Comparative Studies for Cryopreservation of Agave Shoot Tips by Droplet-Vitrification
Abstract
1. Introduction
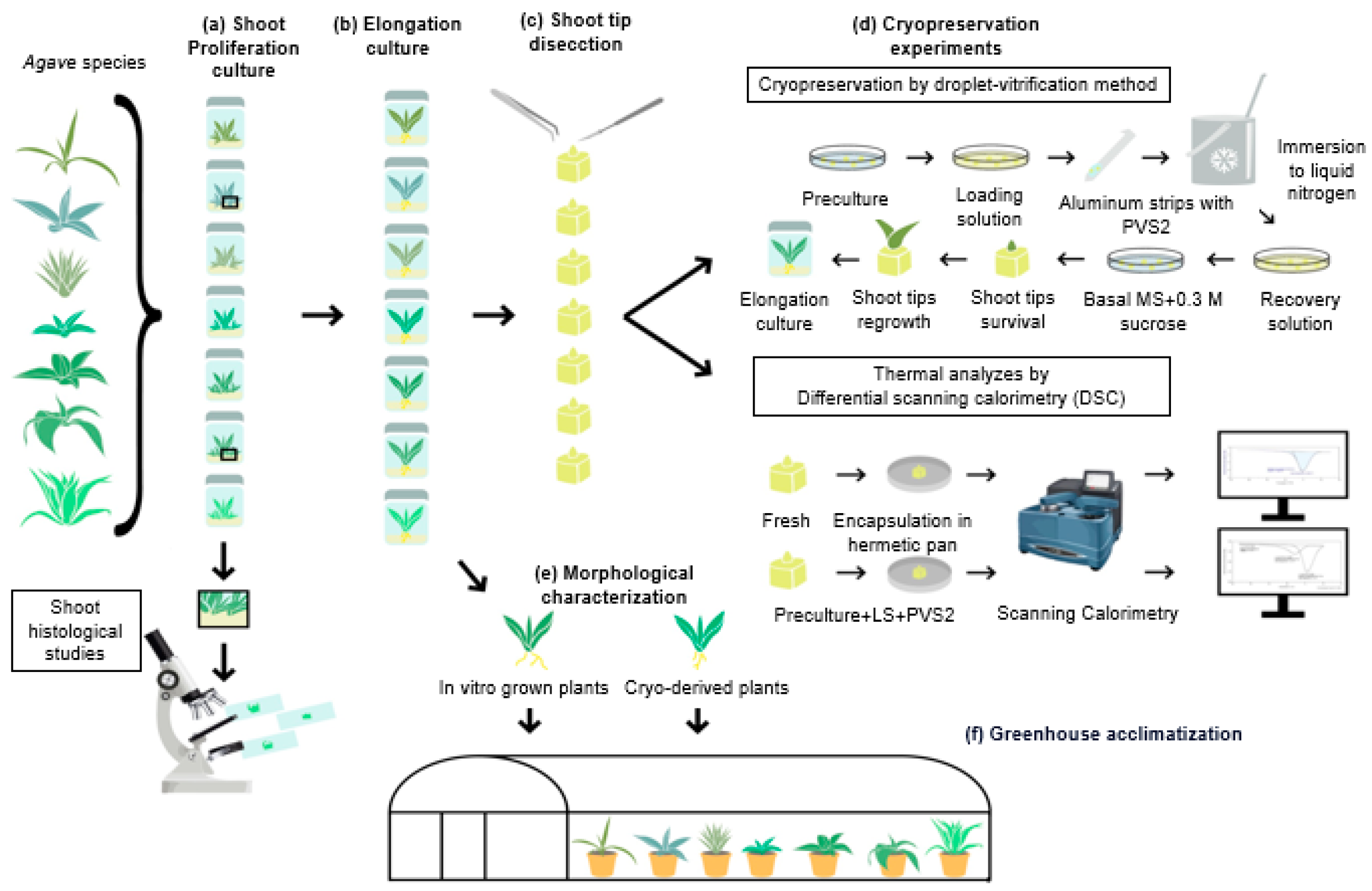
2. Materials and Methods
2.1. Plant Material
2.2. Cryopreservation of Agave Shoot Tips
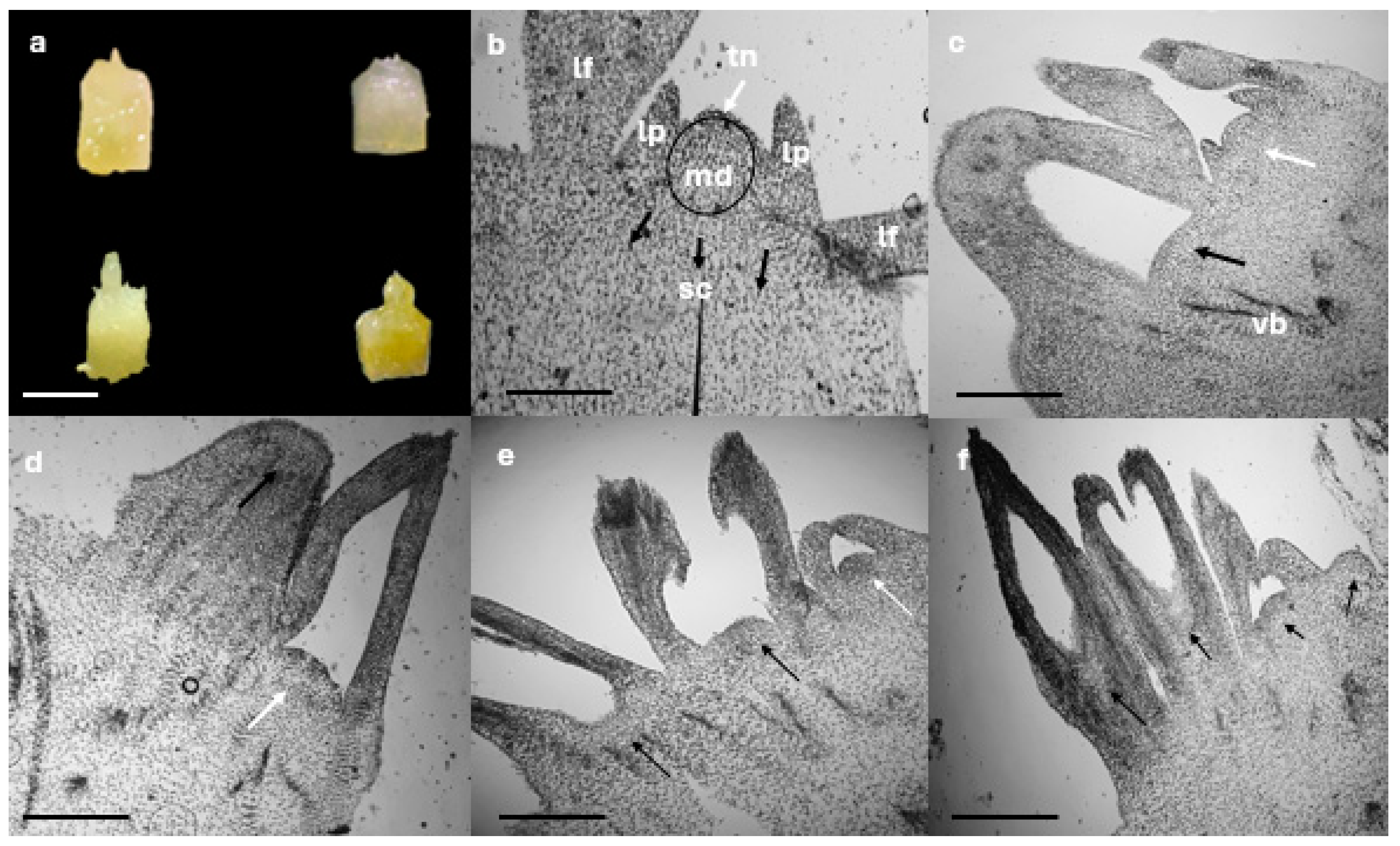
2.3. Shoot Histological Studies
2.4. Differential Scanning Calorimetry
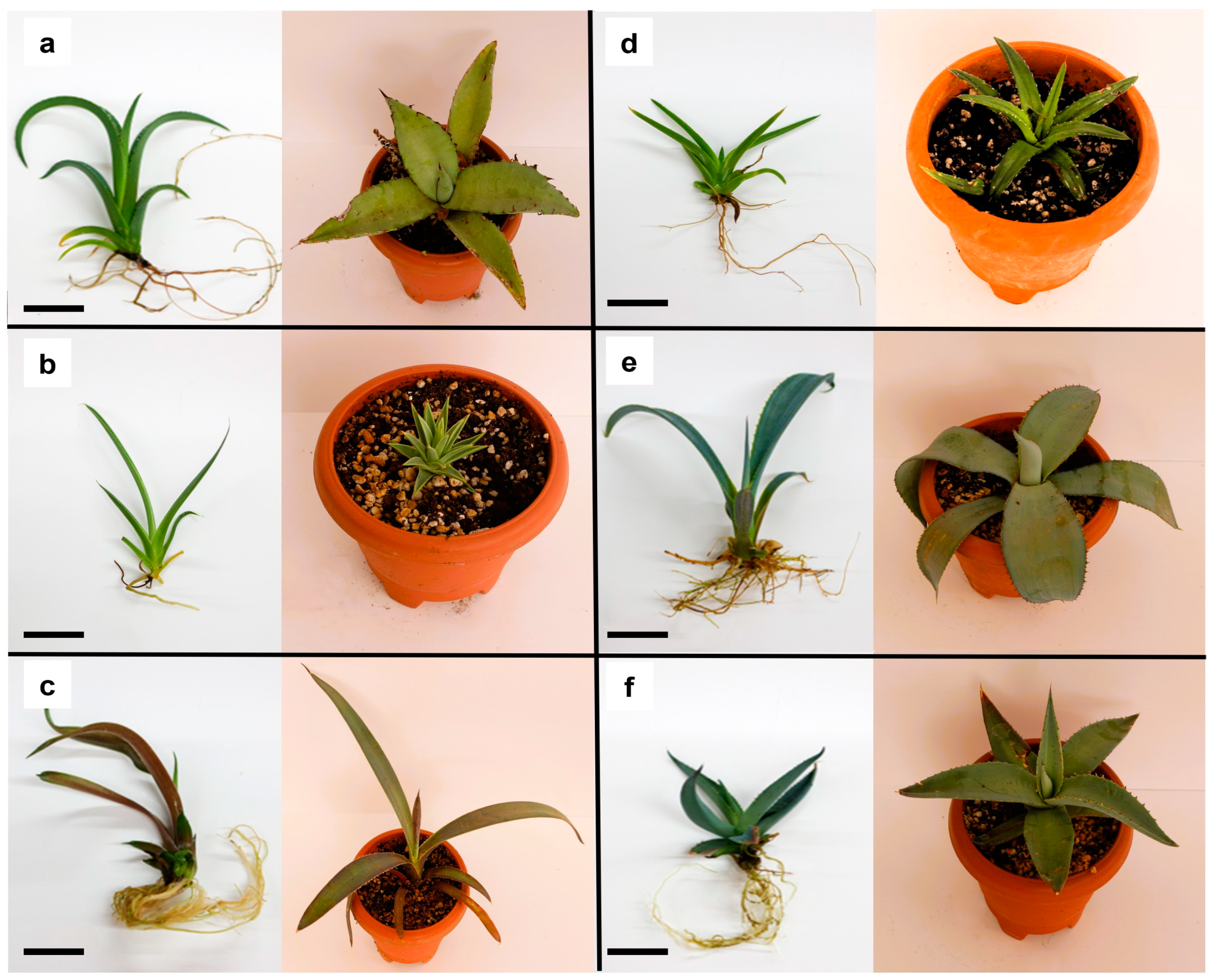
2.5. Morphology Characterization of Agave In Vitro Plants
2.6. Acclimatization
2.7. Statistical Analyses
3. Results and Discussion
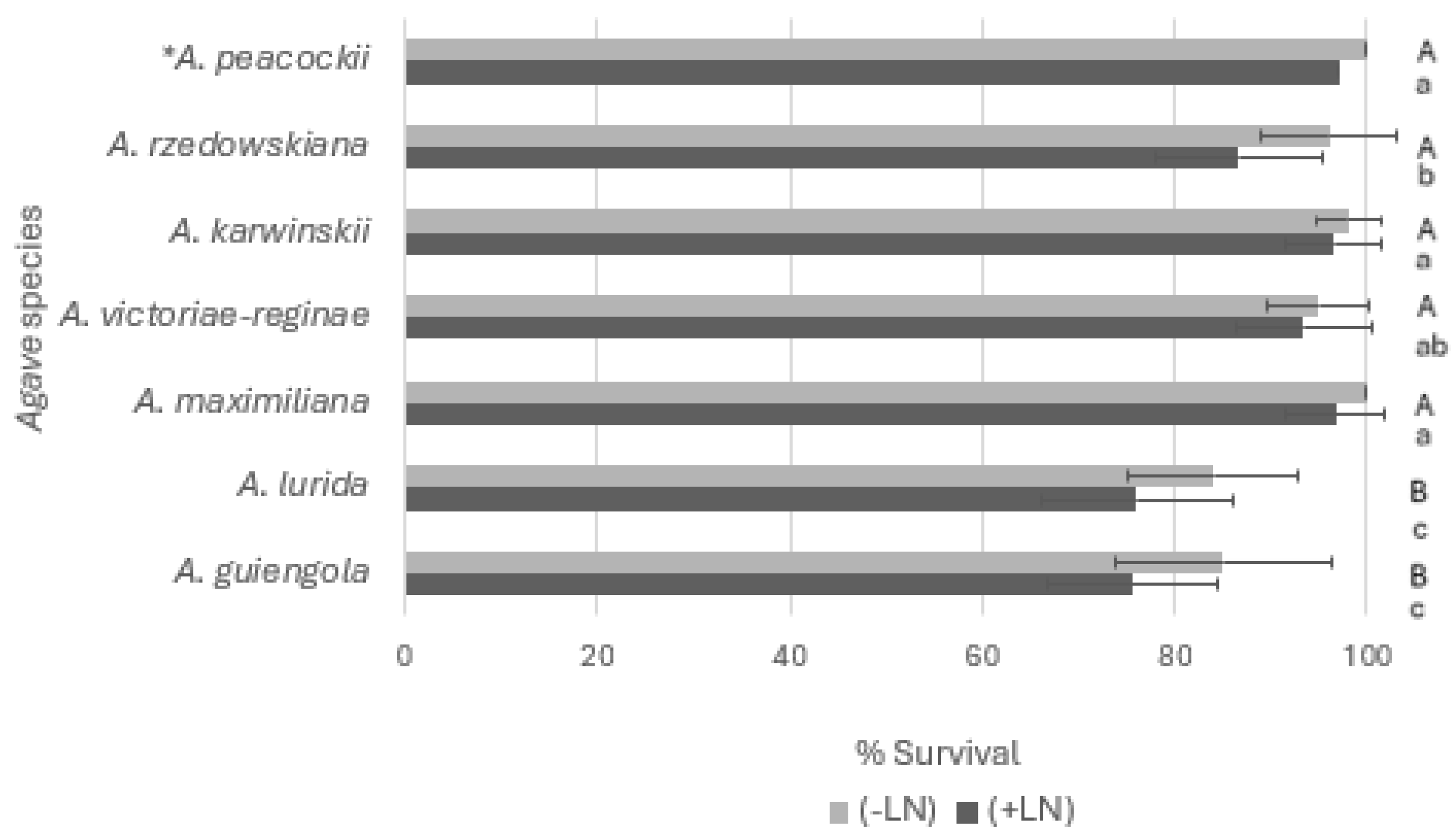
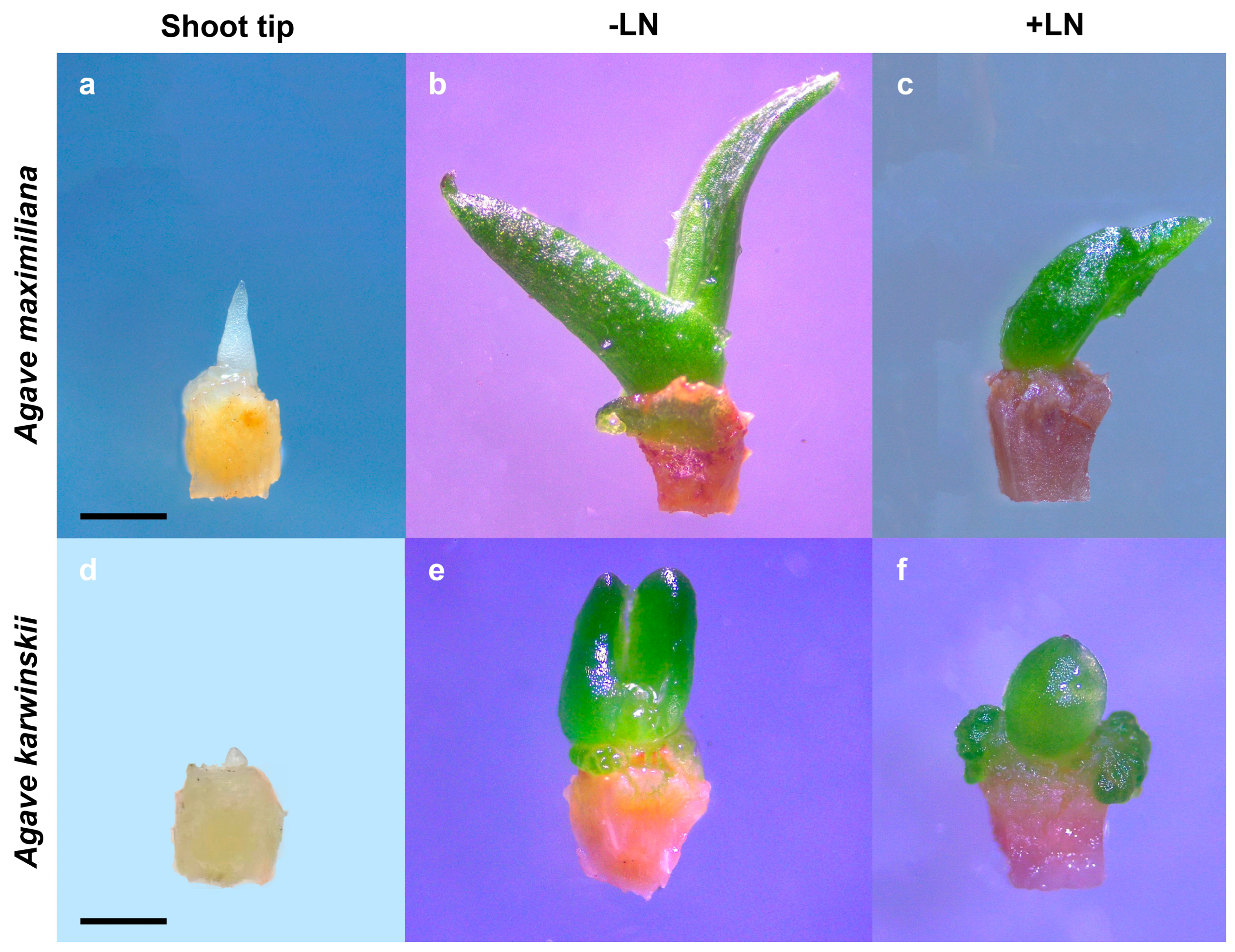
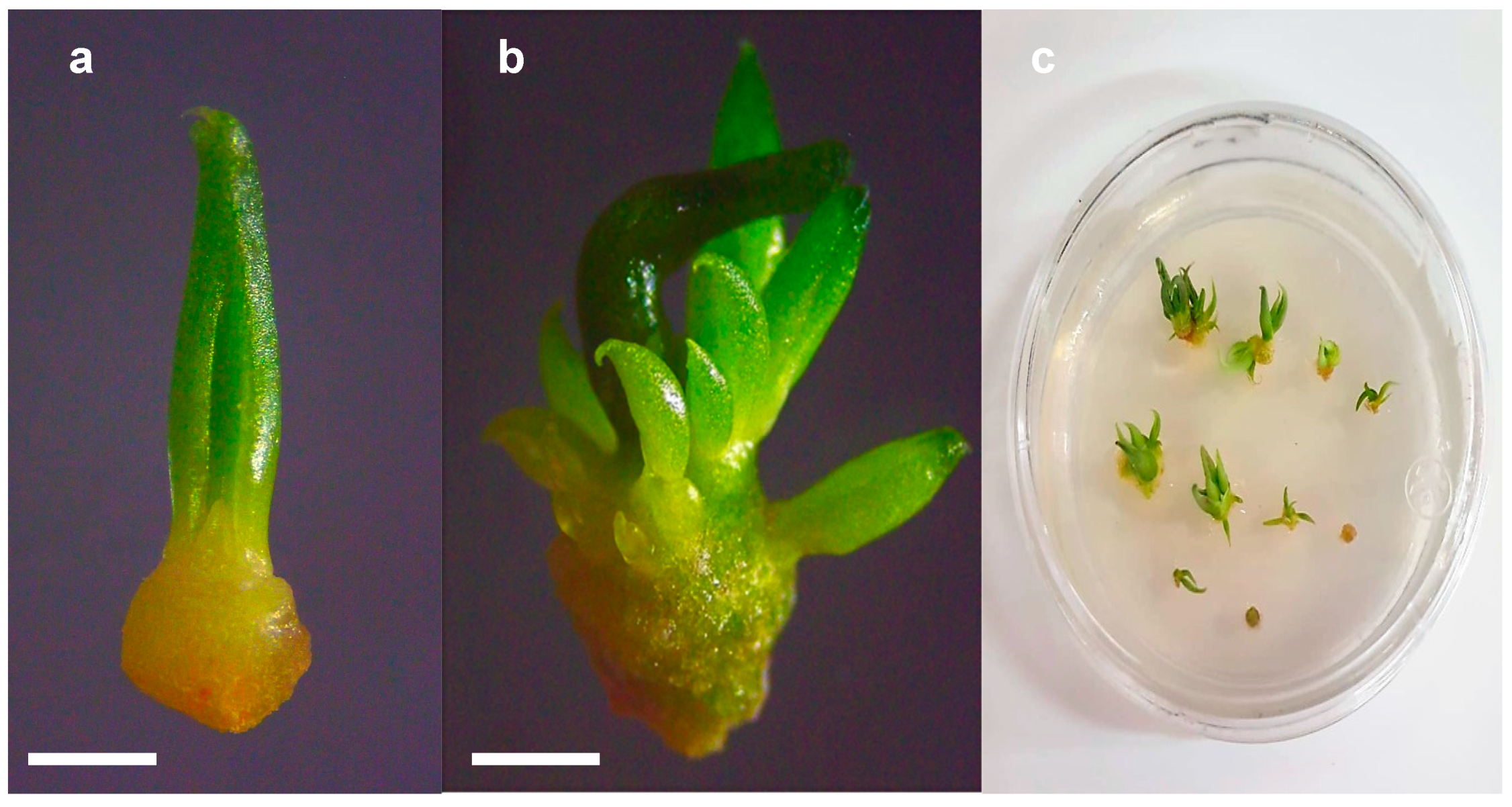
3.1. Cryopreservation of Agave Species Shoot Tips
3.2. Shoot Histological Studies
3.3. Differential Scanning Calorimetry
3.4. Morphological Characterization of Agave In Vitro Plants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- García-Mendoza, A.; Nieto-Sotelo, J.; Sánchez-Teyer, L.; Tapia, E.; Gómez-Leyva, J.; Tamayo-Ordoñez, M.C. Panorama del Aprovechamiento de los Agaves en Mexico. In AGARED—Red Temática Mexicana Aprovechamiento Integral Sustentable y Biotechnología de los Agaves; CONACYT: Mexico City, Mexico; CIATEJ: Guadalajara, Mexico; AGARED: Guadalajara, Mexico, 2017; ISBN 978-607-97548-5-3. [Google Scholar]
- Pérez-Zavala, M.D.L.; Hernández-Arzaba, J.C.; Bideshi, D.K.; Barboza-Corona, J.E. Agave: A natural renewable resource with multiple applications. J. Sci. Food Agric. 2020, 100, 5324–5433. [Google Scholar] [CrossRef] [PubMed]
- Alducin-Martínez, C.; Ruiz Mondragón, K.Y.; Jiménez-Barrón, O.; Aguirre-Planter, E.; Gasca-Pineda, J.; Eguiarte, L.E.; Medellin, R.A. Uses, Knowledge and Extinction Risk Faced by Agave Species in Mexico. Plants 2023, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Colunga-GarcíaMarín, P.; Zizumbo-Villarreal, D. Tequila and other Agave spirits from west-central Mexico: Current germplasm diversity, conservation and origin. Biodivers. Conserv. 2007, 16, 1653–1667. [Google Scholar] [CrossRef]
- Torres, I.; Casas, A.; Vega, E.; Martínez-Ramos, M.; Delgado-Lemus, A. Population dynamics and sustainable management of mescal Agaves in central Mexico: Agave potatorum in the Tehuacán Cuicatlán valley. Econ. Bot. 2015, 69, 26–41. [Google Scholar] [CrossRef]
- Delgado-Aceves, M.L. Desarrollo de Estrategias Para el Cultivo In Vitro y Crioconservación de Especies de Agave (A. tequilana cultivar ‘Chato’, A. peacockii y A. cupreata). Ph.D. Thesis, University of Guadalajara, Zapopan, Mexico, March 2022. [Google Scholar]
- Wang, M.-R.; Bi, W.; Shukla, M.R.; Ren, L.; Hamborg, Z.; Blystad, D.-R.; Saxena, P.K.; Wang, Q.-C. Epigenetic and Genetic Integrity, Metabolic Stability, and Field Performance of Cryopreserved Plants. Plants 2021, 10, 1889. [Google Scholar] [CrossRef]
- Pence, V.C.; Bruns, E.B. Scratching the surface: The in vitro research that will be critical for conserving exceptional plants to scale. In Vitro Cell. Dev. Biol.-Plant 2024, 60, 266–282. [Google Scholar] [CrossRef]
- González-Arnao, M.T.; Martínez-Montero, M.; Cruz-Cruz, C.A.; Engelmann, F. Advances in cryogenic techniques for the long-term preservation of plant biodiversity. In Biotechnology and Biodiversity, 1st ed.; Ahuja, M.R., Ramawat, K.G., Eds.; Springer: Rajasthan, India, 2014; Volume 4, pp. 129–170. [Google Scholar]
- Benelli, C. Plant Cryopreservation: A Look at the Present and the Future. Plants 2021, 10, 2744. [Google Scholar] [CrossRef]
- González-Arnao, M.T.; Gámez, R.; Martínez, Y.; Valdés, S.; Mascorro, J.O.; Osorio, A.; Pastelín, M.; Guevara, M.; Cruz, C.A. Estado actual de la Crioconservación vegetal en México. In Crioconservación de Plantas en América Latina y el Caribe, 1st ed.; González-Arnao, M.T., Engelmann, F., Eds.; IICA: San José, Costa Rica, 2013; pp. 161–173. [Google Scholar]
- Kaviani, B.; Kulus, D. Cryopreservation of Endangered Ornamental Plants and Fruit Crops from Tropical and Subtropical Regions. Biology 2022, 11, 847. [Google Scholar] [CrossRef]
- Delgado-Aceves, L.; González-Arnao, M.T.; Santacruz-Ruvalcaba, F.; Folgado, R.; Portillo, L. Indirect Somatic Embryogenesis and Cryopreservation of Agave tequilana Weber Cultivar ‘Chato’. Plants 2021, 10, 249. [Google Scholar] [CrossRef]
- Delgado-Aceves, M.L.; Portillo, L.; Folgado, R.; Romo-Paz, F.J.; González-Arnao, M.T. New approaches for micropropagation and cryopreservation of Agave peacockii, an endangered species. Plant Cell Tissue Organ Cult. 2022, 150, 85–95. [Google Scholar] [CrossRef]
- Gentry, H. Agaves of Continental North America; The University of Arizona Press: Tucson, AZ, USA, 1982. [Google Scholar]
- International Union for the Conservation of Nature. The IUCN Red List of Threatened Species. Version 2024-1. 2024. Available online: https://www.iucnredlist.org (accessed on 10 September 2024).
- Zámečník, J.; Faltus, M.; Bilavčík, A.; Kotková, R. Comparison of cryopreservation methods of vegetatively propagated crops based on thermal analysis. In Current Frontiers in Cryopreservation; Katkov, I., Ed.; InTech: London, UK, 2012; pp. 333–358. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Castro-Concha, L.; Loyola-Vargas, V.M.; Chan, J.L.; Robert, M.L. Glutamate dehydrogenase activity in normal and vitrified plants of Agave tequilana Weber propagated in vitro. Plant Cell Tissue Organ Cult. 1990, 22, 147–151. [Google Scholar] [CrossRef]
- Sakai, A.; Kobayashi, S.; Oiyama, I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tnaka) by vitrification. Plant Cell Rep. 1990, 9, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Burger, L.; Richter, H. Anatomia da Madeira, 1st ed.; Livraria Nobel: São Paulo, Brazil, 1991; pp. 97–105. [Google Scholar]
- Zhang, R.; Chen, Y.; Yang, L.; Xie, Y.; Yan, W.; He, Z. Study on the relationship between mucilage cells and low temperature tolerance of Sedum aizoon L. IOP Conf. Ser. Earth Environ. Sci. 2020, 461, 012055. [Google Scholar] [CrossRef]
- Popova, E.; Kulichenko, I.; Kim, H.-H. Critical Role of Regrowth Conditions in Post-Cryopreservation of In Vitro Plant Germplasm. Biology 2023, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Palacios, H.; Portillo, L. Histologic origin of micropropagated shoots of Agave tequilana. In Sustainable and Integral Exploitation of Agave; Gutiérrez-Mora, A., Ed.; CIATEJ: Jalisco, Mexico, 2014; pp. 36–39. [Google Scholar]
- Delgado-Aceves, L.; Palacios, H.; Romo-Paz, F.; Portillo, L. Micropropagation systems in Agave spp.: Common errors. In Integral and Sustainable Use of Agave; Gutiérrez-Mora, A., Ed.; CIATEJ: Jalisco, Mexico, 2019; pp. 51–54. [Google Scholar]
- Bettoni, J.; Chen, K.; Volk, G. An Overview of Shoot Tip Cryopreservation. In Training in Plant Genetic Resources: Cryopreservation of Clonal Propagules; Volk, G., Balunek, E., Chen, K., Eds.; Colorado State University: Fort Collins, CO, USA, 2020. [Google Scholar]
- Bettoni, J.C.; Wang, M.R.; Li, J.W.; Fan, X.; Fazio, G.; Hurtado-Gonzales, O.P.; Volk, G.M.; Wang, Q.C. Application of biotechniques for in vitro virus and viroid elimination in pome fruit crops. Phytopathology 2024, 114, 930–954. [Google Scholar] [CrossRef]
- Guerra, P.A.; Souza, E.H.; Max, D.A.S.; Rossi, M.L.; Villalobos-Olivera, A.; Ledo, C.A.S.; Martinez-Montero, M.E.; Souza, F.V.D. Morphoanatomical aspects of the starting material for the improvement of pineapple cryopreservation by the droplet-vitrification technique. An. Acad. Bras. Cienc. 2021, 93, e20190555. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol. Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Souza, F.V.D.; Kaya, E.; Vieira, L.J.; Souza, E.H.; Amorim, V.B.O.; Skogerboe, D.; Matsumoto, T.; Alves, A.A.C.; Ledo, C.A.S.; Jenderek, M.M. Droplet-vitrification and morphohistological studies of cryopreserved shoot tips of cultivated and wild pineapple genotypes. Plant Cell Tissue Organ Cult. 2016, 124, 351–360. [Google Scholar] [CrossRef]
- Faltus, M.; Domkářová, J.; Svoboda, P.; Horáčková, V.; Nesvadba, V.; Klička, V.; Ptáček, J.; Bilavcik, A.; Zamecnik, J. Analysis of Thermal Characteristics of Potato and Hop Pollen for Their Cryopreservation and Cross-Breeding. Plants 2024, 13, 1578. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. CryoLetters 2007, 28, 151–172. [Google Scholar] [PubMed]
- Benson, E.E.; Reed, B.M.; Brennan, R.M.; Clacher, K.A.; Ross, D.A. Use of Thermal Analysis in the Evaluation of Cryopreservation Protocols for Ribes nigrum L. Germplasm. CryoLetters 1996, 17, 347–362. [Google Scholar]
- Smulders, M.J.M.; de Klerk, G.J. Epigenetics in plant tissue culture. Plant Growth Regul. 2011, 63, 137–146. [Google Scholar] [CrossRef]
- Carrillo-Reyes, P.; Aviña, R.V.; Ramírez-delgadillo, R. Agave rzedowskiana, a new species in subgenus Littaea (Agavaceae) from western Mexico. Brittonia 2003, 55, 240–244. [Google Scholar] [CrossRef]
- Rodríguez-Garay, B.; Gutiérrez-Mora, A.; Acosta-Dueñas, B. Somatic embryogenesis of Agave victoria-reginae Moore. Plant Cell Tissue Organ Cult. 1996, 46, 85–87. [Google Scholar] [CrossRef]
- Normah, M.N.; Sulong, N.; Reed, B.M. Cryopreservation of shoot tips of recalcitrant and tropical species: Advances and strategies. Cryobiology 2019, 87, 1–14. [Google Scholar] [CrossRef]
- Senula, A.; Nagel, M. Cryopreservation of Plant Shoot Tips of Potato, Mint, Garlic, and Shallot Using Plant Vitrification Solution 3. Methods Mol. Biol. 2021, 2180, 647–661. [Google Scholar]
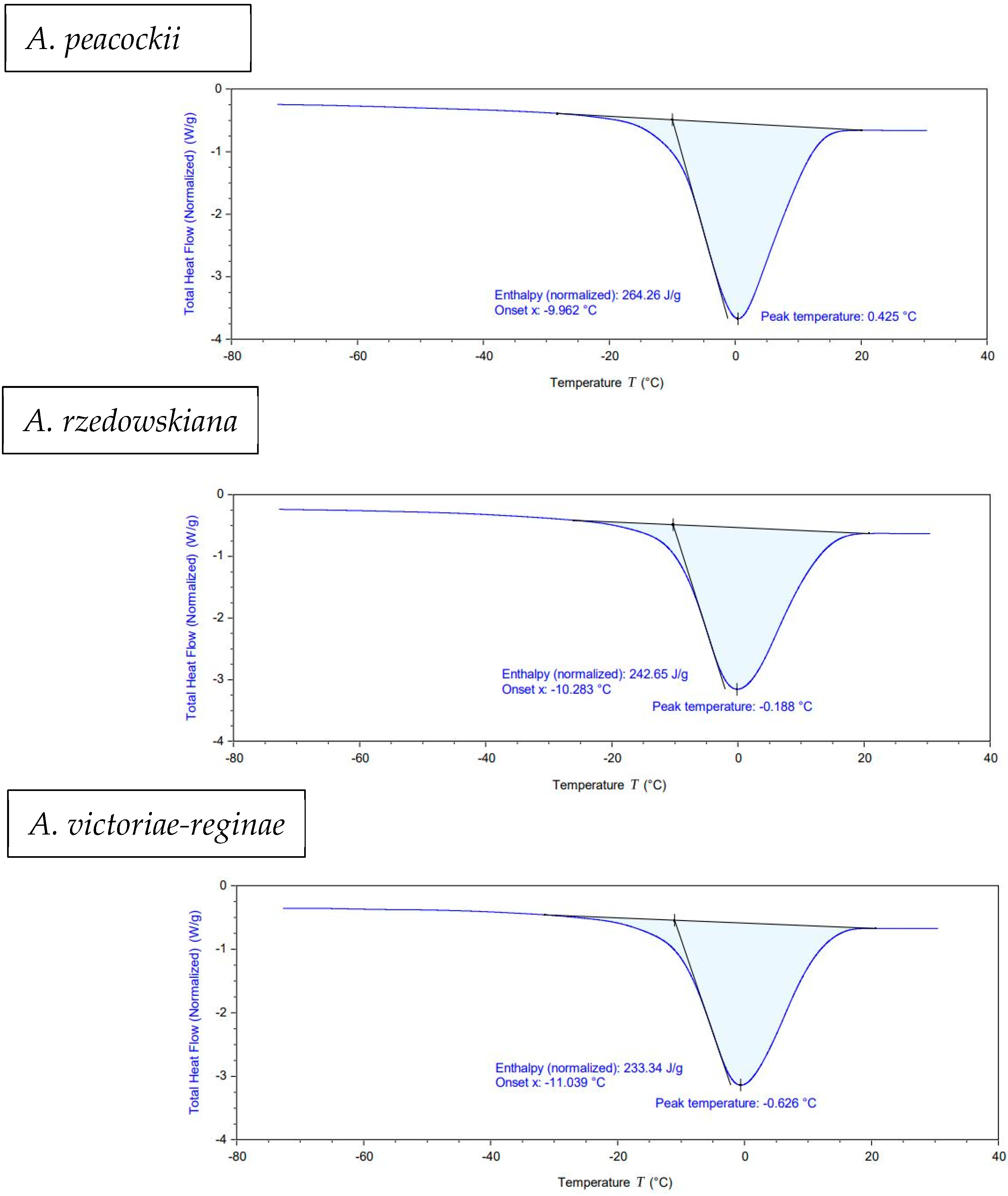
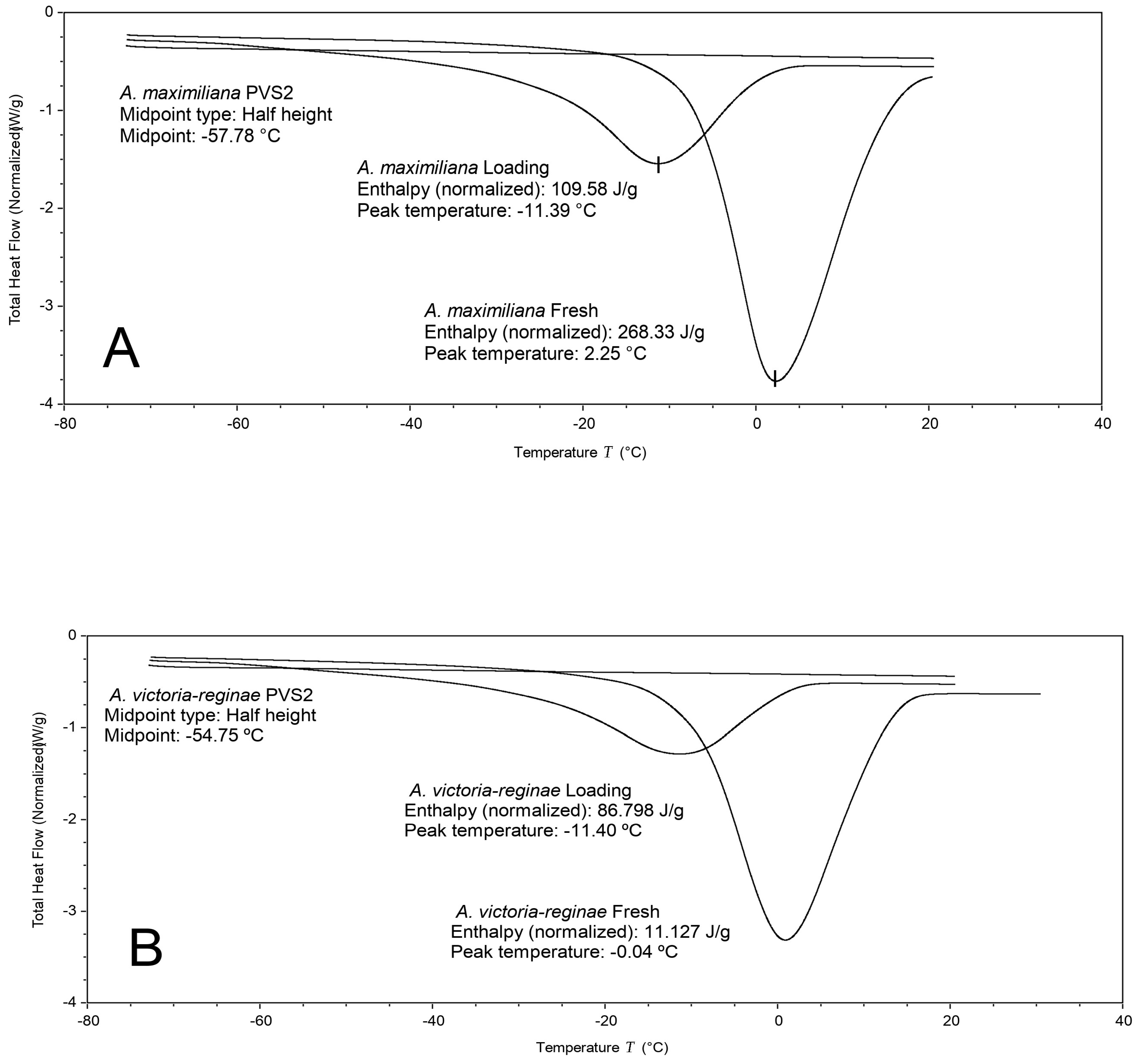
| Species | * (°C) | ** (J/g) | Moisture Content (%) |
|---|---|---|---|
| A. peacockii | −0.332 ± 1.07 | 251.23 ± 18.42 | 75.32 ± 5.52 |
| A. rzedwiskiana | 0.423 ± 0.86 | 238.72 ± 5.55 | 71.57 ± 1.66 |
| A. karwinskii | 0.131 ± 0.65 | 244.52 ± 2.99 | 73.08 ± 0.89 |
| A. victoriae-reginae | −0.408 ± 0.30 | 226.42 ± 9.78 | 67.88 ± 2.93 |
| A. maximiliana | 1.885 ± 1.04 | 267.32 ± 1.42 | 80.14 ± 0.42 |
| A. lurida | 0.236 ± 0.24 | 255.60 ± 6.56 | 76.63 ± 1.96 |
| A. guiengola | 0.500 ± 0.22 | 254.23 ± 5.13 | 76.21 ± 1.53 |
| Species | Treatment | * (°C) | ** (J/g) | Moisture Content (%) |
|---|---|---|---|---|
| A. peacockii | Fresh | −0.332 ± 1.07 | 251.23 ± 18.42 | 75.32 ± 5.52 |
| Pregrowth + Loading | −10.64 ± 0.73 | 117.86 ± 20.73 | 35.33 ± 6.21 | |
| Pregrowth + Loading + PVS2 | - | ND | - | |
| A. rzedwiskiana | Fresh | 0.423 ± 0.86 | 238.72 ± 5.55 | 71.57 ± 1.66 |
| Pregrowth + Loading | −6.16 ± 0.50 | 122.50 ± 12.02 | 36.72 ± 3.60 | |
| Pregrowth + Loading + PVS2 | - | ND | - | |
| A. karwinskii | Fresh | 0.131 ± 0.65 | 244.52 ± 2.99 | 73.08 ± 0.89 |
| Pregrowth + Loading | −8.10 ± 1.23 | 146.30 ± 28.14 | 43.86 ± 8.43 | |
| Pregrowth + Loading + PVS2 | - | ND | - | |
| A. victoriae-reginae | Fresh | 0.100 ± 1.00 | 240.60 ± 10.27 | 72.12 ± 3.08 |
| Pregrowth + Loading | −10.25 ± 1.62 | 97.97 ± 15.79 | 29.37 ± 4.73 | |
| Pregrowth + Loading + PVS2 | - | ND | - |
| Agave Species | Condition | Shoot Length (cm) ** | Leaf Shape | W/V * | Root Length (cm) ** |
|---|---|---|---|---|---|
| A. peacockii | −LN | 5.97 ± 0.90 a | Linear-lanceolate | 2.66 ± 0.66 a | 16.19 ± 2.58 a |
| +LN | 5.28 ± 1.11 a | 1.83 ± 0.73 b | 10.33 ± 1.64 b | ||
| A. rzedowskiana | −LN | 6.08 ± 1.01 a | Linear-triangular | 0.23 ± 0.05 b | 7.00 ± 2.37 a |
| +LN | 4.78 ± 0.67 b | 0.82 ± 0.25 a | 3.53 ± 1.94 b | ||
| A. karwinskii | −LN | 9.15 ± 2.02 a | Linear-lanceolate | 5.27 ± 1.57 a | 6.06 ± 2.53 a |
| +LN | 5.94 ± 1.09 b | 2.77 ± 1.08 b | 3.91 ± 1.69 b | ||
| A. victoriae-reginae | −LN | 4.79 ± 0.90 a | 1.25 ± 0.41 a | 14.22 ± 5.37 a | |
| +LN | 2.15 ± 0.98 b | Lineal-ovate | 0.34 ± 0.21 b | 3.78 ± 1.60 b | |
| A. maximiliana | −LN | 9.22 ± 2.26 a | Lanceolate or oblacnceolate | 11.45 ± 5.48 a | 4.25 ± 1.87 a |
| +LN | 5.46 ± 1.11 b | 3.43 ± 1.27 b | 3.10 ± 0.73 b | ||
| A. guiengola | −LN | 4.38 ± 0.75 a | Ovate-ovate lanceolate | 1.97 ± 0.51 a | 9.47 ± 2.44 a |
| +LN | 1.17 ± 0.60 b | 0.13 ± 0.97 b | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Aceves, L.; Corona, S.; Marin-Castro, U.R.; Rascón-Díaz, M.P.; Portillo, L.; Gutiérrez-Mora, A.; González-Arnao, M.T. Comparative Studies for Cryopreservation of Agave Shoot Tips by Droplet-Vitrification. Plants 2024, 13, 2609. https://doi.org/10.3390/plants13182609
Delgado-Aceves L, Corona S, Marin-Castro UR, Rascón-Díaz MP, Portillo L, Gutiérrez-Mora A, González-Arnao MT. Comparative Studies for Cryopreservation of Agave Shoot Tips by Droplet-Vitrification. Plants. 2024; 13(18):2609. https://doi.org/10.3390/plants13182609
Chicago/Turabian StyleDelgado-Aceves, Lourdes, Santiago Corona, Ubaldo Richard Marin-Castro, Martha Paola Rascón-Díaz, Liberato Portillo, Antonia Gutiérrez-Mora, and María Teresa González-Arnao. 2024. "Comparative Studies for Cryopreservation of Agave Shoot Tips by Droplet-Vitrification" Plants 13, no. 18: 2609. https://doi.org/10.3390/plants13182609
APA StyleDelgado-Aceves, L., Corona, S., Marin-Castro, U. R., Rascón-Díaz, M. P., Portillo, L., Gutiérrez-Mora, A., & González-Arnao, M. T. (2024). Comparative Studies for Cryopreservation of Agave Shoot Tips by Droplet-Vitrification. Plants, 13(18), 2609. https://doi.org/10.3390/plants13182609






