Overexpression of Auxin/Indole-3-Acetic Acid Gene TrIAA27 Enhances Biomass, Drought, and Salt Tolerance in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
2.1. Sequence Characteristics of TrIAA27
2.2. Expression Pattern of White Clover Gene TrIAA27 in Response to Different Stimuli
2.3. Subcellular Localization of TrIAA27
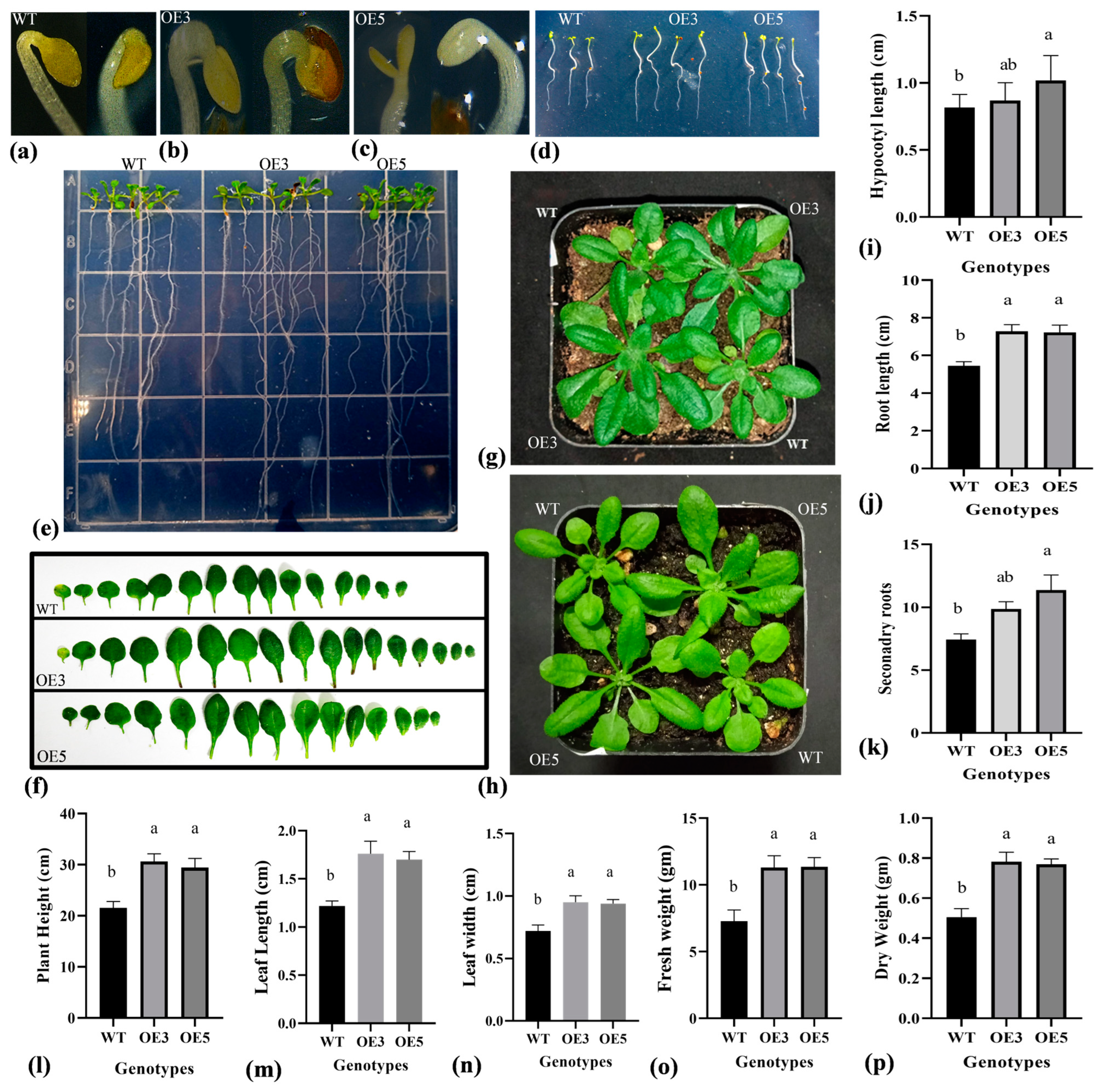
2.4. Overexpression of TrIAA27 Improves Plant Size and Roots of Transgenic Arabidopsis thaliana Plants
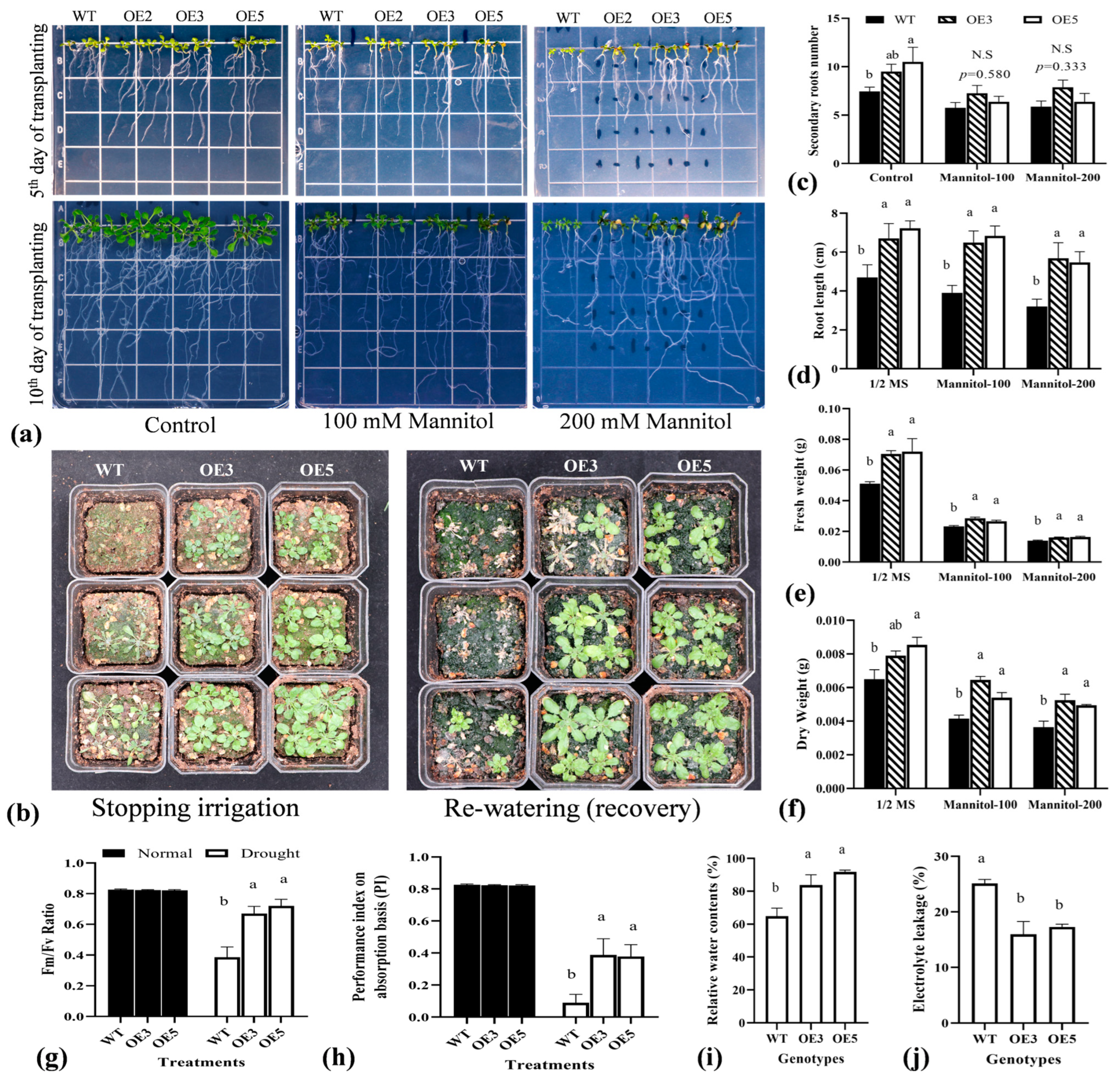
2.5. Overexpression of TrIAA27 in A. thaliana Improved Drought Stress Tolerance of Transgenic Plants
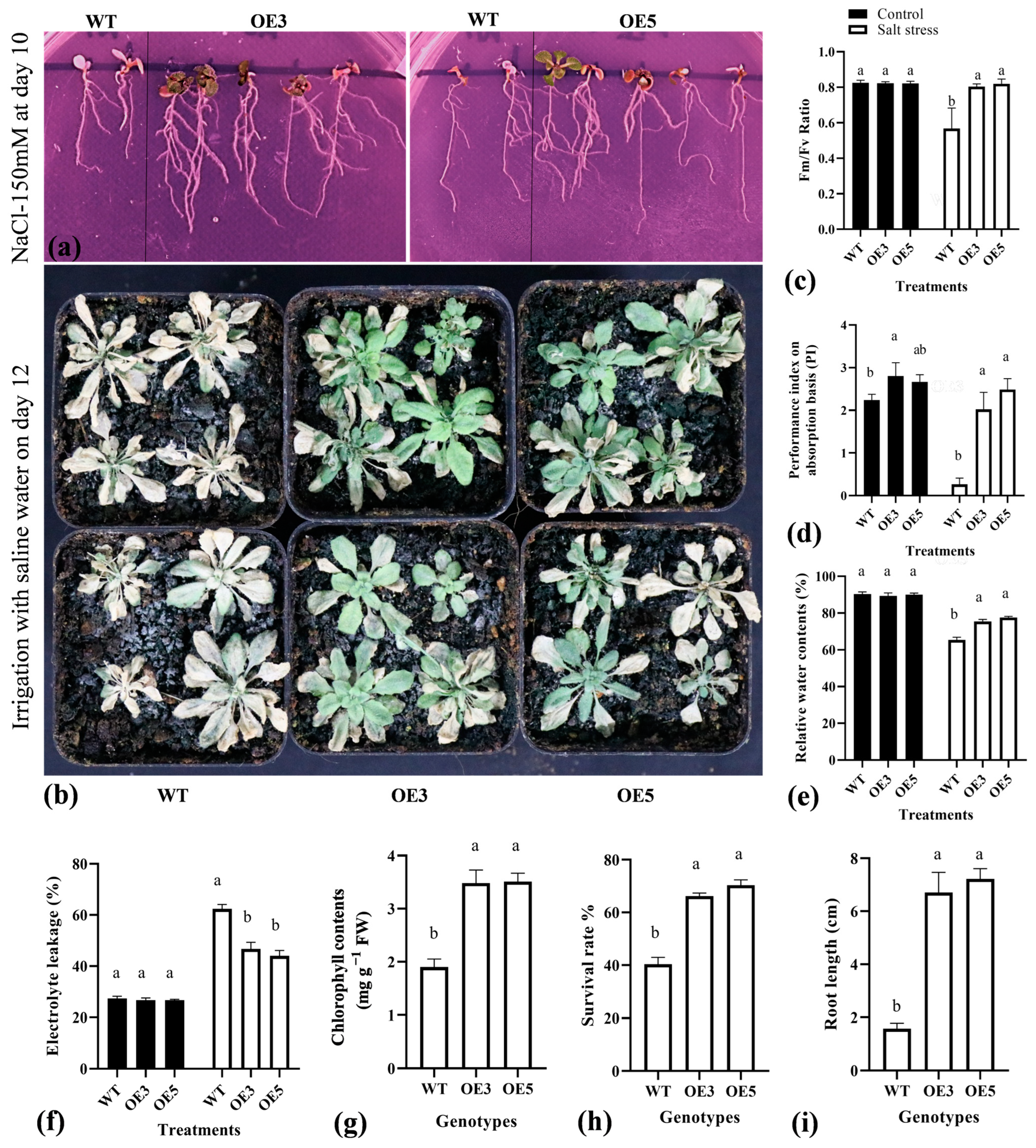
2.6. Overexpression of TrIAA27 Improved the Salt Stress Resistance of Transgenic Arabidopsis
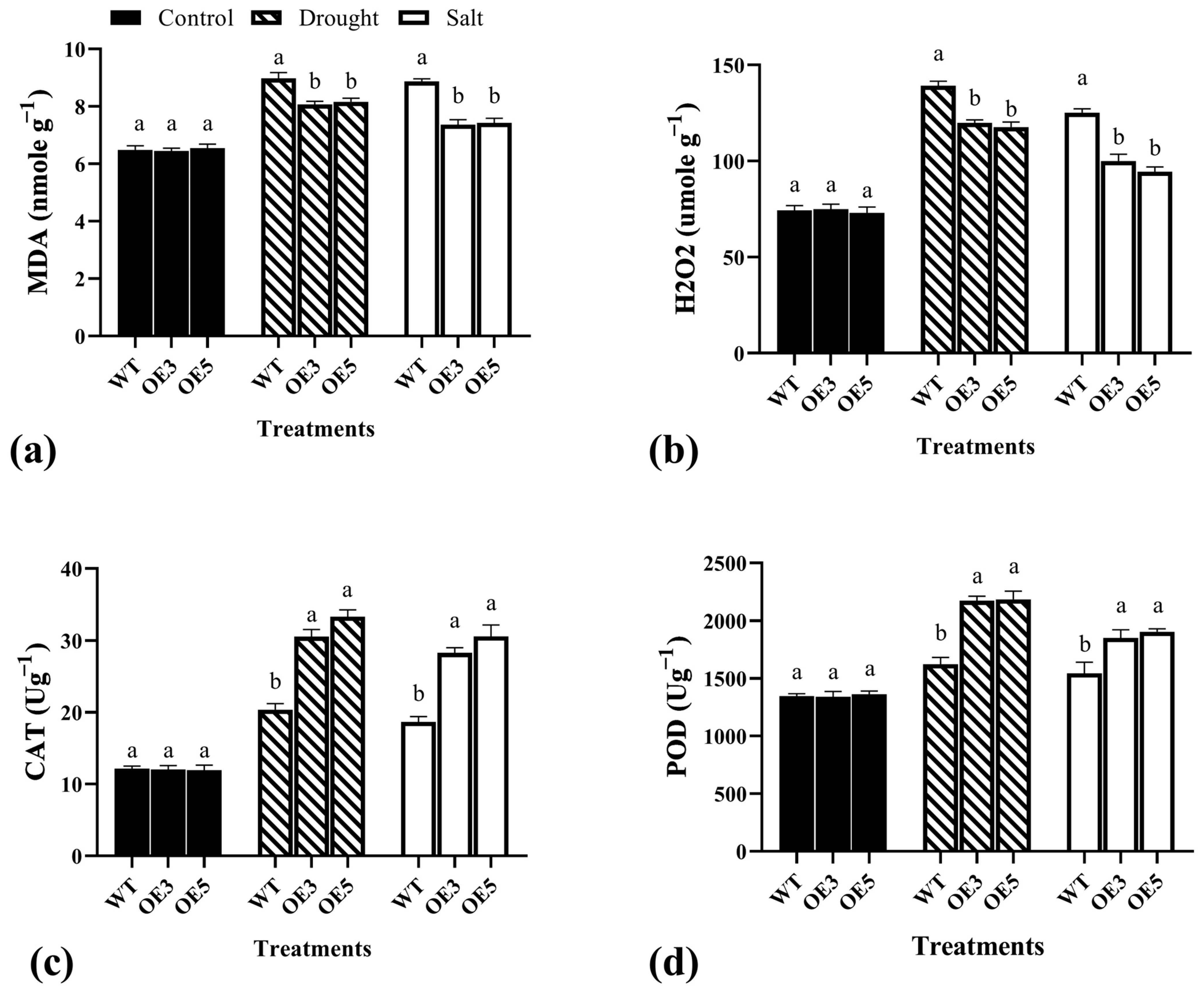
2.7. Physiological Indicators and Enzyme Activity under Drought and Salt Stress in Wild and Overexpression Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Exogenous Treatment of Heavy Metal, Abiotic Stress, Signalling Molecules, and Phytohormones to White Clover and Sample Collection
4.3. Drought and Salt Stress Treatments in Arabidopsis
4.4. Isolation of the IAA27 Gene from White Clover
4.5. Bioinformatics Analysis
4.6. Total RNA Isolation, Construction of cDNA, and qRT-PCR Analysis
4.7. Plasmid Construction and Genetic Transformation
4.8. Subcellular Localization
4.9. Determination of Relative Water Contents (RWC)
4.10. Determination of Relative Electrical Conductivity (EL)
4.11. Determination of Chlorophyll Fluorescence Parameters
4.12. Determination of Malondialdehyde (MDA) Content
4.13. Measuring H2O2, CAT, and POD
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caradus, J.; Woodfield, D.; Stewart, A. Overview and vision for white clover. NZGA Res. Pract. Ser. 1995, 6, 1–6. [Google Scholar] [CrossRef]
- Caradus, J.R.; Roldan, M.; Voisey, C.; Woodfield, D.R. White Clover (Trifolium repens L.) Benefits in Grazed Pastures and Potential Improvements; IntechOpen: New York, NY, USA, 2023. [Google Scholar]
- Sanderson, M.; Byers, R.; Skinner, R.; Elwinger, G. Growth and complexity of white clover stolons in response to biotic and abiotic stress. Crop Sci. 2003, 43, 2197–2205. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X.; Huang, L.; Yan, Y. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 16, 3039–3052. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Hassan, M.J.; Li, Z.; Peng, Y. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol. 2020, 20, 150. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Li, Y.-P.; Zhang, X.-Q.; Ma, X.; Huang, L.-K.; Yan, Y.-H.; Peng, Y. Chitosan and spermine enhance drought resistance in white clover, associated with changes in endogenous phytohormones and polyamines, and antioxidant metabolism. Funct. Plant Biol. 2018, 45, 1205–1222. [Google Scholar] [CrossRef]
- Han, J. Effect of Water Stress on the Water-using Capacity of White Clover. J. Anhui Agric. Sci. 2010, 38, 5580–5582. [Google Scholar]
- Shi, H.; Chen, L.; Ye, T.; Liu, X.; Ding, K.; Chan, Z. Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol. Biochem. 2014, 82, 209–217. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T.J. Aux/IAA Proteins Contain a Potent Transcriptional Repression Domain. Plant Cell 2004, 16, 533–543. [Google Scholar] [CrossRef]
- Dharmasiri, N.; Estelle, M. Auxin signaling and regulated protein degradation. Trends Plant Sci. 2004, 9, 302–308. [Google Scholar] [CrossRef]
- Winkler, M.; Niemeyer, M.; Hellmuth, A.; Janitza, P.; Christ, G.; Samodelov, S.L.; Wilde, V.; Majovsky, P.; Trujillo, M.; Zurbriggen, M.D. Variation in auxin sensing guides AUX/IAA transcriptional repressor ubiquitylation and destruction. Nat. Commun. 2017, 8, 15706. [Google Scholar] [CrossRef]
- Niemeyer, M.; Moreno Castillo, E.; Ihling, C.H.; Iacobucci, C.; Wilde, V.; Hellmuth, A.; Hoehenwarter, W.; Samodelov, S.L.; Zurbriggen, M.D.; Kastritis, P.L. Flexibility of intrinsically disordered degrons in AUX/IAA proteins reinforces auxin co-receptor assemblies. Nat. Commun. 2020, 11, 2277. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.; Guilfoyle, T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Szemenyei, H.; Hannon, M.; Long, J.A. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 2008, 319, 1384–1386. [Google Scholar] [CrossRef]
- Morgan, K.E.; Zarembinski, T.I.; Theologis, A.; Abel, S. Biochemical characterization of recombinant polypeptides corresponding to the predicted βαα fold in Aux/IAA proteins. FEBS Lett. 1999, 454, 283–287. [Google Scholar]
- Remington, D.L.; Vision, T.J.; Guilfoyle, T.J.; Reed, J.W. Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Physiol. 2004, 135, 1738–1752. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cao, X.; Shi, S.; Ma, Y.; Wang, K.; Liu, S.; Chen, D.; Chen, Q.; Ma, H. Genome-wide survey of Aux/IAA gene family members in potato (Solanum tuberosum): Identification, expression analysis, and evaluation of their roles in tuber development. Biochem. Biophys. Res. Commun. 2016, 471, 320–327. [Google Scholar] [CrossRef]
- Wu, J.; Peng, Z.; Liu, S.; He, Y.; Cheng, L.; Kong, F.; Wang, J.; Lu, G. Genome-wide analysis of Aux/IAA gene family in Solanaceae species using tomato as a model. Mol. Genet. Genom. 2012, 287, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yuan, C.; Feng, S.; Zhong, S.; Li, H.; Zhong, J.; Shen, C.; Liu, J. Genome-wide analysis and characterization of Aux/IAA family genes related to fruit ripening in papaya (Carica papaya L.). BMC Genom. 2017, 18, 351. [Google Scholar] [CrossRef]
- Boer, D.R.; Freire-Rios, A.; van den Berg, W.A.M.; Saaki, T.; Manfield, I.W.; Kepinski, S.; López-Vidrieo, I.; Franco-Zorrilla, J.M.; Vries, S.C.d.; Solano, R.; et al. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell 2014, 156, 577–589. [Google Scholar] [CrossRef]
- Nanao, M.H.; Vinos-Poyo, T.; Brunoud, G.; Thévenon, E.; Mazzoleni, M.; Mast, D.; Lainé, S.; Wang, S.; Hagen, G.; Li, H.; et al. Structural basis for oligomerization of auxin transcriptional regulators. Nat. Commun. 2014, 5, 3617. [Google Scholar] [CrossRef]
- Hamann, T.; Benkova, E.; Bäurle, I.; Kientz, M.; Jürgens, G. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev. 2002, 16, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Qi, L.; Li, Y.; Zhai, Q.; Li, C. PIF4 and PIF5 transcription factors link blue light and auxin to regulate the phototropic response in Arabidopsis. Plant Cell 2013, 25, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jones, B.; Li, Z.; Frasse, P.; Delalande, C.; Regad, F.; Chaabouni, S.; Latché, A.; Pech, J.-C.; Bouzayen, M. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 2005, 17, 2676–2692. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.-J.; Zhang, J.-Z. Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef]
- Fukaki, H.; Tameda, S.; Masuda, H.; Tasaka, M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 2002, 29, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Rogg, L.E.; Bartel, B. Auxin signaling: Derepression through regulated proteolysis. Dev. Cell 2001, 1, 595–604. [Google Scholar] [CrossRef]
- Nagpal, P.; Walker, L.M.; Young, J.C.; Sonawala, A.; Timpte, C.; Estelle, M.; Reed, J.W. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 2000, 123, 563–574. [Google Scholar] [CrossRef]
- Rouse, D.; Mackay, P.; Stirnberg, P.; Estelle, M.; Leyser, O. Changes in auxin response from mutations in an AUX/IAA gene. Science 1998, 279, 1371–1373. [Google Scholar] [CrossRef]
- Ouellet, F.; Overvoorde, P.J.; Theologis, A. IAA17/AXR3: Biochemical insight into an auxin mutant phenotype. Plant Cell 2001, 13, 829–841. [Google Scholar] [CrossRef]
- Hamann, T.; Mayer, U.; Jürgens, G. The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development 1999, 126, 1387–1395. [Google Scholar] [CrossRef]
- Ploense, S.E.; Wu, M.-F.; Nagpal, P.; Reed, J.W. A gain-of-function mutation in IAA18 alters Arabidopsis embryonic apical patterning. Development 2009, 136, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Guo, H.-S. Cleavage of INDOLE-3-ACETIC ACID INDUCIBLE28 mRNA by microRNA847 upregulates auxin signaling to modulate cell proliferation and lateral organ growth in Arabidopsis. Plant Cell 2015, 27, 574–590. [Google Scholar] [CrossRef]
- Dreher, K.A.; Brown, J.; Saw, R.E.; Callis, J. The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell 2006, 18, 699–714. [Google Scholar] [CrossRef]
- Krogan, N.T.; Berleth, T. The identification and characterization of specific ARF-Aux/IAA regulatory modules in plant growth and development. Plant Signal. Behav. 2015, 10, e992748. [Google Scholar] [CrossRef]
- Weijers, D.; Benkova, E.; Jäger, K.E.; Schlereth, A.; Hamann, T.; Kientz, M.; Wilmoth, J.C.; Reed, J.W.; Jürgens, G. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J. 2005, 24, 1874–1885. [Google Scholar] [CrossRef]
- Lakehal, A.; Chaabouni, S.; Cavel, E.; Le Hir, R.; Ranjan, A.; Raneshan, Z.; Novák, O.; Păcurar, D.I.; Perrone, I.; Jobert, F.; et al. A Molecular Framework for the Control of Adventitious Rooting by TIR1/AFB2-Aux/IAA-Dependent Auxin Signaling in Arabidopsis. Mol. Plant 2019, 12, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.-X.; Wang, L.; Yang, H.-Q. A gain-of-function mutation in IAA7/AXR2 confers late flowering under short-day light in Arabidopsis. J. Integr. Plant Biol. 2011, 53, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Reed, J.W. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 1999, 126, 711–721. [Google Scholar] [CrossRef]
- Arase, F.; Nishitani, H.; Egusa, M.; Nishimoto, N.; Sakurai, S.; Sakamoto, N.; Kaminaka, H. IAA8 involved in lateral root formation interacts with the TIR1 auxin receptor and ARF transcription factors in Arabidopsis. PLoS ONE 2012, 7, e43414. [Google Scholar] [CrossRef]
- Timpte, C.; Lincoln, C.; Pickett, F.B.; Turner, J.; Estelle, M. The AXR1 and AUX1 genes of Arabidopsis function in separate auxin-response pathways. Plant J. 1995, 8, 561–569. [Google Scholar] [CrossRef]
- Yang, X.; Lee, S.; So, J.H.; Dharmasiri, S.; Dharmasiri, N.; Ge, L.; Jensen, C.; Hangarter, R.; Hobbie, L.; Estelle, M. The IAA1 protein is encoded by AXR5 and is a substrate of SCFTIR1. Plant J. 2004, 40, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Rogg, L.E.; Lasswell, J.; Bartel, B. A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell 2001, 13, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019, 10, 4021. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Sui, C.; Li, J.; Wan, K.; Sun, H.; Wang, S.; Liu, X.; Guo, S. The Aux/IAA protein TaIAA15-1A confers drought tolerance in Brachypodium by regulating abscisic acid signal pathway. Plant Cell Rep. 2023, 42, 385–394. [Google Scholar] [CrossRef]
- Wang, F.; Niu, H.; Xin, D.; Long, Y.; Wang, G.; Liu, Z.-L.; Li, G.; Zhang, F.; Qi, M.; Ye, Y.; et al. OsIAA18, an Aux/IAA Transcription Factor Gene, Is Involved in Salt and Drought Tolerance in Rice. Front. Plant Sci. 2021, 12, 738660. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, X.; Lu, J.; Song, F.; Sun, J.; Wang, C.; Lian, J.; Zhao, L.; Zhao, B. OsIAA20, an Aux/IAA protein, mediates abiotic stress tolerance in rice through an ABA pathway. Plant Sci. 2021, 308, 110903. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Zhang, Y.; Cheng, B.; Peng, Y.; Zhang, X.; Ma, X.; Huang, L.; Yan, Y. Indole-3-acetic acid modulates phytohormones and polyamines metabolism associated with the tolerance to water stress in white clover. Plant Physiol. Biochem. 2018, 129, 251–263. [Google Scholar] [CrossRef]
- Bassa, C.; Mila, I.; Bouzayen, M.; Audran-Delalande, C. Phenotypes associated with down-regulation of Sl-IAA27 support functional diversity among Aux/IAA family members in tomato. Plant Cell Physiol. 2012, 53, 1583–1595. [Google Scholar] [CrossRef]
- Guillotin, B.; Etemadi, M.; Audran, C.; Bouzayen, M.; Bécard, G.; Combier, J.-P. Sl-IAA27 regulates strigolactone biosynthesis and mycorrhization in tomato (var. MicroTom). New Phytol. 2017, 213, 1124–1132. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Chen, Y.; Shin, J.H.; Mila, I.; Audran, C.; Zouine, M.; Pirrello, J.; Bouzayen, M. The tomato Ethylene Response Factor Sl-ERF. B3 integrates ethylene and auxin signaling via direct regulation of Sl-Aux/IAA 27. New Phytol. 2018, 219, 631–640. [Google Scholar] [CrossRef]
- Liu, W.; Kohlen, W.; Lillo, A.; Op den Camp, R.; Ivanov, S.; Hartog, M.; Limpens, E.; Jamil, M.; Smaczniak, C.; Kaufmann, K. Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell 2011, 23, 3853–3865. [Google Scholar] [CrossRef]
- Hou, Y.; Li, H.; Zhai, L.; Xie, X.; Li, X.; Bian, S. Identification and functional characterization of the Aux/IAA gene VcIAA27 in blueberry. Plant Signal. Behav. 2020, 15, 1700327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, X.; Xu, X.; Han, Z.; Qiu, C. Transcription Factor IAA27 Positively Regulates P Uptake through Promoted Adventitious Root Development in Apple Plants. Int. J. Mol. Sci. 2022, 23, 14029. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Hou, J.; Iqbal, M.Z.; Zhang, Y.; Cheng, B.; Feng, H.; Li, Z.; Liu, L.; Zhou, J.; Feng, G. Overexpression of the white clover TrSAMDC1 gene enhanced salt and drought resistance in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 165, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, Z.; Lu, S. Genome-wide identification and expression analysis of the Aux/IAA and auxin response factor gene family in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 10494. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; To, V.-T.; Shi, J.; Zhang, D.; Cai, W. Genome-wide characterization and expression analyses of the auxin/indole-3-acetic acid (Aux/IAA) gene family in barley (Hordeum vulgare L.). Sci. Rep. 2020, 10, 10242. [Google Scholar] [CrossRef]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef]
- Demirkol, G. PopW enhances drought stress tolerance of alfalfa via activating antioxidative enzymes, endogenous hormones, drought related genes and inhibiting senescence genes. Plant Physiol. Biochem. 2021, 166, 540–548. [Google Scholar] [CrossRef]
- Shahzad, Z.; Eaglesfield, R.; Carr, C.; Amtmann, A. Cryptic variation in RNA-directed DNA-methylation controls lateral root development when auxin signalling is perturbed. Nat. Commun. 2020, 11, 218. [Google Scholar] [CrossRef]
- Ren, H.; Gray, W.M. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef]
- Liu, W.; Yu, J.; Ge, Y.; Qin, P.; Xu, L. Pivotal role of LBD1 6 in root and root-like organ initiation. Cell. Mol. Life Sci. 2018, 75, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, H.; Murali, M.; Pal, M.A.C.; Melvin, P.; Sharada, M. Salicylic acid seed priming instigates defense mechanism by inducing PR-Proteins in Solanum melongena L. upon infection with Verticillium dahliae Kleb. Plant Physiol. Biochem. 2017, 117, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Müllender, M.; Varrelmann, M.; Savenkov, E.I.; Liebe, S. Manipulation of auxin signalling by plant viruses. Mol. Plant Pathol. 2021, 22, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Fu, S.; Yang, Y.; Sun, Q.; Wang, J.; Dong, Y.; Gu, X.; Wang, T.; Xie, X.; Mo, X. Comparative Microscopic, Transcriptome and IAA Content Analyses Reveal the Stem Growth Variations in Two Cultivars Ilex verticillata. Plants 2023, 12, 1941. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, J.; Qi, X.; Wang, J.; Chen, H.; Zhang, Z.; Huang, J.; Liang, Y.; Lin, W. Differential expression and function analysis of proteins in flag leaves of rice during grain filling. Acta Agron. Sin. 2009, 35, 132–139. [Google Scholar]
- Su, B.; Wu, H.; Guo, Y.; Gao, H.; Wei, Z.; Zhao, Y.; Qiu, L. GmIAA27 encodes an AUX/IAA protein involved in dwarfing and multi-branching in soybean. Int. J. Mol. Sci. 2022, 23, 8643. [Google Scholar] [CrossRef]
- Blum, A.; Sullivan, C.Y.; Nguyen, H.T. The effect of plant size on wheat response to agents of drought stress. II. Water deficit, heat and ABA. Aust. J. Plant Physiol. 1997, 24, 43–48. [Google Scholar] [CrossRef]
- Ma, C.; Yuan, S.; Xie, B.; Li, Q.; Wang, Q.D.; Shao, M. IAA Plays an Important Role in Alkaline Stress Tolerance by Modulating Root Development and ROS Detoxifying Systems in Rice Plants. Int. J. Mol. Sci. 2022, 23, 14817. [Google Scholar] [CrossRef] [PubMed]
- Motte, H.; Beeckman, T. The evolution of root branching: Increasing the level of plasticity. J. Exp. Bot. 2019, 70, 785–793. [Google Scholar] [CrossRef]
- Farooq, M.A.; Farooq, M.A.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2011, 29, 185–212. [Google Scholar] [CrossRef]
- Jung, H.; Lee, D.-K.; Do Choi, Y.; Kim, J.-K. OsIAA6, a member of the rice Aux/IAA gene family, is involved in drought tolerance and tiller outgrowth. Plant Sci. 2015, 236, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, G.; Yang, Z.; Wu, J.; Liu, B.; Xu, X.; Nie, G.; Huang, L.; Zhang, X. Genome-Wide Characterization of the Aux/IAA Gene Family in Orchardgrass and a Functional Analysis of DgIAA21 in Responding to Drought Stress. Int. J. Mol. Sci. 2023, 24, 16184. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.Z.; Jia, T.; Tang, T.; Anwar, M.; Ali, A.; Hassan, M.J.; Zhang, Y.; Tang, Q.; Peng, Y. A Heat Shock Transcription Factor TrHSFB2a of White Clover Negatively Regulates Drought, Heat and Salt Stress Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2022, 23, 12769. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Shani, E.; Salehin, M.; Zhang, Y.; Sanchez, S.E.; Doherty, C.; Wang, R.; Mangado, C.C.; Song, L.; Tal, I.; Pisanty, O. Plant stress tolerance requires auxin-sensitive Aux/IAA transcriptional repressors. Curr. Biol. 2017, 27, 437–444. [Google Scholar] [CrossRef]
- Orosa-Puente, B.; Leftley, N.; Von Wangenheim, D.; Banda, J.; Srivastava, A.K.; Hill, K.; Truskina, J.; Bhosale, R.; Morris, E.; Srivastava, M. Root branching toward water involves posttranslational modification of transcription factor ARF7. Science 2018, 362, 1407–1410. [Google Scholar] [CrossRef]
- Wang, S.; Bai, Y.; Shen, C.; Wu, Y.; Zhang, S.; Jiang, D.; Guilfoyle, T.J.; Chen, M.; Qi, Y. Auxin-related gene families in abiotic stress response in Sorghum bicolor. Funct. Integr. Genom. 2010, 10, 533–546. [Google Scholar] [CrossRef]
- Park, J.-E.; Park, J.-Y.; Kim, Y.-S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.-Y.; Kim, J.; Lee, Y.-H.; Park, C.-M. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef]
- Li, G.; Ye, Y.; Ren, X.; Qi, M.; Zhao, H.; Zhou, Q.; Chen, X.; Wang, J.; Yuan, C.; Wang, F. The rice Aux/IAA transcription factor gene OsIAA18 enhances salt and osmotic tolerance in Arabidopsis. Biol. Plant 2020, 64, 454–464. [Google Scholar] [CrossRef]
- Li, W.; Dang, C.; Ye, Y.; Wang, Z.; Hu, L.; Zhang, F.; Zhang, Y.; Qian, X.; Shi, J.; Guo, Y. Overexpression of grapevine VvIAA18 gene enhanced salt tolerance in tobacco. Int. J. Mol. Sci. 2020, 21, 1323. [Google Scholar] [CrossRef]
- Lavy, M.; Estelle, M. Mechanisms of auxin signaling. Development 2016, 143, 3226–3229. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat 1. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, X.; Wang, Y.; Lebwohl, M. Inhibition of ultraviolet light-induced oxidative events in the skin and internal organs of hairless mice by isoflavone genistein. Cancer Lett. 2002, 185, 21–29. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Kirungu, J.N.; Lu, P.; Yang, X.; Dong, Q.; Cai, X.; Xu, Y.; Wang, X.; Zhou, Z.; Hou, Y. Genome wide identification of the trihelix transcription factors and overexpression of Gh_A05G2067 (GT-2), a novel gene contributing to increased drought and salt stresses tolerance in cotton. Physiol. Plant. 2019, 167, 447–464. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, M.Z.; Liang, Y.; Anwar, M.; Fatima, A.; Hassan, M.J.; Ali, A.; Tang, Q.; Peng, Y. Overexpression of Auxin/Indole-3-Acetic Acid Gene TrIAA27 Enhances Biomass, Drought, and Salt Tolerance in Arabidopsis thaliana. Plants 2024, 13, 2684. https://doi.org/10.3390/plants13192684
Iqbal MZ, Liang Y, Anwar M, Fatima A, Hassan MJ, Ali A, Tang Q, Peng Y. Overexpression of Auxin/Indole-3-Acetic Acid Gene TrIAA27 Enhances Biomass, Drought, and Salt Tolerance in Arabidopsis thaliana. Plants. 2024; 13(19):2684. https://doi.org/10.3390/plants13192684
Chicago/Turabian StyleIqbal, Muhammad Zafar, Yuzhou Liang, Muhammad Anwar, Akash Fatima, Muhammad Jawad Hassan, Asif Ali, Qilin Tang, and Yan Peng. 2024. "Overexpression of Auxin/Indole-3-Acetic Acid Gene TrIAA27 Enhances Biomass, Drought, and Salt Tolerance in Arabidopsis thaliana" Plants 13, no. 19: 2684. https://doi.org/10.3390/plants13192684
APA StyleIqbal, M. Z., Liang, Y., Anwar, M., Fatima, A., Hassan, M. J., Ali, A., Tang, Q., & Peng, Y. (2024). Overexpression of Auxin/Indole-3-Acetic Acid Gene TrIAA27 Enhances Biomass, Drought, and Salt Tolerance in Arabidopsis thaliana. Plants, 13(19), 2684. https://doi.org/10.3390/plants13192684






