Spatio-Temporal Variations of Volatile Metabolites as an Eco-Physiological Response of a Native Species in the Tropical Forest
Abstract
1. Introduction
2. Material and Methods
2.1. Botanical Material
2.2. Essential Oil Extraction and Analysis
2.3. Data Processing and Statistical Analysis
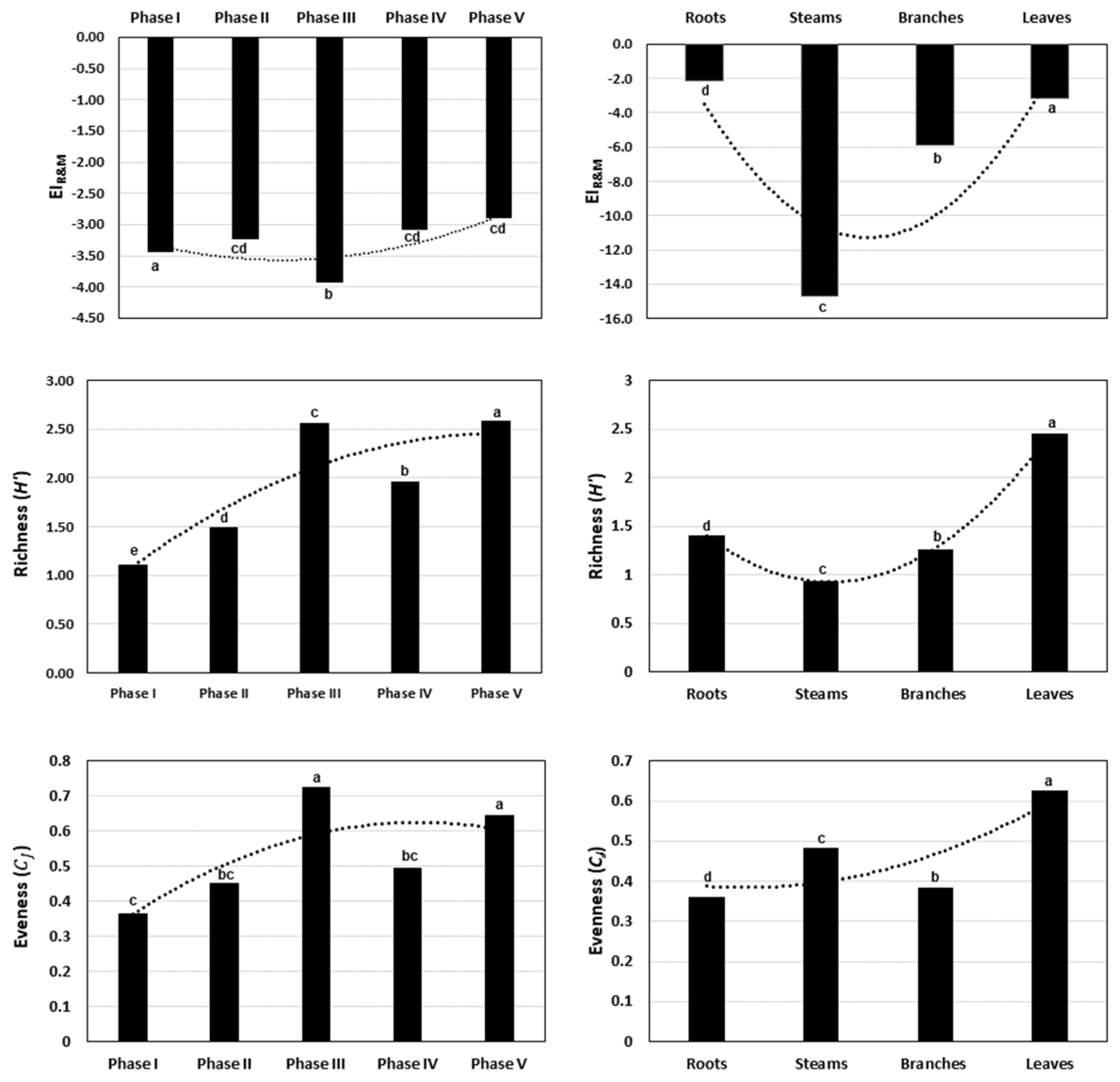
2.4. Chemodiversity and Advanced Chemophenetics Approaches
3. Results
3.1. Chemical Composition and Yields of Essential Oils from P. rivinoides
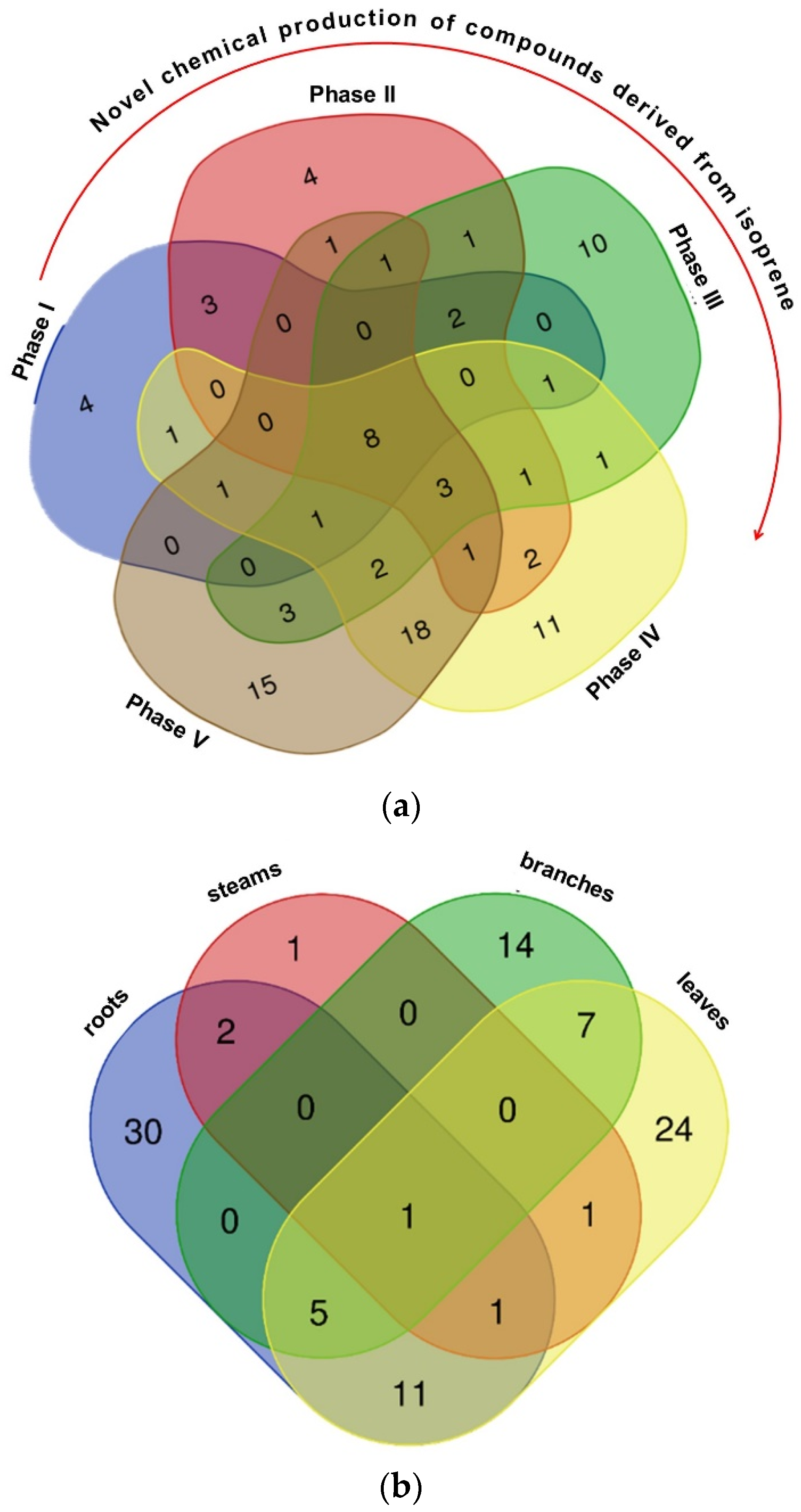
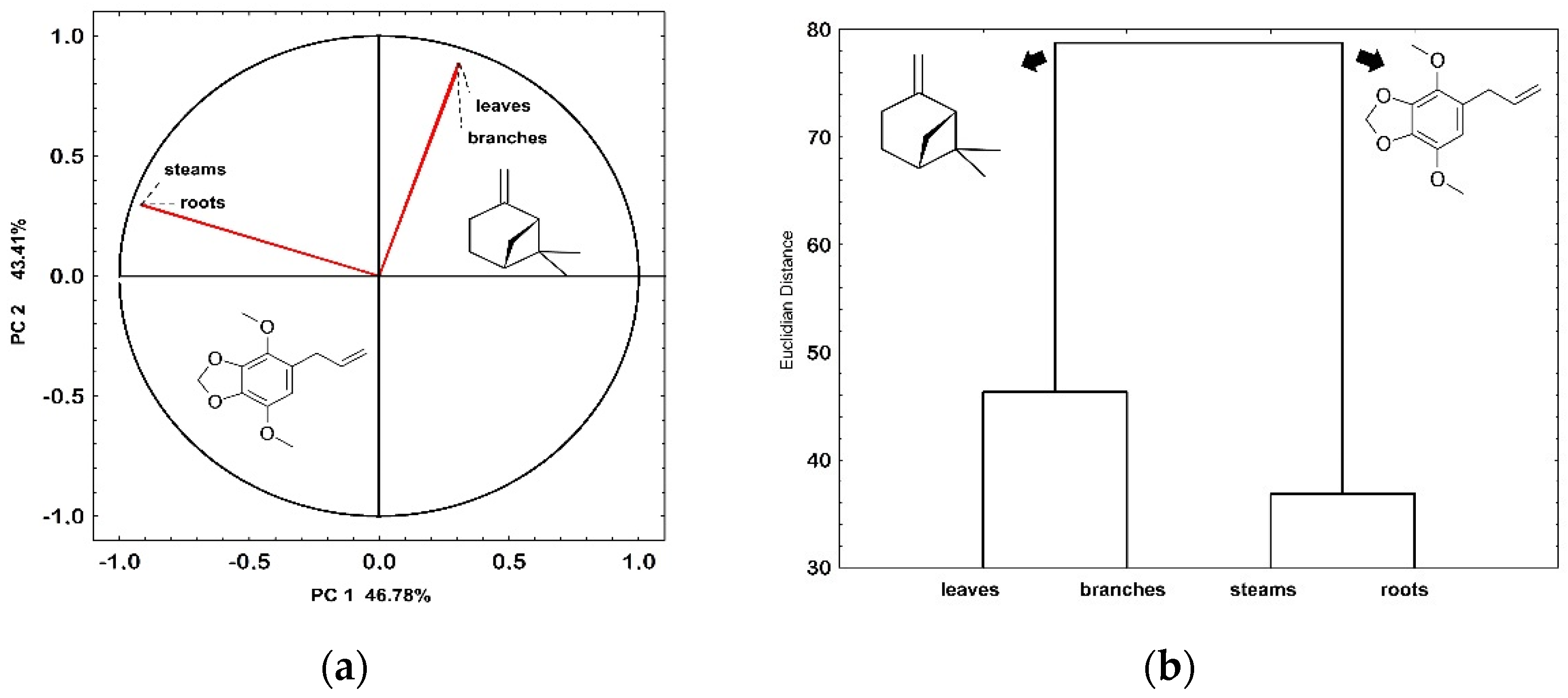
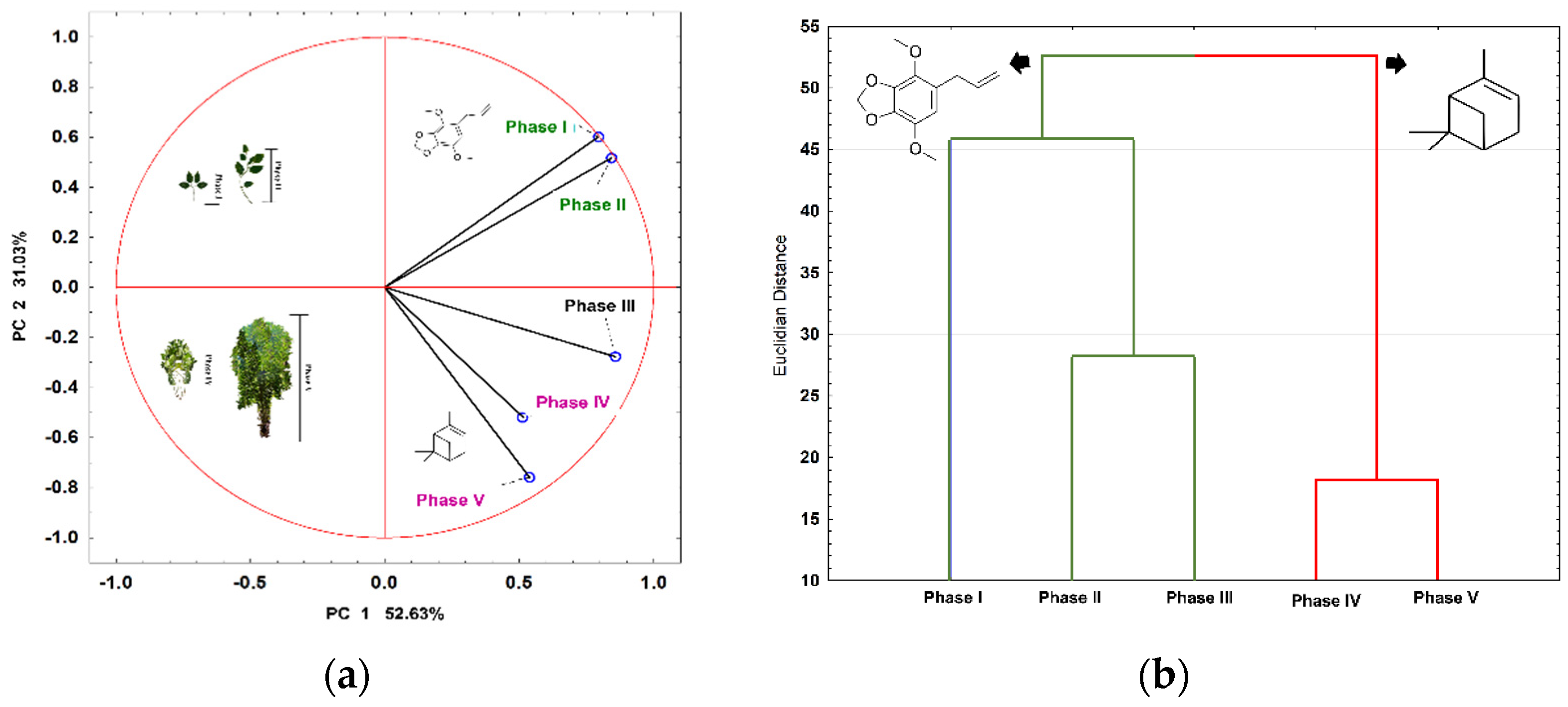
3.2. Chemodiversity and Chemophenetic Index
4. Discussion
4.1. Chemical Composition and Yields of Essential Oils from P. rivinoides
4.2. Chemodiversity and Chemophenetic Indices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salvi, L.; Cecilia, B.; Eleonora, C.; Paolo, S.; Giovan, B.M. Eco-physiological traits and phenylpropanoid profiling on potted Vitis vinifera L. cv Pinot noir subjected to Ascophyllum nodosum treatments under post-veraison low water availability. Appl. Sci. 2020, 10, 13. [Google Scholar] [CrossRef]
- Paul, I.; Sarkar, M.P.; Bhadoria, P.B.S. Floral secondary metabolites in context of biotic and abiotic stress factors. Chemoecology 2022, 32, 49–68. [Google Scholar] [CrossRef]
- Ashra, H.; Nair, S. Trait plasticity during plant-insect interactions: From molecular mechanisms to impact on community dynamics. Plant Sci. 2022, 317, 111188. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Joshi, R.; Kumar, R. Metabolic signatures provide novel insights to Picrorhizakurroa adaptation along the altitude in Himalayan region. Metabolomics 2020, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Y.J.; da Silva, L.J.; de Oliveira, R.S.; Gomes, T.L.M.; Pott, A. Chemical composition of the essential oils of circadian rhythm and of different vegetative parts from Piper mollicomum Kunth-A medicinal plant from Brazil. Biochem. Syst. Ecol. 2020, 92, 104116. [Google Scholar] [CrossRef]
- Ramos, Y.J.; Gouvêa-Silva, J.G.; de Brito Machado, D.; Felisberto, J.S.; Pereira, R.C.; Sadgrove, N.J.; de Lima Moreira, D. Chemophenetic and Chemodiversity Approaches: New Insights on Modern Study of Plant Secondary Metabolite Diversity at Different Spatiotemporal and Organizational Scales. Rev. Bras. Farmacogn. 2023, 33, 49–72. [Google Scholar] [CrossRef]
- Raposo, J.D.A.; Figueiredo, P.L.B.; Santana, R.L.; da Silva Junior, A.Q.; Suemitsu, C.; da Silva, R.; Mourão, R.H.V.; Maia, J.G.S. Seasonal and circadian study of the essential oil of Myrcia sylvatica (G. Mey) DC., a valuable aromatic species occurring in the Lower Amazon River region. Biochem. Syst. Ecol. 2018, 79, 21–29. [Google Scholar] [CrossRef]
- Németh-Zámbori, É. Natural variability of essential oil components. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2020; pp. 85–124. [Google Scholar]
- Cornara, L.; Sgrò, F.; Raimondo, F.M.; Ingegnieri, M.R.; Mastracci, L.; D’Angelo, V.; Germanò, M.P.; Trombetta, D.; Smeriglio, A. Pedoclimatic Conditions Influence the Morphological, Phytochemical and Biological Features of Mentha pulegium L. Plants 2023, 12, 24. [Google Scholar] [CrossRef]
- Müller, C.; Junker, R.R. Chemical phenotype as important and dynamic niche dimension of plants. New Phytol. 2022, 234, 1168–1174. [Google Scholar] [CrossRef]
- Thon, F.M.; Müller, C.; Wittmann, M.J. The evolution of chemodiversity in plants—From verbal to quantitative models. Ecol. Lett. 2024, 27, e14365. [Google Scholar] [CrossRef]
- Flexas, J.; Gago, J. A role for ecophysiology in the’omics’ era. Plant J. 2018, 96, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.E. The ontogenetic dimension of plant functional ecology. Funct. Ecol. 2024, 38, 98–113. [Google Scholar] [CrossRef]
- Hanski, I. Dynamics of regional distribution: The core and satellite species hypothesis. Oikos 1982, 38, 210–221. [Google Scholar] [CrossRef]
- Mehranvar, L.; Jackson, D.A. History and taxonomy: Their roles in the core-satellite hypothesis. Oecologia 2001, 127, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Gerber, D.; Thompson, B.B.; Kato, M.J.; Yamaguchi, L.F.; Bechara, F.C. Óleo essencial de Piper aduncum L. para atração de morcegos-da-fruta para restauração ecológica. Rev. Bras. Agroecol. 2022, 17, 384–393. [Google Scholar]
- De Souza, S.P.; Valverde, S.S.; Costa, N.F.; Calheiros, A.S.; Lima, K.S.; Frutuoso, V.S.; Lima, A.L. Chemical composition and antinociceptive activity of the essential oil of Piper mollicomum and Piper rivinoides. J. Med. Plants Res. 2014, 8, 788–793. [Google Scholar]
- Bernuci, K.Z.; Iwanaga, C.C.; Fernandez-Andrade, C.M.M.; Lorenzetti, F.B.; Torres-Santos, E.C.; Faiões, V.D.S.; Gonçalves, J.E.; do Amaral, W.; Deschamps, C.; de Lima Scodro, R.B.; et al. Evaluation of chemical composition and antileishmanial and antituberculosis activities of essential oils of Piper species. Molecules 2016, 21, 1698. [Google Scholar] [CrossRef]
- Alves Borges Leal, A.L.; Fonseca Bezerra, C.; Ferreira e Silva, A.K.; Everson da Silva, L.; Bezerra, L.L.; Almeida-Neto, F.W.; Marinho, E.M.; Fernandes, C.F.C.; da Rocha, M.N.; Marinho, M.M.; et al. Seasonal variation of the composition of essential oils from Piper cernuum Vell and Piper rivinoides Kunth, ADMET study, DFT calculations, molecular docking and dynamics studies of major components as potent inhibitors of the heterodimer methyltransferase complex NSP16-NSP10 SARS-CoV-2 protein. J. Biomol. Struct. Dyn. 2023, 41, 6326–6344. [Google Scholar]
- Felisberto, J.R.S.; Ramos, Y.J.E.; de Queiroz, G.A.; Guimarães, E.F.; Marques, A.E.M.; de Lima Moreira, D. Piper rivinoides Kunth: A medicinal plant that preserves bioactive chemical compounds in its essential oil throughout the seasons. J. Med. Plants Res. 2022, 16, 258–268. [Google Scholar]
- Barros, A.J.B. Ossaim-O Orixá E Nossos Chás-Volume Único; Clube de Autores: Joinville, Brazil, 2010. [Google Scholar]
- Barros, J.F.P.D.; Napoleão, E. EwéÒrìsà: Uso Litúrgico e Terapêutico dos Vegetais nas Casas de Candomblé Jêje-Nagô; Bertrand Brasil: Rio de Janeiro, Brazil, 2003. [Google Scholar]
- Leal, A.L.A.B.; Machado, A.J.T.; Bezerra, C.F.; Inácio, C.E.S.; Rocha, J.E.; Sales, D.L.; de Freitas, T.S.; Almeida, W.O.; do Amaral, W.; da Silva, L.E.; et al. Chemical identification and antimicrobial potential of essential oil of Piper rivinoides kunth (BETIS-WHITE). Food Chem. Toxicol. 2019, 131, 110559. [Google Scholar] [CrossRef]
- Moreira, D.; de Paiva, R.A.; Marques, A.M.; Borges, R.M.; Barreto, A.L.S.; da Rocha Curvelo, J.A.; Cavalcanti, J.F.; Romanos, T.V.; de Araujo Soares, R.M.; Kaplan, M.A.C. Bioactive neolignans from the leaves of Piper rivinoides Kunth (Piperaceae). Rec. Nat. Prod. 2016, 10, 472. [Google Scholar]
- Fonseca, A.C.C.D.; de Queiroz, L.N.; Sales Felisberto, J.; Jesse Ramos, Y.; Mesquita Marques, A.; Wermelinger, G.F.; Pontes, B.; de Lima Moreira, D.; Robbs, B.K. Cytotoxic effect of pure compounds from Piper rivinoides Kunth against oral squamous cell carcinoma. Nat. Prod. Res. 2021, 35, 6163–6167. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.Q.; Felisberto, J.R.S.; Guimarães, E.F.; Queiroz, G.A.D.; Fonseca, A.C.C.D.; Ramos., Y.J.; Marques, A.M.; de Lima Moreira., D.; Robbs, B.K. Apoptotic effect of β-pinene on oral squamous cell carcinoma as one of the major compounds from essential oil of medicinal plant Piper rivinoides Kunth. Nat. Prod. Res. 2022, 36, 1636–1640. [Google Scholar] [CrossRef]
- INEA—Instituto Estadual do Meio Ambiente. Unidades de Conservação. Parque Estadual da Pedra Branca e Parque Estadual da Ilha Grande. Available online: http://www.inea.rj.gov.br/biodiversidade-territorio/conheca-as-unidades-de-conservacao/ (accessed on 5 June 2022).
- Dayrell, R.L.; Arruda, A.J.; Pierce, S.; Negreiros, D.; Meyer, P.B.; Lambers, H.; Silveira, F.A. Ontogenetic shifts in plant ecological strategies. Funct. Ecol. 2018, 32, 2730–2741. [Google Scholar] [CrossRef]
- Gatsuk, L.E.; Smirnova, O.V.; Vorontzova, L.I.; Zaugolnova, L.B.; Zhukova, L.A. Age states of plants of various growth forms: A review. J. Ecol. 1980, 68, 675–696. [Google Scholar] [CrossRef]
- Ramos, Y.J.; Moreira, D.L. Seasonal study of essential oil from aerial parts of Peperomia galioides kunth (piperaceae). Rev. Virtual Quím. 2019, 11, 1540–1550. [Google Scholar] [CrossRef]
- Oliveira, G.L.; Moreira, D.D.L.; Mendes, A.D.R.; Guimarães, E.F.; Figueiredo, L.S.; Kaplan, M.A.C.; Martins, E.R. Growth study and essential oil analysis of Piper aduncum from two sites of Cerrado biome of Minas Gerais State, Brazil. Rev. Bras. Farmacogn. 2013, 23, 743–753. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017; ISBN 978-0-9981557-2-2. [Google Scholar]
- Costa-Oliveira, C.D.; Gouvêa-Silva, J.G.; Brito Machado, D.D.; Felisberto, J.R.S.; Queiroz, G.A.D.; Guimarães, E.F.; Ramos, Y.J.; De Lima Moreira, D. Chemical Diversity and Redox Values Change as a Function of Temporal Variations of the Essential Oil of a Tropical Forest Shrub. Diversity 2023, 15, 715. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Jones, G.L. Cytogeography of essential oil chemotypes of Eremophila longifolia F. Muell (Scrophulariaceae). Phytochemistry 2014, 105, 43–51. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Andrade, F.D.D.; Medeiros, M.D.C.D.; Camboim, A.D.S.; Pereira Júnior, F.A.; Athayde, A.C.; Rodrigues, O.G.; Silva, W.W. Estudo da atividade anti-helmíntica do extrato etanólico de Jatrophamollissima (Pohl) Baill. (Euphorbiaceae) sob Haemonchuscontortus em ovinos no semiárido paraibano. Pesq. Vet. Bras. 2014, 34, 1051–1055. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, W.; Wu, W.; Bai, R.; Kuang, S.; Shi, B.; Li, D. Chemical composition and diversity of the essential oils of Juniperus rigida along the elevations in Helan and Changbai Mountains and correlation with the soil characteristics. Ind. Crops Prod. 2021, 159, 113032. [Google Scholar] [CrossRef]
- Pielou, E. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol. 1966, 10, 370–383. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Ramos, Y.J.; Costa-Oliveira, C.D.; Candido-Fonseca, I.; Queiroz, G.A.D.; Guimarães, E.F.; Defaveri, A.C.A.E.; Sadgrove, N.J.; de Lima Moreira, D. Advanced chemophenetic analysis of essential oil from leaves of Piper gaudichaudianum Kunth (piperaceae) using a new reduction-oxidation index to explore seasonal and circadian rhythms. Plants 2021, 10, 2116. [Google Scholar] [CrossRef]
- Aboutabl, E.A.; El-Tantawy, M.; Shams, M.M. Chemical composition and antimicrobial activity of volatile constituents from the roots, leaves, and seeds of Arctium lappa L. (Asteraceae) grown in Egypt. Egypt. Pharm. J. 2013, 12, 163–172. [Google Scholar] [CrossRef]
- Han, F.; Liu, X.; Li, R.; Zhao, Y.; Li, C.; Li, Y. Chemical composition and antioxidant activities of essential oils from different parts of the oregano. J. Zhejiang Univ. Sci. B 2017, 18, 79. [Google Scholar] [CrossRef]
- Park, C.; Garland, S.M.; Close, D.C. The Effect of the Height of Coppicing and Harvest Season on the Yield and Quality of the Essential Oil of Kunzeaambigua. Plants 2023, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.A.; Magalhães, M.C.F.; Oliveira, R.B.; Sartori, A.L.B. Avaliação do rendimento dos óleos essenciais em espécies de Piper. Mostra Nac. Iniciaç. Cient. Tecnol. Interdiscip. (MICTI) 2020, 1, 13. [Google Scholar]
- Bizzo, H.R.; Rezende, C.M. O mercado de óleos essenciais no Brasil e no mundo na última década. Quím. Nova. 2022, 45, 949–958. [Google Scholar]
- Khalid, K.A.; Darwesh, O.M.; Ahmed, A.M. Peel essential oils of citrus types and their antimicrobial activities in response to various growth locations. J. Essent. Oil Bear. Plants 2021, 24, 480–499. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole., S. Extrinsic factors influencing production of secondary metabolites in plants. In Insect-Plant Interactions; CRC Press: Boca Raton, FL, USA, 2019; pp. 107–134. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Puglielli, G.; Llorens, L.; Pedranzani, H.E.; Chaneton, E.J. Woody plant adaptations to multiple abiotic stressors: Where are we? Flora 2023, 299, 152221. [Google Scholar] [CrossRef]
- Octavia, N.D.; Puspitawati, R.P.; Bashri, A. Characteristics of Anatomical Structure and Essential Oil Glands of Leaf Peppermint (Mentha Piperita) and Spearmint (Mentha Spicata). J. World Sci. 2023, 2, 1314–1329. [Google Scholar] [CrossRef]
- Ergin, K.N.; Erol, E.; Aksoy, H.; Karakaya, S.; Yılmaz, M.A. Anatomical and phytochemical characteristics of different parts of Hypericum scabrum L. Extracts, essential oils, and their antimicrobial potential. Molecules 2022, 27, 1228. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Popescu, D.I.; Ciocan, M.A.; Moldovan, C.; Benedec, D.; Filip, L. Volatile compounds and antioxidant and antifungal activity of bud and needle extracts from three populations of Pinus mugo Turra growing in Romania. Horticulturae 2022, 8, 952. [Google Scholar] [CrossRef]
- Yiğit Hanoğlu, D.; Hanoğlu, A.; Adediran, S.B.; Baser, K.H.C.; Özkum Yavuz, D. The essential oil compositions of two Eucalyptus sp.(E. camaldulensis D ehnh. and E. torquata L uehm.) naturalized to Cyprus. J. Essent. Oil Res. 2022, 35, 136–142. [Google Scholar] [CrossRef]
- da Silva Rivas, A.C.; Lopes, P.M.; de Azevedo Barros, M.M.; Costa Machado, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar]
- Kamaitytė-Bukelskienė, L.; Ložienė, K.; Labokas, J. Dynamics of isomeric and enantiomeric fractions of pinene in essential oil of Picea abies annual needles during growing season. Molecules 2021, 26, 2138. [Google Scholar] [CrossRef]
- Leisner, C.P.; Potnis, N.; Sanz-Saez, A. Crosstalk and trade-offs: Plant responses to climate change-associated abiotic and biotic stresses. Plant Cell Environ. 2022, 46, 2946–2963. [Google Scholar] [CrossRef]
- Dewick, P.M. The biosynthesis of C5–C25 terpenoid compounds. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef] [PubMed]
- Langsi, J.D.; Kuete, V.; Fokou, P.V.T.; Tchoumbougnang, F.; Kengne, A.B. Evaluation of the insecticidal activities of α-pinene and 3-carene on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Insects 2020, 11, 540. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Kuang, S.; Qing, M.; Ma, Y.; Yang, T.; Wang, T.; Li, D. Chemical composition, antioxidant, antibacterial and cholinesterase inhibitory activities of three Juniperus species. Nat. Prod. Res. 2020, 34, 3531–3535. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Gandhi, K.J.; Oliver, J.B.; Schultz, P.B.; Cañas, L.; Herms, D.A. Species dependent influence of (−)-α-pinene on attraction of ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) to ethanol-baited traps in nursery agroecosystems. J. Econ. Entomol. 2011, 104, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Rosengaus, R.B.; Lefebvre, M.L.; Traniello, J.F.A. Inhibition of fungal spore germination by Nasutitermes: Evidence for a possible antiseptic role of soldier defensive secretions. J. Chem. Ecol. 2000, 26, 21–39. [Google Scholar] [CrossRef]
- Jensen, T.G.; Holmstrup, M.; Madsen, R.B.; Glasius, M.; Trac, L.N.; Mayer, P.; Ehlers, B.; Slotsbo, S. Effects of α-pinene on life history traits and stress tolerance in the springtail Folsomia candida. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 229, 108681. [Google Scholar] [CrossRef]
- Jun-Wen, C.H.E.N.; Cao, K.F. Plant VOCs emission: A new strategy of thermotolerance. J. For. Res. 2005, 16, 323–326. [Google Scholar] [CrossRef]
- Chen, M.; Cao, Y.; Zhang, J.; Han, Y. Physiological. biochemical and phytohormone responses of Elymus nutans to α-pinene-induced allelopathy. PeerJ 2022, 10, e14100. [Google Scholar] [CrossRef]
- Singh, H.P.; Kaur, S.; Mittal, S.; Batish, D.R.; Kohli, R.K. α-Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Ishii-Iwamoto, L.E.; Oliveira, V.F.; Gualtieri, S.C.J. Effects of monoterpenes on physiological processes during seed germination and seedling growth. Curr. Bioact. Compd. 2012, 8, 50–64. [Google Scholar] [CrossRef]
- Erb, M.; Köllner, T.G.; Degenhardt, J.; Zwahlen, C.; Hibbard, B.E.; Turlings, T.C. Synergies and trade-offs between insect and pathogen resistance in maize leaves and roots. Plant Cell Environ. 2011, 34, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Kooyers, N.J.; Blackman, B.K.; Holeski, L.M. Optimal defense theory explains deviations from latitudinal herbivory defense hypothesis. Ecology. 2017, 98, 1036–1048. [Google Scholar] [CrossRef]
- Ramos, Y.J.; Felisberto, J.S.; Gouvêa-Silva, J.G.; de Souza., U.C.; da Costa-Oliveira, C.; de Queiroz., G.A.; Guimarães, E.F.; Sadgrove, N.J.; de Lima Moreira., D. Phenoplasticity of essential oils from two species of Piper (Piperaceae): Comparing wild specimens and bi-generational monoclonal cultivars. Plants 2022, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Ullah, C. Plants protect themselves from herbivores by optimizing the distribution of chemical defenses. Proc. Natl. Acad. Sci. USA 2022, 119, e2120277119. [Google Scholar] [CrossRef]
- Ohnmeiss, T.E.; Baldwin, I.T. Optimal defense theory predicts the ontogeny of an induced nicotine defense. Ecology 2000, 81, 1765–1783. [Google Scholar] [CrossRef]
- Gottlieb, O.R. Phytochemicals: Differentiation and function. Phytochemistry 1990, 29, 1715–1724. [Google Scholar] [CrossRef]
- Richards, L.A.; Dyer, L.A.; Forister, M.L.; Smilanich, A.M.; Dodson, C.D.; Leonard, M.D.; Jeffrey, C.S. Phytochemical diversity drives plant–insect community diversity. Proc. Natl. Acad. Sci. USA 2015, 112, 10973–10978. [Google Scholar] [CrossRef]
- Firn, R.D.; Jones, C.G. Natural products–a simple model to explain chemical diversity. Nat. Prod. Rep. 2003, 20, 382–391. [Google Scholar] [CrossRef]
- Zhan, X.; Shen, C. Environmental and genetic factors involved in plant protection-associated secondary metabolite biosynthesis pathways. Front. Plant Sci. 2022, 13, 877304. [Google Scholar] [CrossRef]
- Lee, Y.L.; Ding, P. Production of essential oil in plants: Ontogeny, secretory structures, and seasonal variations. Pertanika J. Scholar. Res. Rev. 2016, 2, 1–10. [Google Scholar]
- Sakthi, A.R.; Selvi, C.; Poorniammal, R. Role of phytohormones in plant defence against insects: Signalling and crosstalk. In Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology; Springer: Singapore, 2021; pp. 215–231. [Google Scholar]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Neilson, E.H.; Goodger, J.Q.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef]
- Ehlers, B.K.; Berg, M.P.; Staudt, M.; Holmstrup, M.; Glasius, M.; Ellers, J.; Tomiolo, S.; Madsen, R.B.; Slotsbo, S.; Penuelas, J. Plant secondary compounds in soil and their role in belowground species interactions. Trends Ecol. Evol. 2020, 35, 716–730. [Google Scholar] [CrossRef]
- Block, A.K.; Hunter, C.T.; Sattler, S.E.; Rering, C.; McDonald, S.; Basset, G.J.; Christensen, S.A. Fighting on two fronts: Elevated insect resistance in flooded maize. Plant Cell Environ. 2020, 1, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Rojas, J.M.; Bassar, R.D.; Matthews, B.; Goldberg, J.F.; King, L.; Reznick, D.; Travis, J. Does the evolution of ontogenetic niche shifts favour species coexistence? An empirical test in Trinidadian streams. J. Anim. Ecol. 2023, 92, 1601–1612. [Google Scholar] [CrossRef]
- de Roos, A.M.; Leonardsson, K.; Persson, L.; Mittelbach, G.G. Ontogenetic niche shifts and flexible behavior in size-structured populations. Ecol. Monogr. 2002, 72, 271–292. [Google Scholar] [CrossRef]
- Fokkema, W.; van der Jeugd, H.P.; Lameris, T.K.; Dokter, A.M.; Ebbinge, B.S.; de Roos, A.M.; Nolet, B.A.; Piersma, T.; Olff, H. Ontogenetic niche shifts as a driver of seasonal migration. Oecologia 2020, 193, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Glassmire, A.E.; Jeffrey, C.S.; Forister, M.L.; Parchman, T.L.; Nice, C.C.; Jahner, J.P.; Wilson, J.S.; Walla, T.R.; Richards, L.A.; Smilanich, A.M.; et al. Intraspecific phytochemical variation shapes community and population structure for specialist caterpillars. New Phytol. 2016, 212, 208–219. [Google Scholar] [CrossRef]
- Schneider, G.F.; Salazar, D.; Hildreth, S.B.; Helm, R.F.; Whitehead, S.R. Comparative metabolomics of fruits and leaves in a hyperdiverse lineage suggests fruits are a key incubator of phytochemical diversification. Front. Plant Sci. 2021, 12, 693739. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.A. Evolutionary diversification of primary metabolism and its contribution to plant chemical diversity. Front. Plant Sci. 2019, 10, 469456. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions. biosynthesis. modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef] [PubMed]
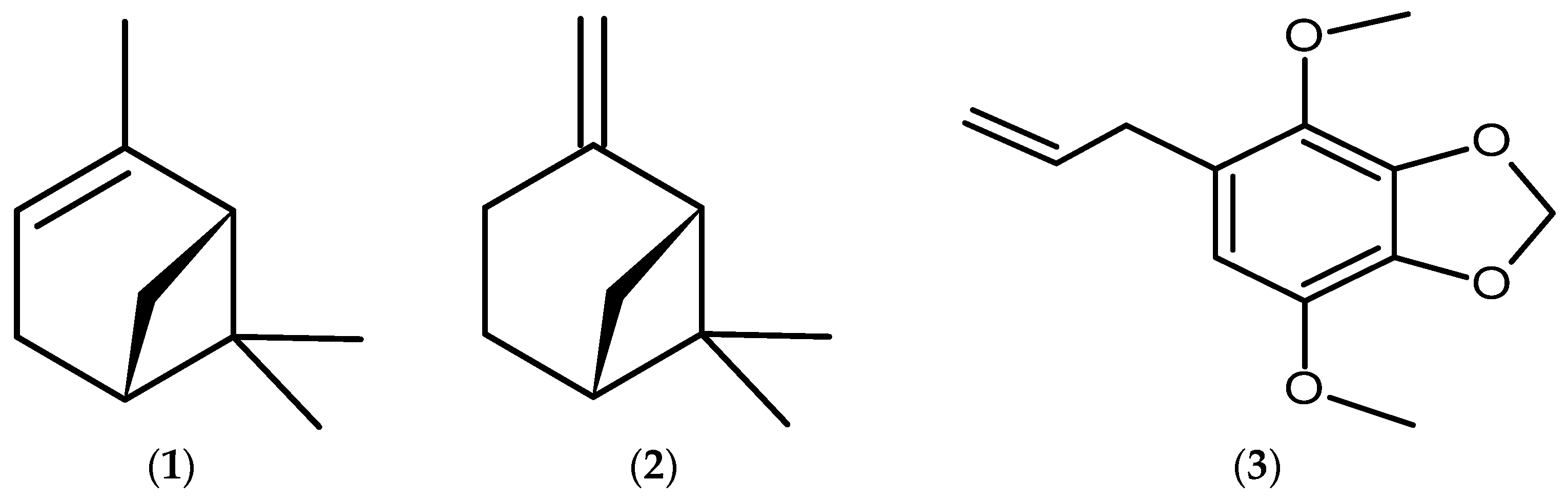
| Class | Compounds # | RIlit | RIcal | Molecular Formula | Relative Percentage (Mean ± Standard Deviation) * | |||
|---|---|---|---|---|---|---|---|---|
| Roots | Steams | Branches | Leaves | |||||
| NO | Santoline triene | 906 | 906 | C10H16 | - | - | 0.15 ± 0.10 | - |
| NO | α-Thujene | 924 | 924 | C10H16 | 2.68 ± 0.03 | - | - | 0.47 ± 0.03 |
| NO | α-Pinene | 932 | 933 | C10H16 | 1.07 ± 0.05 | 1.47 ± 0.06 | 20.87 ± 0.21 | 31.90 ± 0.44 |
| NO | Camphene | 946 | 945 | C10H16 | 0.04 ± 0.02 | - | 0.24 ± 0.40 | 1.68 ± 0.22 |
| NO | Sabinene | 969 | 969 | C10H16 | 0.51 ± 0.03 | - | - | 0.37 ± 0.03 |
| NO | β-Pinene | 974 | 973 | C10H16 | 0.23 ± 0.01 | - | 64.61 ± 0.22 | 20.96 ± 0.47 |
| NO | Myrcene | 988 | 987 | C10H16 | 0.43 ± 0.02 | - | - | 3.23 ± 0.50 |
| NO | α-Phelandrene | 1002 | 1000 | C10H16 | 0.12 ± 0.04 | - | - | 0.63 ± 0.18 |
| NO | α-Terpinene | 1014 | 1012 | C10H16 | - | - | 0.03 ± 0.04 | 0.24 ± 0.17 |
| NO | p-Cymene | 1020 | 1020 | C10H16 | 0.64 ± 0.03 | - | - | 0.35 ± 0.61 |
| NO | Limonene | 1024 | 1022 | C10H16 | 0.59 ± 0.03 | - | 3.76 ± 0.45 | 1.80 ± 0.19 |
| NO | β-Phellandrene | 1025 | 1024 | C10H16 | 0.13 ± 0.07 | - | - | 1.06 ± 0.05 |
| NO | Z-β-Ocimene | 1032 | 1034 | C10H16 | - | - | 0.29 ± 0.04 | - |
| NO | E-β-Ocimene | 1044 | 1048 | C10H16 | - | - | 0.42 ± 0.02 | 0.20 ± 0.10 |
| NO | γ-Terpinene | 1054 | 1055 | C10H16 | 0.1 ± 0.01 | - | 0.52 ± 0.03 | 0.35 ± 0.02 |
| OM | Z-Sabinene hydrate | 1065 | 1068 | C10H18O | - | - | 0.99 ± 0.22 | 0.08 ± 0.01 |
| OT | 3-Isopropyl-2-methoxypyrazine | 1090 | 1094 | C8H12N2O | 0.05 ± 0.01 | - | - | - |
| OM | Linalool | 1095 | 1096 | C10H22O | - | - | 1.73 ± 0.05 | 1.83 ± 0.04 |
| OM | E-Sabinene hydrate | 1098 | 1097 | C12H20O2 | - | - | - | 0.24 ± 0.01 |
| OM | iso-3-Turjanol | 1134 | 1132 | C10H18O | 0.04 ± 0.01 | - | - | - |
| OM | Z-p-Menth-2-en-ol | 1136 | 1136 | C10H18O | - | - | - | 0.14 ± 0.01 |
| OM | Camphor | 1141 | 1142 | C10H16O | 0.76 ± 0.02 | - | - | - |
| OM | α-Terpineol | 1186 | 1187 | C10H18O | - | - | 0.93 ± 0.13 | - |
| OM | Verbenone | 1204 | 1208 | C10H14O | - | - | 0.12 ± 0.07 | - |
| OM | Piperitone | 1249 | 1251 | C10H16O | 0.17 ± 0.04 | - | - | - |
| AR | E-Anetole | 1282 | 1285 | C10H12O | - | - | 0.53 ± 0.04 | 0.15 ± 0.34 |
| AR | Safrole | 1285 | 1289 | C10H10O2 | 0.08 ± 0.01 | - | - | - |
| OM | Z-Sabinyl acetate | 1289 | 1294 | C12H18O2 | - | - | 0.12 ± 0.02 | - |
| OM | Tujanol acetate | 1295 | 1298 | C12H20O2 | - | - | 0.44 ± 0.03 | - |
| NS | δ-Elemene | 1335 | 1339 | C15H24 | - | - | 0.33 ± 0.01 | 0.14 ± 0.17 |
| OS | α-Terpinyl acetate | 1346 | 1348 | C12H20O2 | - | - | - | 1.54 ± 0.04 |
| OS | Neryl acetate | 1359 | 1362 | C12H20O2 | - | - | 0.21 ± 0.04 | 0.35 ± 0.02 |
| NS | α-Copaene | 1374 | 1375 | C15H24 | - | - | - | 0.40 ± 0.22 |
| NS | Isoledene | 1374 | 1378 | C15H25 | 1.02 ± 0.03 | - | - | 1.48 ± 0.32 |
| NS | α-Cubebene | 1376 | 1380 | C15H26 | 0.17 ± 0.02 | - | - | - |
| OS | Mirtanol acetate | 1385 | 1386 | C12H20O2 | - | - | 0.26 ± 0.05 | - |
| NS | β-Cubebene | 1387 | 1392 | C15H24 | 0.05 ± 0.01 | - | - | - |
| NS | Ciperene | 1398 | 1403 | C15H24 | - | - | - | 0.21 ± 0.21 |
| NS | Sibirene | 1400 | 1409 | C15H24 | 0.10 ± 0.01 | - | - | - |
| NS | E-Caryophyllene | 1417 | 1426 | C15H24 | 0.13 ± 0.03 | - | 1.30 ± 0.04 | 3.03 ± 0.24 |
| NS | β-Copaene | 1430 | 1448 | C15H24 | 0.04 ± 0.02 | - | - | - |
| NS | β-Gurjunene | 1433 | 1450 | C15H24 | 0.28 ± 0.01 | - | - | - |
| NS | Aromadendrene | 1439 | 1452 | C15H24 | - | - | 0.28 ± 0.02 | 2.41 ± 0.18 |
| NS | Myltayl-4(12)-ene | 1445 | 1444 | C15H24 | - | - | - | 0.16 ± 0.02 |
| NS | Muurola-3,5-diene | 1448 | 1452 | C15H24 | - | - | 0.10 ± 0.01 | 1.31 ± 0.13 |
| NS | α-Humulene | 1452 | 1454 | C15H24 | - | - | - | 0.24 ± 0.02 |
| AR | Croweacin | 1457 | 1563 | C11H12O3 | 0.05 ± 0.01 | - | - | - |
| NS | allo-Aromadendrene | 1458 | 1465 | C15H24 | - | - | 0.17 ± 0.03 | - |
| NS | Z-Cadina-1(6),4-diene | 1461 | 1467 | C15H24 | - | - | - | 0.28 ± 0.03 |
| NS | γ-Gurjunene | 1475 | 1478 | C15H24 | 0.27 ± 0.02 | - | - | - |
| NS | γ-Muurolene | 1479 | 1481 | C15H24 | 0.25 ± 0.02 | - | - | - |
| NS | Germacrene D | 1480 | 1490 | C15H24 | 0.10 ± 0.01 | - | - | - |
| NS | E-Muurola-4(14),5-diene | 1493 | 1492 | C15H24 | 0.26 ± 0.01 | - | - | - |
| OS | epi-Cubebol | 1493 | 1499 | C15H26O | 1.06 ± 0.04 | - | - | 1.33 ± 0.32 |
| NS | Bicyclogermacrene | 1500 | 1507 | C15H24 | - | - | 0.23 ± 0.02 | 5.90 ± 0.68 |
| NS | E-β-Guaienol | 1502 | 1509 | C15H24 | 0.46 ± 0.03 | - | - | - |
| NS | δ-Amorphene | 1511 | 1512 | C15H24 | 0.46 ± 0.01 | - | 0.12 ± 0.01 | 0.10 ± 0.08 |
| NS | γ-Cadinene | 1513 | 1512 | C15H24 | 9.73 ± 0.07 | - | - | 0.70 ± 0.02 |
| OS | Cubebol | 1514 | 1514 | C15H26O | - | - | - | 0.57 ± 0.06 |
| AR | Myristicin | 1517 | 1519 | C11H12O3 | 0.05 ± 0.01 | - | - | - |
| NS | E-Calamene | 1521 | 1525 | C15H22 | - | - | - | 4.04 ± 0.21 |
| NS | β-Sesquifelandrene | 1521 | 1529 | C15H24 | - | 0.94 ± 0.12 | 0.42 ± 0.03 | - |
| NS | δ-Cadinene | 1522 | 1530 | C15H24 | - | - | - | 0.56 ± 0.22 |
| NS | E-Cadina-1,4-diene | 1533 | 1540 | C15H24 | 0.06 ± 0.01 | - | - | - |
| NS | α-Cadinene | 1537 | 1542 | C15H24 | - | - | - | 0.56 ± 0.09 |
| OS | Elemol | 1548 | 1550 | C15H26O | - | - | - | 0.07 ± 0.01 |
| NS | β-Calacorene | 1564 | 1566 | C15H20 | 0.59 ± 0.02 | - | - | - |
| AR | Isoelemicin | 1568 | 1569 | C12H16O3 | 0.03 ± 0.01 | - | - | - |
| AR | γ-Asarone | 1572 | 1574 | C12H16O3 | 0.05 ± 0.01 | - | - | - |
| OS | Spathulenol | 1577 | 1581 | C15H24O | 0.03 ± 0.01 | - | - | 2.30 ± 0.15 |
| OS | Caryophyllene oxyde | 1582 | 1580 | C15H24O | 0.17 ± 0.03 | - | - | 0.15 ± 0.01 |
| OS | Globulol | 1590 | 1585 | C15H26O | - | - | 0.42 ± 0.04 | 2.76 ± 0.55 |
| OS | Viridiflorol | 1592 | 1594 | C15H26O | - | - | - | 0.34 ± 0.02 |
| OS | Cubeban-11-ol | 1595 | 1596 | C15H26O | - | - | - | 0.17 ± 0.01 |
| AR | 6-Methoxyelemicin | 1595 | 1599 | C13H18O4 | 0.64 ± 0.03 | - | - | - |
| OS | Rosifoliol | 1600 | 1600 | C15H26O | - | 3.03 ± 0.03 | - | 0.41 ± 0.37 |
| OS | Guaiol | 1600 | 1603 | C15H26O | 3.89 ± 0.06 | - | - | - |
| OS | 1,10-di-epi-Cubenol | 1618 | 1619 | C15H26O | - | - | - | 0.26 ± 0.10 |
| AR | Dillapiole | 1620 | 1628 | C12H14O4 | 0.83 ± 0.03 | 34.12 ± 0.46 | - | - |
| OS | α-Muurolol | 1644 | 1640 | C15H26O | 0.17 ± 0.02 | 1.11 ± 0.19 | - | 0.94 ± 0.03 |
| OS | Cubenol | 1645 | 1647 | C15H26O | 0.86 ± 0.02 | - | - | 0.14 ± 0.02 |
| OS | Agarospirol | 1646 | 1654 | C15H26O | 0.12 ± 0.01 | - | - | - |
| OS | α-Cadinol | 1652 | 1659 | C15H26O | 0.04 ± 0.03 | - | - | 0.29 ± 0.01 |
| OS | Selin-11-en-4-α-ol | 1658 | 1667 | C15H26O | - | - | - | - |
| AR | Apiole | 1677 | 1689 | C12H14O4 | 69.68 ± 0.18 | 59.32 ± 0.24 | - | - |
| OS | Amorfa-4,9-dien-2-ol | 1700 | 1718 | C15H24O | 0.14 ± 0.01 | - | - | - |
| OS | 5-Hydroxy-Z-calamenene | 1713 | 1722 | C15H22O | 0.03 ± 0.01 | - | - | 0.11 ± 0.02 |
| Number of compounds identified | 49 | 6 | 27 | 50 | ||||
| Total Quantified Compounds | 99.45 | 99.99 | 99.59 | 98.93 | ||||
| Non-Oxygenated Monoterpenes (NO) | 6.54 | 1.47 | 90.89 | 63.24 | ||||
| Oxygenated Monoterpenes (OM) | 0.97 | 0.00 | 4.33 | 2.29 | ||||
| Non-Oxygenated Sesquiterpenes (NS) | 13.97 | 0.94 | 2.95 | 21.52 | ||||
| Oxigenated Sesquiterpenes (OS) | 6.51 | 4.14 | 0.89 | 11.73 | ||||
| Arylpropanoids (AR) | 71.41 | 93.44 | 0.53 | 0.15 | ||||
| Other (OT) | 0.05 | 0.00 | 0.00 | 0.00 | ||||
| Yield of EO% (w/w) | 0.57 | 0.05 | 0.31 | 0.82 | ||||
| Class | Constituintes # | RIlit | RIcal | Molecular Formula | Relative Percentage (Mean ± Standard Deviation) * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase I | Phase II | Phase III | Phase IV | Phase V | ||||||
| NO | Tricyclene | 921 | 921 | C10H16 | - | - | 1.2 ± 0.06 | - | - | |
| NO | Artemisiatiene | 923 | 923 | C10H16 | - | - | 2.03 ± 0.04 | - | - | |
| NO | α-Thujene | 924 | 926 | C10H16 | - | 0.02 ± 0.63 | 5.90 ± 0.53 | 0.21 ± 0.01 | 0.54 ± 0.06 | |
| NO | α-Pinene | 932 | 933 | C10H16 | 0.62 ± 0.66 | 1.68 ± 0.93 | 20.88 ± 0.73 | 20.03 ± 0.14 | 35.09 ± 0.68 | |
| NO | Camphene | 946 | 945 | C10H16 | 0.12 ± 0.02 | 0.29 ± 0.22 | 1.27 ± 0.13 | 0.70 ± 0.53 | 1.79 ± 0.20 | |
| NO | Sabinene | 969 | 970 | C10H16 | - | - | - | 1.03 ± 0.05 | 0.77 ± 0.20 | |
| NO | β-Pinene | 974 | 975 | C10H16 | 3.58 ± 0.32 | 7.88 ± 0.28 | 11.45 ± 0.12 | 16.72 ± 0.62 | 14.48 ± 0.44 | |
| NO | Myrcene | 988 | 990 | C10H16 | 0.33 ± 0.07 | 0.84 ± 0.04 | 1.54 ± 0.06 | 2.55 ± 0.99 | 3.30 ± 0.89 | |
| NO | α-Phellandrene | 1002 | 1002 | C10H16 | - | 0.39 ± 0.33 | 0.95 ± 0.23 | - | - | |
| NO | δ-2-Carene | 1008 | 1003 | C10H16 | 7.18 ± 0.17 | 16.07 ± 0.06 | 0.32 ± 0.03 | 42.07 ± 0.78 | 0.80 ± 0.60 | |
| NO | δ-3-Carene | 1008 | 1010 | C10H16 | - | 0.17 ± 0.92 | 9.91 ± 0.50 | 0.20 ± 1.21 | 0.38 ± 1.12 | |
| NO | α-Terpinene | 1014 | 1015 | C10H16 | - | - | - | - | - | |
| NO | p-Cymene | 1020 | 1022 | C10H16 | 0.12 ± 0.02 | 0.14 ± 0.01 | 0.14 ± 0.21 | 1.17 ± 0.01 | 0.58 ± 0.59 | |
| NO | Limonene | 1024 | 1024 | C10H16 | 0.45 ± 0.06 | 0.16 ± 0.01 | 1.55 ± 0.33 | 1.85 ± 0.09 | 1.34 ± 0.38 | |
| NO | β-Phellandrene | 1025 | 1026 | C10H16 | - | - | 0.10 ± 0.42 | 0.83 ± 0.02 | 1.27 ± 0.16 | |
| NO | β-Ocimene | 1032 | 1029 | C10H16 | - | - | 0.14 ± 0.62 | - | - | |
| NO | Z-β-Ocimene | 1037 | 1033 | C10H16 | - | 0.61 ± 0.87 | - | 0.04 ± 0.01 | - | |
| NO | E-β-Ocimene | 1044 | 1040 | C10H16 | - | 0.40 ± 0.71 | - | 0.07 ± 0.01 | - | |
| NO | γ-Terpinene | 1054 | 1059 | C10H16 | - | - | - | 0.20 ± 0.01 | 0.40 ± 0.16 | |
| OM | Z-Sabinenehydrate | 1065 | 1061 | C10H18O | - | - | - | 0.02 ± 0.23 | 0.17 ± 0.25 | |
| NO | p-Mentha-2,4(8)-diene | 1085 | 1080 | C10H16 | - | - | - | 0.08 ± 0.91 | 0.33 ± 0.16 | |
| OM | Terpinolene | 1086 | 1088 | C10H18O | - | - | - | 1.58 ± 0.11 | - | |
| NO | p-Cymenene | 1089 | 1094 | C10H12 | - | - | - | 0.16 ± 0.72 | - | |
| OM | Linalool | 1095 | 1096 | C10H22O | - | - | - | 0.84 ± 0.83 | 1.67 ± 0.16 | |
| OM | cis-p-Menth-2-en-1-ol | 1118 | 1112 | C10H18O | - | - | - | 0.04 ± 0.01 | - | |
| OM | E-Sabinol | 1137 | 1134 | C10H16O | - | - | - | 0.03 ± 0.02 | - | |
| OM | Isopulegol | 1155 | 1153 | C10H18O | - | - | - | 0.75 ± 0.01 | - | |
| OM | p-Mentha-1,5-dien-8-ol | 1166 | 1163 | C10H16O | - | - | - | 0.05 ± 0.98 | - | |
| OM | Terpinen-4-ol | 1174 | 1172 | C10H18O | - | - | - | 0.09 ± 0.42 | 0.34 ± 0.16 | |
| OM | α-Terpineol | 1186 | 1188 | C10H18O | 0.27 ± 1.05 | - | - | 0.03 ± 0.10 | - | |
| OM | Piperitol | 1195 | 1192 | C10H18O | - | - | - | 0.09 ± 0.32 | - | |
| OM | Pulegenol | 1233 | 1230 | C10H18O | - | - | - | 0.07 ± 0.91 | - | |
| AR | E-Anethole | 1282 | 1280 | C10H12O | - | - | - | 0.39 ± 0.42 | 0.45 ± 0.16 | |
| OM | α-Terpinylacetate | 1346 | 1340 | C12H20O2 | - | - | - | - | 3.19 ± 0.23 | |
| AR | Eugenol | 1356 | 1351 | C10H12O2 | - | 0.54 ± 0.63 | - | - | - | |
| NS | δ-Elemene | 1335 | 1335 | C15H24 | 0.18 ± 0.71 | - | 0.24 ± 0.82 | 0.07 ± 0.75 | - | |
| NI | - | - | - | - | - | - | 0.54 ± 1.91 | 0.11 ± 0.02 | ||
| NS | α-Cubebene | 1348 | 1346 | C15H24 | - | - | - | - | 0.76 ± 0.10 | |
| OS | Nerylacetate | 1359 | 1356 | C12H20O2 | - | - | - | 0.08 ± 0.91 | 0.17 ± 0.05 | |
| NS | α-Copaene | 1374 | 1370 | C15H24 | 0.16 ± 0.43 | - | 0.32 ± 1.31 | 0.03 ± 0.76 | 0.15 ± 0.06 | |
| NS | β-Cubebene | 1387 | 1384 | C15H24 | - | - | - | - | 0.10 ± 0.81 | |
| NS | β-Elemene | 1389 | 1386 | C15H24 | - | - | 0.78 ± 0.02 | - | - | |
| NS | α-Gurjunene | 1409 | 1416 | C15H24 | - | - | 0.08 ± 0.01 | - | 0.24 ± 0.02 | |
| NS | E-Caryophyllene | 1417 | 1420 | C15H24 | 3.42 ± 0.41 | 3.04 ± 0.06 | 6.68 ± 0.12 | 1.53 ± 0.17 | 5.27 ± 0.40 | |
| NS | β-Cedrene | 1419 | 1023 | C15H24 | - | 0.12 ± 0.05 | 0.17 ± 0.71 | - | 0.10 ± 0.91 | |
| NS | β-Copaene | 1430 | 1428 | C15H24 | 0.18 ± 0.04 | 0.1 ± 0.91 | - | - | - | |
| NS | β-Gurjunene | 1431 | 1429 | C15H24 | - | 0.08 ± 0.91 | - | - | 0.12 ± 0.31 | |
| NS | γ-Elemene | 1434 | 1431 | C15H24 | - | 0.11 ± 0.83 | - | - | - | |
| NS | Aromadendrene | 1439 | 1440 | C15H24 | - | - | - | 0.20 ± 0.71 | 2.47 ± 0.34 | |
| NS | β-Barbatene | 1440 | 1440 | C15H24 | - | - | - | - | 0.24 ± 0.54 | |
| NS | 6,9-Guaiadiene | 1442 | 1441 | C15H24 | - | - | - | 0.09 ± 0.99 | 0.50 ± 0.22 | |
| NS | α-Humulene | 1452 | 1455 | C15H24 | - | - | - | 0.07 ± 0.76 | 0.42 ± 0.98 | |
| NS | allo-Aromadendrene | 1458 | 1460 | C15H24 | - | - | - | - | 0.19 ± 0.73 | |
| NS | cis-Cadina-1(6),4-diene | 1461 | 1463 | C15H24 | - | - | - | 0.09 ± 0.62 | ||
| NS | trans-Cadina-1(6),4-diene | 1475 | 1477 | C15H24 | - | - | - | - | 0.19 ± 0.81 | |
| NS | Muurola-4(14),5-diene | 1465 | 1464 | C15H24 | - | - | - | 0.10 ± 0.61 | - | |
| NS | Cumacrene | 1470 | 1466 | C15H24 | - | 0.98 ± 0.91 | - | - | - | |
| NS | γ-Gurjunene | 1475 | 1485 | C15H24 | - | - | - | - | 0.60 ± 0.13 | |
| NS | γ-Muurolene | 1478 | 1486 | C15H24 | - | - | - | - | 11.15 ± 0.28 | |
| NS | Amopha-4,7(11)-diene | 1479 | 1489 | C15H24 | - | - | 0.17 ± 0.21 | - | 0.17 ± 0.88 | |
| NS | Germacrene D | 1480 | 1479 | C15H24 | - | - | 2.09 ± 0.72 | - | 0.49 ± 0.73 | |
| NS | δ-Selinene | 1492 | 1495 | C15H24 | - | - | - | - | - | |
| NS | Bicyclogermacrene | 1500 | 1506 | C15H24 | - | - | 5.58 ± 0.22 | 0.81 ± 0.31 | - | |
| NS | δ-Amorphene | 1511 | 1514 | C15H24 | - | - | - | 0.09 ± 0.71 | - | |
| NS | γ-Cadinene | 1513 | 1515 | C15H24 | - | - | - | 0.07 ± 0.93 | 0.55 ± 0.87 | |
| NS | β-Curcumene | 1514 | 1419 | C15H24 | - | - | - | 0.26 ± 0.99 | - | |
| NS | Calamene | 1521 | 1523 | C15H24 | - | - | 2.28 ± 0.12 | - | - | |
| NS | cis-Calamenene | 1528 | 1528 | C15H24 | - | - | - | 0.76 ± 0.01 | 1.18 ± 0.03 | |
| NS | δ-Cadinene | 1522 | 1528 | C15H24 | - | - | - | - | - | |
| NS | Cadina-1,4-diene | 1528 | 1533 | C15H24 | - | 0.11 ± 0.22 | 0.09 ± 0.21 | 0.06 ± 0.97 | - | |
| NS | γ-Cuprenene | 1532 | 1535 | C15H24 | - | - | - | - | 0.29 ± 0.03 | |
| NS | Selina-3,7(11)-diene | 1545 | 1548 | C15H24 | - | - | - | 0.10 ± 0.21 | 0.19 ± 0.04 | |
| NS | trans-Dauca-4(11),7-diene | 1557 | 1563 | C15H24 | - | - | - | - | 0.52 ± 0.03 | |
| OS | Spathulenol | 1577 | 1579 | C15H24O | - | - | 0.32 ± 0.41 | 0.77 ± 0.41 | 2.12 ± 0.05 | |
| OS | CaryophylleneOxide | 1582 | 1580 | C15H24O | - | - | - | 0.04 ± 0.65 | 0.10 ± 0.02 | |
| OS | Globulol | 1590 | 1587 | C15H26O | - | 0.16 ± 0.05 | 0.12 ± 0.21 | 0.95 ± 0.37 | 2.25 ± 0.08 | |
| OS | Carotol | 1594 | 1598 | C15H26O | - | - | - | 0.07 ± 0.81 | 0.48 ± 0.93 | |
| OS | Rosifoliol | 1600 | 1605 | C15H26O | - | - | - | 0.06 ± 0.78 | 0.33 ± 0.55 | |
| OS | 5-epi-7-epi-α-Eudesmol | 1607 | 1606 | C15H26O | - | - | 0.10 ± 0.02 | 0.22 ± 0.56 | ||
| OS | 1,10-di-epi-Cubenol | 1618 | 1616 | C15H26O | 0.14 ± 0.02 | - | - | 0.35 ± 0.48 | 0.08 ± 0.75 | |
| OS | 1-epi-Cubenol | 1627 | 1631 | C15H26O | - | 0.31 ± 1.01 | - | 0.05 ± 0.91 | 0.10 ± 0.35 | |
| OS | epi-α-Muurolol | 1640 | 1639 | C15H26O | - | - | - | 0.18 ± 1.21 | 0.55 ± 0.92 | |
| OS | α-Muurolol | 1644 | 1640 | C15H26O | - | - | - | - | 0.08 ± 0.05 | |
| OS | Cubenol | 1645 | 1641 | C15H26O | - | - | - | - | 0.29 ± 0.05 | |
| OS | Nerolidylacetate | 1676 | 1679 | C17H28O2 | - | - | 0.1 ± 0.04 | - | - | |
| AR | Croweacin | 1457 | 1454 | C11H12O3 | - | 0.14 ± 0.05 | - | - | - | |
| AR | Myristicin | 1517 | 1518 | C11H12O3 | - | - | 2.79 ± 0.57 | - | - | |
| AR | E-Carpacin | 1593 | 1591 | C11H12O3 | 0.27 ± 0.05 | - | - | - | - | |
| AR | 6-Methoxy-elemicin | 1595 | 1593 | C13H18O4 | 0.70 ± 0.03 | - | - | - | - | |
| AR | Isomyristicin | 1616 | 1614 | C11H12O3 | 3.59 ± 0.17 | 2.69 ± 0.78 | - | - | - | |
| AR | Z-Asarone | 1617 | 1615 | C12H16O3 | 0.12 ± 0.04 | 0.31 ± 0.22 | - | - | - | |
| OT | Butylanthranilate | 1617 | 1615 | C11H15NO2 | 0.13 ± 0.92 | - | - | - | - | |
| AR | Dillapiole | 1620 | 1618 | C12H14O4 | 2.76 ± 0.17 | 2.29 ± 0.18 | 0.85 ± 0.83 | - | - | |
| AR | Apiole | 1677 | 1674 | C12H14O4 | 74.69 ± 0.48 | 59.59 ± 0.56 | 15.65 ± 0.47 | - | - | |
| AR | Niranin | 1715 | 1713 | C11H15NOS | - | - | 0.49 ± 0.39 | - | - | |
| OT | NI | 1600 | 1597 | C16H34 | 0.24 ± 0.02 | - | - | - | - | |
| OT | 4-epi-Abietol | 2343 | 2345 | C20H32O | - | - | 0.16 ± 0.51 | - | - | |
| OT | Libocedrol | 2344 | 2348 | C22H30O4 | - | - | 0.08 ± 0.41 | - | - | |
| OT | Heyderiol | 2390 | 2397 | C22H30O4 | - | - | 0.02 ± 0.71 | - | - | |
| Number of compounds identified | 20 | 27 | 34 | 51 | 54 | |||||
| Total Quantified Compounds | 99.25 | 99.22 | 96.34 | 99.06 | 99.23 | |||||
| Non-Oxygenated Monoterpenes (NO) | 12.40 | 28.65 | 57.38 | 87.91 | 61.07 | |||||
| Oxygenated Monoterpenes (OM) | 0.27 | 0.00 | 0.00 | 3.59 | 5.37 | |||||
| Oxigenated Sesquiterpenes (OS) | 0.14 | 4.54 | 18.38 | 4.78 | 25.98 | |||||
| Non-Oxygenated Sesquiterpenes (NS) | 3.94 | 0.47 | 0.54 | 2.65 | 6.77 | |||||
| Arilpropanoids (AR) | 82.13 | 65.56 | 19.78 | 0.39 | 0.45 | |||||
| Others (OT) | 0.37 | 0.00 | 0.26 | 0.00 | 0.00 | |||||
| Yield of EO (w/w) | 0.89 | 0.93 | 0.76 | 0.92 | 0.87 | |||||
| Sample Compared | Jaccard Index | Sorensen Index |
|---|---|---|
| roots × steams | 5.77 | 10.91 |
| roots × branches | 10.14 | 18.00 |
| roots × leaves | 28.57 | 44.00 |
| steams × branches | 6.00 | 12.00 |
| steams × leaves | 4.00 | 7.00 |
| branches × leaves | 31.00 | 20.50 |
| phase I × phase II | 6.82 | 12.77 |
| phase I × phase III | 3.85 | 7.00 |
| phase I × phase IV | 18.33 | 31.00 |
| phase I × phase V | 15.63 | 27.00 |
| phase II × phase III | 36.00 | 52.00 |
| phase II × phase IV | 24.00 | 38.00 |
| phase II × phase IV | 21.00 | 35.00 |
| phase III × phase IV | 25.00 | 40.00 |
| phase III × phase V | 26.00 | 41.00 |
| phase IV × phase V | 75.00 | 86.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felisberto, J.S.; Machado, D.B.; Assunção, J.A.S.; Massau, S.A.S.; Queiroz, G.A.d.; Guimarães, E.F.; Ramos, Y.J.; Moreira, D.d.L. Spatio-Temporal Variations of Volatile Metabolites as an Eco-Physiological Response of a Native Species in the Tropical Forest. Plants 2024, 13, 2599. https://doi.org/10.3390/plants13182599
Felisberto JS, Machado DB, Assunção JAS, Massau SAS, Queiroz GAd, Guimarães EF, Ramos YJ, Moreira DdL. Spatio-Temporal Variations of Volatile Metabolites as an Eco-Physiological Response of a Native Species in the Tropical Forest. Plants. 2024; 13(18):2599. https://doi.org/10.3390/plants13182599
Chicago/Turabian StyleFelisberto, Jéssica Sales, Daniel B. Machado, Jeferson A. S. Assunção, Samik A. S. Massau, George A. de Queiroz, Elsie F. Guimarães, Ygor J. Ramos, and Davyson de Lima Moreira. 2024. "Spatio-Temporal Variations of Volatile Metabolites as an Eco-Physiological Response of a Native Species in the Tropical Forest" Plants 13, no. 18: 2599. https://doi.org/10.3390/plants13182599
APA StyleFelisberto, J. S., Machado, D. B., Assunção, J. A. S., Massau, S. A. S., Queiroz, G. A. d., Guimarães, E. F., Ramos, Y. J., & Moreira, D. d. L. (2024). Spatio-Temporal Variations of Volatile Metabolites as an Eco-Physiological Response of a Native Species in the Tropical Forest. Plants, 13(18), 2599. https://doi.org/10.3390/plants13182599







