Enhanced Antioxidant, Antifungal, and Herbicidal Activities through Bioconversion of Diosgenin by Yarrowia lipolytica P01a
Abstract
1. Introduction
2. Results
2.1. Production of Protodioscin and Soyasaponin I
2.2. Antioxidant Activity
2.3. Antifungal Activity
2.4. Herbicidal Activity
3. Discussion
4. Materials and Methods
4.1. Microorganism
4.2. Chemicals
4.3. Whole-Cell Bioconversion
4.4. Sample Preparation
4.5. Product Analysis by HPLC-UV
4.6. Determination of Antioxidant Activity
4.7. Determination of Antifungal Activity
4.8. Determination of Herbicidal Activity
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and Quantification of Saponins: A Review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Góngora Chi, G.J.; Lizardi Mendoza, J.; López Franco, Y.; López Mata, M.; Quihui Cota, L. Métodos de Extracción, Funcionalidad y Bioactividad de Saponinas de Yucca: Una Revisión. Biotecnia 2022, 25, 147–155. [Google Scholar] [CrossRef]
- Reichert, C.L.; Salminen, H.; Weiss, J. Quillaja Saponin Characteristics and Functional Properties. Annu. Rev. Food Sci. Technol. 2019, 10, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, A.; Ortega, A.; Chito, D.; Benítez, R. Saponinas de Quinua (Chenopodium quinoa Willd.): Un Subproducto Con Alto Potencial Biológico. Rev. Colomb. Cienc. Químico-Farm. 2016, 45, 438–469. [Google Scholar] [CrossRef]
- Guclu-Ustundag, Ö.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological Activities and Distribution of Plant Saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Musende, A.G.; Eberding, A.; Wood, C.; Adomat, H.; Fazli, L.; Hurtado-Coll, A.; Jia, W.; Bally, M.B.; Guns, E.T. Pre-Clinical Evaluation of Rh2 in PC-3 Human Xenograft Model for Prostate Cancer in Vivo: Formulation, Pharmacokinetics, Biodistribution and Efficacy. Cancer Chemother. Pharmacol. 2009, 64, 1085–1095. [Google Scholar] [CrossRef]
- Bandara, K.R.V.; Padumadasa, C.; Peiris, D.C. Potent Antibacterial, Antioxidant and Toxic Activities of Extracts from Passiflora suberosa L. Leaves. PeerJ 2018, 6, e4804. [Google Scholar] [CrossRef]
- Ashour, A.S.; El Aziz, M.M.A.; Gomha Melad, A.S. A Review on Saponins from Medicinal Plants: Chemistry, Isolation, and Determination. J. Nanomed. Res. 2019, 7, 282–288. [Google Scholar] [CrossRef]
- Gurfinkel, D.M.; Rao, A.V. Soyasaponins: The Relationship Between Chemical Structure and Colon Anticarcinogenic Activity. Nutr. Cancer 2003, 47, 24–33. [Google Scholar] [CrossRef]
- Alves, E.C.; Sartor, E.d.B.; Miguel, O.G.; Miguel, M.D.; Dalarmi, L.; Dias, J.d.F.G.; Montrucchio, D.P. Screening Fitoquímico e Potencial Alelopático Dos Extratos de Partes Aéreas de Phoradendron Ensifolium. Res. Soc. Dev. 2022, 11, e468111638585. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Yu, D.; Huo, J.; Wu, J.; Chen, Y.; Du, X.; Wang, X. Study of Saponin Components after Biotransformation of Dioscorea Nipponica by Endophytic Fungi C39. J. Anal. Methods Chem. 2022, 2022, 2943177. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Lee, S.S. Chemical Constituents from Dendropanax Dentiger. Nat. Prod. Commun. 2013, 8, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.G.; Benito, J.; Macías, F.A.; Simonet, A.M. Agave Steroidal Saponins as Potential Bioherbicides. Agronomy 2021, 11, 2404. [Google Scholar] [CrossRef]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-Derived Saponins: A Review of Their Surfactant Properties and Applications. Sci 2021, 3, 44. [Google Scholar] [CrossRef]
- Hernández Lauzardo, A.N.; Bautista Baños, S.; Velázquez del Valle, M.G. Prospectiva de extractos vegetales para controlar enfermedades postcosecha hortofrutícolas. Rev. Fitotec. Mex. 2007, 30, 119. [Google Scholar] [CrossRef]
- Guzman, B.; Tenorio, R.; Cruz, D.L.; Espinal, C.; Alvarado, J.A.; Mollinedo, P. Saponins from Chenopodium quinoa Willd and Chenopodium Pallidicaule Aellen as Biocontrollers of Phytopathogen Fungi and Hemolysis Agents. Rev. Boliv. Química 2015, 32, 8–14. [Google Scholar]
- Stuardo, M.; San Martín, R. Antifungal Properties of Quinoa (Chenopodium quinoa Willd) Alkali Treated Saponins against Botrytis cinerea. Ind. Crops Prod. 2008, 27, 296–302. [Google Scholar] [CrossRef]
- San Martín, R.; Ndjoko, K.; Hostettmann, K. Novel Molluscicide against Pomacea Canaliculata Based on Quinoa (Chenopodium quinoa) Saponins. Crop Prot. 2008, 27, 310–319. [Google Scholar] [CrossRef]
- Apaza, R.; Smeltekop, H.; Flores, Y.; Ii, G.A.; Salcedo, L. Efecto de Saponinas de Chenopodium quinoa Willd Contra El Fitopatógeno Cercospora Beticola Sacc. Rev. Prot. Veg. 2016, 31, 63–69. [Google Scholar]
- Hernández-Guzmán, C.; Prado-Barragán, A.; Gimeno, M.; Román-Guerrero, A.; Rutiaga-Quiñones, O.M.; Rocha Guzmán, N.E.; Huerta-Ochoa, S. Whole-Cell Bioconversion of Naringenin to High Added Value Hydroxylated Compounds Using Yarrowia lipolytica 2.2ab in Surface and Liquid Cultures. Bioprocess Biosyst. Eng. 2020, 43, 1219–1230. [Google Scholar] [CrossRef]
- Villagrasa, M.; Eljarrat, E.; Barceló, D. Analysis of Benzoxazinone Derivatives in Plant Tissues and Their Degradation Products in Agricultural Soils. TrAC Trends Anal. Chem. 2009, 28, 1103–1114. [Google Scholar] [CrossRef]
- Chen, K.; Tong, W.Y.; Wei, D.Z.; Jiang, W. The 11β-Hydroxylation of 16,17α-Epoxyprogesterone and the Purification of the 11β-Hydroxylase from Absidia coerulea IBL02. Enzym. Microb. Technol. 2007, 41, 71–79. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, L.M.; Wang, X.N.; Shen, T.; Ji, M.; Lou, H.X. Hydroxylation of Diosgenin by Absidia coerulea. Nat. Prod. Commun. 2010, 5, 373–376. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Nicaud, J.M. Metabolic Engineering for Expanding the Substrate Range of Yarrowia lipolytica. Trends Biotechnol. 2016, 34, 798–809. [Google Scholar] [CrossRef]
- Coelho, M.A.Z.; Amaral, P.F.F.; Belo, I. Yarrowia lipolytica: An Industrial Workhorse. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 930–944. [Google Scholar]
- Mauersberger, S. Cytochromes P450 of the Alkane-Utilising Yeast Yarrowia lipolytica. In Yarrowia lipolytica: Biotechnological Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 227–262. [Google Scholar] [CrossRef]
- Babour, A.; Beckerich, J.M.; Gaillardin, C. Identification of an UDP-Glc:Glycoprotein Glucosyltransferase in the Yeast Yarrowia lipolytica. Yeast 2004, 21, 11–24. [Google Scholar] [CrossRef]
- de Pourcq, K.; Tiels, P.; van Hecke, A.; Geysens, S.; Vervecken, W.; Callewaert, N. Engineering Yarrowia lipolytica to Produce Glycoproteins Homogeneously Modified with the Universal Man3GlcNAc2 N-Glycan Core. PLoS ONE 2012, 7, e39976. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hu, Q.; Ye, Q.; Zhang, H.; Bao, Z.; Li, Y.; Mo, L.J. Diosgenin Biosynthesis Pathway and Its Regulation in Dioscorea cirrhosa L. PeerJ 2024, 12, e16702. [Google Scholar] [CrossRef]
- Holland, H.L.; Weber, H.K. Enzymatic Hydroxylation Reactions. Curr. Opin. Biotechnol. 2000, 11, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, Q.; Li, C.; Liu, M.; Zhang, Y. Comparative Transcriptome Analysis Identifies Putative Genes Involved in Dioscin Biosynthesis in Dioscorea Zingiberensis. Molecules 2018, 23, 454. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yang, G.; Ye, J.; Yao, Y.; Lu, G.; Chen, J.; Fang, L.; Lu, S.; Zhou, J. Dioscin Elicits Anti-Tumour Immunity by Inhibiting Macrophage M2 Polarization via JNK and STAT3 Pathways in Lung Cancer. J. Cell. Mol. Med. 2020, 24, 9217–9230. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.L.; Yuan, D.; Zhou, Z.Y.; Wan, J.Z.; Zhang, C.C.; Liu, C.Q.; Dun, Y.Y.; Zhao, H.X.; Zhao, B.; Yang, Y.J.; et al. Saponins from Panax japonicus Attenuate Age-Related Neuroinflammation via Regulation of the Mitogenactivated Protein Kinase and Nuclear Factor Kappa B Signaling Pathways. Neural Regen. Res. 2017, 12, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Lei, C.; Lu, D.; Wang, Y. Direct Biotransformation of Dioscin into Diosgenin in Rhizome of Dioscorea Zingiberensis by Penicillium Dioscin. Indian J. Microbiol. 2015, 55, 200–206. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.S.; Qi, S.S.; Wang, H.; Xiu, Z.L. Biotransformation of Steriodal Saponins in Dioscorea Zingiberensis C. H. Wright to Diosgenin by Trichoderma harzianum. Appl. Microbiol. Biotechnol. 2010, 85, 933–940. [Google Scholar] [CrossRef]
- Wu, G.W.; Gao, J.M.; Shi, X.W.; Zhang, Q.; Wei, S.P.; Ding, K. Microbial Transformations of Diosgenin by the White-Rot Basidiomycete Coriolus Versicolor. J. Nat. Prod. 2011, 74, 2095–2101. [Google Scholar] [CrossRef]
- Dong, T.; Wu, G.W.; Wang, X.N.; Gao, J.M.; Chen, J.G.; Lee, S.S. Microbiological Transformation of Diosgenin by Resting Cells of Filamentous Fungus, Cunninghamella echinulata CGMCC 3.2716. J. Mol. Catal. B Enzym. 2010, 67, 251–256. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.K.; Fu, S.B.; Sun, D.A. Microbial Transformation of Diosgenin by Filamentous Fungus Cunninghamella echinulata. J. Asian Nat. Prod. Res. 2011, 13, 270–275. [Google Scholar] [CrossRef]
- Wang, F.Q.; Li, B.; Wang, W.; Zhang, C.G.; Wei, D.Z. Biotransformation of Diosgenin to Nuatigenin-Type Steroid by a Newly Isolated Strain, Streptomyces Virginiae IBL-14. Appl. Microbiol. Biotechnol. 2007, 77, 771–777. [Google Scholar] [CrossRef]
- Dinham, B.; Malik, S. Pesticides and Human Rights. Int. J. Occup. Environ. Health 2003, 9, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Célis, A.; Mendoza, C.; Pachón, M.; Cardona, J.; Delgado, W.; Cuca, L.E. Extractos vegetales utilizados como biocontroladores con énfasis en la familia Piperaceae. Una revisión. Agron. Colomb. 2008, 26, 97–106. [Google Scholar]
- Santander, M.; Cerón, L.; Hurtado, A. Acción Biocida Del Jugo de Fique (Furcraea gigantea Vent.) Sobre Colletotrichum Gloeosporioides Aislado de Tomate de Árbol (Solanum betaceum Cav.). Agro Sur 2014, 42, 13–17. [Google Scholar] [CrossRef]
- Hata, Y.; Reguero, M.T.; Arteaga de García, L.; Buitrago, G.; Álvarez, A. Evaluación Del Contenido de Sapogeninas En Variedades Nativas de Ñame (Dioscorea Spp.), Provenientes de La Colección de La Universidad de Córdoba. Rev. Colomb. Cienc. Químico-Farm. 2003, 32, 149–157. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; García-Lara, S.; Gutiérrez-Uribe, J.A. Enhancement of Saponins and Flavonols by Micropropagation of Agave Salmiana. Ind. Crops Prod. 2017, 105, 225–230. [Google Scholar] [CrossRef]
- González, J.G.; Flies, C.N.; Navarrete, A.M.; López, J.G.; Troncoso, C.T. Bioherbicida a Partir de Extracto Fenólico Obtenido de Residuos de Almazaras. Sci. Agropecu. 2019, 10, 497–503. [Google Scholar] [CrossRef]
- Osadebe, P.O.; Okoye, F.B.C.; Uzor, P.F.; Nnamani, N.R.; Adiele, I.E.; Obiano, N.C. Phytochemical Analysis, Hepatoprotective and Antioxidant Activity of Alchornea Cordifolia Methanol Leaf Extract on Carbon Tetrachloride-Induced Hepatic Damage in Rats. Asian Pac. J. Trop. Med. 2012, 5, 289–293. [Google Scholar] [CrossRef]
- Wang, P.; Ma, C.; Chen, S.; Zhu, S.; Lou, Z.; Wang, H. Conversion of Steroid Saponins into Diosgenin by Catalytic Hydrolysis Using Acid-Functionalized Ionic Liquid under Microwave Irradiation. J. Clean. Prod. 2014, 79, 265–270. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH.Free Radical Method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alara, O.R.; Abayomi, O.O. Extraction, Characterization and Antioxidant Activity of Fenugreek (Trigonella-Foenum Graecum) Seed Oil. Mater. Sci. Energy Technol. 2019, 2, 349–355. [Google Scholar] [CrossRef]
- Chau Miranda, G.; Herrera Calderón, O.; Condorhuamán Figueroa, M. Actividad antioxidante in vitro, de diferentes extractos del fruto de Physalis peruviana L. (aguaymanto). Rev. Peru. Med. Integr. 2019, 4, 22–27. [Google Scholar] [CrossRef]
- Betancur, Y.L.; Mosquera, O.M. Cuantificación de Tioles Libres y Superoxido Dismutasa (SOD) En Extractos Metanolicos de Plantas de Las Familias Asteraceae, Euphorbiaceae y Piperaceae. Rev. Fac. Cienc. Básicas 2017, 13, 117–122. [Google Scholar] [CrossRef]
- Pantoja Pulido, K.D.; Colmenares Dulcey, A.J.; Isaza Martínez, J.H. New Caffeic Acid Derivative from Tithonia diversifolia (Hemsl.) A. Gray Butanolic Extract and Its Antioxidant Activity. Food Chem. Toxicol. 2017, 109, 1079–1085. [Google Scholar] [CrossRef]
- Cerna, M.; Mencias, F.; Salazar, T.; Gutiérrez, S. Estudio Fitoquímico, Actividad Antioxidante de Especies de Orquídeas de Los Géneros Epidemdrum, Oncidium y Caucaea. Bionatura 2018, 1. [Google Scholar] [CrossRef]
- Mosquera, O.M.; González, L.M.; Cortés, Y.J.; Camargo, J.C. Caracterización Fitoquímica, Determinación Del Contenido de Lignina y La Actividad Antioxidante de Los Culmos de Guadua Angustifolia Kunth. Rev. Fac. Cienc. Básicas 2015, 11, 124–135. [Google Scholar] [CrossRef]
- Jiménez, E.V.; Mosquera, O.M. Actividad Antifúngica In Vitro de Tres Extractos de Plantas Frente a Botrytis cinerea (Moho gris). Salud Soc. Uptc 2014, 1. [Google Scholar]
- Quiroga, E.N.; Sampietro, A.R.; Vattuone, M.A. Screening Antifungal Activities of Selected Medicinal Plants. J. Ethnopharmacol. 2001, 74, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Salcedo, H.E.; Barrientos-Ramírez, L.; Vargas-Radillo, J.J.; Rodríguez-Macías, R.; Ruíz-López, M.A.; Virgen-Calleros, G.; Ramírez-Salcedo, H.E.; Barrientos-Ramírez, L.; Vargas-Radillo, J.J.; Rodríguez-Macías, R.; et al. Inhibición de Colletotrichum Gloeosporioides y Botrytis cinerea Con Extractos de Guazuma Ulmifolia Lam. Rev. Mex. Fitopatol. 2019, 37, 330–344. [Google Scholar] [CrossRef]
- Rodriguez-Maturino, A.; Troncoso-Rojas, R.; Sánchez-Estrada, A.; González-Mendoza, D.; Ruiz-Sanchez, E.; Zamora-Bustillos, R.; Ceceña-Duran, C.; Grimaldo-Juarez, O.; Aviles-Marin, M. Efecto Antifúngico de Extractos Fenólicos y de Carotenoides de Chiltepín (Capsicum annum Var. Glabriusculum) En Alternaria alternata y Fusarium Oxysporum. Rev. Argent. Microbiol. 2015, 47, 72–77. [Google Scholar] [CrossRef]
- Marques, M.E.M.; de Carvalho, A.C.; Yendo, A.C.A.; Magedans, Y.V.S.; Zachert, E.; Fett-Neto, A.G. Phytotoxicity of Quillaja Lancifolia Leaf Saponins and Their Bioherbicide Potential. Plants 2023, 12, 663. [Google Scholar] [CrossRef]
- Mahmoud, L.A.M.; Dos Reis, R.A.; Chen, X.; Ting, V.P.; Nayak, S. Metal-Organic Frameworks as Potential Agents for Extraction and Delivery of Pesticides and Agrochemicals. ACS Omega 2022, 7, 45910–45934. [Google Scholar] [CrossRef] [PubMed]
- Brühl, C.A.; Zaller, J.G. Biodiversity Decline as a Consequence of an Inappropriate Environmental Risk Assessment of Pesticides. Front. Environ. Sci. 2019, 7, 464007. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural Compounds as Next-Generation Herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef]
- Díaz-Mota, M.d.l.Á.; García-Mateos, M.R.; Martínez-Solís, J.; Acosta-Ramos, M.; Serrato-Cruz, M.Á.; Colinas-León, M.T.; Magdaleno-Villar, J. Fitotoxicidad de Los Extractos de Dieffenbachia amoena, Nerium oleander, Raphanus sativus y Brassica napobrassica. Rev. Fac. Cienc. Agrarias. Univ. Nac. Cuyo 2017, 49, 303–318. [Google Scholar]
- Khadka, G.B.; Neupane, S.; Phunyal, S.; Khanal, S. Phytochemical Screening of Selected Plants and Their Allelopathic Effect on Bean and Radish. Fundam. Appl. Agric. 2023, 8, 415–422. [Google Scholar] [CrossRef]
- Taupik, S.A.M.; Aani, S.N.A.; Chia, P.W.; Chuah, T.S. Phytotoxic Compounds of Cassava Leaf Extracts for Weed Inhibition in Aerobic Rice. S. Afr. J. Bot. 2023, 159, 563–570. [Google Scholar] [CrossRef]
- Hoffmann, C.E.F.; Neves, L.A.S.d.; Bastos, C.F.; Wallau, G.d.L. Allelopathic Activity of Nerium Oleander L. and Dieffenbachia Picta Schott in Seeds of Lactuca sativa L. and Bidens pilosa L. Rev. Ciências Agroveterinárias 2007, 6, 11–21. [Google Scholar]
- Swiech, J.N.D.; Folquitto, D.G.; Bobek, V.B.; Urban, A.M.; Betim, F.C.M.; Oliveira, L.F.; Pereira, C.B.; Merino, F.J.Z.; Dias, J.d.F.G.; da Silva, R.Z.; et al. Phytotoxic and Enzymatic Study of Philodendron Meridionale on Seeds of Lactuca sativa L. Res. Soc. Dev. 2021, 10, e5610111336. [Google Scholar] [CrossRef]
- Araniti, F.; Berestetskiy, A. Modern Approaches for the Development of New Herbicides Based on Natural Compounds. Plants 2023, 12, 234. [Google Scholar] [CrossRef]
- de Gaulejac NS, C.; Vivas, N.; de Freitas, V.; Bourgeois, G. The influence of various phenolic compounds on scavenging activity assessed by an enzymatic method. J. Sci. Food Agric. 1999, 79, 1081–1090. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]

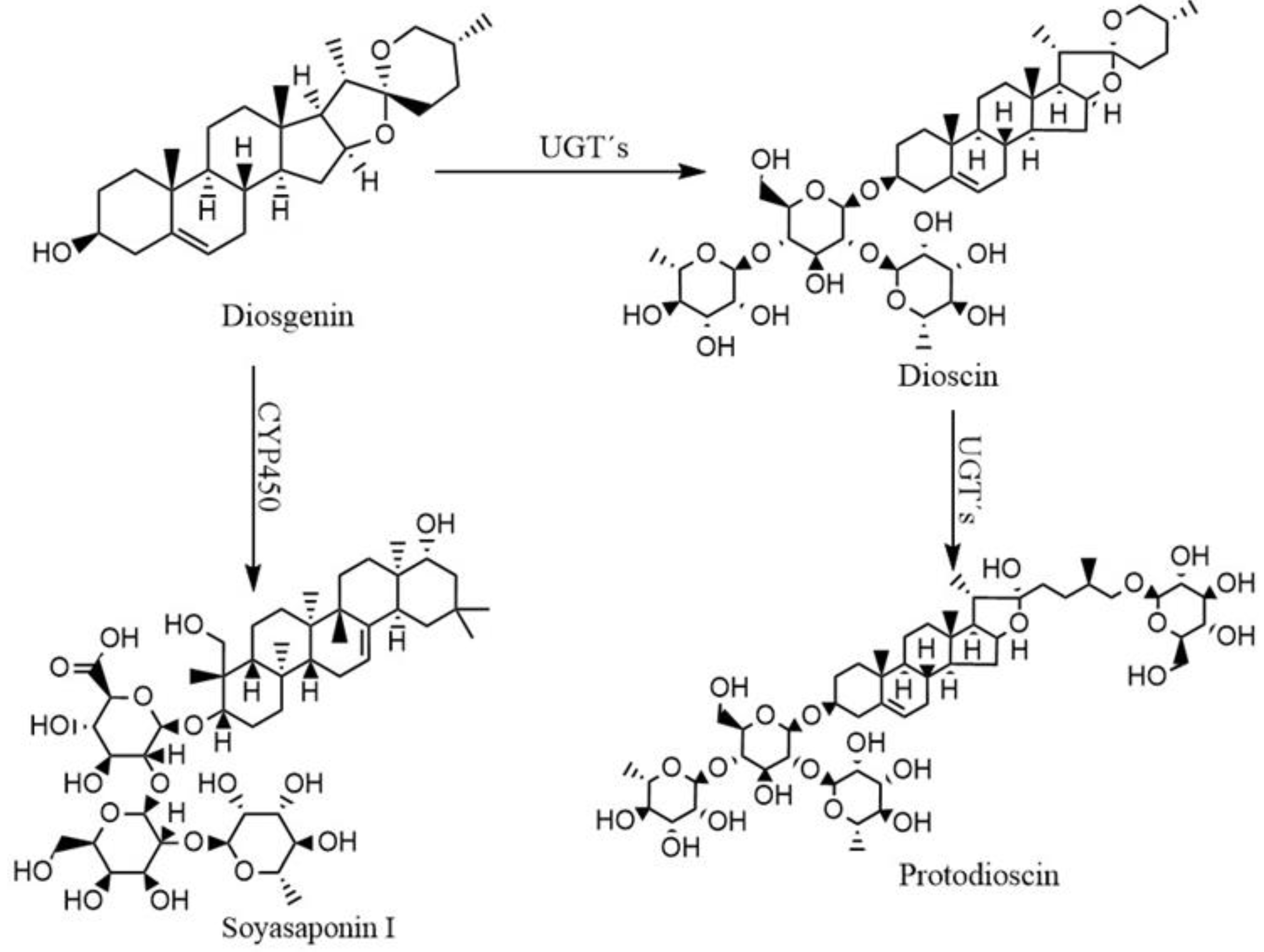
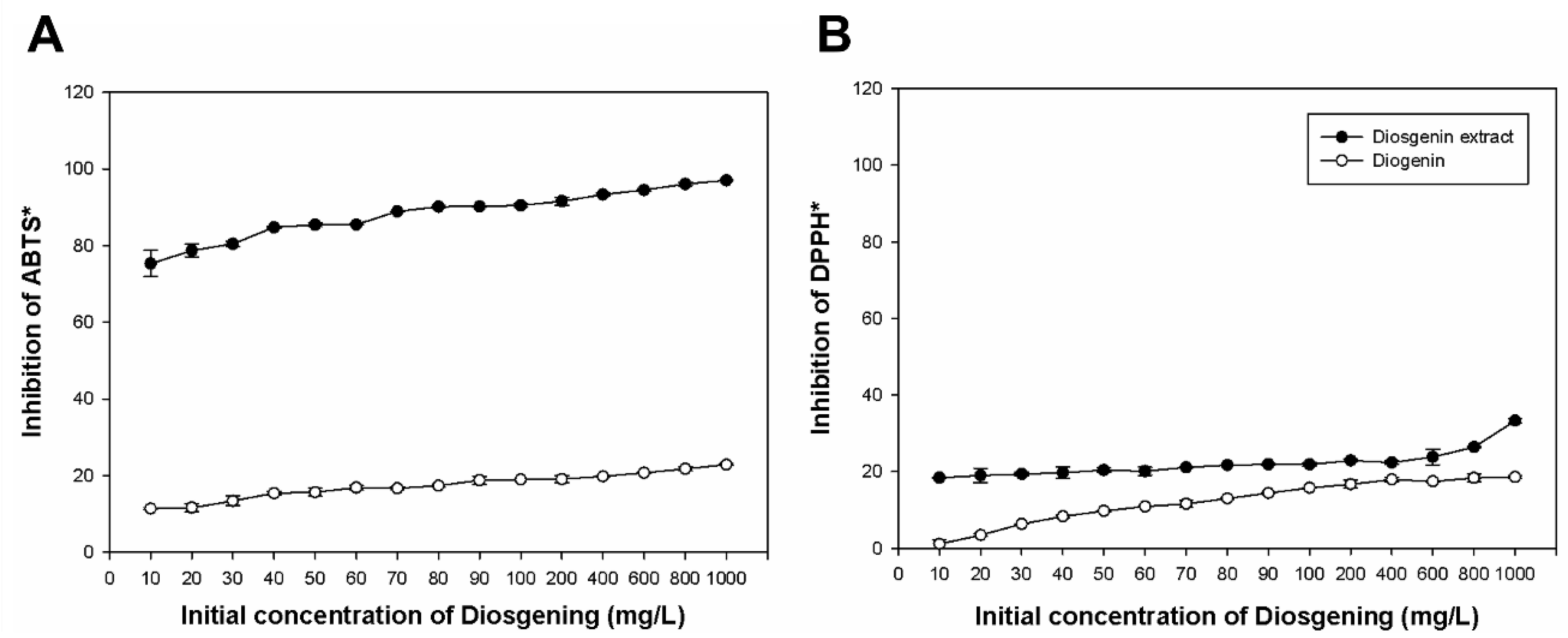
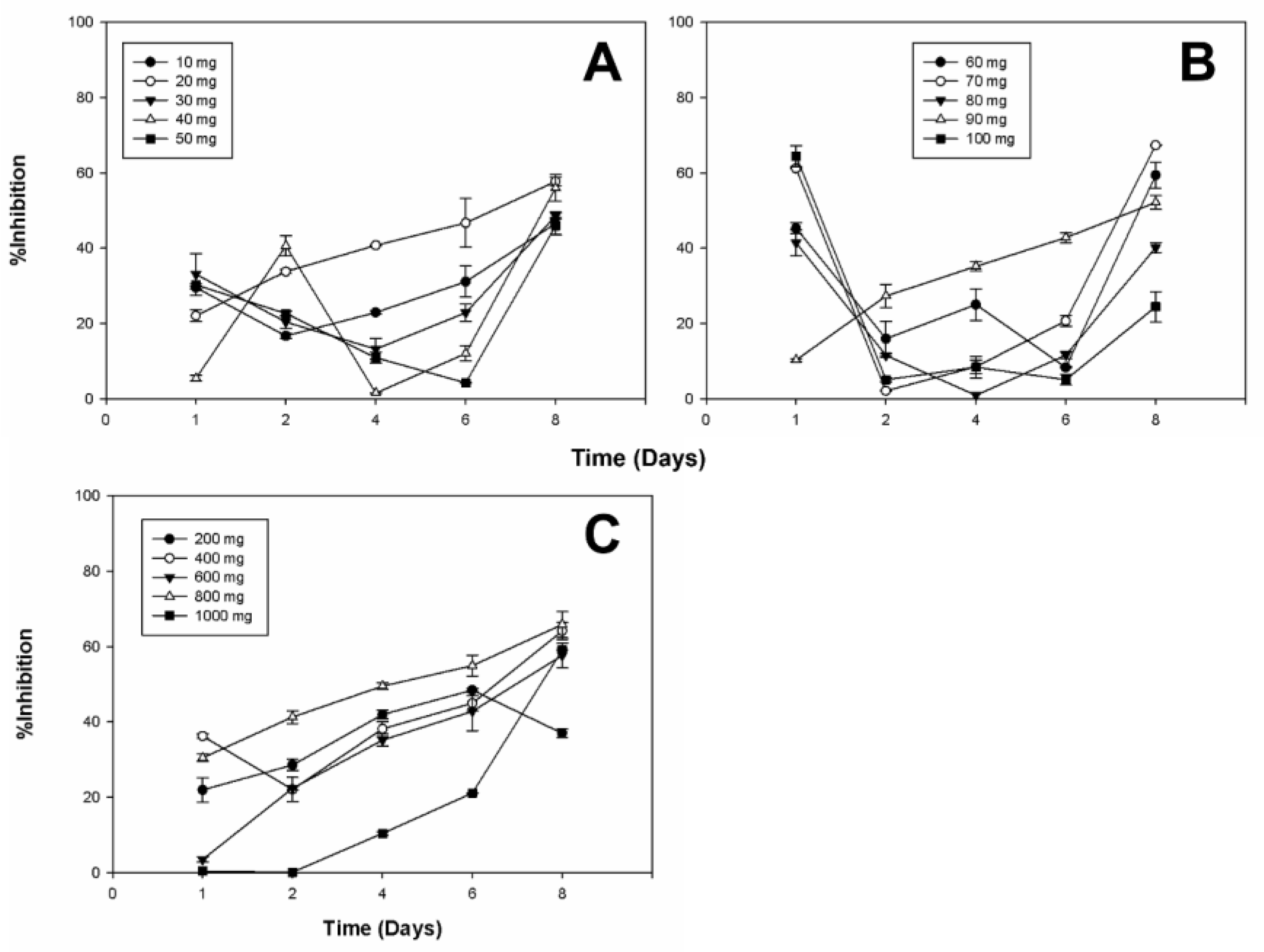

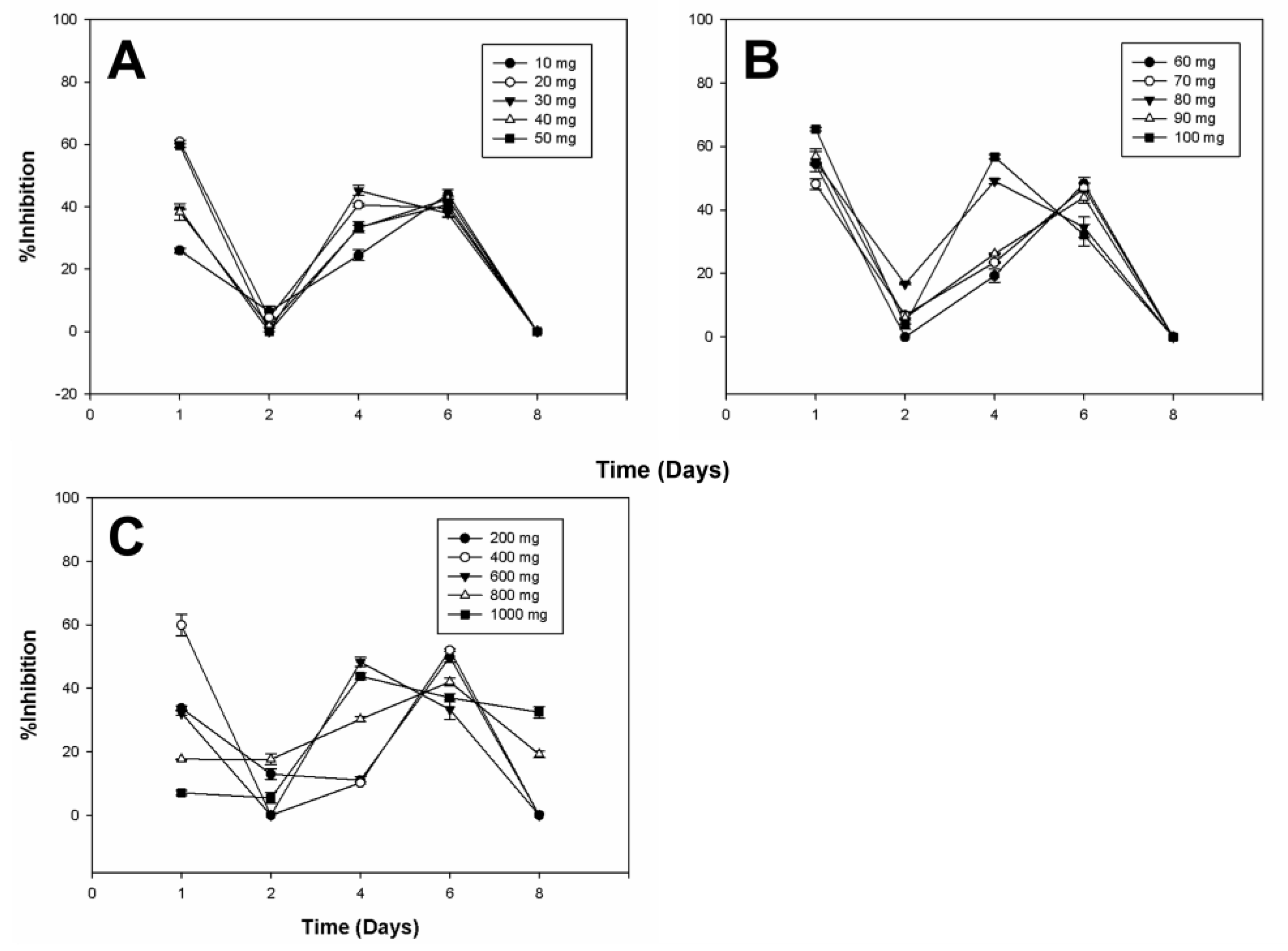
| Initial Concentration of Diosgenin (mg L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80–1000 | |
| Protodioscin (mg L−1) | 4.25 ± 0.00 | 16.53 ± 2.50 | 3.45 ± 0.01 | 5.31 ± 0.21 | 2.87 ± 0.01 | ND | ND | ND |
| Soyasaponin I (mg L−1) | 3.47 ± 0.00 | 2.86 ± 0.00 | 1.75 ± 0.00 | 2.94 ± 0.00 | 10.97 ± 0.59 | 7.70 ± 0.01 | 18.04 ± 0.01 | ND |
| YP/S | 0.772 | 0.969 | 0.173 | 0.206 | 0.276 | 0.128 | 0.257 | ND |
| Initial Concentration of Diosgenin (mg L−1) | Corn | Wheat | Barley | Bean | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Inhibition | ||||||||||||
| Diosgenin Extract | Diosgenin | Herbicide (Picloram)+2,4-D) | Diosgenin Extract | Diosgenin | Herbicide (Picloram+2,4-D) | Diosgenin Extract | Diosgenin | Herbicide (Picloram+2,4-D) | Diosgenin Extract | Diosgenin | Herbicide (Picloram+2,4-D) | |
| 10 | 100 ± 0.00 | 68.88 ± 3.25 | 100 ± 0.00 | 100 ± 0.00 | 86.66 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 87.11 ± 1.72 | 100 ± 0.00 | 100 ± 0.00 | 92.44 ± 2.34 | 100 ± 0.00 |
| 20 | 100 ± 0.00 | 79.55 ± 1.72 | 100 ± 0.00 | 100 ± 0.00 | 93.33 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 92.44 ± 2.34 | 100 ± 0.00 | 100 ± 0.00 | 93.33 ± 0.00 | 100 ± 0.00 |
| 30 | 100 ± 0.00 | 72.00 ± 2.76 | 100 ± 0.00 | 100 ± 0.00 | 88.88 ± 3.25 | 100 ± 0.00 | 100 ± 0.00 | 92.00 ± 2.76 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 40 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 50 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 60 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 70 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 80 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 90 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 100 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 200 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 400 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 600 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 800 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| 1000 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
| Study | Microbial System | Initial Substrate | Metabolites Produced | Saponin Yield (mg/L) | Enhanced Bioactivity |
|---|---|---|---|---|---|
| This study | Yarrowia lipolytica P01a | Diosgenin | Protodioscin, Soyasaponin I | Up to 19.39 | Significant increase in antioxidant, antifungal, and herbicidal activities |
| Dong et al., 2015 [36] | Penicillium dioscin | Dioscin | Diosgenin | >90% conversion yield | High bioactivity, precursor for steroidal hormones |
| Liu et al., 2010 [37] | Trichoderma harzianum | Steroidal saponins | Diosgenin | 30.05 mg/g | High yield via optimized biotransformation; potential for eco-friendly diosgenin production. |
| Wu et al., 2011 [38] | Coriolus versicolor | Diosgenin | Diosgenin Derivatives | 25 mg from compound 5 8 mg from compound 8 | Novel hydroxylated diosgenin derivatives, indicating new pathways for steroidal modifications. |
| Dong et al., 2010 [39] | Cunninghamella echinulata CGMCC | Diosgenin | Diosgenin derivatives | 20 mg from derivative 2 12 mg from compound 3 80 mg from compound 4 100 mg from compound 5 | Hydroxylation products with no significant cytotoxicity; potential for further pharmaceutical applications. |
| Xiao et al., 2011 [40] | Cunninghamella echinulata CGMCC | Diosgenin | Diosgenin derivatives | Not specified | Hydroxylation products contributing to novel steroidal structures with potential bioactivities. |
| Wang et al., 2007 [41] | Streptomyces virginiae IBL-14 | Diosgenin, Diosgenone | Isonuatigenone, 25β-hydroxy derivatives | 28.4% of Diosgenin bioconversion | Pathway elucidation for converting diosgenin to isonuatigenone suggests potential drug synthesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Guzmán, C.; Hernández-Montiel, L.G.; Velázquez-Lizarraga, A.E.; Ríos-González, L.J.; Huerta-Ochoa, S.; Cervantes-Güicho, V.d.J.; Morales-Martínez, T.K.; Mejía-Ruíz, C.H.; Reyes, A.G. Enhanced Antioxidant, Antifungal, and Herbicidal Activities through Bioconversion of Diosgenin by Yarrowia lipolytica P01a. Plants 2024, 13, 2629. https://doi.org/10.3390/plants13182629
Hernández-Guzmán C, Hernández-Montiel LG, Velázquez-Lizarraga AE, Ríos-González LJ, Huerta-Ochoa S, Cervantes-Güicho VdJ, Morales-Martínez TK, Mejía-Ruíz CH, Reyes AG. Enhanced Antioxidant, Antifungal, and Herbicidal Activities through Bioconversion of Diosgenin by Yarrowia lipolytica P01a. Plants. 2024; 13(18):2629. https://doi.org/10.3390/plants13182629
Chicago/Turabian StyleHernández-Guzmán, Christian, Luis G. Hernández-Montiel, Adrian E. Velázquez-Lizarraga, Leopoldo J. Ríos-González, Sergio Huerta-Ochoa, Vianey de J. Cervantes-Güicho, Thelma K. Morales-Martínez, Claudio H. Mejía-Ruíz, and Ana G. Reyes. 2024. "Enhanced Antioxidant, Antifungal, and Herbicidal Activities through Bioconversion of Diosgenin by Yarrowia lipolytica P01a" Plants 13, no. 18: 2629. https://doi.org/10.3390/plants13182629
APA StyleHernández-Guzmán, C., Hernández-Montiel, L. G., Velázquez-Lizarraga, A. E., Ríos-González, L. J., Huerta-Ochoa, S., Cervantes-Güicho, V. d. J., Morales-Martínez, T. K., Mejía-Ruíz, C. H., & Reyes, A. G. (2024). Enhanced Antioxidant, Antifungal, and Herbicidal Activities through Bioconversion of Diosgenin by Yarrowia lipolytica P01a. Plants, 13(18), 2629. https://doi.org/10.3390/plants13182629









