (Melothria pendula L.) for In Situ and Ex Situ Conservation in Mexico. Plants. 2024; 13(18):2572. https://doi.org/10.3390/plants13182572
Chicago/Turabian StyleGonzález-Santos, Rosalinda, Luis Hernández-Sandoval, and Mauricio Parra-Quijano. 2024. "Spatial Analysis of the Ecogeographic Diversity of Wild Creeping Cucumber (Melothria pendula L.) for In Situ and Ex Situ Conservation in Mexico" Plants 13, no. 18: 2572. https://doi.org/10.3390/plants13182572
APA StyleGonzález-Santos, R., Hernández-Sandoval, L., & Parra-Quijano, M. (2024). Spatial Analysis of the Ecogeographic Diversity of Wild Creeping Cucumber (Melothria pendula L.) for In Situ and Ex Situ Conservation in Mexico. Plants, 13(18), 2572. https://doi.org/10.3390/plants13182572







