Design and Characterization of Liposomal-Based Carriers for the Encapsulation of Rosa canina Fruit Extract: In Vitro Gastrointestinal Release Behavior
Abstract
:1. Introduction
2. Results and Discussion
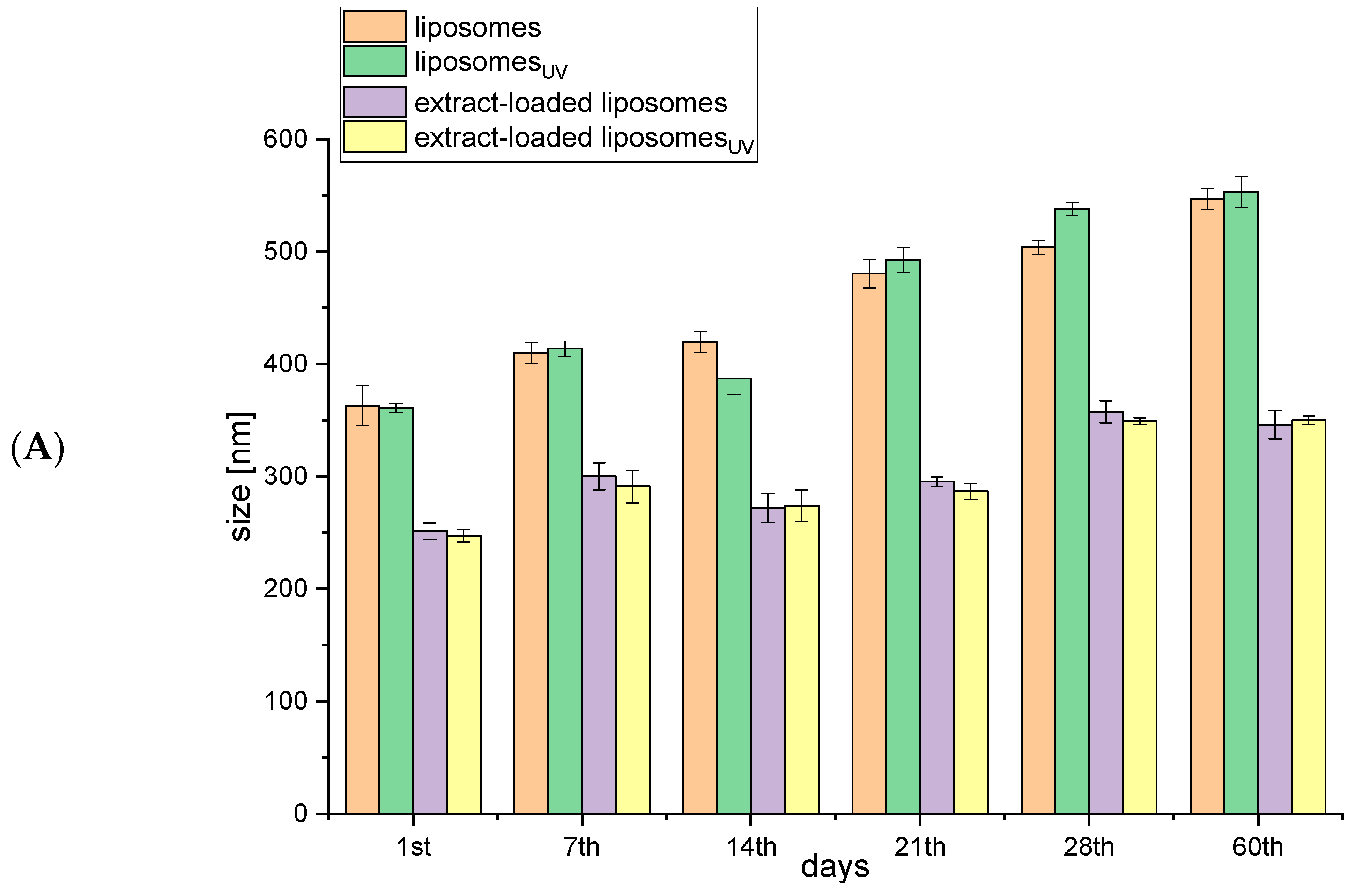
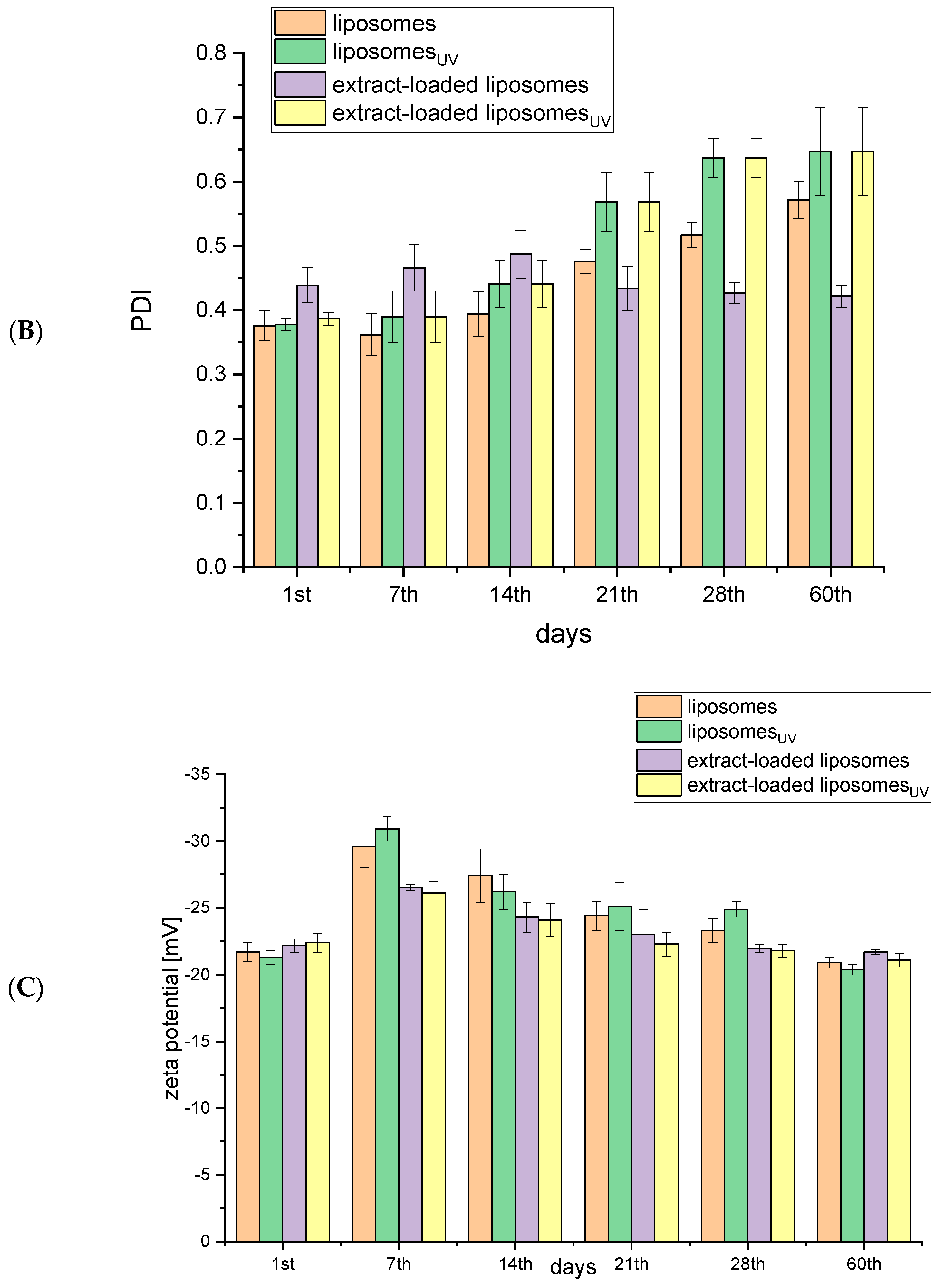
2.1. Storage Stability of Liposomes
2.2. Physical Properties of Rosa canina Fruit Extract and Liposomes
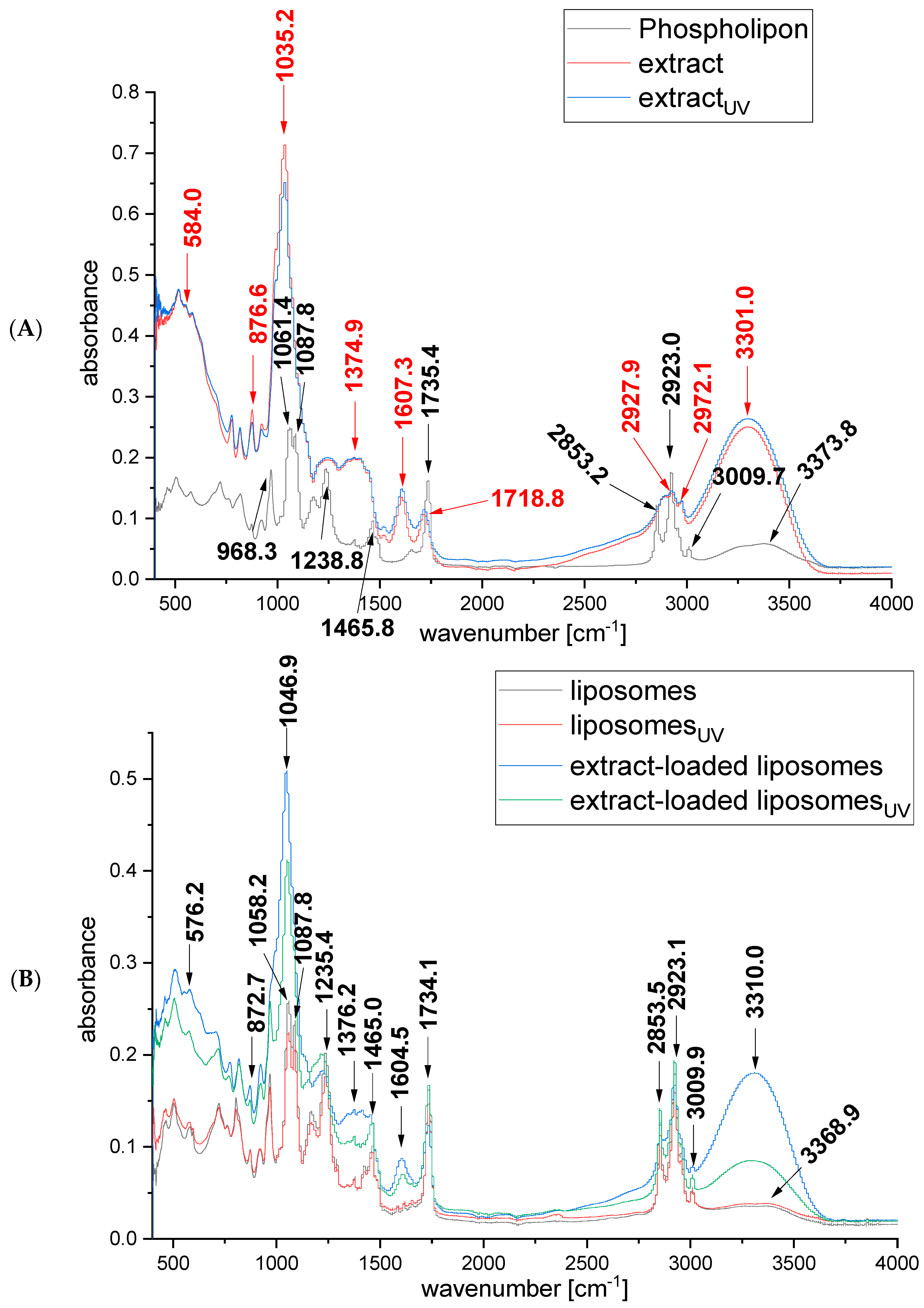
2.3. FTIR Spectra of Rosa canina Fruit Extract and Liposomes
2.4. Polyphenol Profile of Rosa canina Fruit Extract and Extract-Loaded Liposomes
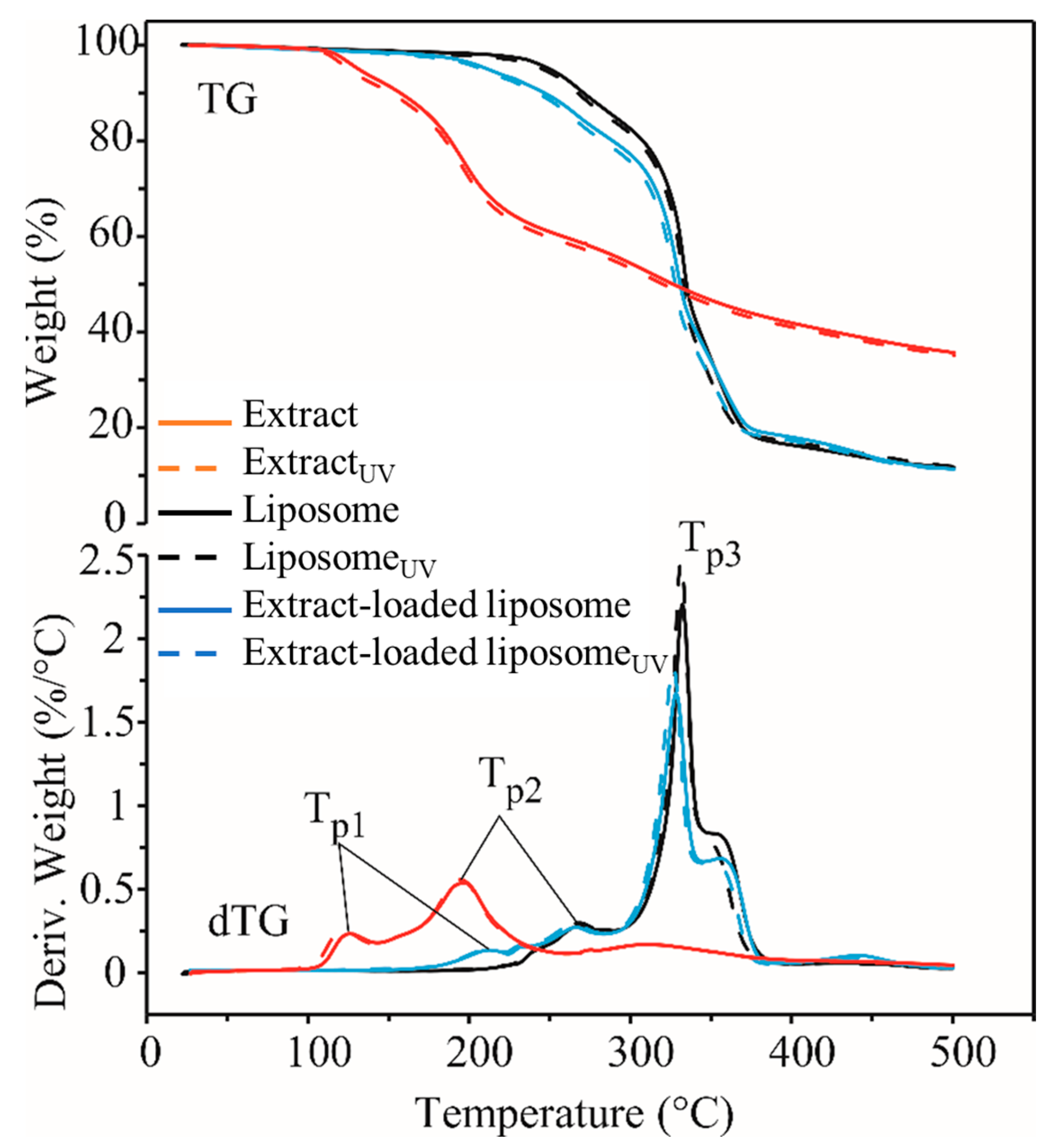
2.5. Thermal Stability of Rosa canina Fruit Extract and Liposomes
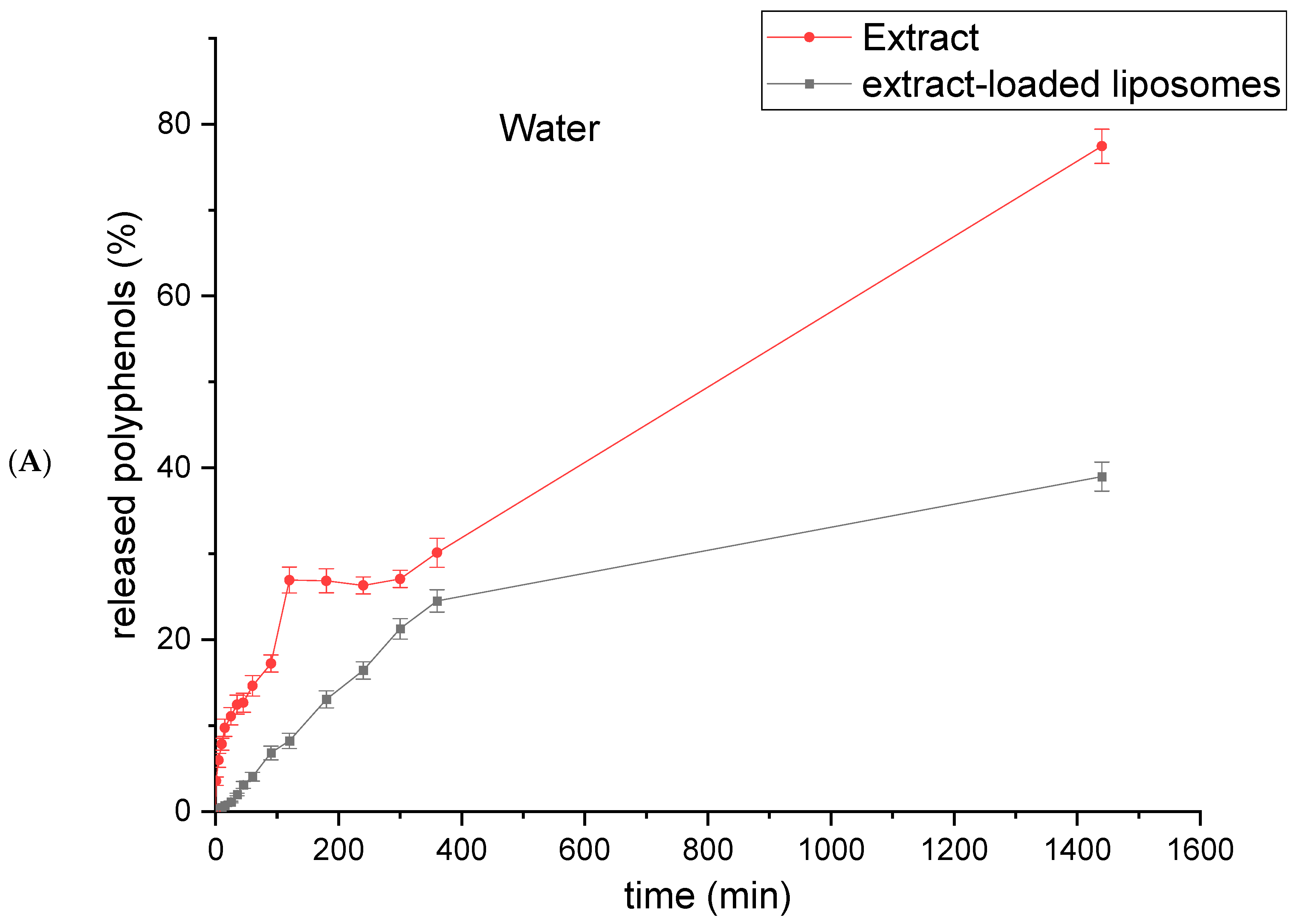
2.6. Polyphenol Release from Rosa canina Fruit Extract and Extract-Loaded Liposomes
3. Materials and Methods
3.1. Plant Material and Reagents
3.2. Preparation of Rosa Canina Fruit Extract and Determination of the Extraction Yield
3.3. Preparation of Liposomal Vesicles and Lyophilization
3.4. Storage-Stability Study
3.5. UV-Stability Study
3.6. Density, Surface Tension, and Viscosity Analyses
3.7. Fourier-Transform Infrared (FTIR) Analysis
3.8. HPLC Analysis
3.9. Thermogravimetric Analysis
3.10. In Vitro Release Study
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jones, P.J.; Jew, S. Functional food development: Concept to reality. Trends Food Sci. Technol. 2007, 18, 387–390. [Google Scholar] [CrossRef]
- Tur, J.A.; Bibiloni, M.M. Functional foods. In Encyclopedia of Food and Health; Caballero, P.M., Ed.; Academic Press: Oxford, UK, 2016; pp. 157–161. [Google Scholar]
- Ortega-Ramirez, L.A.; Rodriguez-Garcia, I.; Leyva, J.M.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Siddiqui, M.W.; Ayala-Zavala, J.F. Potential of medicinal plants as antimicrobial and antioxidant agents in food industry: A hypothesis. J. Food Sci. 2014, 79, R129–R137. [Google Scholar] [CrossRef] [PubMed]
- García-Aguilar, A.; Palomino, O.; Benito, M.; Guillén, C. Dietary polyphenols in metabolic and neurodegenerative diseases: Molecular targets in autophagy and biological effects. Antioxidants 2021, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Dabić Zagorac, D.Č.; Fotirić Akšić, M.M.; Glavnik, V.; Gašić, U.M.; Vovk, I.; Tešić, Ž.L.; Natić, M.M. Establishing the chromatographic fingerprints of flavan-3-ols and proanthocyanidins from rose hip (Rosa sp.) species. J. Sep. Sci. 2020, 43, 1431–1439. [Google Scholar] [CrossRef]
- Demir, F.; Özcan, M. Chemical and technological properties of rose (Rosa canina L.) fruits grown wild in Turkey. J. Food Eng. 2001, 47, 333–336. [Google Scholar] [CrossRef]
- Popović-Djordjević, J.; Špirović-Trifunović, B.; Pećinar, I.; de Oliveira, L.F.C.; Krstić, Đ.; Mihajlović, D.; Akšić-Fotirić, D.; Simal-Gandara, J.. Fatty acids in seed oil of wild and cultivated rosehip (Rosa canina L.) from different locations in Serbia. Ind. Crops Prod. 2023, 191, 115797. [Google Scholar] [CrossRef]
- Özdemir, N.; Pashazadeh, H.; Zannou, O.; Koca, I. Phytochemical content, and antioxidant activity, and volatile compounds associated with the aromatic property, of the vinegar produced from rosehip fruit (Rosa canina L.). Lwt 2022, 154, 112716. [Google Scholar] [CrossRef]
- Nedović, V.; Kalusević, A.; Manojlović, V.; Lević, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols–a review. Trends Food Sci. Technol 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Takahashi, M.; Inafuku, K.I.; Miyagi, T.; Oku, H.; Wada, K.; Imura, T.; Kitamoto, D. Efficient preparation of liposomes encapsulating food materials using lecithins by a mechanochemical method. J. Oleo Sci. 2007, 56, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S. UV Light in Food Preservation. In Handbook of Food Preservation; Rahman, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 769–778. [Google Scholar]
- Rastogi, R.P.; Richa, N.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids. 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed]
- Sallustio, V.; Farruggia, G.; Di Cagno, M.P.; Tzanova, M.M.; Marto, J.; Ribeiro, H.; Goncalves, L.M.; Mandrone, M.; Chiocchio, I.; Cerchiara, T.; et al. Design and characterization of an ethosomal gel encapsulating rosehip extract. Gels 2023, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Bryła, A.; Lewandowicz, G.; Juzwa, W. Encapsulation of elderberry extract into phospholipid nanoparticles. J. Food Eng. 2015, 167, 189–195. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Aliakbarian, B.; Perego, P.; Reverchon, E. Supercritical assisted process for the encapsulation of olive pomace extract into liposomes. J. Supercrit. Fluids 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Dag, D.; Oztop, M.H. Formation and characterization of green tea extract loaded liposomes. J. Food Sci. 2017, 82, 463–470. [Google Scholar] [CrossRef]
- Taylor, T.M.; Weiss, J.; Davidson, P.M.; Bruce, B.D. Liposomal nanocapsules in food science and agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef]
- Hu, S.; Niu, M.; Hu, F.; Lu, Y.; Qi, J.; Yin, Z.; Wu, W. Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int. J. Pharm. 2013, 441, 693–700. [Google Scholar] [CrossRef]
- Pravilović, R.N.; Balanč, B.D.; Trifković, K.T.; Đorđević, V.B.; Bošković-Vragolović, N.M.; Bugarski, B.M.; Pjanović, R.V. Comparative effects of span 20 and span 40 on liposomes release properties. Int. J. Food Eng. 2017, 13, 20170339. [Google Scholar] [CrossRef]
- Kerwin, B.A. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways. J. Pharm. Sci. 2008, 97, 2924–2935. [Google Scholar] [CrossRef]
- Ji, X.; Gao, Y.; Chen, L.; Zhang, Z.; Deng, Y.; Li, Y. Nanohybrid systems of non-ionic surfactant inserting liposomes loading paclitaxel for reversal of multidrug resistance. Int. J. Pharm. 2012, 422, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A.; Petrović, P.; Ćujić, D.; Stepanovicć, S.; Gnjatović, M.; Marinković, A.; Bugarski, B. The stability of liposomes with ergosterol and Thymus serpyllum L. extract. In Proceedings of the VIII International Congress “Engineering, Environment and Materials in Process Industry”, Jahorina, Bosnia and Herzegovina, 20–23 March 2023. [Google Scholar]
- Gibis, M.; Ruedt, C.; Weiss, J. In Vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.Y.; Gan, L.J.; Zhang, H.; Shin, J.A.; Lee, K.T.; Hong, S.T. Effects of flavonoid glycosides obtained from a Ginkgo biloba extract fraction on the physical and oxidative stabilities of oil-in-water emulsions prepared from a stripped structured lipid with a low omega-6 to omega-3 ratio. Food Chem. 2015, 174, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Guldiken, B.; Gibis, M.; Boyacioglu, D.; Capanoglu, E.; Weiss, J. Physical and chemical stability of anthocyanin-rich black carrot extract-loaded liposomes during storage. Food Res. Int. 2018, 108, 491–497. [Google Scholar] [CrossRef]
- Duffy, C.F.; Gafoor, S.; Richards, D.P.; Admadzadeh, H.; O’Kennedy, R.; Arriaga, E.A. Determination of properties of individual liposomes by capillary electrophoresis with postcolumn laser-induced fluorescence detection. Anal. Chem. 2001, 73, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Lidgate, D.M.; Hegde, S.G.; Maskiewicz, R. Conductivity measurement as a convenient technique for determination of liposome capture volume. Int. J. Pharm. 1993, 96, 51–58. [Google Scholar] [CrossRef]
- Azarbayjani, A.F.; Jouyban, A.; Chan, S.Y. Impact of surface tension in pharmaceutical sciences. Int. J. Pharm. Pharm. Sci. 2009, 12, 218–228. [Google Scholar] [CrossRef]
- Kunastitchai, S.; Pichert, L.; Sarisuta, N.; Müller, B.W. Application of aerosol solvent extraction system (ASES) process for preparation of liposomes in a dry and reconstitutable form. Int. J. Pharm. 2006, 316, 93–101. [Google Scholar] [CrossRef]
- Luo, Z.; Murray, B.S.; Yusoff, A.; Morgan, M.R.; Povey, M.J.; Day, A.J. Particle-stabilizing effects of flavonoids at the oil−water interface. J. Agric. Food. Chem. 2011, 59, 2636–2645. [Google Scholar] [CrossRef]
- Demirbay, B.; Akaoğlu, C.; Ulusaraç, İ.; Acar, F.G. Thermal and UV radiation effects on dynamic viscosity of gelatin-based riboflavin solutions. J. Mol. Liq. 2017, 225, 147–150. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Santiago-Adame, R.; Calderas, F.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Núñez-Ramírez, D.M.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation by spray drying of laurel infusions (Litsea glaucescens) with maltodextrin. Ind. Crops Prod. 2016, 90, 1–8. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; Oliveira, F.C.S.D.; Passos, T.M.; Quilty, B.; Thiré, R.M.D.S.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Veisi, H.; Rashtiani, A.; Barjasteh, V. Biosynthesis of palladium nanoparticles using Rosa canina fruit extract and their use as a heterogeneous and recyclable catalyst for Suzuki-Miyaura coupling reactions in water. Appl. Organometal. Chem. 2016, 30, 231–235. [Google Scholar] [CrossRef]
- Noh, C.H.C.; Azmin, N.F.M.; Amid, A. Principal component analysis application on flavonoids characterization. Adv. Sci. Technol. Eng. Syst. J. 2017, 2, 435–440. [Google Scholar] [CrossRef]
- Trifunschi, S.; Munteanu, M.F.; Agotici, V.; Pintea, S.; Gligor, R. Determination of flavonoid and polyphenol compounds in Viscum album and Allium sativum extracts. Int. Curr. Pharm. J. 2015, 4, 382–385. [Google Scholar] [CrossRef]
- Cardoso-Avila, P.E.; Patakfalvi, R.; Rodríguez-Pedroza, C.; Aparicio-Fernández, X.; Loza-Cornejo, S.; Villa-Cruz, V.; Martínez-Cano, E. One-pot green synthesis of gold and silver nanoparticles using Rosa canina L. extract. RSC Adv. 2021, 11, 14624–14631. [Google Scholar] [CrossRef]
- Saygi, K.O.; Usta, C. Rosa canina waste seed extract-mediated synthesis of silver nanoparticles and the evaluation of its antimutagenic action in Salmonella typhimurium. Mater. Chem. Phys. 2021, 266, 124537. [Google Scholar] [CrossRef]
- Jafarirad, S.; Mehrabi, M.; Divband, B.; Kosari-Nasab, M. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Mater. Sci. Eng. C 2016, 59, 296–302. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Lević, S.; Kalušević, A.; Špoljarić, I.; Đorđević, V.; Komes, D.; Mršić, G.; Nedović, V. Efficiency assessment of natural biopolymers as encapsulants of green tea (Camellia sinensis L.) bioactive compounds by spray drying. Food Bioprocess Technol. 2015, 8, 2444–2460. [Google Scholar] [CrossRef]
- Pawlikowska-Pawlęga, B.; Misiak, L.E.; Zarzyka, B.; Paduch, R.; Gawron, A.; Gruszecki, W.I. FTIR, 1H NMR and EPR spectroscopy studies on the interaction of flavone apigenin with dipalmitoylphosphatidylcholine liposomes. Biochim. Biophys. Acta Biomembr. 2013, 1828, 518–527. [Google Scholar] [CrossRef]
- Fetni, S.; Bertella, N.; Ouahab, A.; Zapater, J.M.M.; Fernandez, S.D.P.T. Composition and biological activity of the Algerian plant Rosa canina L. by HPLC-UV-MS. Arabian J. Chem. 2020, 13, 1105–1119. [Google Scholar] [CrossRef]
- Hvattum, E. Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection. Rapid Commun. Mass Spectrom. 2002, 16, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Lee, H.J.; Bahn, K.N.; Seo, I.W.; Lee, Y.J.; Lee, J.H.; Park, J.M.; Kang, T.S. Development of Analytical Methods of Hyperoside from Rosa canina L. J. Food Hzg. Saf. 2015, 30, 173–177. [Google Scholar] [CrossRef]
- Tayefi-Nasrabadi, H.; Sadigh-Eteghad, S.; Aghdam, Z. The effects of the hydroalcohol extract of Rosa canina L. fruit on experimentally nephrolithiasic Wistar rats. Phytother. Res 2012, 26, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Bąkowska, A.; Kucharska, A.Z.; Oszmiański, J. The effects of heating, UV irradiation, and storage on stability of the anthocyanin–polyphenol copigment complex. Food Chem. 2003, 81, 349–355. [Google Scholar] [CrossRef]
- Narantuya, L.; Ahn, C. UV protection effect of cotton dyed with Flos Sophorae (Sophora japonica L.) extracted with acid hydrolysis. Fashion Text. 2022, 9, 28. [Google Scholar] [CrossRef]
- Kitagawa, S.; Yoshii, K.; Morita, S.Y.; Teraoka, R. Efficient topical delivery of chlorogenic acid by an oil-in-water microemulsion to protect skin against UV-induced damage. Chem. Pharm. Bull. 2011, 59, 793–796. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. sinica B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Liu, C.; Liu, W.; Singh, H. Structure and integrity of liposomes prepared from milk-or soybean-derived phospholipids during In Vitro digestion. Food Res. Int. 2012, 48, 499–506. [Google Scholar] [CrossRef]
- Sun, J.; Deng, Y.; Wang, S.; Cao, J.; Gao, X.; Dong, X. Liposomes incorporating sodium deoxycholate for hexamethylmelamine (HMM) oral delivery: Development, characterization, and in vivo evaluation. Drug Delivery 2010, 17, 164–170. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Lou, X.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.; Xia, S. Liposomes as delivery systems for carotenoids: Comparative studies of loading ability, storage stability and In Vitro release. Food Funct. 2014, 5, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Pjanović, R.; Bošković-Vragolović, N.; Veljković-Giga, J.; Garić-Grulović, R.; Pejanović, S.; Bugarski, B. Diffusion of drugs from hydrogels and liposomes as drug carriers. J. Chem. Technol. Biotechnol. 2010, 85, 693–698. [Google Scholar] [CrossRef]
- Isailović, B.D.; Kostić, I.T.; Zvonar, A.; Đorđević, V.B.; Gašperlin, M.; Nedović, V.A.; Bugarski, B.M. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.D.; Djordjević, V.B.; Ota, A.; Skrt, M.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Ulrih, N.P. Effect of gentisic acid on the structural-functional properties of liposomes incorporating β-sitosterol. Colloids Surf. B 2019, 183, 110422. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.D.; Ota, A.; Ahlin Grabnar, P.; Djordjević, V.B.; Šavikin, K.P.; Bugarski, B.M.; Nedović, V.A.; Poklar Ulrih, N. Comparative effects of cholesterol and β-sitosterol on the liposome membrane characteristics. Eur. J. Lipid Sci. Technol. 2018, 120, 1800039. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.; Volić, M.; Pećinar, I.; Živković, J.; Šavikin, K.P. Rosehip extract-loaded liposomes for potential skin application: Physicochemical properties of non-and UV-irradiated liposomes. Plants 2023, 12, 3063. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Balanč, B.; Živković, J.; Šavikin, K.P.; Čutović, N.; Gnjatović, M.; Bugarski, B. The influence of UV irradiation on physicochemical properties of ethanol Rosa canina L. extract. In Proceedings of the 7th International Food Safety Congress, Istanbul, Turkey, 3–4 November 2022; p. 120. [Google Scholar]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.C.; Silva, M.C.; Freitas, M.Q.; et al. Ultraviolet radiation: An interesting technology to preserve quality and safety of milk and dairy foods. Trends Food Sci. Technol 2020, 102, 146–154. [Google Scholar] [CrossRef]
- Jovanović, A.; Djordjević, V.; Petrović, P.; Pljevljakušić, D.; Zdunić, G.; Šavikin, K.; Bugarski, B. The influence of different extraction conditions on polyphenol content, antioxidant and antimicrobial activities of wild thyme. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100328. [Google Scholar] [CrossRef]
- Kaeswurm, J.A.; Scharinger, A.; Teipel, J.; Buchweitz, M. Absorption coefficients of phenolic structures in different solvents routinely used for experiments. Molecules 2021, 26, 4656. [Google Scholar] [CrossRef]


| Scheme | ρ [g/mL] | γ [mN/m] | η [mPa·s] |
|---|---|---|---|
| extract | 0.931 ± 0.001 c* | 25.4 ± 0.2 a | 4.21 ± 0.01 c |
| extractuv | 0.969 ± 0.001 c | 23.3 ± 0.4 c | 5.49 ± 0.07 b |
| liposomes | 0.998 ± 0.000 b | 24.1 ± 0.1 b | 3.13 ± 0.01 d |
| liposomesUV | 0.997 ± 0.002 b | 24.4 ± 0.2 b | 3.14 ± 0.02 d |
| extract-loaded liposomes | 1.010 ± 0.002 a | 18.7 ± 0.3 d | 6.75 ± 0.05 a |
| extract-loaded liposomesUV | 1.012 ± 0.002 a | 18.5 ± 0.1 d | 6.71 ± 0.02 a |
| Sample | Chlorogenic Acid | Rutin | Quercetin Derivatives * |
|---|---|---|---|
| µg/mL | |||
| Extract | 67.63 ± 1.42 | 157.81 ± 1.78 | 247.71 ± 2.63 |
| ExtractUV | 57.42 ± 1.01 | 56.47 ± 1.14 | 135.63 ± 1.74 |
| Extract-loaded liposomes | 5.33 ± 0.65 | 18.83 ± 1.34 | 46.53 ± 1.86 |
| Extract-loaded liposomesUV | 6.32 ± 0.72 | 17.65 ± 1.22 | 48.14 ± 1.29 |
| Extract | ExtractUV | Liposomes | LiposomesUV | Extract-Loaded Liposomes | Extract-Loaded Liposomesuv | |
|---|---|---|---|---|---|---|
| WL until TD | 0.5 ± 0.1 b | 0.5 ± 0.2 b | 1.8 ± 0.4 a | 2.2 ± 0.5 a | 1.8 ± 0.4 a | 1.9 ± 0.6 a |
| TD | ||||||
| Ton (°C) | 108.5 ± 1.5 c | 104.1 ± 1.9 d | 228.4 ± 0.5 a | 225.3 ± 1.7 a | 177.8 ± 0.6 b | 177.4 ± 1.1 b |
| Toff (°C) | 386.2 ± 1.1 a | 386.2 ± 1.8 a | 376.5 ± 0.6 bc | 372.4 ± 1.7 d | 377.6 ± 1.6 b | 373.7 ± 1.0 cd |
| WL (%) | 58.4 ± 0.6 b | 59.0 ± 1.3 b | 81.8 ± 0.9 a | 80.7 ± 0.9 a | 80.0 ± 0.7 a | 80.0 ± 1.3 a |
| Tp1 (°C) | 125.9 ± 1.7 b | 121.8 ± 0.8 c | - | - | 212.0 ± 1.9 a | 214.1 ± 1.4 a |
| Tp2 (°C) | 197.0 ± 1.0 c | 195.0 ± 0.5 c | 268.2 ± 1.2 a | 268.4 ± 1.4 a | 266.6 ± 1.0 ab | 265.5 ± 0.6 b |
| Tp3 (°C) | 309.2 ± 0.9 c | 310.4 ± 1.5 c | 332.3 ± 1.1 a | 331.4 ± 1.6 a | 328.4 ± 1.8 ab | 326.5 ± 1.5 b |
| Residue 500 °C | 35.8 ± 0.9 a | 35.2 ± 0.9 a | 11.8 ± 0.9 b | 12.0 ± 0.6 b | 11.4 ± 0.7 b | 11.3 ± 1.0 b |
| Conditions | Sample | D [m2/s] | R [s/m] |
|---|---|---|---|
| Water, 25 °C | Extract | 2.07 × 10−9 | 1.47 × 106 |
| Extract-loaded liposomes | 3.35 × 10−10 | 1.90 × 107 | |
| SGF, pH 1.2, 37 °C | Extract | 2.54 × 10−8 | 1.20 × 105 |
| Extract-loaded liposomes | 1.67 × 10−9 | 3.80 × 106 | |
| SIF, pH 6.8, 37 °C | Extract | 7.50 × 10−9 | 4.07 × 105 |
| Extract-loaded liposomes | 4.88 × 10−9 | 1.30 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, A.A.; Balanč, B.; Petrović, P.M.; Volić, M.; Micić, D.; Živković, J.; Šavikin, K.P. Design and Characterization of Liposomal-Based Carriers for the Encapsulation of Rosa canina Fruit Extract: In Vitro Gastrointestinal Release Behavior. Plants 2024, 13, 2608. https://doi.org/10.3390/plants13182608
Jovanović AA, Balanč B, Petrović PM, Volić M, Micić D, Živković J, Šavikin KP. Design and Characterization of Liposomal-Based Carriers for the Encapsulation of Rosa canina Fruit Extract: In Vitro Gastrointestinal Release Behavior. Plants. 2024; 13(18):2608. https://doi.org/10.3390/plants13182608
Chicago/Turabian StyleJovanović, Aleksandra A., Bojana Balanč, Predrag M. Petrović, Mina Volić, Darko Micić, Jelena Živković, and Katarina P. Šavikin. 2024. "Design and Characterization of Liposomal-Based Carriers for the Encapsulation of Rosa canina Fruit Extract: In Vitro Gastrointestinal Release Behavior" Plants 13, no. 18: 2608. https://doi.org/10.3390/plants13182608









