Prion–like Proteins in Plants: Key Regulators of Development and Environmental Adaptation via Phase Separation
Abstract
:1. Introduction
2. Structural Features of PrLPs and Mechanisms in Forming Phase Separation
3. The Molecular Function of PrLP Condensates
3.1. Transcriptional Regulation
3.2. RNA Processing
3.3. Formation of Cytoplasmic RNP Granules
3.4. Membrane−Related Regulation
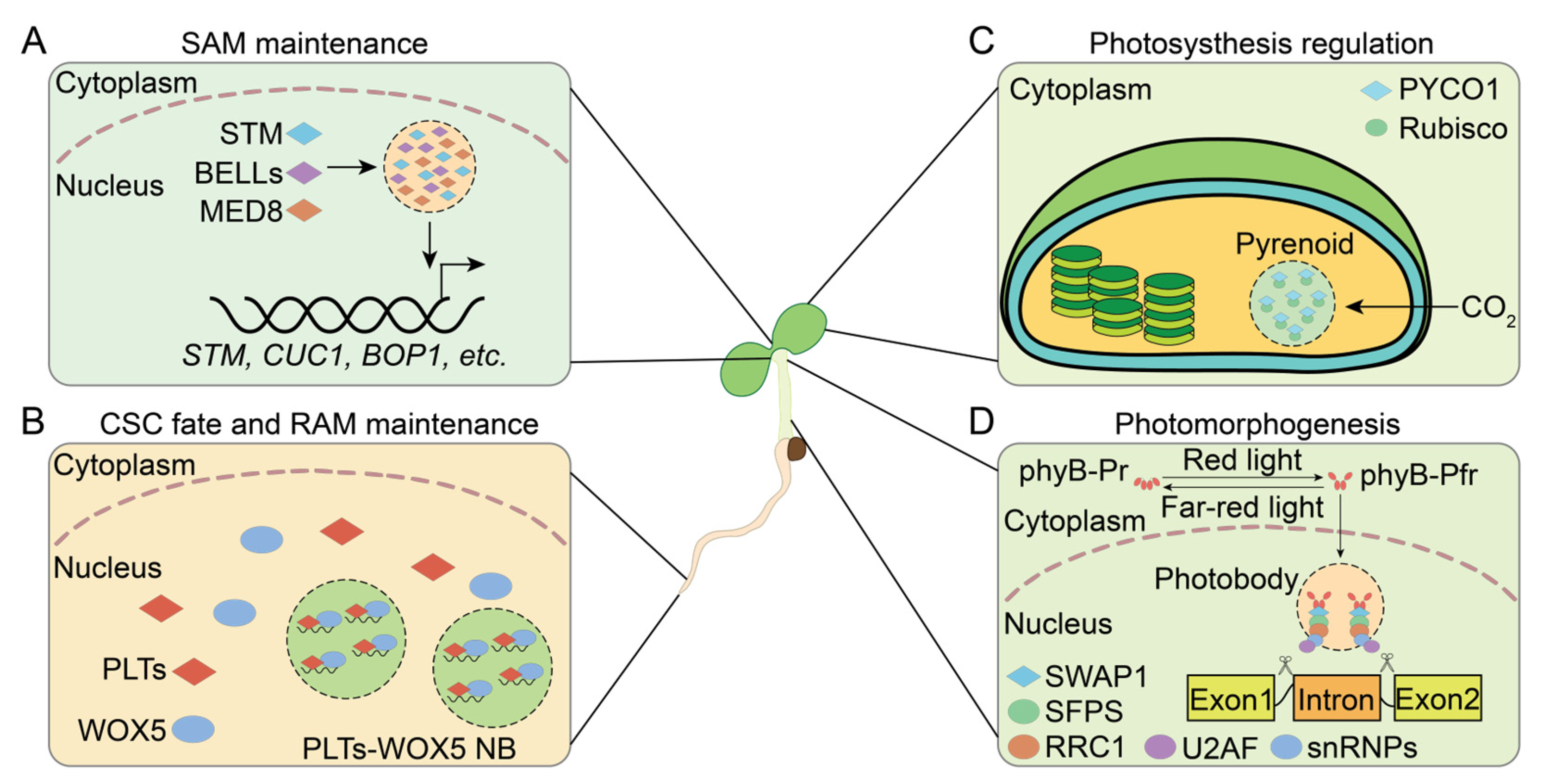
4. Functional Roles of PrLPs in Plant Development
4.1. Meristem Maintenance
4.2. Light Signaling Regulation
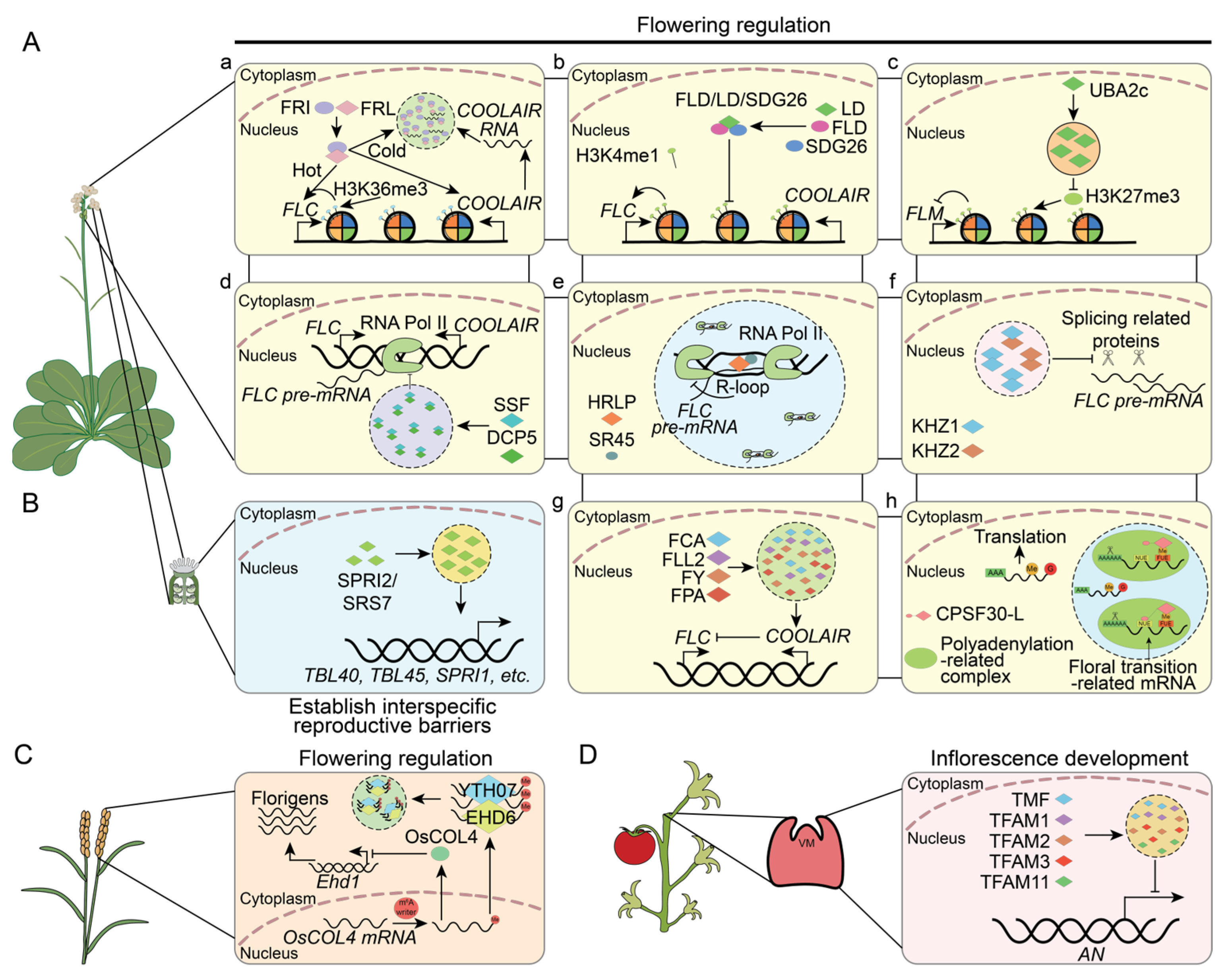
4.3. Reproductive Growth
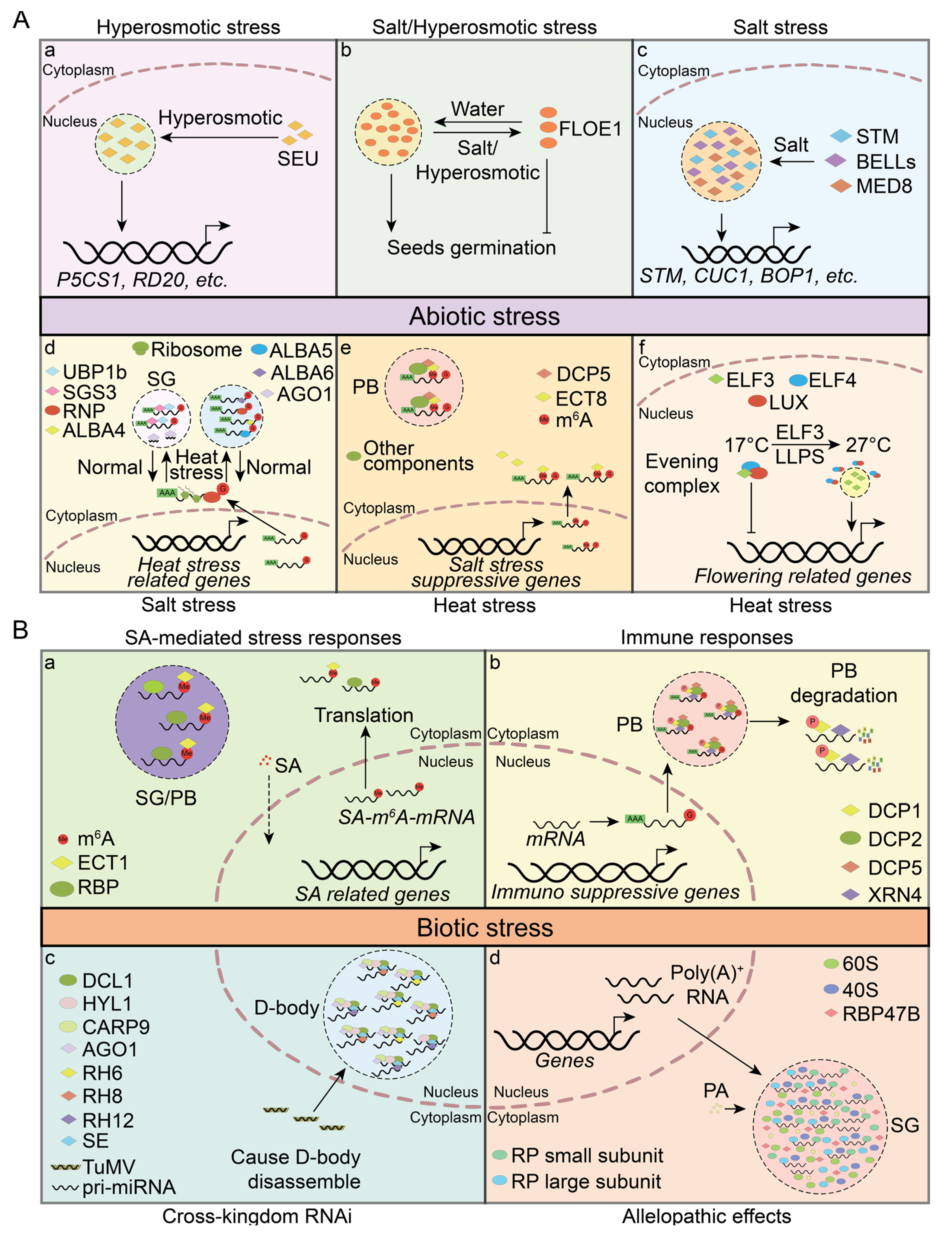
5. PrLPs Involved in Environmental Perception
5.1. Abiotic Stress
5.2. Biotic Stress
6. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. The Methods and Materials of Gene Ontology Enrichment Analysis
References
- Prusiner, S.B. Novel proteinaceous infectious particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Shorter, J.; Lindquist, S. Prions as adaptive conduits of memory and inheritance. Nat. Rev. Genet. 2005, 6, 435–450. [Google Scholar] [CrossRef]
- Riesner, D. Biochemistry and structure of PrPC and PrPSc. Br. Med. Bull. 2003, 66, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef] [PubMed]
- Eghiaian, F.; Grosclaude, J.; Lesceu, S.; Debey, P.; Doublet, B.; Tréguer, E.; Rezaei, H.; Knossow, M. Insight into the PrPC → PrPSc conversion from the structures of antibody–bound ovine prion scrapie–susceptibility variants. Proc. Natl. Acad. Sci. USA 2004, 101, 10254–10259. [Google Scholar] [CrossRef]
- Prusiner, S.B. A unifying role for prions in neurodegenerative diseases. Science 2012, 336, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- King, O.D.; Gitler, A.D.; Shorter, J. The tip of the iceberg: RNA–binding proteins with prion–like domains in neurodegenerative disease. Brain Res. 2012, 1462, 61–80. [Google Scholar] [CrossRef]
- Krishnan, R.; Lindquist, S.L. Structural insights into a yeast prion illuminate nucleation and strain diversity. Nature 2005, 435, 765–772. [Google Scholar] [CrossRef]
- Santoso, A.; Chien, P.; Osherovich, L.Z.; Weissman, J.S. Molecular basis of a yeast prion species barrier. Cell 2000, 100, 277–288. [Google Scholar] [CrossRef]
- Sabate, R.; Rousseau, F.; Schymkowitz, J.; Ventura, S. What makes a protein sequence a prion? PLoS Comput. Biol. 2015, 11, e1004013. [Google Scholar] [CrossRef]
- Alberti, S.; Halfmann, R.; King, O.; Kapila, A.; Lindquist, S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 2009, 137, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Gil-Garcia, M.; Iglesias, V.; Pallarès, I.; Ventura, S. Prion–like proteins: From computational approaches to proteome–wide analysis. FEBS Open Biol. 2021, 11, 2400–2417. [Google Scholar] [CrossRef] [PubMed]
- Liebman, S.W.; Chernoff, Y.O. Prions in yeast. Genetics 2012, 191, 1041–1072. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortee, S.; Kayatekin, C.; Newby, G.A.; Mendillo, M.L.; Lancaster, A.; Lindquist, S. Luminidependens (LD) is an Arabidopsis protein with prion behavior. Proc. Natl. Acad. Sci. USA 2016, 113, 6065–6070. [Google Scholar] [CrossRef]
- Garai, S.; Citu; Singla-Pareek, S.L.; Sopory, S.K.; Kaur, C.; Yadav, G. Complex networks of prion–like proteins reveal cross talk between stress and memory pathways in plants. Front. Plant Sci. 2021, 12, 707286. [Google Scholar] [CrossRef]
- Emenecker, R.J.; Holehouse, A.S.; Strader, L.C. Biological phase separation and biomolecular condensates in plants. Annu. Rev. Plant Biol. 2021, 72, 17–46. [Google Scholar] [CrossRef]
- Boeynaems, S.; Alberti, S.; Fawzi, N.L.; Mittag, T.; Polymenidou, M.; Rousseau, F.; Schymkowitz, J.; Shorter, J.; Wolozin, B.; Van Den Bosch, L.; et al. Protein phase separation: A new phase in cell biology. Trends Cell Biol. 2018, 28, 420–435. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, X.; Li, P.; Liu, C.; Lou, J.; Wang, Z.; Wen, W.; Xiao, Y.; Zhang, M.; Zhu, X. Liquid–liquid phase separation in biology: Mechanisms, physiological functions and human diseases. Sci. China Life Sci. 2020, 63, 953–985. [Google Scholar] [CrossRef]
- Wiedner, H.J.; Giudice, J. It’s not just a phase: Function and characteristics of RNA–binding proteins in phase separation. Nat. Struct. Mol. Biol. 2021, 28, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Gotor, N.L.; Armaos, A.; Calloni, G.; Torrent Burgas, M.; Vabulas, R.M.; De Groot, N.S.; Tartaglia, G.G. RNA–binding and prion domains: The Yin and Yang of phase separation. Nucleic Acids Res. 2020, 48, 9491–9504. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Choi, J.M.; Holehouse, A.S.; Lee, H.O.; Zhang, X.; Jahnel, M.; Maharana, S.; Lemaitre, R.; Pozniakovsky, A.; Drechsel, D.; et al. A molecular grammar governing the driving forces for phase separation of prion–like RNA binding proteins. Cell 2018, 174, 688–699.e16. [Google Scholar] [CrossRef] [PubMed]
- Fomicheva, A.; Ross, E.D. From prions to stress granules: Defining the compositional features of prion–like domains that promote different types of assemblies. Int. J. Mol. Sci. 2021, 22, 1251. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.D.; Edskes, H.K.; Terry, M.J.; Wickner, R.B. Primary sequence independence for prion formation. Proc. Natl. Acad. Sci. USA 2005, 102, 12825–12830. [Google Scholar] [CrossRef]
- Ross, E.D.; Baxa, U.; Wickner, R.B. Scrambled prion domains form prions and amyloid. Mol. Cell. Biol. 2004, 24, 7206–7213. [Google Scholar] [CrossRef]
- Lancaster, A.K.; Nutter-Upham, A.; Lindquist, S.; King, O.D. PLAAC: A web and command–line application to identify proteins with prion–like amino acid composition. Bioinformatics 2014, 30, 2501–2502. [Google Scholar] [CrossRef]
- Li, P.; Banjade, S.; Cheng, H.-C.; Kim, S.; Chen, B.; Guo, L.; Llaguno, M.; Hollingsworth, J.V.; King, D.S.; Banani, S.F.; et al. Phase transitions in the assembly of multivalent signalling proteins. Nature 2012, 483, 336–340. [Google Scholar] [CrossRef]
- Farag, M.; Borcherds, W.M.; Bremer, A.; Mittag, T.; Pappu, R.V. Phase separation of protein mixtures is driven by the interplay of homotypic and heterotypic interactions. Nat. Commun. 2023, 14, 5527. [Google Scholar] [CrossRef]
- Oh, Z.G.; Ang, W.S.L.; Poh, C.W.; Lai, S.-K.; Sze, S.K.; Li, H.-Y.; Bhushan, S.; Wunder, T.; Mueller-Cajar, O. A linker protein from a red–type pyrenoid phase separates with Rubisco via oligomerizing sticker motifs. Proc. Natl. Acad. Sci. USA 2023, 120, e2304833120. [Google Scholar] [CrossRef]
- Martin, E.W.; Holehouse, A.S.; Peran, I.; Farag, M.; Incicco, J.J.; Bremer, A.; Grace, C.R.; Soranno, A.; Pappu, R.V.; Mittag, T. Valence and patterning of aromatic residues determine the phase behavior of prion–like domains. Science 2020, 367, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Bremer, A.; Farag, M.; Borcherds, W.M.; Peran, I.; Martin, E.W.; Pappu, R.V.; Mittag, T. Deciphering how naturally occurring sequence features impact the phase behaviours of disordered prion–like domains. Nat. Chem. 2021, 14, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Holehouse, A.S.; Ginell, G.M.; Griffith, D.; Böke, E. Clustering of aromatic residues in prion–like domains can tune the formation, state, and organization of biomolecular condensates. Biochemistry 2021, 60, 3566–3581. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.N.; Rubinstein, M. Thermoreversible gelation in solutions of associative polymers. 1. Statics. Macromolecules 1998, 31, 1373–1385. [Google Scholar] [CrossRef]
- Dougherty, D.A. Cation–pi interactions in chemistry and biology: A new view of benzene, Phe, Tyr, and Trp. Science 1996, 271, 163–168. [Google Scholar] [CrossRef]
- Dougherty, D.A. The cation–pi interaction. Acc. Chem. Res. 2013, 46, 885–893. [Google Scholar] [CrossRef]
- Gallivan, J.P.; Dougherty, D.A. Cation–pi interactions in structural biology. Proc. Natl. Acad. Sci. USA 1999, 96, 9459–9464. [Google Scholar] [CrossRef]
- Langdon, E.M.; Qiu, Y.; Ghanbari Niaki, A.; McLaughlin, G.A.; Weidmann, C.A.; Gerbich, T.M.; Smith, J.A.; Crutchley, J.M.; Termini, C.M.; Weeks, K.M.; et al. mRNA structure determines specificity of a polyQ–driven phase separation. Science 2018, 360, 922–927. [Google Scholar] [CrossRef]
- Maharana, S.; Wang, J.; Papadopoulos, D.K.; Richter, D.; Pozniakovsky, A.; Poser, I.; Bickle, M.; Rizk, S.; Guillén-Boixet, J.; Franzmann, T.M.; et al. RNA buffers the phase separation behavior of prion–like RNA binding proteins. Science 2018, 360, 918–921. [Google Scholar] [CrossRef]
- Gomes, E.; Shorter, J. The molecular language of membraneless organelles. J. Biol. Chem. 2019, 294, 7115–7127. [Google Scholar] [CrossRef]
- Sharp, P.A.; Chakraborty, A.K.; Henninger, J.E.; Young, R.A. RNA in formation and regulation of transcriptional condensates. RNA 2022, 28, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Barbosa, A.D.; Hutin, S.; Kumita, J.R.; Gao, M.; Derwort, D.; Silva, C.S.; Lai, X.; Pierre, E.; Geng, F.; et al. A prion–like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 2020, 585, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Du, Q.; Guo, Y.; Wang, Y.; Jiao, Y. Condensation of STM is critical for shoot meristem maintenance and salt tolerance in Arabidopsis. Mol. Plant 2023, 16, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huo, X.; Pei, G.; Jia, Z.; Yan, Y.; Yu, J.; Qu, H.; Xie, Y.; Yuan, J.; Zheng, Y.; et al. Dual–role transcription factors stabilize intermediate expression levels. Cell 2024, 187, 2746–2766.e25. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Wang, Y.; Ma, J.; Wang, T.; Tao, Z.; Liu, P.; Li, S.; Hu, Y.; Gu, A.; et al. The P–body component DECAPPING5 and the floral repressor SISTER OF FCA regulate FLOWERING LOCUS C transcription in Arabidopsis. Plant Cell 2023, 35, 3303–3324. [Google Scholar] [CrossRef]
- Demmerle, J.; Hao, S.; Cai, D. Transcriptional condensates and phase separation: Condensing information across scales and mechanisms. Nucleus 2023, 14, 2213551. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Hua, C.; Teo, Z.W.N.; Kiang, J.X.; Shen, L.; Yu, H. Phase separation of HRLP regulates flowering time in Arabidopsis. Sci. Adv. 2022, 8, eabn5488. [Google Scholar] [CrossRef]
- Garcia-Muse, T.; Aguilera, A. R loops: From physiological to pathological roles. Cell 2019, 179, 604–618. [Google Scholar] [CrossRef]
- Stortz, M.; Presman, D.M.; Levi, V. Transcriptional condensates: A blessing or a curse for gene regulation? Commun. Biol. 2024, 7, 187. [Google Scholar] [CrossRef]
- March, Z.M.; King, O.D.; Shorter, J. Prion–like domains as epigenetic regulators, scaffolds for subcellular organization, and drivers of neurodegenerative disease. Brain Res. 2016, 1647, 9–18. [Google Scholar] [CrossRef]
- Song, P.; Yang, J.; Wang, C.; Lu, Q.; Shi, L.; Tayier, S.; Jia, G. Arabidopsis N(6)–methyladenosine reader CPSF30–L recognizes FUE signals to control polyadenylation site choice in liquid–like nuclear bodies. Mol. Plant 2021, 14, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, L.; Ishikawa, R.; Li, Y.; Fiedler, M.; Liu, F.; Calder, G.; Rowan, B.; Weigel, D.; Li, P.; et al. Arabidopsis FLL2 promotes liquid–liquid phase separation of polyadenylation complexes. Nature 2019, 569, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, N.; Liu, Q.; Zheng, X.; Lu, L.; Gao, W.; Liu, Y.; Liu, Y.; Zhang, S.; Wang, Q.; et al. DEAD–box helicases modulate dicing body formation in Arabidopsis. Sci. Adv. 2021, 7, eabc6266. [Google Scholar] [CrossRef]
- Yan, Z.; Shi, H.; Liu, Y.; Jing, M.; Han, Y. KHZ1 and KHZ2, novel members of the autonomous pathway, repress the splicing efficiency of FLC pre–mRNA in Arabidopsis. J. Exp. Bot. 2020, 71, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Ren, Z.; Sun, L.; Zhou, S.; Yuan, W.; Hui, Y.; Ci, D.; Wang, W.; Fan, L.M.; Wu, Z.; et al. ALBA proteins confer thermotolerance through stabilizing HSF messenger RNAs in cytoplasmic granules. Nat. Plants 2022, 8, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Kathare, P.K.; Xin, R.; Ganesan, A.S.; June, V.M.; Reddy, A.S.N.; Huq, E. SWAP1–SFPS–RRC1 splicing factor complex modulates pre–mRNA splicing to promote photomorphogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2022, 119, e2214565119. [Google Scholar] [CrossRef]
- Xie, D.; Chen, M.; Niu, J.; Wang, L.; Li, Y.; Fang, X.; Li, P.; Qi, Y. Phase separation of SERRATE drives dicing body assembly and promotes miRNA processing in Arabidopsis. Nat. Cell Biol. 2021, 23, 32–39. [Google Scholar] [CrossRef]
- Li, C.F.; Pontes, O.; El-Shami, M.; Henderson, I.R.; Bernatavichute, Y.V.; Chan, S.W.; Lagrange, T.; Pikaard, C.S.; Jacobsen, S.E. An ARGONAUTE4–containing nuclear processing center colocalized with Cajal bodies in Arabidopsis thaliana. Cell 2006, 126, 93–106. [Google Scholar] [CrossRef]
- Tan, H.; Luo, W.; Yan, W.; Liu, J.; Aizezi, Y.; Cui, R.; Tian, R.; Ma, J.; Guo, H. Phase separation of SGS3 drives siRNA body formation and promotes endogenous gene silencing. Cell Rep. 2023, 42, 111985. [Google Scholar] [CrossRef]
- Weber, C.; Nover, L.; Fauth, M. Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J. 2008, 56, 517–530. [Google Scholar] [CrossRef]
- Sorenson, R.; Bailey-Serres, J. Selective mRNA sequestration by OLIGOURIDYLATE–BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.R.; Matheny, T.; Jain, S.; Abrisch, R.; Parker, R. Distinct stages in stress granule assembly and disassembly. Elife 2016, 5, e18413. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.Y.; Dyakov, B.J.A.; Zhang, J.; Knight, J.D.R.; Vernon, R.M.; Forman-Kay, J.D.; Gingras, A.C. Properties of stress granule and P–body proteomes. Mol. Cell 2019, 76, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Chantarachot, T.; Bailey-Serres, J. Polysomes, stress granules, and processing bodies: A dynamic triumvirate controlling cytoplasmic mRNA fate and function. Plant Physiol. 2018, 176, 254–269. [Google Scholar] [CrossRef]
- Anderson, P.; Kedersha, N. RNA granules: Post–transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 2009, 10, 430–436. [Google Scholar] [CrossRef]
- Vanderweyde, T.; Youmans, K.; Liu-Yesucevitz, L.; Wolozin, B. Role of stress granules and RNA–binding proteins in neurodegeneration: A mini–review. Gerontology 2013, 59, 524–533. [Google Scholar] [CrossRef]
- Buchan, J.R.; Parker, R. Eukaryotic stress granules: The ins and outs of translation. Mol. Cell 2009, 36, 932–941. [Google Scholar] [CrossRef]
- Iserman, C.; Desroches Altamirano, C.; Jegers, C.; Friedrich, U.; Zarin, T.; Fritsch, A.W.; Mittasch, M.; Domingues, A.; Hersemann, L.; Jahnel, M.; et al. Condensation of Ded1p promotes a translational switch from housekeeping to stress protein production. Cell 2020, 181, 818–831.e819. [Google Scholar] [CrossRef]
- Jang, G.J.; Yang, J.Y.; Hsieh, H.L.; Wu, S.H. Processing bodies control the selective translation for optimal development of Arabidopsis young seedlings. Proc. Natl. Acad. Sci. USA 2019, 116, 6451–6456. [Google Scholar] [CrossRef]
- Decker, C.J.; Parker, R. P–bodies and stress granules: Possible roles in the control of translation and mRNA degradation. Cold Spring Harb. Perspect. Biol. 2012, 4, a012286. [Google Scholar] [CrossRef]
- Aizer, A.; Kalo, A.; Kafri, P.; Shraga, A.; Ben-Yishay, R.; Jacob, A.; Kinor, N.; Shav-Tal, Y. Quantifying mRNA targeting to P–bodies in living human cells reveals their dual role in mRNA decay and storage. J. Cell Sci. 2014, 127, 4443–4456. [Google Scholar] [CrossRef] [PubMed]
- Schiaffini, M.; Chicois, C.; Pouclet, A.; Chartier, T.; Ubrig, E.; Gobert, A.; Zuber, H.; Mutterer, J.; Chicher, J.; Kuhn, L.; et al. A NYN domain protein directly interacts with DECAPPING1 and is required for phyllotactic pattern. Plant Physiol. 2022, 188, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mentzelopoulou, A.; Muhammad, A.; Volkov, A.; Weijers, D.; Gutierrez-Beltran, E.; Moschou, P.N. An actin remodeling role for Arabidopsis processing bodies revealed by their proximity interactome. EMBO J. 2023, 42, e111885. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, B.; Jang, G.J.; Jiang, S.; Jiang, D.; Jang, J.C.; Wu, S.H.; Shan, L.; He, P. Orchestration of processing body dynamics and mRNA decay in Arabidopsis immunity. Cell Rep. 2019, 28, 2194–2205.e6. [Google Scholar] [CrossRef]
- Jang, G.J.; Jang, J.C.; Wu, S.H. Dynamics and functions of stress granules and processing bodies in plants. Plants 2020, 9, 1122. [Google Scholar] [CrossRef]
- Maruri-Lopez, I.; Figueroa, N.E.; Hernandez-Sanchez, I.E.; Chodasiewicz, M. Plant stress granules: Trends and beyond. Front. Plant Sci. 2021, 12, 722643. [Google Scholar] [CrossRef]
- Chantarachot, T.; Sorenson, R.S.; Hummel, M.; Ke, H.; Kettenburg, A.T.; Chen, D.; Aiyetiwa, K.; Dehesh, K.; Eulgem, T.; Sieburth, L.E.; et al. DHH1/DDX6–like RNA helicases maintain ephemeral half–lives of stress–response mRNAs. Nat. Plants 2020, 6, 675–685. [Google Scholar] [CrossRef]
- Kim, E.Y.; Wang, L.; Lei, Z.; Li, H.; Fan, W.; Cho, J. Ribosome stalling and SGS3 phase separation prime the epigenetic silencing of transposons. Nat. Plants 2021, 7, 303–309. [Google Scholar] [CrossRef]
- Schiano Lomoriello, I.; Sigismund, S.; Day, K.J. Biophysics of endocytic vesicle formation: A focus on liquid–liquid phase separation. Curr. Opin. Cell Biol. 2022, 75, 102068. [Google Scholar] [CrossRef]
- Hatzianestis, I.H.; Mountourakis, F.; Stavridou, S.; Moschou, P.N. Plant condensates: No longer membrane–less? Trends Plant Sci. 2023, 28, 1101–1112. [Google Scholar] [CrossRef]
- Snead, W.T.; Gladfelter, A.S. The control centers of biomolecular phase separation: How membrane surfaces, PTMs, and active processes regulate condensation. Mol. Cell 2019, 76, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Zhang, H. Phase separation in membrane biology: The interplay between membrane–bound organelles and membraneless condensates. Dev. Cell 2020, 55, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.R.; Kolaitis, R.M.; Taylor, J.P.; Parker, R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 2013, 153, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Smith, S.M.; Li, J. Genetic regulation of shoot architecture. Annu. Rev. Plant Biol. 2018, 69, 437–468. [Google Scholar] [CrossRef] [PubMed]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis root development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef]
- van den Berg, C.; Willemsen, V.; Hendriks, G.; Weisbeek, P.; Scheres, B. Short–range control of cell differentiation in the Arabidopsis root meristem. Nature 1997, 390, 287–289. [Google Scholar] [CrossRef]
- Lu, R.; Canher, B.; Bisht, A.; Heyman, J.; De Veylder, L. Three–dimensional quantitative analysis of the Arabidopsis quiescent centre. J. Exp. Bot. 2021, 72, 6789–6800. [Google Scholar] [CrossRef]
- Burkart, R.C.; Strotmann, V.I.; Kirschner, G.K.; Akinci, A.; Czempik, L.; Dolata, A.; Maizel, A.; Weidtkamp-Peters, S.; Stahl, Y. PLETHORA–WOX5 interaction and subnuclear localization control Arabidopsis root stem cell maintenance. EMBO Rep. 2022, 23, e54105. [Google Scholar] [CrossRef]
- Dolan, L.; Janmaat, K.; Willemsen, V.; Linstead, P.; Poethig, S.; Roberts, K.; Scheres, B. Cellular organisation of the Arabidopsis thaliana root. Development 1993, 119, 71–84. [Google Scholar] [CrossRef]
- Benfey, P.N.; Scheres, B. Root development. Curr. Biol. 2000, 10, R813–R815. [Google Scholar] [CrossRef]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.-S.; Amasino, R.; Scheres, B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Forzani, C.; Aichinger, E.; Sornay, E.; Willemsen, V.; Laux, T.; Dewitte, W.; Murray, J.A.H. WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Curr. Biol. 2014, 24, 1939–1944. [Google Scholar] [CrossRef]
- Pi, L.; Aichinger, E.; van der Graaff, E.; Llavata-Peris, C.I.; Weijers, D.; Hennig, L.; Groot, E.; Laux, T. Organizer–derived WOX5 signal maintains root columella stem cells through chromatin–mediated repression of CDF4 expression. Dev. Cell 2015, 33, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.; Tsiantis, M. KNOX genes: Versatile regulators of plant development and diversity. Development 2010, 137, 3153–3165. [Google Scholar] [CrossRef]
- Long, J.A.; Barton, M.K. The development of apical embryonic pattern in Arabidopsis. Development 1998, 125, 3027–3035. [Google Scholar] [CrossRef]
- Long, J.A.; Moan, E.I.; Medford, J.I.; Barton, M.K. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 1996, 379, 66–69. [Google Scholar] [CrossRef]
- Meyer, M.T.; Whittaker, C.; Griffiths, H. The algal pyrenoid: Key unanswered questions. J. Exp. Bot. 2017, 68, 3739–3749. [Google Scholar] [CrossRef]
- Mackinder, L.C.M.; Meyer, M.T.; Mettler-Altmann, T.; Chen, V.K.; Mitchell, M.C.; Caspari, O.; Freeman Rosenzweig, E.S.; Pallesen, L.; Reeves, G.; Itakura, A.; et al. A repeat protein links Rubisco to form the eukaryotic carbon–concentrating organelle. Proc. Natl. Acad. Sci. USA 2016, 113, 5958–5963. [Google Scholar] [CrossRef]
- Freeman Rosenzweig, E.S.; Xu, B.; Kuhn Cuellar, L.; Martinez-Sanchez, A.; Schaffer, M.; Strauss, M.; Cartwright, H.N.; Ronceray, P.; Plitzko, J.M.; Förster, F.; et al. The eukaryotic CO2–concentrating organelle is liquid–like and exhibits dynamic reorganization. Cell 2017, 171, 148–162.e19. [Google Scholar] [CrossRef]
- He, S.; Chou, H.-T.; Matthies, D.; Wunder, T.; Meyer, M.T.; Atkinson, N.; Martinez-Sanchez, A.; Jeffrey, P.D.; Port, S.A.; Patena, W.; et al. The structural basis of Rubisco phase separation in the pyrenoid. Nat. Plants 2020, 6, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Wunder, T.; Cheng, S.L.H.; Lai, S.-K.; Li, H.-Y.; Mueller-Cajar, O. The phase separation underlying the pyrenoid–based microalgal Rubisco supercharger. Nat. Commun. 2018, 9, 5076. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.; Huq, E. Plant photoreceptors: Multi–functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Legris, M.; Ince, Y.C.; Fankhauser, C. Molecular mechanisms underlying phytochrome–controlled morphogenesis in plants. Nat. Commun. 2019, 10, 5219. [Google Scholar] [CrossRef] [PubMed]
- Xin, R.; Zhu, L.; Salome, P.A.; Mancini, E.; Marshall, C.M.; Harmon, F.G.; Yanovsky, M.J.; Weigel, D.; Huq, E. SPF45–related splicing factor for phytochrome signaling promotes photomorphogenesis by regulating pre–mRNA splicing in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E7018–E7027. [Google Scholar] [CrossRef]
- Xin, R.; Kathare, P.K.; Huq, E. Coordinated regulation of pre–mRNA splicing by the SFPS–RRC1 complex to promote photomorphogenesis. Plant Cell 2019, 31, 2052–2069. [Google Scholar] [CrossRef]
- Cho, L.H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef]
- Song, Y.H.; Ito, S.; Imaizumi, T. Flowering time regulation: Photoperiod– and temperature–sensing in leaves. Trends Plant Sci. 2013, 18, 575–583. [Google Scholar] [CrossRef]
- Pin, P.A.; Nilsson, O. The multifaceted roles of FLOWERING LOCUS T in plant development. Plant Cell Environ. 2012, 35, 1742–1755. [Google Scholar] [CrossRef]
- Wickland, D.P.; Hanzawa, Y. The FLOWERING LOCUS T/TERMINAL FLOWER 1 gene family: Functional evolution and molecular mechanisms. Mol. Plant 2015, 8, 983–997. [Google Scholar] [CrossRef]
- Whittaker, C.; Dean, C. The FLC locus: A platform for discoveries in epigenetics and adaptation. Annu. Rev. Cell Dev. Biol. 2017, 33, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Michaels, S.D.; Amasino, R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999, 11, 949–956. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Amasino, R.M. Role of chromatin modification in flowering–time control. Trends Plant Sci. 2005, 10, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, C.A.; Anderssen, R.S.; Robertson, M.; Finnegan, E.J. How is FLC repression initiated by cold? Trends Plant Sci. 2015, 20, 76–82. [Google Scholar] [CrossRef]
- Choi, K.; Kim, J.; Hwang, H.-J.; Kim, S.; Park, C.; Kim, S.Y.; Lee, I. The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors. Plant Cell 2011, 23, 289–303. [Google Scholar] [CrossRef]
- Ko, J.-H.; Mitina, I.; Tamada, Y.; Hyun, Y.; Choi, Y.; Amasino, R.M.; Noh, B.; Noh, Y.-S. Growth habit determination by the balance of histone methylation activities in Arabidopsis. EMBO J. 2010, 29, 3208–3215. [Google Scholar] [CrossRef]
- Zhu, P.; Lister, C.; Dean, C. Cold–induced Arabidopsis FRIGIDA nuclear condensates for FLC repression. Nature 2021, 599, 657–661. [Google Scholar] [CrossRef]
- Fang, X.; Wu, Z.; Raitskin, O.; Webb, K.; Voigt, P.; Lu, T.; Howard, M.; Dean, C. The 3′ processing of antisense RNAs physically links to chromatin–based transcriptional control. Proc. Natl. Acad. Sci. USA 2020, 117, 15316–15321. [Google Scholar] [CrossRef]
- Posé, D.; Verhage, L.; Ott, F.; Yant, L.; Mathieu, J.; Angenent, G.C.; Immink, R.G.H.; Schmid, M. Temperature–dependent regulation of flowering by antagonistic FLM variants. Nature 2013, 503, 414–417. [Google Scholar] [CrossRef]
- Lee, J.H.; Ryu, H.-S.; Chung, K.S.; Posé, D.; Kim, S.; Schmid, M.; Ahn, J.H. Regulation of temperature–responsive flowering by MADS–box transcription factor repressors. Science 2013, 342, 628–632. [Google Scholar] [CrossRef]
- Zhao, N.; Su, X.M.; Liu, Z.W.; Zhou, J.X.; Su, Y.N.; Cai, X.W.; Chen, L.; Wu, Z.; He, X.J. The RNA recognition motif–containing protein UBA2c prevents early flowering by promoting transcription of the flowering repressor FLM in Arabidopsis. New Phytol. 2021, 233, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Fang, X.; Zhu, D.; Dean, C. Autonomous pathway: FLOWERING LOCUS C repression through an antisense–mediated chromatin–silencing mechanism. Plant Physiol. 2020, 182, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Csorba, T.; Questa, J.I.; Sun, Q.; Dean, C. Antisense COOLAIR mediates the coordinated switching of chromatin states at FLC during vernalization. Proc. Natl. Acad. Sci. USA 2014, 111, 16160–16165. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Song, P.; Wang, C.; Chen, S.; Hao, B.; Xu, Z.; Cai, L.; Chen, X.; Zhu, S.; Gan, X.; et al. The RNA binding protein EHD6 recruits the m(6)A reader YTH07 and sequesters OsCOL4 mRNA into phase–separated ribonucleoprotein condensates to promote rice flowering. Mol. Plant 2024, 17, 935–954. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, N.; Zou, Y.; Xie, Y.; Tang, L.; Zhang, Y.; Yu, Y.; Li, Y.; Xu, C. Heterotypic transcriptional condensates formed by prion–like paralogous proteins canalize flowering transition in tomato. Genome Biol. 2022, 23, 78. [Google Scholar] [CrossRef]
- Tsuchimatsu, T.; Fujii, S. The selfing syndrome and beyond: Diverse evolutionary consequences of mating system transitions in plants. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20200510. [Google Scholar] [CrossRef]
- Moreira-Hernández, J.I.; Muchhala, N. Importance of pollinator–mediated interspecific pollen transfer for angiosperm evolution. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 191–217. [Google Scholar] [CrossRef]
- Fujii, S.; Yamamoto, E.; Ito, S.; Tangpranomkorn, S.; Kimura, Y.; Miura, H.; Yamaguchi, N.; Kato, Y.; Niidome, M.; Yoshida, A.; et al. SHI family transcription factors regulate an interspecific barrier. Nat. Plants 2023, 9, 1862–1873. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and challenges in studying liquid–liquid phase separation and biomolecular condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, C.; Lu, D.; Song, C.P.; Zhang, L. Phase separation in plants: New insights into cellular compartmentalization. J. Integr. Plant Biol. 2021, 63, 1835–1855. [Google Scholar] [CrossRef]
- Emenecker, R.J.; Holehouse, A.S.; Strader, L.C. Emerging roles for phase separation in plants. Dev. Cell 2020, 55, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, H.; Huai, J.; Peng, F.; Wu, J.; Lin, R.; Fang, X. Condensation of SEUSS promotes hyperosmotic stress tolerance in Arabidopsis. Nat. Chem. Biol. 2022, 18, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying alive: Molecular aspects of seed longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Dorone, Y.; Boeynaems, S.; Flores, E.; Jin, B.; Hateley, S.; Bossi, F.; Lazarus, E.; Pennington, J.G.; Michiels, E.; De Decker, M.; et al. A prion–like protein regulator of seed germination undergoes hydration–dependent phase separation. Cell 2021, 184, 4284–4298.e27. [Google Scholar] [CrossRef] [PubMed]
- Penfield, S. Water sensing in seeds by FLOE1 phase transitions. Dev. Cell 2021, 56, 2140–2141. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Riaz, A.; Chachar, S.; Ding, Y.; Du, H.; Gu, X. Epigenetic modifications of mRNA and DNA in plants. Mol. Plant 2020, 13, 14–30. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, G.; Tang, R.; Wang, W.; Wang, Y.; Tian, S.; Qin, G. m6A–mediated regulation of crop development and stress responses. Plant Biotechnol. J. 2022, 20, 1447–1455. [Google Scholar] [CrossRef]
- Tang, J.; Chen, S.; Jia, G. Detection, regulation, and functions of RNA N6–methyladenosine modification in plants. Plant Commun. 2023, 4, 100546. [Google Scholar] [CrossRef]
- Cai, Z.; Tang, Q.; Song, P.; Tian, E.; Yang, J.; Jia, G. The m6A reader ECT8 is an abiotic stress sensor that accelerates mRNA decay in Arabidopsis. Plant Cell 2024, 36, 2908–2926. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Molecular regulation of plant responses to environmental temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.-H.; Ma, X.; Luo, D.-X.; Gong, Z.-H.; Lu, M.-H. The plant heat stress transcription factors (HSFs): Structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.C.; Nakaminami, K.; Matsui, A.; Kobayashi, S.; Kurihara, Y.; Toyooka, K.; Tanaka, M.; Seki, M. Oligouridylate binding protein 1b plays an integral role in plant heat stress tolerance. Front. Plant Sci. 2016, 7, 853. [Google Scholar] [CrossRef] [PubMed]
- Blagojevic, A.; Baldrich, P.; Schiaffini, M.; Lechner, E.; Baumberger, N.; Hammann, P.; Elmayan, T.; Garcia, D.; Vaucheret, H.; Meyers, B.C.; et al. Heat stress promotes Arabidopsis AGO1 phase separation and association with stress granule components. Iscience 2024, 27, 109151. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Ashraf, M.Y.; Khaliq, B.; Sun, M.; Hussain, S.; Gao, Z.-Q.; Noor, H.; Alam, S. Mechanisms and adaptation strategies to improve heat tolerance in rice. A review. Plants 2019, 8, 508. [Google Scholar] [CrossRef]
- Tun, W.; Yoon, J.; Jeon, J.-S.; An, G. Influence of climate change on flowering time. J. Plant Biol. 2021, 64, 193–203. [Google Scholar] [CrossRef]
- Nieto, C.; López-Salmerón, V.; Davière, J.-M.; Prat, S. ELF3–PIF4 interaction regulates plant growth independently of the evening complex. Curr. Biol. 2015, 25, 187–193. [Google Scholar] [CrossRef]
- Nusinow, D.A.; Helfer, A.; Hamilton, E.E.; King, J.J.; Imaizumi, T.; Schultz, T.F.; Farré, E.M.; Kay, S.A. The ELF4–ELF3–LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 2011, 475, 398–402. [Google Scholar] [CrossRef]
- Wang, Y.; Pruitt, R.N.; Nürnberger, T.; Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef]
- Pokotylo, I.; Hodges, M.; Kravets, V.; Ruelland, E. A menage a trois: Salicylic acid, growth inhibition, and immunity. Trends Plant Sci. 2022, 27, 460–471. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Radojičić, A.; Li, X.; Zhang, Y. Salicylic acid: A double–edged sword for programed cell death in plants. Front. Plant Sci. 2018, 9, 1133. [Google Scholar] [CrossRef]
- Lee, K.P.; Liu, K.; Kim, E.Y.; Medina-Puche, L.; Dong, H.; Di, M.; Singh, R.M.; Li, M.; Qi, S.; Meng, Z.; et al. The m6A reader ECT1 drives mRNA sequestration to dampen salicylic acid–dependent stress responses in Arabidopsis. Plant Cell 2024, 36, 746–763. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Niu, R.; Zhou, Y.; Tang, Z.; Xu, G.; Zhou, G. ECT9 condensates with ECT1 and regulates plant immunity. Front. Plant Sci. 2023, 14, 1140840. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Vance, R.E.; Dangl, J.L. Intracellular innate immune surveillance devices in plants and animals. Science 2016, 354, aaf6395. [Google Scholar] [CrossRef]
- Lai, Y.; Eulgem, T. Transcript–level expression control of plant NLR genes. Mol. Plant Pathol. 2018, 19, 1267–1281. [Google Scholar] [CrossRef]
- Parker, M.T.; Knop, K.; Zacharaki, V.; Sherwood, A.V.; Tomé, D.; Yu, X.; Martin, P.G.P.; Beynon, J.; Michaels, S.D.; Barton, G.J.; et al. Widespread premature transcription termination of Arabidopsis thaliana NLR genes by the spen protein FPA. Elife 2021, 10, e65537. [Google Scholar] [CrossRef]
- Zhao, J.H.; Guo, H.S. Trans–kingdom RNA interactions drive the evolutionary arms race between hosts and pathogens. Curr. Opin. Genet. Dev. 2019, 58–59, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-W. RNA–based antiviral immunity. Nat. Rev. Immunol. 2010, 10, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, X.; Du, R.; Shan, X.; Xie, D. The phase separation of SGS3 regulates antiviral immunity and fertility in Arabidopsis. Sci. China Life Sci. 2023, 66, 1938–1941. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA–induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, S.; Li, Y.; Deng, Y.; Shi, Y.; Chen, X.; Li, Y.; Li, H.; Chen, C.; Wang, X.; et al. Phenolic acid–induced phase separation and translation inhibition mediate plant interspecific competition. Nat. Plants 2023, 9, 1481–1499. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO–slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. Expansion of the gene ontology knowledgebase and resources. Nucleic Acids Res. 2017, 45, D331–D338. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol update for large–scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Ginestet, C. ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2011, 174, 245–246. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Li, Y. Prion–like Proteins in Plants: Key Regulators of Development and Environmental Adaptation via Phase Separation. Plants 2024, 13, 2666. https://doi.org/10.3390/plants13182666
Wu P, Li Y. Prion–like Proteins in Plants: Key Regulators of Development and Environmental Adaptation via Phase Separation. Plants. 2024; 13(18):2666. https://doi.org/10.3390/plants13182666
Chicago/Turabian StyleWu, Peisong, and Yihao Li. 2024. "Prion–like Proteins in Plants: Key Regulators of Development and Environmental Adaptation via Phase Separation" Plants 13, no. 18: 2666. https://doi.org/10.3390/plants13182666
APA StyleWu, P., & Li, Y. (2024). Prion–like Proteins in Plants: Key Regulators of Development and Environmental Adaptation via Phase Separation. Plants, 13(18), 2666. https://doi.org/10.3390/plants13182666






