Genome-Wide Identification of Rubber Tree SCAMP Genes and Functional Characterization of HbSCAMP3
Abstract
1. Introduction
2. Results
2.1. Whole-Genome Characterization of SCAMP Proteins in Rubber Trees
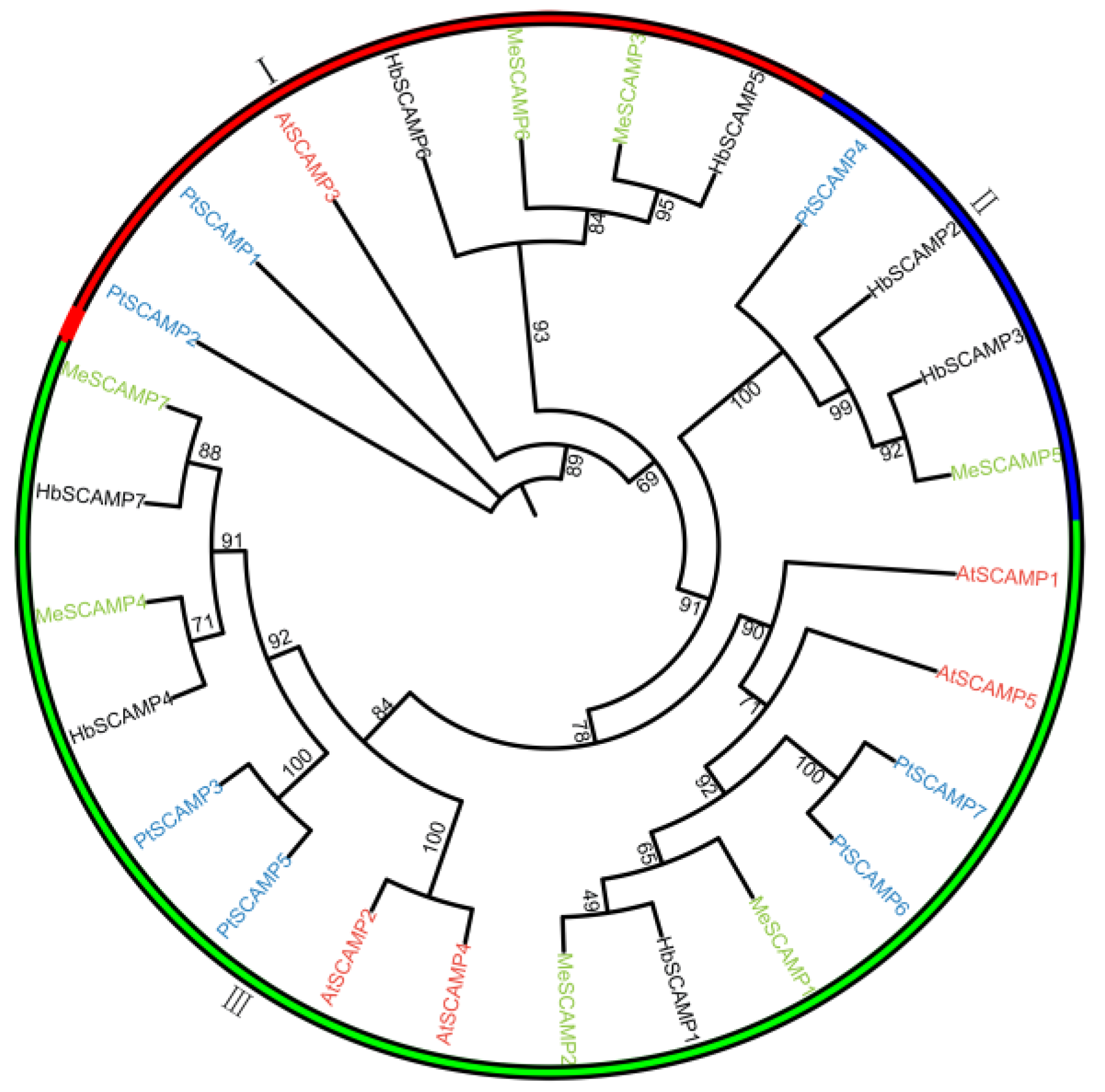
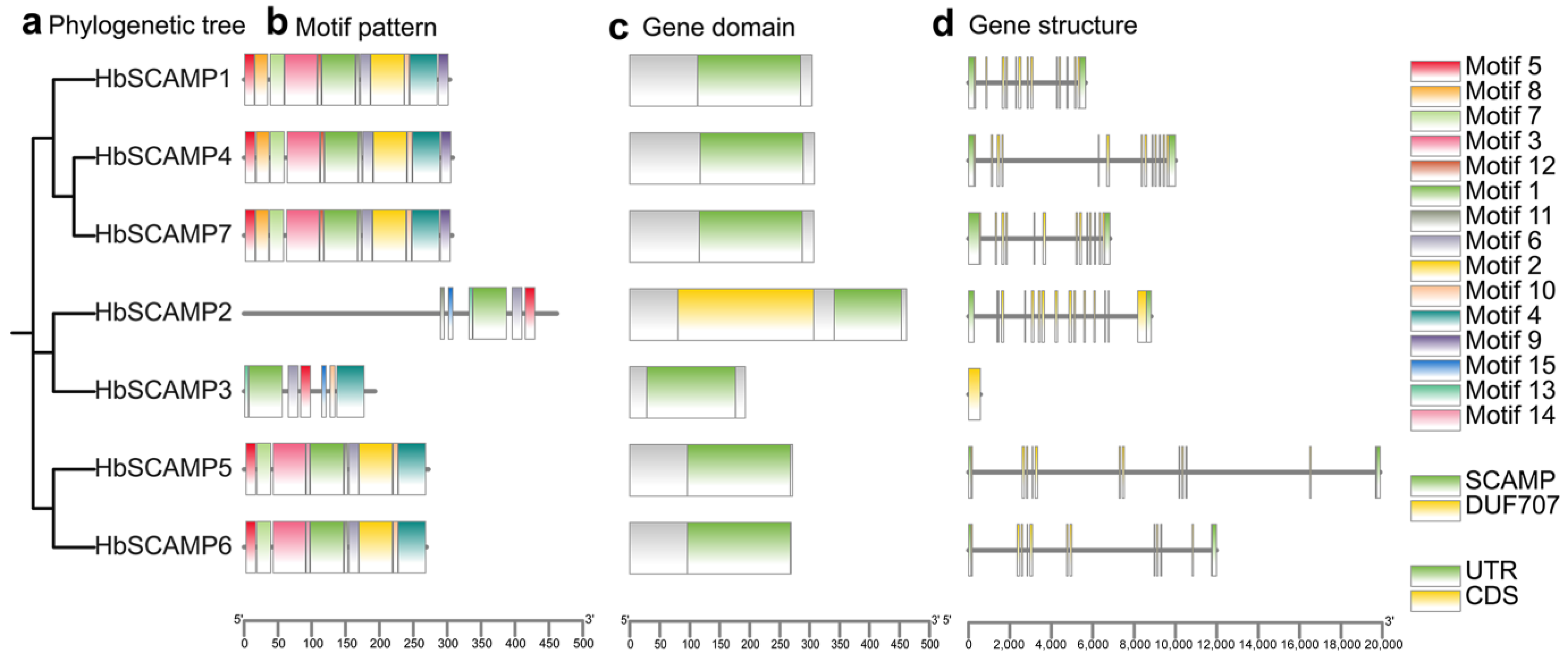
2.2. Phylogenetic Relationship, Gene Structure, and Conserved Motif Analysis of HbSCAMPs
2.3. Evolutionary Analyses of the HbSCAMPs within and between Species
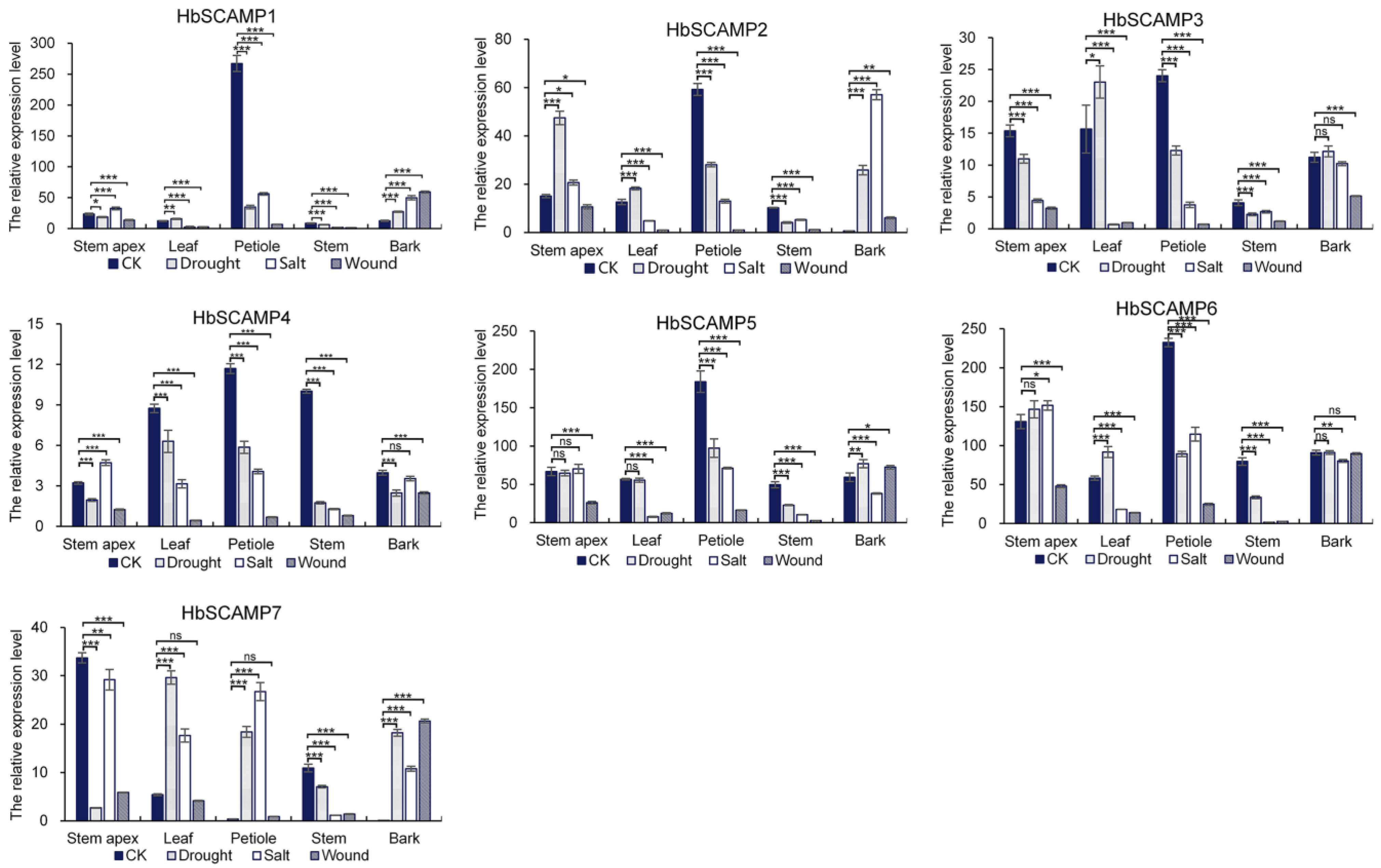
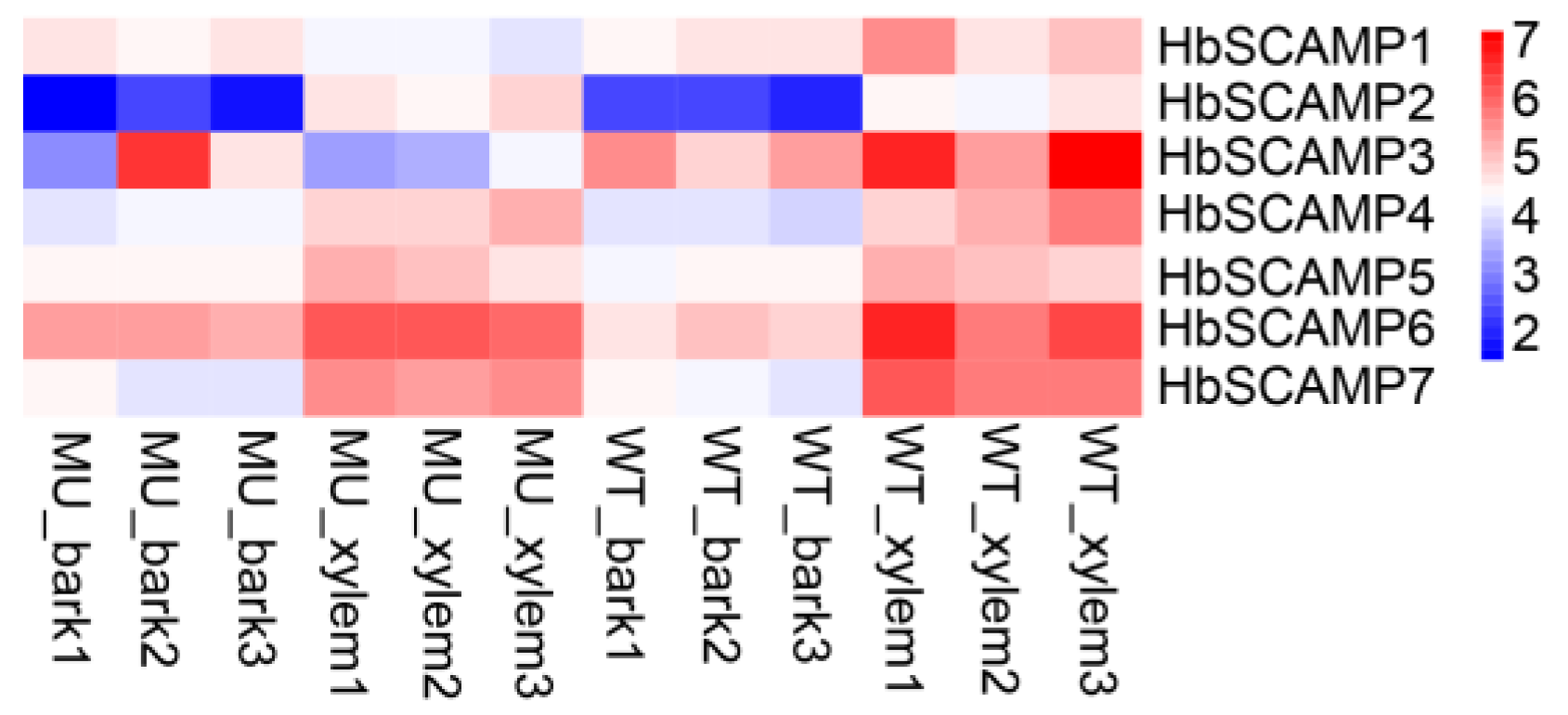
2.4. Expression Profiles of HbSCAMPs under Stress
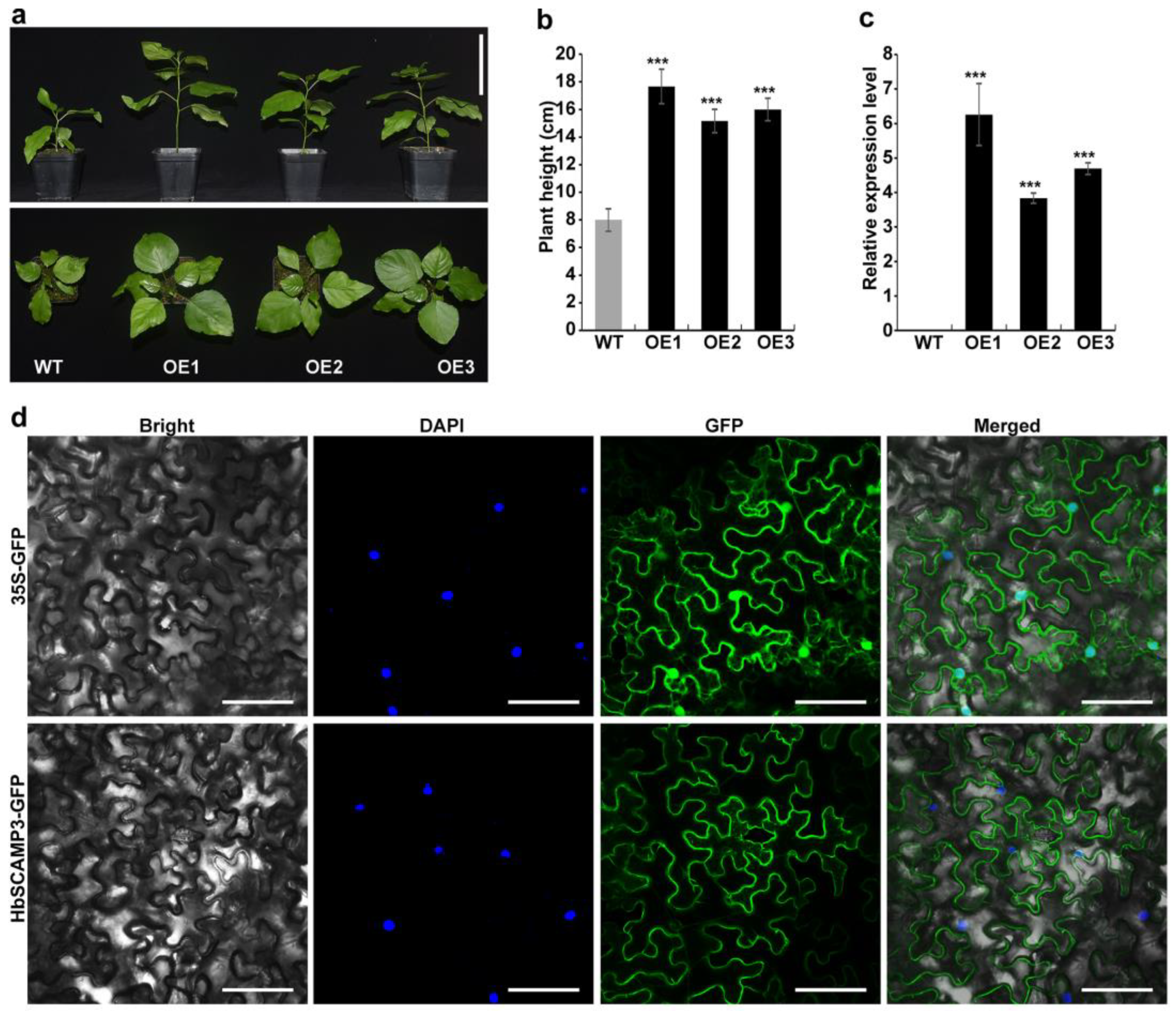
2.5. Overexpressing HbSCAMP3 Increased Plant Height in Poplar
3. Discussion
3.1. SCAMP Gene Family Analysis in Rubber Trees
3.2. Regulation of Plant Stress Response by HbSCAMPs
3.3. Regulation of Plant Growth by HbSCAMP3
4. Materials and Methods
4.1. Identification and Sequence Analysis of SCAMP Genes of Rubber Trees
4.2. Phylogenetic Relationship, Gene Structure, and Conserved Motif and Syntenic Analysis of SCAMPs
4.3. Subcellular Localization of HbSCAMP3
4.4. Construction and Transformation of HbSCAMP3 Overexpression Vector in Poplar
4.5. Plant Stress Treatment and qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mantello, C.C.; Cardoso-Silva, C.B.; da Silva, C.C.; de Souza, L.M.; Scaloppi Junior, E.J.; de Souza Gonçalves, P.; Vicentini, R.; de Souza, A.P. De Novo assembly and transcriptome analysis of the rubber tree (Hevea brasiliensis) and SNP markers development for rubber biosynthesis pathways. PLoS ONE 2014, 9, e102665. [Google Scholar] [CrossRef] [PubMed]
- Sathik, M.M.; Kuruvilla, L. Developments in research on abiotic stress responsive microRNAs of Hevea brasiliensis. Ind. J. Plant Physiol. 2017, 22, 470–483. [Google Scholar] [CrossRef]
- Yu, W.; Kong, G.; Chao, J.; Yin, T.; Tian, H.; Ya, H.; He, L.; Zhang, H. Genome-wide identification of the rubber tree superoxide dismutase (SOD) gene family and analysis of its expression under abiotic stress. PeerJ 2022, 10, e14251. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.B.; Aono, A.H.; Francisco, F.R.; da Silva, C.C.; Souza, L.M.; Souza, A.P. The rubber tree kinome: Genome-wide characterization and insights into coexpression patterns associated with abiotic stress responses. Front. Plant Sci. 2023, 14, 1068202. [Google Scholar] [CrossRef]
- Koryati, T.; Yunidawati, W.; Tistama, R.; Nurhayati, N.; Mazlina, M.; Rosmaiti, R. Study of five clones with combinations of growth regulators based on growth and anatomical characteristics of rubber plant (Hevea brasiliensis L.). AGRIVITA J. Agric. Sci. 2023, 45, 371–380. [Google Scholar] [CrossRef]
- Wang, Y.X.; Teng, R.M.; Wang, W.L.; Wang, Y.; Shen, W.; Zhuang, J. Identification of genes revealed differential expression profiles and lignin accumulation during leaf and stem development in tea plant (Camellia sinensis (L.) O. Kuntze). Protoplasma 2019, 256, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, Y.; Li, J.; Jiao, N.; Zhang, X.; Zhang, Y.; Chen, J.; Tu, Z. RNA sequencing reveals phenylpropanoid biosynthesis genes and transcription factors for Hevea brasiliensis reaction wood formation. Front. Genet. 2021, 12, 763841. [Google Scholar] [CrossRef]
- Francisco, F.R.; Aono, A.H.; da Silva, C.C.; Gonçalves, P.S.; Scaloppi Junior, E.J.; Le Guen, V.; Fritsche-Neto, R.; Souza, L.M.; Souza, A.P. Unravelling rubber tree growth by integrating GWAS and biological network-based approaches. Front. Plant Sci. 2021, 12, 768589. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, X.; Deng, Z.; An, Z.; Huang, H.; Li, W.; Cheng, H. Ultrahigh-density genetic map construction and identification of quantitative trait loci for growth in rubber tree (Hevea brasiliensis). Ind. Crop. Prod. 2022, 178, 114560. [Google Scholar] [CrossRef]
- Apostolova, E.L. Molecular mechanisms of plant defense against abiotic stress. Int. J. Mol. Sci. 2023, 24, 10339. [Google Scholar] [CrossRef]
- Pecherina, A.; Grinberg, M.; Ageyeva, M.; Zanegina, D.; Akinchits, E.; Brilkina, A.; Vodeneev, V. Salt-induced changes in cytosolic ph and photosynthesis in tobacco and potato leaves. Int. J. Mol. Sci. 2022, 24, 491. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.J.; Williams, W.P.; Luu, Y.; Molday, R.S.; Orlowski, J.; Numata, M. Secretory carrier membrane proteins interact and regulate trafficking of the organellar (Na+,K+)/H+ exchanger NHE7. J. Cell Sci. 2005, 118, 1885–1897. [Google Scholar] [CrossRef]
- Wang, M.; He, C.; Shi, G.; Yin, Q.; Zhang, H.; Yang, W.; Yue, A.; Wang, L.; Du, W. Genome-wide analysis of the SCAMPs gene family of soybean and functional identification of GmSCAMP5 in salt tolerance. BMC Plant Biol. 2023, 23, 628. [Google Scholar] [CrossRef] [PubMed]
- Law, A.H.Y.; Shen, J.; Jiang, L. SCAMP, VSR, and Plant Endocytosis. In Endocytosis in Plants; Šamaj, J., Ed.; Springer: Berlin, Germany, 2012; pp. 217–231. [Google Scholar]
- Obudulu, O.; Mähler, N.; Skotare, T.; Bygdell, J.; Abreu, I.N.; Ahnlund, M.; Gandla, M.L.; Petterle, A.; Moritz, T.; Hvidsten, T.R.; et al. A multi-omics approach reveals function of secretory carrier-associated membrane proteins in wood formation of Populus6 trees. BMC Genom. 2018, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- He, C.X.; Li, J.Y.; Zhou, P.; Guo, M.; Zheng, Q.S. Changes of leaf morphological, anatomical structure and carbon isotope ratio with the height of the wangtian tree (Parashorea chinensis) in Xishuangbanna, China. J. Integr. Plant Biol. 2008, 50, 168–173. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Su, X.; Tao, S.; Feng, X.; Wang, Q.; Xu, X.; Liu, Y.; Michaletz, S.T.; Shrestha, N.; et al. Patterns and ecological determinants of woody plant height in eastern Eurasia and its relation to primary productivity. J. Plant Ecol. 2019, 12, 791–803. [Google Scholar] [CrossRef]
- Toyooka, K.; Matsuoka, K. Exo-and endocytotic trafficking of SCAMP2. Plant Signal. Behav. 2009, 4, 1196–1198. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Chen, X.; Huang, T.; Jiang, X.; Zhou, Y. Cloning and bioinformatics analysis of SCAMP genes from Arabidopsis thaliana under salt stress. J. Trop. Ecol. 2020, 11, 138–144. [Google Scholar]
- Vaschetto, L.M.; Ortiz, N. The role of sequence duplication in transcriptional regulation and genome evolution. Curr. Genom. 2019, 20, 405–408. [Google Scholar] [CrossRef]
- Nekrutenko, A.; Makova, K.D.; Li, W.H. The KA/KS ratio test for assessing the protein-coding potential of genomic regions: An empirical and simulation study. Genome Res. 2002, 12, 198–202. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Penna, S. Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol. Plant 2021, 171, 739–755. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Delhaize, E.; Frommer, W.B.; Guerinot, M.L.; Harrison, M.J.; Herrera-Estrella, L.; Horie, T.; Kochian, L.V.; Munns, R.; Nishizawa, N.K.; et al. Using membrane transporters to improve crops for sustainable food production. Nature 2013, 497, 60–66. [Google Scholar] [CrossRef]
- Gill, R.A.; Ahmar, S.; Ali, B.; Saleem, M.H.; Khan, M.U.; Zhou, W.; Liu, S. The Role of Membrane Transporters in Plant Growth and Development, and Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 12792. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Kaldenhoff, R. Cell-specific expression of the mercury-insensitive plasma-membrane aquaporin NtAQP1 from Nicotiana tabacum. Planta 2000, 211, 167–172. [Google Scholar] [PubMed]
- Tang, L.; Dong, J.; Tan, L.; Ji, Z.; Li, Y.; Sun, Y.; Chen, C.; Lv, Q.; Mao, B.; Hu, Y.; et al. Overexpression of OsLCT2, a Low-Affinity Cation Transporter Gene, Reduces Cadmium Accumulation in Shoots and Grains of Rice. Rice 2021, 14, 89. [Google Scholar] [CrossRef]
- Luo, D.; Liu, J.; Wu, Y.; Zhang, X.; Zhou, Q.; Fang, L.; Liu, Z. NUCLEAR TRANSPORT FACTOR 2-LIKE improves drought tolerance by modulating leaf water loss in alfalfa (Medicago sativa L.). Plant J. 2022, 112, 429–450. [Google Scholar] [CrossRef]
- Gupta, A.; Shaw, B.P.; Sahu, B.B. Post-translational regulation of the membrane transporters contributing to salt tolerance in plants. Funct. Plant Biol. 2021, 48, 1199–1212. [Google Scholar] [CrossRef]
- Hassan, S.; Qadir, J.; Bhat, E.A.; Maqbool, F.; Jan, M.; Sajjad, N.; Ali, R. Regulation of membrane transporters in plants in response to drought stress. In Transporters and Plant Osmotic Stress; Roychoudhury, A., Tripathi, D.K., Deshmukh, R., Eds.; Academic Press: New York, NY, USA, 2012; pp. 261–272. [Google Scholar]
- Jacobs, B. Exploring Mechanisms of Xylem Cell Wall Patterning with Dynamic Models. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, 2022. [Google Scholar]
- Cai, Y.; Jia, T.; Lam, S.K.; Ding, Y.; Gao, C.; San, M.W.Y.; Pimpl, P.; Jiang, L. Multiple cytosolic and transmembrane determinants are required for the trafficking of SCAMP1 via an ER-Golgi-TGN-PM pathway. Plant J. 2011, 65, 882–896. [Google Scholar] [CrossRef]
- Cheng, H.; Song, X.; Hu, Y.; Wu, T.; Yang, Q.; An, Z.; Feng, S.; Deng, Z.; Wu, W.; Zeng, X.; et al. Chromosome-level wild Hevea brasiliensis genome provides new tools for genomic-assisted breeding and valuable loci to elevate rubber yield. Plant Biotechnol. J. 2023, 21, 1058–1072. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Huang, X.; Zhang, Y.; Gao, X.; Ding, S.; Qi, J.; Wang, X. Genome-Wide Identification of Rubber Tree SCAMP Genes and Functional Characterization of HbSCAMP3. Plants 2024, 13, 2729. https://doi.org/10.3390/plants13192729
Yang B, Huang X, Zhang Y, Gao X, Ding S, Qi J, Wang X. Genome-Wide Identification of Rubber Tree SCAMP Genes and Functional Characterization of HbSCAMP3. Plants. 2024; 13(19):2729. https://doi.org/10.3390/plants13192729
Chicago/Turabian StyleYang, Baoyi, Xiao Huang, Yuanyuan Zhang, Xinsheng Gao, Shitao Ding, Juncang Qi, and Xiangjun Wang. 2024. "Genome-Wide Identification of Rubber Tree SCAMP Genes and Functional Characterization of HbSCAMP3" Plants 13, no. 19: 2729. https://doi.org/10.3390/plants13192729
APA StyleYang, B., Huang, X., Zhang, Y., Gao, X., Ding, S., Qi, J., & Wang, X. (2024). Genome-Wide Identification of Rubber Tree SCAMP Genes and Functional Characterization of HbSCAMP3. Plants, 13(19), 2729. https://doi.org/10.3390/plants13192729





