Comparative Analysis of Chemical Profiles and Biological Activities of Essential Oils Derived from Torreya grandis Arils and Leaves: In Vitro and In Silico Studies
Abstract
1. Introduction
2. Results
2.1. Chemical Compositions and Yield of EOs from Aril and Leaves of T. grandis
2.2. Biological Activity Comparison of EOs
2.2.1. In Vitro Anti-Melanogenic Activity
2.2.2. In Vitro Antioxidant Activity
2.2.3. In Vitro Anti-Inflammatory Activity
2.3. Network Pharmacology Prediction
2.3.1. Putative Targets of EOs for the Treatment of Skin Pigmentation and Inflammation
2.3.2. Compound–Target Interaction Network
2.3.3. PPI Network Construction
2.3.4. Bioinformatic Enrichment
2.3.5. Molecular Docking Analysis
3. Discussion
3.1. EO Yield and Composition between Torreya Aril and Leaves
3.2. Biological Activities of EOs from Torreya Leaves and Aril
3.3. Molecular Mechanisms of Torreya EO Treatment for Skin Pigmentation and Inflammation
4. Materials and Methods
4.1. Plant Material and EO Hydrodistillation
4.2. Chemical Analysis
4.3. Determination of Antioxidant Activity with β-Carotene Bleaching Assay
4.4. Cell Line Culture and Cell Viability Assay
4.5. Cellular Melanin Content and Tyrosinase Activity Assay
4.6. Cellular NO, TNF-α, and IL-6 Expression Assay
4.7. Network Pharmacology
4.7.1. Screening the Active Compounds and Targets of AEO and LEO
4.7.2. Construction of Active Compound–Target Network
4.7.3. Protein–Protein Interaction Network Construction
4.7.4. GO and KEGG Enrichment Analysis
4.7.5. Molecular Docking and Visualization
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Jin, H. Review of cultivation and development of Chinese torreya in China. For. Trees Livelihoods 2018, 28, 68–78. [Google Scholar] [CrossRef]
- Chen, H.; Yue, X.; Yang, J.; Lv, C.; Dong, S.; Luo, X.; Sun, Z.; Zhang, Y.; Li, B.; Zhang, F.; et al. Pyrolysis molecule of Torreya grandis bark for potential biomedicine. Saudi J. Biol. Sci. 2019, 26, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shu, Q.; Niu, Y.; Jiao, Y.; Chen, Q. Preparation, characterization, and antibacterial effects of chitosan nanoparticles embedded with essential oils synthesized in an ionic liquid containing system. J. Agric. Food Chem. 2018, 66, 7006–7014. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Bao, J.; Mo, J.; Zhang, Y. Chemical composition and mosquito (Aedes aegypti) repellent activity of essential oil extracted from the aril of Torreya grandis. J. Essent. Oil Bear. Plants 2010, 13, 594–602. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Ni, S.; Wu, F.; Sang, W.-G. Chemical composition and antioxidant activity of essential oil from Torreya grandis cv. ‘merrillii’ arils. J. Essent. Oil Bear. Plants 2016, 19, 1170–1180. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Dong, Y.; Wang, Y. Effect of chitosan coating incorporated with Torreya grandis essential oil on the quality and physiological attributes of loquat fruit. J. Food Meas. Charact. 2022, 16, 2820–2830. [Google Scholar] [CrossRef]
- Feng, T.; Hu, Z.S.; Song, S.Q.; Yao, L.Y.; Sun, M.; Zhu, X.; Lu, J. The antioxidant and tyrosinase inhibition properties of essential oil from the peel of Chinese Torreya grandis Fort. RSC Adv. 2019, 9, 42360–42366. [Google Scholar] [CrossRef]
- Saeed, M.K.; Deng, Y.; Dai, R.; Li, W.; Yu, Y.; Iqbal, Z. Appraisal of antinociceptive and anti-inflammatory potential of extract and fractions from the leaves of Torreya grandis Fort Ex. Lindl. J. Ethnopharmacol. 2010, 127, 414–418. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, A.; Wang, J.; Huang, D.; Deng, Y.; Zhang, X.; Qu, Q.; Ma, W.; Xiong, R.; Zhu, M.; et al. Potential application of natural bioactive compounds as skin-whitening agents: A review. J. Cosmet. Dermatol-Us 2022, 21, 6669–6687. [Google Scholar] [CrossRef]
- Munoz-Munoz, J.L.; García-Molina, F.; Varón, R.; Tudela, J.; García-Cánovas, F.; Rodríguez-López, J.N. Generation of hydrogen peroxide in the melanin biosynthesis pathway. BBA-Proteins Proteom. 2009, 1794, 1017–1029. [Google Scholar] [CrossRef]
- Chao, W.-W.; Su, C.-C.; Peng, H.-Y.; Chou, S.-T. Melaleuca quinquenervia essential oil inhibits α-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Phytomedicine 2017, 34, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, K.; Kazimierczak, U.; Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Contemp. Oncol. 2022, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-T.; Lai, C.-C.; Lai, C.-P.; Chao, W.-W. Chemical composition, antioxidant, anti-melanogenic and anti-inflammatory activities of Glechoma hederacea (Lamiaceae) essential oil. Ind. Crop. Prod. 2018, 122, 675–685. [Google Scholar] [CrossRef]
- Fu, C.; Chen, J.; Lu, J.; Yi, L.; Tong, X.; Kang, L.; Pei, S.; Ouyang, Y.; Jiang, L.; Ding, Y.; et al. Roles of inflammation factors in melanogenesis (Review). Mol. Med. Rep. 2020, 21, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, E.; Karaman-Jurukovska, N.; Mammone, T.; Idowu, O.C. Post-inflammatory hyperpigmentation in dark skin: Molecular mechanism and skincare implications. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2555–2565. [Google Scholar] [CrossRef]
- You, Z.; Li, Y.; Chen, M.; Wong, V.K.W.; Zhang, K.; Zheng, X.; Liu, W. Inhibition of plant essential oils and their interaction in binary combinations against tyrosinase. Food Nutr. Res. 2022, 66, 8466. [Google Scholar] [CrossRef]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Caudullo, G.; Carrara, M. Essential oil phytocomplex activity, a review with a focus on multivariate analysis for a network pharmacology-informed phytogenomic approach. Molecules 2020, 25, 1833. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Gutiérrez-Praena, D.; Jos, A.; Cameán, A.M. In vitro toxicological evaluation of essential oils and their main compounds used in active food packaging: A review. Food Chem. Toxicol. 2015, 81, 9–27. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Németh, Z.E. Sources of variability of wormwood (Artemisia absinthium L.) essential oil. J. Appl. Res. Med. Aromat. Plants 2016, 3, 143–150. [Google Scholar] [CrossRef]
- Dodos, T.; Jankovic, S.; Marin, P.D.; Rajcevic, N. Essential oil composition and micromorphological traits of Satureja montana L., S. subspicata Bartel ex Vis., and S. kitaibelii Wierzb. Ex Heuff. plant organs. Plants 2021, 10, 511. [Google Scholar] [CrossRef]
- Llorens-Molina, J.A.; Vacas, S. Seasonal variations in essential oil of aerial parts and roots of an Artemisia absinthium L. population from a Spanish area with supramediterranean climate (Teruel, Spain). J. Essent. Oil Res. 2015, 27, 395–405. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Frag. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Tech. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Kim, D.-O.; Heo, H.J. Melanogenesis regulatory activity of the ethyl acetate fraction from Arctium lappa L. leaf on α-MSH–induced B16/F10 melanoma cells. Ind. Crop. Prod. 2019, 138, 111581. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Vani, M.G.; Wu, P.C.; Lee, H.J.; Tseng, Y.H.; Wang, S.Y. Essential oils of Alpinia nantoensis retard forskolin-induced melanogenesis via ERK1/2-mediated proteasomal degradation of MITF. Plants 2020, 9, 1672. [Google Scholar] [CrossRef]
- Mansour, R.B.; Wasli, H.; Bourgou, S.; Khamessi, S.; Ksouri, R.; Megdiche-Ksouri, W.; Cardoso, S.M. Insights on Juniperus phoenicea essential oil as potential anti-proliferative, anti-tyrosinase, and antioxidant candidate. Molecules 2023, 28, 7547. [Google Scholar] [CrossRef]
- El Omari, N.; Mrabti, H.N.; Benali, T.; Ullah, R.; Alotaibi, A.; Abdullah, A.D.I.; Goh, K.W.; Bouyahya, A. Expediting multiple biological properties of Limonene and α-pinene: Main bioactive compounds of Pistacia lentiscus L., essential oils. Front. Biosci. (Landmark Ed.) 2023, 28, 229. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, S.Y.; Jang, S.K.; Kim, K.J.; Park, M.J. Inhibition of melanogenesis by essential oils from the citrus cultivars peels. Int. J. Mol. Sci. 2023, 24, 4207. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Boffa, L.; Genzini, L.; Garella, D. Phytotherapeutics: An evaluation of the potential of 1000 plants. J. Clin. Pharm. Ther. 2010, 35, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system-A Review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Frag. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Tian, M.; Xie, D.; Yang, Y.; Tian, Y.; Jia, X.; Wang, Q.; Deng, G.; Zhou, Y. Hedychium flavum flower essential oil: Chemical composition, anti-inflammatory activities and related mechanisms in vitro and in vivo. J. Ethnopharmacol. 2023, 301, 115846. [Google Scholar] [CrossRef]
- de Cássia da Silveira e Sá, R.; Andrade, L.; de Sousa, D. A review on anti-Inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, L.; Wang, S.; Gao, Y.; Jin, F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: A systematic review. J. Ethnopharmacol. 2023, 301, 115829. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, S.-Y.; Jang, S.-K.; Kim, K.-J.; Park, M.-J. Anti-inflammatory effects of essential oils from the peels of citrus cultivars. Pharmaceutics 2023, 15, 1595. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zheng, Y.; Feng, Y.; Chen, H.; Dai, S.X.; Wang, Y.; Xu, M. Comparative analysis of active ingredients and potential bioactivities of essential oils from Artemisia argyi and A. verlotorum. Molecules 2023, 28, 3927. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Takeuchi, Y.; Hirota, K. The pathogenicity of Th17 cells in autoimmune diseases. Semin. Immunopathol. 2019, 41, 283–297. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Shin, S.Y.; Gil, H.N.; Choi, J.H.; Lim, Y.; Lee, Y.H. Agerarin inhibits α-MSH-induced TYR gene transcription via STAT3 suppression independent of CREB-MITF pathway. J. Dermatol. Sci. 2018, 91, 107–110. [Google Scholar] [CrossRef]
- Yan, X.; Ma, X.; Dai, D.; Yan, X.; Han, X.; Bao, X.; Xie, Q. Potent pigmentation inhibitory activity of incensole-enriched frankincense volatile oil-identification, efficacy and mechanism. J. Cosmet. Dermatol. 2024, 23, 244–255. [Google Scholar] [CrossRef]
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The epidermal growth factor receptor system in skin repair and inflammation. J. Investig. Dermatol. 2008, 128, 1365–1374. [Google Scholar] [CrossRef]
- Matsumura, S.; Terao, M.; Itami, S.; Katayama, I. Local cortisol activation is involved in EGF-induced immunosuppression. Derm. Endocrinol. 2017, 9, e1412018. [Google Scholar] [CrossRef]
- Lin, K.Y.; Chen, C.M.; Lu, C.Y.; Cheng, C.Y.; Wu, Y.H. Regulation of miR-21 expression in human melanoma via UV-ray-induced melanin pigmentation. Environ. Toxicol. 2017, 32, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Techapichetvanich, T.; Wanitphakdeedecha, R.; Iamphonrat, T.; Phothong, W.; Eimpunth, S.; Jane Hidajat, I.; Manuskiatti, W. The effects of recombinant human epidermal growth factor containing ointment on wound healing and post inflammatory hyperpigmentation prevention after fractional ablative skin resurfacing: A split-face randomized controlled study. J. Cosmet. Dermatol. 2018, 17, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Oh, S.; Yang, J.Y.; Sun, H.J.; Jang, M.; Kang, D.; Son, K.H.; Byun, K. Evaluating whether radiofrequency irradiation attenuated UV-B-induced skin pigmentation by increasing melanosomal autophagy and decreasing melanin synthesis. Int. J. Mol. Sci. 2021, 22, 10724. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, T.; Zheng, G.; Zhou, L.; Ma, K.; Xiong, X.; Zheng, C.; Li, J.; Zhu, Y.; Bian, W.; et al. Mechanism exploration of 6-Gingerol in the treatment of atherosclerosis based on network pharmacology, molecular docking and experimental validation. Phytomedicine 2023, 115, 154835. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chouhan, K.B.S.; Chandrakar, M.; Gupta, P.; Lal, K.; Mandal, V. A cross talk based critical analysis of solvent free microwave extraction to accentuate it as the new normal for extraction of essential oil: An attempt to overhaul the science of distillation through a comprehensive tutelage. Crit. Rev. Food Sci. 2023, 63, 6960–6982. [Google Scholar] [CrossRef]
- Lin, Q.M.; Wang, Y.; Yu, J.H.; Liu, Y.L.; Wu, X.; He, X.R.; Zhou, Z.W. Tyrosinase inhibitors from the leaves of Eucalyptus globulus. Fitoterapia 2019, 139, 104418. [Google Scholar] [CrossRef]
- Mondal, M.; Quispe, C.; Sarkar, C.; Bepari, T.C.; Alam, M.J.; Saha, S.; Ray, P.; Rahim, M.A.; Islam, M.T.; Setzer, W.N.; et al. Analgesic and anti-inflammatory potential of essential oil of leaf: In vivo and in silico studies. Nat. Prod. Commun. 2021, 16, 1–16. [Google Scholar] [CrossRef]
- Cardenas Garza, G.R.; Elizondo Luevano, J.H.; Bazaldua Rodriguez, A.F.; Chavez Montes, A.; Perez Hernandez, R.A.; Martinez Delgado, A.J.; Lopez Villarreal, S.M.; Rodriguez Rodriguez, J.; Sanchez Casas, R.M.; Castillo Velazquez, U.; et al. Benefits of Cardamom (Elettaria cardamomum (L.) Maton) and Turmeric (Curcuma longa L.) extracts for their applications as natural anti-inflammatory adjuvants. Plants 2021, 10, 1908. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Sun, Y.L.; Ruan, X.F. Bornyl acetate: A promising agent in phytomedicine for inflammation and immune modulation. Phytomedicine 2023, 114, 154781. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.Y.; Hong, C.Y.; Gwak, K.S.; Park, M.J.; Smith, D.; Choi, I.G. Whitening and antioxidant activities of bornyl acetate and nezukol fractionated from Cryptomeria japonica essential oil. Int. J. Cosmet. Sci. 2013, 35, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Bonjardim, L.R.; Cunha, E.S.; Guimaraes, A.G.; Santana, M.F.; Oliveira, M.G.; Serafini, M.R.; Araujo, A.A.; Antoniolli, A.R.; Cavalcanti, S.C.; Santos, M.R.; et al. Evaluation of the anti-inflammatory and antinociceptive properties of p-cymene in mice. Z. Naturforschung C J. Biosci. 2012, 67, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Sun, Y.; Zhou, J.; Gu, Y.; Li, Y. Baicalein inhibits melanogenesis through activation of the ERK signaling pathway. Int. J. Mol. Med. 2010, 25, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tan, N.; Hu, J.; Wang, H.; Duan, D.; Ma, L.; Xiao, J.; Wang, X. Analysis of the main active ingredients and bioactivities of essential oil from Osmanthus fragrans Var. thunbergii using a complex network approach. BMC Syst. Biol. 2017, 11, 144. [Google Scholar] [CrossRef]
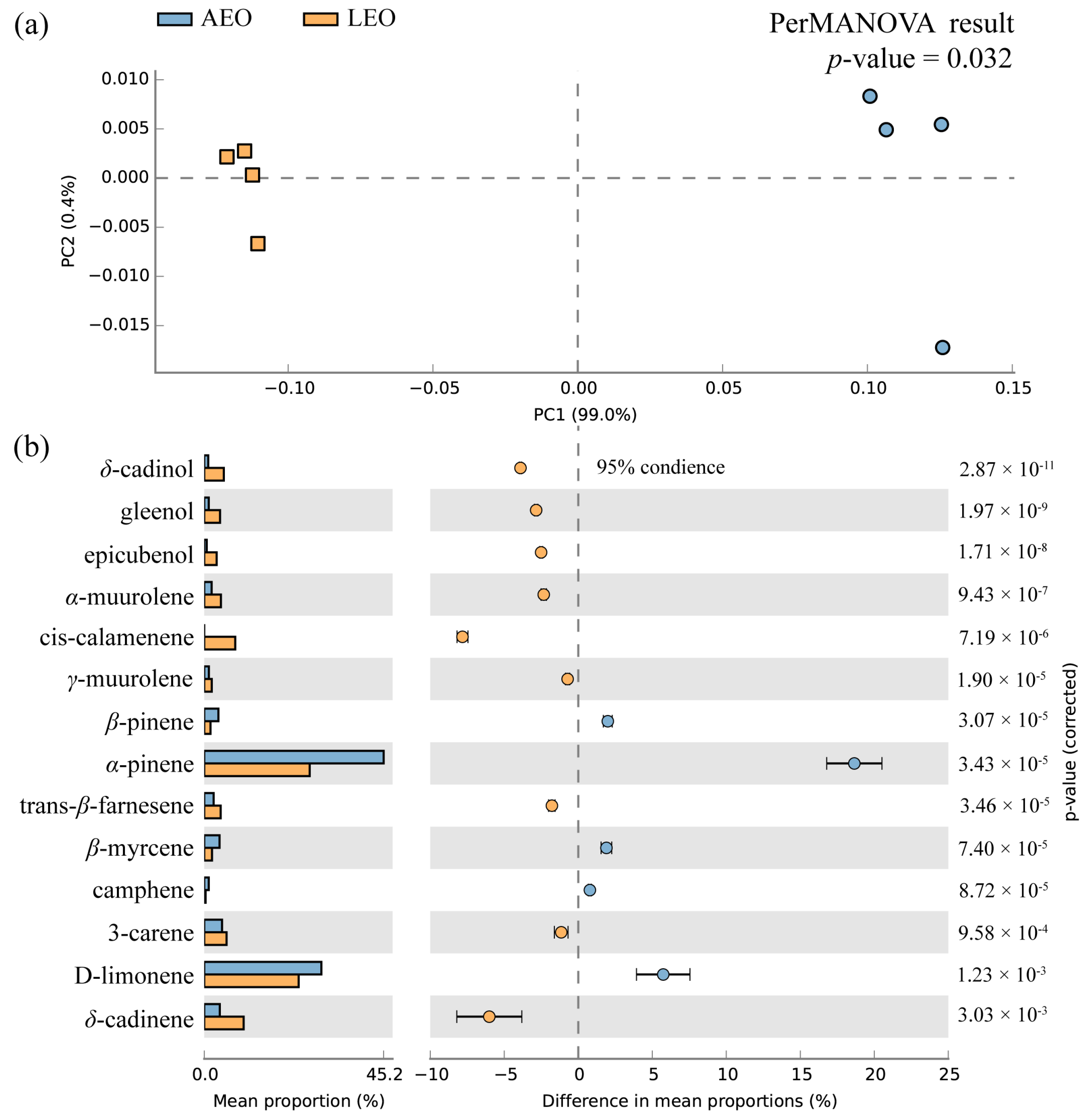


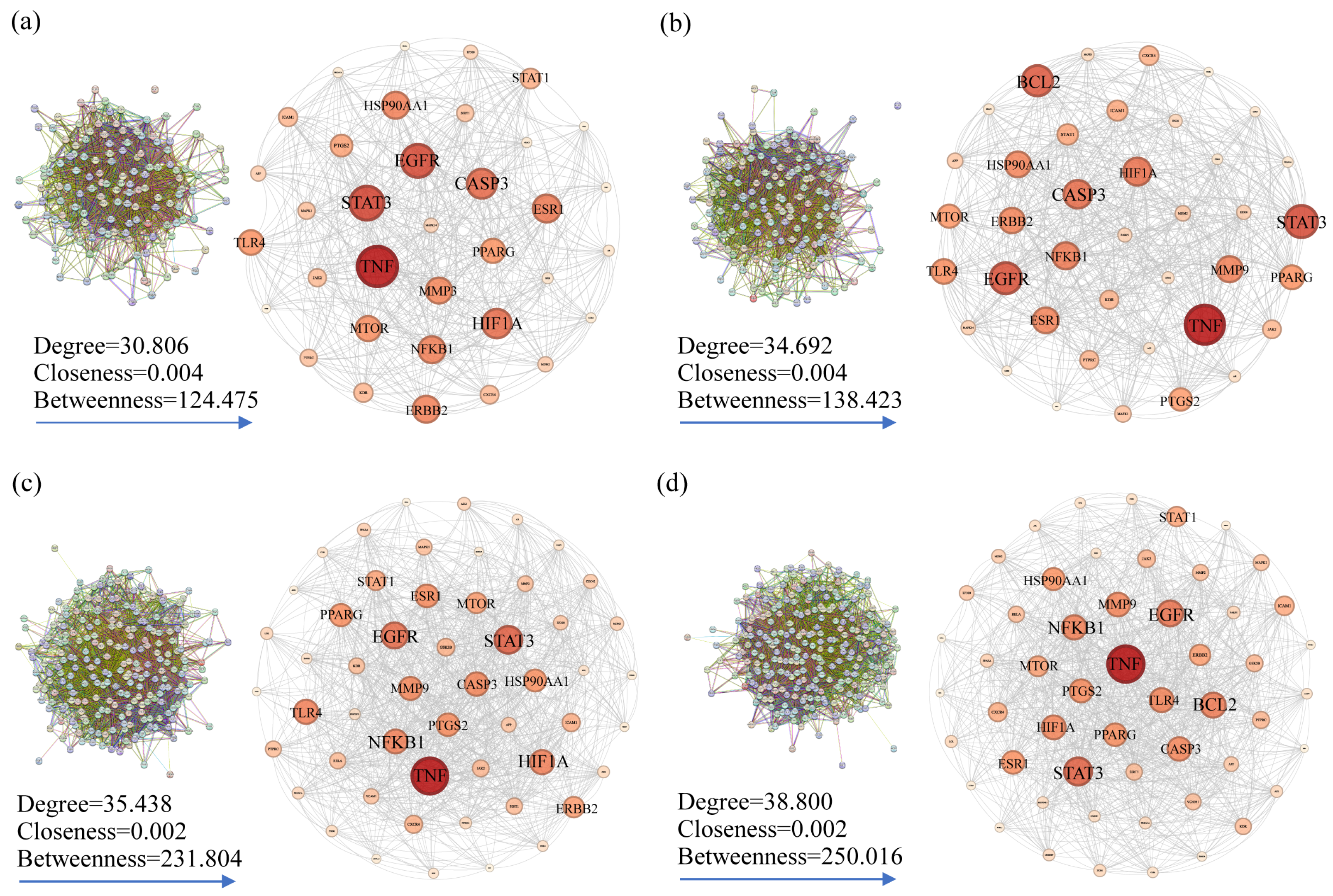
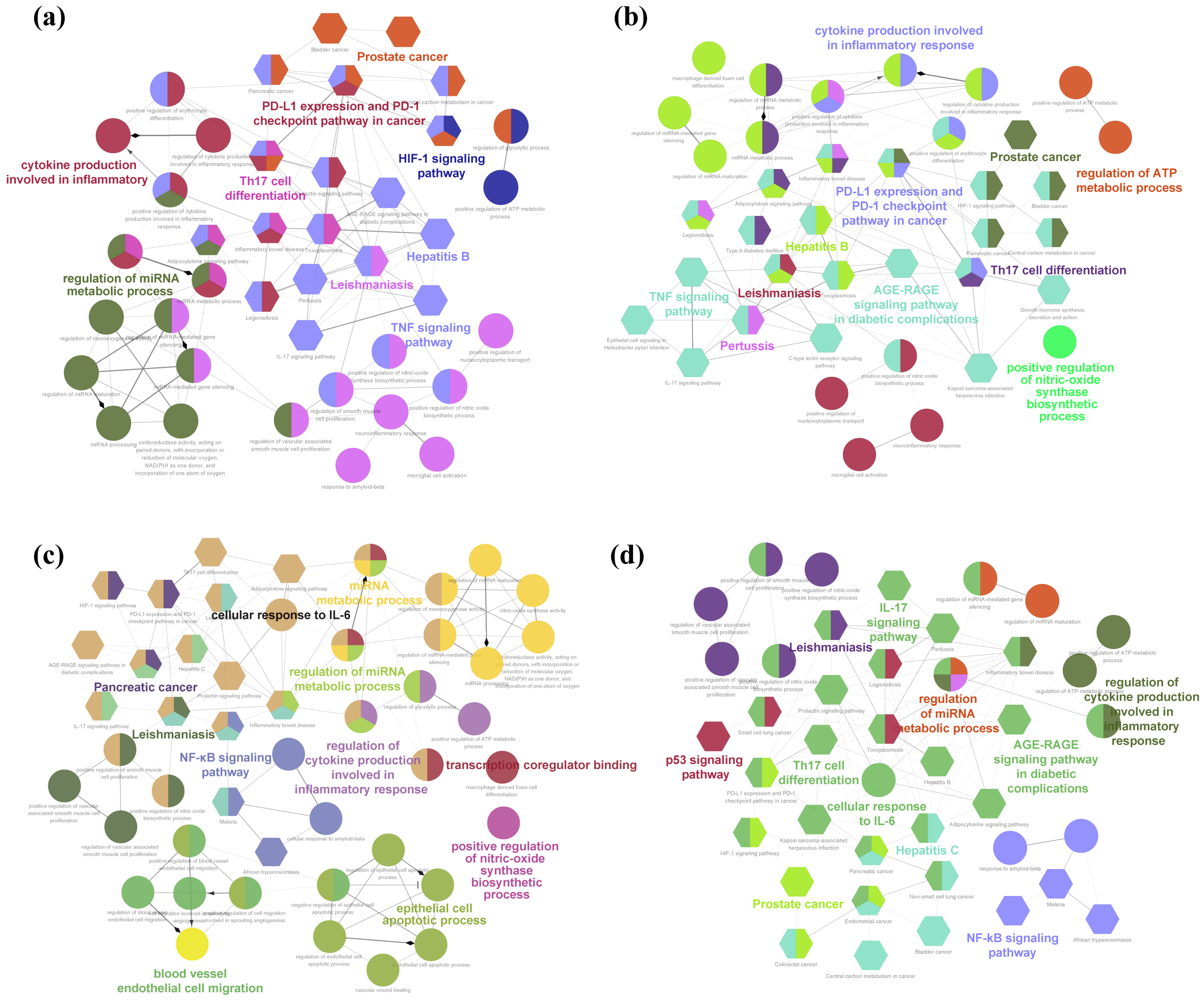
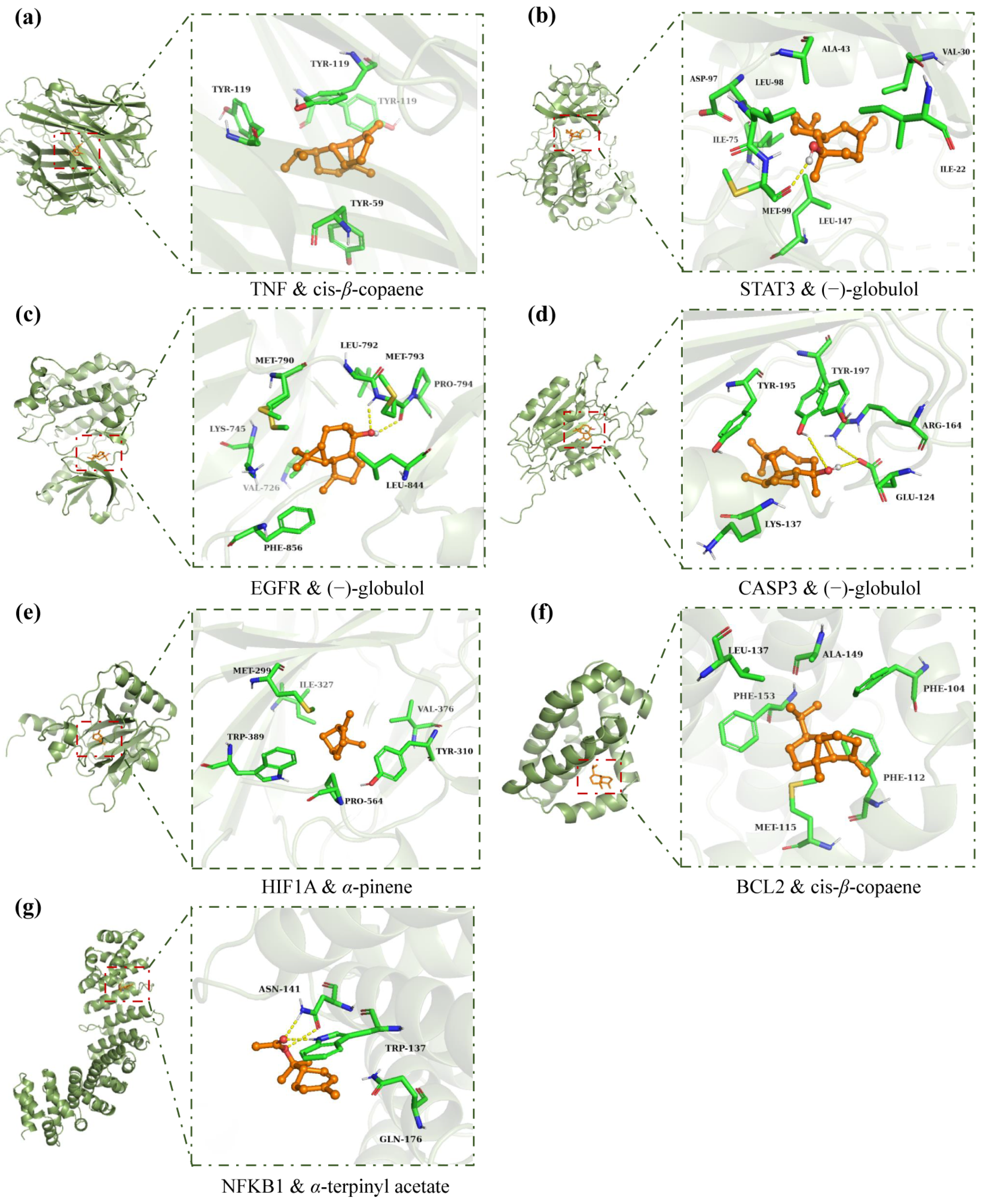
| No. | Compounds (a) | Formula | RT (b) | RI (c) | Library RI (d) | AEO | LEO |
|---|---|---|---|---|---|---|---|
| Relative Area Percentage (%) | |||||||
| 1 | tricyclene | C10H16 | 9.28 | 920 | 925 | 0.25 ± 0.02 | N/A |
| 2 | α-pinene | C10H16 | 9.74 | 933 | 937 | 39.15 ± 1.23 | 21.26 ± 0.27 |
| 3 | α-fenchene | C10H16 | 10.19 | 945 | 950 | 0.12 ± 0.07 | 0.19 ± 0.01 |
| 4 | camphene | C10H16 | 10.23 | 947 | 952 | 0.99 ± 0.05 | 0.29 ± 0.01 |
| 5 | sabinen | C10H16 | 11.16 | 972 | 974 | 0.96 ± 0.05 | 0.35 ± 0.02 |
| 6 | β-pinene | C10H16 | 11.26 | 975 | 979 | 3.10 ± 0.05 | 1.27 ± 0.07 |
| 7 | 1-octen-3-ol | C8H16O | 11.41 | 979 | 981 | N/A | 1.27 ± 0.02 |
| 8 | β-myrcene | C10H16 | 11.85 | 991 | 991 | 3.33 ± 0.17 | 1.56 ± 0.09 |
| 9 | 3-carene | C10H16 | 12.52 | 1009 | 1011 | 3.89 ± 0.16 | 4.51 ± 0.21 |
| 10 | p-cymene | C10H14 | 13.05 | 1024 | 1025 | 0.76 ± 0.04 | 0.43 ± 0.03 |
| 11 | D-limonene | C10H16 | 13.25 | 1029 | 1029 | 25.57 ± 0.53 | 19.04 ± 1.04 |
| 12 | β-ocimene | C10H16 | 13.59 | 1038 | 1037 | 0.17 ± 0.01 | N/A |
| 13 | trans-β-ocimene | C10H16 | 13.97 | 1049 | 1049 | 0.20 ± 0.01 | N/A |
| 14 | α-terpinolene | C10H16 | 15.44 | 1088 | 1088 | 0.38 ± 0.02 | 0.26 ± 0.01 |
| 15 | 4-terpineol | C10H18O | 18.67 | 1178 | 1177 | 0.35 ± 0.02 | 0.14 ± 0.01 |
| 16 | p-cymen-8-ol | C10H14O | 18.93 | 1185 | 1183 | 0.27 ± 0.01 | 0.14 ± 0.07 |
| 17 | α-terpineol | C10H18O | 19.14 | 1191 | 1189 | 0.30 ± 0.01 | 0.10 ± 0.01 |
| 18 | citronellol | C10H20O | 20.46 | 1228 | 1228 | 0.40 ± 0.02 | 0.10 ± 0.01 |
| 19 | bornyl acetate | C12H20O2 | 22.49 | 1286 | 1285 | 0.15 ± 0.01 | 0.27 ± 0.01 |
| 20 | δ-eIemene | C15H24 | 24.40 | 1341 | 1338 | 0.08 ± 0.04 | 0.14 ± 0.01 |
| 21 | α-terpinyl acetate | C12H20O2 | 24.79 | 1352 | 1350 | N/A | 0.40 ± 0.01 |
| 22 | α-cubebene | C15H24 | 24.83 | 1353 | 1351 | 0.41 ± 0.02 | 0.62 ± 0.01 |
| 23 | copaene | C15H24 | 25.73 | 1379 | 1376 | 0.19 ± 0.01 | 0.16 ± 0.01 |
| 24 | β-cubebene | C15H24 | 26.20 | 1393 | 1389 | 0.17 ± 0.01 | 0.32 ± 0.02 |
| 25 | β-elemene | C15H24 | 26.25 | 1394 | 1391 | 0.11 ± 0.01 | 0.19 ± 0.01 |
| 26 | caryophyllene | C15H24 | 27.14 | 1423 | 1419 | 0.29 ± 0.01 | 0.9 ± 0.02 |
| 27 | cis-β-copaene | C15H24 | 27.43 | 1433 | 1432 | 0.83 ± 0.05 | 1.53 ± 0.07 |
| 28 | aromandendrene | C15H24 | 27.74 | 1443 | 1440 | 0.20 ± 0.01 | 0.13 ± 0.01 |
| 29 | trans-β-farnesene | C15H24 | 28.21 | 1459 | 1457 | 2.05 ± 0.10 | 3.32 ± 0.03 |
| 30 | isocadinene | C15H24 | 28.75 | 1478 | 1477 | N/A | 0.43 ± 0.01 |
| 31 | γ-muurolene | C15H24 | 28.84 | 1481 | 1481 | 1.01 ± 0.05 | 1.51 ± 0.06 |
| 32 | germacrene D | C15H24 | 28.98 | 1485 | 1485 | 0.64 ± 0.04 | 2.48 ± 0.08 |
| 33 | β-selinene | C15H24 | 29.13 | 1491 | 1486 | 0.06 ± 0.01 | 0.13 ± 0.01 |
| 34 | γ-amorphene | C15H24 | 29.29 | 1496 | 1493 | 0.51 ± 0.03 | 2.01 ± 0.07 |
| 35 | viridiflorene | C15H24 | 29.37 | 1499 | 1496 | 0.25 ± 0.02 | 0.52 ± 0.01 |
| 36 | α-muurolene | C15H24 | 29.51 | 1504 | 1499 | 1.61 ± 0.08 | 3.36 ± 0.04 |
| 37 | γ-cadinene | C15H24 | 29.89 | 1519 | 1513 | 0.60 ± 0.03 | 0.81 ± 0.03 |
| 38 | cis-calamenene | C15H24 | 30.14 | 1528 | 1523 | N/A | 6.25 ± 0.16 |
| 39 | δ-cadinene | C15H24 | 30.15 | 1529 | 1524 | 3.42 ± 1.24 | 7.95 ± 0.08 |
| 40 | germacrene B | C15H24 | 31.06 | 1563 | 1557 | N/A | 0.21 ± 0.01 |
| 41 | trans-nerolidol | C15H26O | 31.14 | 1566 | 1564 | N/A | 0.25 ± 0.02 |
| 42 | (−)-globulol | C15H26O | 31.35 | 1574 | 1576 | N/A | 0.10 ± 0.01 |
| 43 | spathulenol | C15H26O | 31.59 | 1583 | 1580 | 0.27 ± 0.02 | 0.57 ± 0.01 |
| 44 | gleenol | C15H26O | 31.76 | 1590 | 1586 | 0.99 ± 0.05 | 3.19 ± 0.05 |
| 45 | epicubenol | C15H26O | 32.86 | 1634 | 1627 | 0.54 ± 0.03 | 2.50 ± 0.04 |
| 46 | δ-cadinol | C15H26O | 33.28 | 1652 | 1645 | 0.88 ± 0.03 | 3.94 ± 0.04 |
| 47 | α-cadinol | C15H26O | 33.49 | 1661 | 1653 | 0.13 ± 0.01 | 0.71 ± 0.03 |
| 48 | cis-10-hydroxycalamene | C15H26O | 33.58 | 1665 | 1666 | 0.46 ± 0.01 | 0.40 ± 0.01 |
| 49 | trans-10-hydroxycalamenene | C15H26O | 33.79 | 1674 | 1676 | 0.81 ± 0.03 | 0.66 ± 0.01 |
| 50 | trans-farnesol | C15H26O | 34.96 | 1724 | 1713 | N/A | 0.78 ± 0.11 |
| Total identified | 96.86 ± 1.54 | 97.65 ± 0.05 | |||||
| Monoterpene hydrocarbons | 78.43 ± 1.09 | 48.84 ± 0.72 | |||||
| Oxygenated monoterpenes | 1.17 ± 0.04 | 2.32 ± 0.10 | |||||
| Sesquiterpene hydrocarbons | 12.42 ± 1.24 | 32.96 ± 0.67 | |||||
| Oxygenated sesquiterpenes | 4.08 ± 0.15 | 13.10 ± 0.20 | |||||
| Yield of TGEO (v/w) | 2.04 | 0.49 | |||||
| Bioactive Compounds | Binding Energies (kcal/mol) | |||||||
|---|---|---|---|---|---|---|---|---|
| Name | Pubchem ID | TNF | STAT3 | EGFR | CASP3 | HIF1A | BCL2 | NFKB1 |
| cis-β-copaene | 87529 | −9.6 | −7.3 | −7.9 | −6.5 | −5.7 | −7.0 | NA |
| bornyl acetate | 93009 | −7.1 | −6.1 | −6.2 | −5.8 | −5.0 | −6.1 | −5.4 |
| tricyclene | 79035 | −6.8 | −5.6 | −5.1 | −5.6 | −5.4 | NA | −5.5 |
| p-cymen-8-ol | 14529 | −7.6 | −5.9 | −6.7 | −5.9 | −5.8 | −6.1 | −5.3 |
| p-cymene | 7463 | −7.6 | −5.8 | −6.5 | −5.9 | −5.9 | NA | −5.3 |
| 1-octen-3-ol | 18827 | −5.5 | −5.7 | −5.9 | −5.5 | NA | −5.7 | −5.0 |
| (−)-globulol | 12304985 | −9.1 | −7.8 | −8.1 | −6.6 | NA | −6.8 | NA |
| β-eIemene | 6918391 | −8.3 | −6.4 | −7.3 | NA | −5.6 | −6.4 | −5.6 |
| α-terpinyl acetate | 111037 | −8.2 | −6.0 | −6.8 | NA | NA | −6.0 | −5.8 |
| α-pinene | 6654 | −6.6 | −5.6 | −5.3 | −5.9 | −6.0 | −5.7 | −5.1 |
| D-limonene | 440917 | −7.3 | −5.3 | −6.3 | −5.3 | −5.7 | −5.6 | −5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, P.; Wang, H.; Xu, X. Comparative Analysis of Chemical Profiles and Biological Activities of Essential Oils Derived from Torreya grandis Arils and Leaves: In Vitro and In Silico Studies. Plants 2024, 13, 2640. https://doi.org/10.3390/plants13182640
Deng P, Wang H, Xu X. Comparative Analysis of Chemical Profiles and Biological Activities of Essential Oils Derived from Torreya grandis Arils and Leaves: In Vitro and In Silico Studies. Plants. 2024; 13(18):2640. https://doi.org/10.3390/plants13182640
Chicago/Turabian StyleDeng, Pengfei, Huiling Wang, and Xiaoniu Xu. 2024. "Comparative Analysis of Chemical Profiles and Biological Activities of Essential Oils Derived from Torreya grandis Arils and Leaves: In Vitro and In Silico Studies" Plants 13, no. 18: 2640. https://doi.org/10.3390/plants13182640
APA StyleDeng, P., Wang, H., & Xu, X. (2024). Comparative Analysis of Chemical Profiles and Biological Activities of Essential Oils Derived from Torreya grandis Arils and Leaves: In Vitro and In Silico Studies. Plants, 13(18), 2640. https://doi.org/10.3390/plants13182640






