Abstract
The significant reduction in agricultural output and the decline in product quality are two of the most glaring negative impacts caused by plant pathogenic fungi (PPF). Furthermore, contaminated food or transit might introduce mycotoxins produced by PPF directly into the food chain. Eating food tainted with mycotoxin is extremely dangerous for both human and animal health. Using fungicides is the first choice to control PPF or their toxins in food. Fungicide resistance and its effects on the environment and public health are becoming more and more of a concern, despite the fact that chemical fungicides are used to limit PPF toxicity and control growth in crops. Fungicides induce target site alteration and efflux pump activation, and mutations in PPF result in resistance. As a result, global trends are shifting away from chemically manufactured pesticides and toward managing fungal plant diseases using various biocontrol techniques, tactics, and approaches. However, surveillance programs to monitor fungicide resistance and their environmental impact are much fewer compared to bacterial antibiotic resistance surveillance programs. In this review, we discuss the PPF that contributes to disease development in plants, the fungicides used against them, factors causing the spread of PPF and the emergence of new strains, the antifungal resistance mechanisms of PPF, health, the environmental impacts of fungicides, and the use of biocontrol agents (BCAs), antimicrobial peptides (AMPs), and nanotechnologies to control PPF as a safe and eco-friendly alternative to fungicides.
1. Introduction
Globally, phytopathogens represent a major threat to crop productivity. Plant infections caused by fungi, bacteria, viruses, and nematodes seriously impair or destroy crops, significantly lowering the quantity and quality of agricultural products. Each year, these losses pose a danger to the world’s food supply [1,2]. Furthermore, pathogenic infections in the field or during post-harvest storing can be harmful for livestock and people, especially if the disease produces toxins in or on edible goods [2].
In recent decades, fungi have been responsible for an increasing number of epidemics affecting humans, plants, and animals [3]. This issue is intensified by the growing resistance of pathogenic organisms to antifungal medications. When fungi are exposed to fungicides in the environment over time, resistance develops [4]. Fungal diseases such as Aspergillus fumigatus are evolving novel resistant forms, while newly emerging fungal species like Candida auris and Trichophyton indotineae are exhibiting antifungal resistance profiles [3,5].
New disease outbreaks can result from alterations in fungal survival, infectivity, and host vulnerability caused by changes in the climate [6]. Additionally, these changes might encourage the establishment of novel virulent strains, which is extremely concerning for future epidemiological studies [7]. Numerous studies on the relationship between fungal infections and climate change have demonstrated that fungal diseases have become more prevalent as a result of climate change, and they are now widely acknowledged as a global threat to significant crops [6,8,9,10]. Fungi require adaptation or community shifts to survive in the face of land-use change, drought, and climate fluctuation; however, certain fungi with higher thermal optima are more resilient to these changes [11].
Plant pathogens are spreading outside their typical geographical areas due to globalization and worldwide trade, with the potential to adapt to climatic and environmental changes and contemporary agricultural practices, like altered land uses and the widespread use of antifungal agents [12]. Despite their potential to cause severe damage to economically significant crops, emerging fungal diseases are still poorly understood and understudied, posing a growing threat to bionetworks, overall health, food safety, and the economy [13]. Although many questions remain about how these fungal pathogens spread, evolve, adopt novel ecological strategies, switch hosts, and cause infections, these emerging pathogens have the potential to serve as “true reservoirs” for future disease epidemics [10]. Published data suggest that common saprophytic fungi from the Aspergillus, Penicillium, and Cryptococcus genera are emerging as possible plant pathogens. During cultural practices or the post-harvest/storage stages, these fungi can pose a significant hazard to staple crops, including rice, wheat, maize, and potatoes [14]. The potential impact on global food security could be significant if specific disease management policies are not implemented and these infections are not promptly and accurately identified [13].
Controlling various plant pathogens necessitates an effective management system to ensure sufficient food supply for the world’s growing population [15,16]. At present, the main approaches for controlling plant illnesses include the development of disease-resistant crops, the use of chemical pesticides, and the implementation of effective practices. Over the last few decades, these methods have been fundamental in growing yield and quality [17]. Unfortunately, the abuse of chemical pesticides has led to environmental degradation, which has limited their use in agriculture [17,18]. As an eco-friendly and sustainable way of controlling crop diseases, researchers are now considering the use of beneficial microorganisms (e.g., bacterial and fungal endophytes) [17,19]. Different bacteria have shown great potential as BCAs for various plant pathogens. It has also been proven that fungi play a crucial role in preventing serious diseases in essential crops [19,20]. Significant emphasis has also been placed on research into fungal strains such as BCAs for plant disease control. These fungus-based BCAs have the potential to be used as biopesticides in field or greenhouse studies, as they exhibit strong antagonistic activity against various types of soil- and airborne plant pathogens [19,21].
In this review, we briefly discuss the role of PPF in disease development in plants, factors contributing to the spread of PPF in the environment and plants, and the emergence of new pathogenic fungal strains. We also cover the antibiotic resistance mechanisms of PPF, the health and environmental impacts of chemical fungicides, and the use of BCAs to combat PPF as a safe and eco-friendly alternative to antibiotics. Furthermore, we examine the use of AMPs from various natural sources as potential antifungal agents and recent advances in nanotechnology to combat PPF. Nanoparticles (NPs) offer many advantages over chemical antibiotics, such as efficiently reaching target sites and being less prone to developing resistance.
2. Control of PPF by Antibiotics
2.1. Fungal Pathogens and Diseases
There are a lot of PPF infecting plants and crops. Listing or ranking all of them is a troublesome task. However, the journal Molecular Plant Pathology ranked the top 10 fungal pathogens based on a survey conducted with the participation of 495 plant pathologists worldwide [22]. These fungal pathogens include (1) Magnaporthe oryzae, (2) Botrytis cinerea, (3) Puccinia spp., (4) Fusarium graminearum, (5) F. oxysporum, (6) Blumeria graminis, (7) Mycosphaerella graminicola, (8) Colletotrichum spp., (9) Ustilago maydis, and (10) Melampsora lini. Other important PPF that are not included in the above list are Phakopsora pachyrhizi and Rhizoctonia solani [22].
New pathogens brought about by environmental changes and assessment are known as emerging pathogens [10]. One such emerging pathogen is Neopseudocercosporella capsellae, which causes white leaf spots on Brassicaceae plants. Recent reports indicate that this fungus is harming commercially significant Brassicaceae crops, such as oilseed rape, vegetables, condiments, and feed brassicas [23]. Before infection occurs, N. capsellae can transition between two morphologies (septate hyphae and single-celled yeast phase) on various artificial culture mediums (in vitro) or in planta on the host surface. It has been demonstrated that the hyphae-to-yeast transformation involves the development of arthrospores (arthroconidia) and two morphologically distinct types of blastospores (blastoconidia): meso- and micro-blastospores [24].
Numerous crop diseases are caused by Bipolaris sorokiniana [8]. In China, hazelnuts (Corylus heterophylla), a significant nut crop, are reportedly declining due to fungal branch canker and dieback. Three Cytospora and two Diaporthe species were identified as the causative agents through morphological observations combined with multi-locus phylogenetic analysis [25]. Additionally, this epidemic has revealed three new species: C. corylina, C. curvispora, and D. corylicola. Two recognized species, C. leucostoma and D. eres, are also documented in this study [25].
2.2. Factors in Spreading PPF
Food security and agricultural practices are significantly impacted by climate change, which could have detrimental effects on several economically significant crops sooner or later. Perennial crops, like tea, are especially susceptible. Crop-associated fungal infections will likely also be impacted by climate change [9]. For the vector hosts, an intricate combination of several environmental factors (e.g., temperature and moisture) produces the ideal habitat for the pathogens. Global warming and climate change will have catastrophic repercussions on environments that sustain animal, plant, and human life. Pathogens, especially those of overlooked tropical illnesses, are predicted to resurface and develop in several countries, including North America and Europe [9,12]. By 2050, baseline tea-growing areas will no longer be suitable for Camellia sinensis var. assamica (32–34% loss) and C. sinensis var. sinensis (15–32% loss), according to models of the climate-fungal pathogen [9]. In the regions where tea is currently being grown, issues will arise from both known and possibly unknown fungal diseases [9].
Grasslands constitute a significant ecological and economic element of the planet’s vegetation, and the stability of these ecosystems is greatly influenced by the fungal populations that inhabit them. Grass–fungi interactions can be mutualistic or harmful. It is well recognized that fungi, insects, various grassland animals, and herbivorous mammals all contribute significantly to preserving the variety and biomass of grasslands [26]. Because pathogenic fungi alter the physiology and chemical composition of grasses, they have a considerable impact on the population biology of grasses and their contribution to plant communities [26,27].
In addition to the increasing reliance on fossil fuels and water, intensive agriculture has been linked to biodiversity losses [28]. Crop disease protection in our modern agroecosystems is primarily provided by cultivars having single main R genes and single-target-site fungicides. However, this defense mechanism is proving to be temporary, as monocultures encourage the growth of novel fungal strains that may overcome host resistance and resist commonly used fungicides, providing agricultural pathogens with the ideal environment for reproduction and proliferation [29]. Furthermore, when exposed to antibiotics, microbes are likely to develop resistance against them [30,31,32,33]. The widespread use of chemical fungicides is another factor contributing to the environmental development of novel PPF [28,34].
The continual existence of host tissue, especially in temperate zones, also sustains fungal populations [35]. Thus, Zymoseptoria tritici exhibits every trait associated with a high potential for evolution: great variety, frequent sexual reproduction, sizable effective populations, and high rates of recombination and mutation [35]. This promotes the development of novel characteristics that enable Z. tritici to flourish on novel host varieties, in novel environmental circumstances, or in the face of extensive fungicide use [36]. The most damaging of these is soybean rust (SBR), which is brought on by the fungus P. pachyrhizi. In areas where disease conditions are favorable, losses of up to 80% are not uncommon [37,38]. One reason for the severity and spread of SBR is the prolific production of asexual spores, which leads to recurrent cycles of infection [39].
There is ample evidence of the spread of wheat blast to Asia’s wheat-producing regions, especially Bangladesh. Here, crop losses due to wheat blast have reached 50% [40,41]. A common characteristic of disease introduction in a new geographic location is such significant devastation. The reason behind such severe epidemics is frequently the pathogen’s migration to new areas, where it may not be affected by viruses or competitors. Their climate might be more suited to the areas, enabling prolonged intervals of maximal pathogen virulence or enhanced survival in between host growth seasons. Most crucially, though, newly discovered regions contain naive hosts that are not aware of cues to develop an anti-pathogen response since they have not co-evolved with the pathogen [38]. If the velocity of pathogens’ migration is pushed beyond the frequency at which host defense systems evolve, the likelihood of host naivety is significantly raised. This can occur spontaneously when certain diseases move large distances quickly through the atmosphere, as P. pachyrhizi demonstrates [38].
2.3. Antifungal Agrochemicals in PPF Control
By 2050, it is predicted that the world’s population will have increased by roughly 30%, necessitating investments to boost productivity and output in agriculture [42]. Consequently, it is essential to employ effective fungicides to safeguard crops against disease during both the large-scale agricultural production phase and the post-harvest phase [43]. The agricultural industry has faced a number of difficulties recently, including lower crop yields brought on by abiotic stressors, diseases, and pests.
Antifungal agrochemicals are chemicals used to kill PPF in crops. These are frequently employed in agricultural systems to prevent illness and maintain crop quality and output. There are different classes of antifungal agrochemicals used to control PPF. All of these chemicals have specific modes of action and specificity. For example, azoles, especially triazoles, are highly efficient against a broad range of plant diseases such as leaf rust, leaf spots, and powdery mildew, inhibiting fungal ergosterol biosynthesis by impeding 14α-demethylase [44,45,46,47]. Another major class of antibiotics is echinocandins (anidulafungin, caspofungin, and micafungin), which inhibit 1,3-Beta-glucan synthase in fungal cells. As a result, the formation of glucan in the fungal cell wall is blocked, resulting in the death of fungal cells [48]. Other classes of antifungal agrochemicals used in agriculture include polyenes, allylamines, morpholine, and anilinopyrimidine [42]. An organomercurial compound, the first organic fungicide, was created at the start of the 20th century. Several fungicides that are effective against fungi like Fusarium spp. and Dreschlera spp. were commercialized as a result of further research in this area, including 2-methoxyethyl silicate and 2-hydroxyphenyl mercury [49]. Table 1 shows some common fungal diseases of crops and the antibiotics found to be effective against these PPF, while Table 2 shows the mode of action of different fungicides on PPF.

Table 1.
Crops with some common fungal diseases and recent research on antibiotics found to be effective against these PPF.

Table 2.
The main classes of fungicides and their mode of action.
3. Mechanisms of Antifungal Resistance
Every group of fungicides has its mode of action, as shown in Table 3. To develop resistance, fungal pathogens develop their own ways to either block the target sites of fungicides, overexpress efflux pumps, or undergo genetic modifications [5]. One typical mechanism leading to acquired resistance to antifungal chemicals is drug target change. Resistance to azoles is caused by changes in the genes that encode 14-α-demethylase, the target of azole fungicides (cyp51 in molds and erg11 in yeast). Several PPF, such as Penicillium digitatum, Cercospora beticola, Erysiphe necator, B. cinérea, M. graminicola, O. acuformis, and Podosphaera fusca, have been reported to develop resistance against azoles through target modification [5,76,77,78]. Unbelievably, azole-using environments are becoming increasingly contaminated with cyp51A gene promoter duplications paired with specific amino acid substitutions TR34/L98H and TR46/Y121F/T289A. These duplications have also been isolated from hospital patients who have never taken antifungal medications before [79,80,81,82]. Through recombination between resistant and susceptible isolates, a single introduction of a strain bearing the TR34/L98H mutation appears to have resulted in extensive dissemination throughout susceptible Aspergillus populations, according to recent genomic research of A. fumigatus isolates from the USA [83]. Another common way that azole resistance is acquired is through increased expression of the drug target. There are some transcription regulators that contribute to the overexpression of cyp51A/ERG11, resulting in azole resistance. In A. fumigatus and C. neoformans, the sterol regulatory element-binding protein (SREBP), SrbA, and Sre1 control the expression of cyp51A/ERG11 when exposed to azole [84,85]. Additionally, the transcription factors AtrR and SltA also regulate the expression of cyp51A in A. fumigatus, which is responsible for azole resistance [86,87,88]. Interestingly, AtrR induces the expression of cdr1B, an ABC transporter implicated in azole resistance. Therefore, it may be claimed that AtrR, through co-regulating the drug target (Cyp51A) and putative drug efflux pump (Cdr1B), plays a crucial role in a novel azole resistance mechanism [86]. Similarly, mutations in the genes encoding the therapeutic targets FKS1 (Candida, Cryptococcus, and Aspergillus species) and FKS2 (C. glabrata alone) are often responsible for resistance to echinocandin therapy [89,90]. Although polyene resistance is uncommon, changes in the ergosterol production pathway can lead to ergosterol depletion and the build-up of alternative sterols. Numerous ergosterol biosynthesis genes, including ERG2, ERG3, ERG6, and ERG11, have been linked to mutations in Candida species that exhibit resistance to amphotericin B [91,92,93].
A common mechanism of resistance to various antifungal medications is accelerated drug efflux, which operates alongside target modification or overexpression. The overexpression of plasma membrane efflux pumps, reducing intracellular drug accumulation, is frequently associated with resistance to azole therapy. Conversely, efflux-mediated resistance mechanisms are rarely implicated in adaptive resistance to echinocandins and polyenes, as they are weak pump substrates [94]. Constitutive overexpression of the ABC-transporter genes CDR1 and CDR2 results from gain-of-function mutations in the transcriptional activator gene TAC1, while activating mutations in MRR1 cause the upregulation of MDR1 [95,96]. Similarly, the clinical resistance of C. auris to fluconazole is influenced by gain-of-function mutations in TAC1B and MRR1A [97,98]. Thus, various fungal infections demonstrate efflux-mediated resistance to azoles, highlighting the potential utility of targeting drug efflux to overcome resistance.
Genomic modifications are another mechanism by which fungal infections develop resistance to fungicides. Azole-resistant clinical isolates and laboratory-derived resistant strains of Candida albicans have been observed to duplicate the left arm of chromosome 5, forming an isochromosome (i5(L)) [99]. This duplication leads to the overexpression of azole target protein Erg11 and the transcription factor Tac1, which controls drug efflux [100]. Recent studies in C. albicans have revealed that exposure to the endoplasmic reticulum stress-inducing drug tunicamycin or the cancer treatment hydroxyurea selects for trisomy of chromosome 2, conferring tolerance to the echinocandin caspofungin [101,102]. While at least three genes on chromosome 2 (ALG7, RTA2, and RTA3) affect only tunicamycin responses and not caspofungin, extra copies of MKK2 affect tolerance to both fungicides. Other chromosome 2 genes (RNR1 and RNR21) do not impact tunicamycin or caspofungin tolerance, but they do affect hydroxyurea tolerance [101]. Aneuploidy can confer resistance to medications unrelated to cross-tolerance by altering the copy number of numerous genes simultaneously [102]. Genomic rearrangements such as chromosome translocations, segmental duplications, and the sporadic creation of de novo mini-chromosomes underlie azole resistance in the haploid yeast C. glabrata [103]. Heteroresistance in C. neoformans is frequently acquired through disomy formation, with chromosome 1, containing both the ABC transporter genes AFR1 and ERG11, being the most common disomic chromosome [104]. Additionally, recent findings suggest that A. fumigatus develops triazole resistance through horizontal gene transfer (HGT) [105]. Under voriconazole stress alone, A. fumigatus can develop resistance via both chromosomal and plasmid-mediated HGT, indicating that HGT may contribute to antifungal resistance in this pathogen [105]. Table 3 summarizes the antifungal resistance mechanisms of various PPF against different classes of fungicides.

Table 3.
Fungicide resistance mechanisms of PPF.
Table 3.
Fungicide resistance mechanisms of PPF.
| Fungicide Classes | Example | Resistant Fungal Pathogens | Mechanisms of Resistance | References |
|---|---|---|---|---|
| Polyenes | Amphotericin B, Candicidin, Hamycin, Natamycin, Nystatin, Rimocidin | Aspergillus spp., C. albicans, C. glabrata, C. auris, C. neoformans, Fusarium spp. | Target alteration by AmB resistance, ERG gene mutation Mutations in FCY1, FCY2, and FUR1 genes | [5,76] |
| Benzimidazoles | Carbendazim, Thiabendazole | B. cinerea | Point mutations in the β-tubulin gene resulting in altered benzimidazole-binding site | [77,78] |
| Benomyl | Venturia inaequalis, V. pirina, Monilinia fructicola, Sclerotinia homoeocarpa, Penicillium | Point mutations in the β-tubulin gene (mutation at position 198) | [106] | |
| SDHIs | Bixafen, Boscalid, Carboxin, Fluaxapyroxad, Fluopyram, Isopyrazam, Penthiopyrad, Sedaxane | B. cinerea, Corynespora cassicola, D. bryoniae, Botrytis elliptica, Podosphaera xanthii, P. pachyrhizi, Stemphylium botryosum, Aspergillus flavus, Alternaria alternata | Mutations in sdh genes | [73,107,108,109,110,111,112] |
| QoIs | Pyraclostrobin, Azoxystrobin, Mandestrobin, Kresoxim-Methyl, Dimoxystrobin, Famoxadone, Fluoxastrobin, Fenamidone, Pyribencarb | B. cinerea, P. pachyrhizi | Mutation in cytb | [108,111] |
| Morpholines | Aldomorph, Enpropimorph, Dodemorph, Tridemorph, Fenpropimorph, Fenpropidin Spiroxamine, | E. necator, E. graminis, Sphaerotheca fuliginea, | Unknown | [113,114] |
| Anilinopyrimidines | Cyprodinil, Mepanipyrim, Pyrimethanil | B. cinerea, V. inaequalis, | All identified resistance-conferring genes encode proteins that are involved in mitochondrial processes, suggesting that anilinopyrimidines primarily target the mitochondria. | [115,116,117] |
| DMIs | Cyproconazole, Propiconazole, Tebuconazole | P. pachyrhizi | Mutation on cyp51 gene | [111] |
| Azoles | Abafungin, Bifonazole, Butoconazole, Lotrimazole, Econazole, Fenticonazole, Ketoconazole, Miconazole, Oxiconazole, Sulconazole, Albaconazole, Inaconazole, Epoxiconazole, Luconazole, Prothioconazole | Z. tritici, V. inaequalis, P. digitatum, C. beticola, M. fructicola, E. necator, Brumeriela jaapii, B. cinérea, B. graminis, M. graminicola, O. acuformis, O. yallundae, P. fusca | Mutations in cyp51, upregulation of cyp51 and the genes encoding membrane transporters, efflux pump activity, ABC transporter PMR1 over-expression, mutations in erg11 or erg3 | [5,118,119,120] |
| Echinocandins | Anidulafungin, Aspofungin, Micafungin | C. albicans, C. krusei, C. auris, C. tropicalis, A. fumigatus | Mutations in fks1 gene | [4,5] |
| Others | Azoxystrobin, Difenoconazole | Alternaria solani | Mutations in cytb are associated with azoxystrobin resistance | [121] |
| Thiophanate-Methyl | Lasiodiplodia theobromae | Mutations in β-tubulin gene | [122] |
SDHI, succinate dehydrogenase inhibitor; QoI, quinol oxidation inhibitor; DMI, demethylation inhibitor.
4. Impact of Antifungal Chemicals on Humans, Plants, and Environment
Fungicide use has significantly increased in major agricultural countries, including China and the USA, by up to 400%. Most fungicides have a degradation half-life ranging from 45 to 120 days, allowing opportunistic fungal pathogens enough time to develop antifungal resistance [123,124,125].
We frequently live in close quarters with opportunistic pathogenic fungi, many of which are capable of producing large amounts of airborne spores. Consequently, a variety of environmental fungal diseases, known as bioaerosols, regularly expose humans to them. While the majority of ambient fungi do not cause discernible pathophysiological events in healthy individuals, those with weakened immune systems or compromised health are more susceptible to a range of disorders, including invasive fungal diseases (IFDs) that can be fatal, allergic, chronic, or superficial [125]. Patients at risk due to immune-suppression drugs (ISDs) include an increasing number of older adults, those whose immune systems have been weakened by COVID-19 or HIV, those undergoing cancer chemotherapy, those with immune suppression therapy required for transplant recipients, or those with influenza [126,127,128].
Numerous fungal infections are acquired from our surrounding habitats, as demonstrated by numerous molecular epidemiological investigations. This is particularly true for IFDs caused by Coccidioides spp., A. fumigatus, and Cryptococcus spp. [81,129,130,131]. Resistance genotypes may also be passed on by humans suffering from invasive fungal diseases (IFDs), while it is unclear how much human and animal activity contributes to environmental antifungal resistance. The incidence of antifungal resistance is predicted to be influenced by several external variables, such as shifting fungicide usage patterns in waste management and the environment [124].
Furthermore, shifting biotic interactions that may involve xenobiotic chemical analogs of fungicides; shifting endangered human host groups, including infections like COVID-19 or influenza; shifting weathers that may change the geographic area of fungi and adaptive landscape for resistance, along with unique means of infection; and shifting virulence of the fungi themselves due to intrinsic genetic changes or synergies with combinations of the above drivers pose risks regarding the spread of pathogens [132,133].
Another significant risk when utilizing antifungal medications is cross-resistance. A previous study showed that fluconazole was ineffective in treating mice infected by previously benomyl-exposed Cryptococcus gattii cells because benomyl induced the overexpression of efflux pumps in C. gattii [133]. Exposure to fungicides used in agroecosystems has been linked to the establishment of human pathogen isolates resistant to fungicides [43].
There are many helpful microorganisms residing in plants and soil that directly or indirectly support plant growth and provide protection against various infections [2]. However, the use of fungicides on different crops may jeopardize the presence of these microorganisms, especially arbuscular mycorrhizal fungi (AMF) [51]. In a study measuring the effects of azoxystrobin (a systemic broad-spectrum fungicide), flutolanil (a systemic fungicide specific to Basidiomycota), and pencycuron (a contact fungicide specific to Rhizoctonia), along with their corresponding formulations (Amistar, Monarch, and Monceren), on the growth and development of the AMF Rhizophagus irregularis MUCL 41833 (spore germination, root colonization, extraradical mycelium development, and spore production), it was found that at doses used to control R. solani, azoxystrobin and its formulation Amistar inhibited the growth of extraradical mycelium and spore formation at ten times the threshold value [51]. Flutolanil and its derivative Monarch at the suggested dose decreased root colonization and arbuscule development [51].
Antifungal medications enter the environment through various pathways (Figure 1). After excretion in urine or feces, active medicinal components from human preparations may release their metabolites into the environment. Environmental contamination also occurs due to pharmaceutical businesses’ wastewater and the improper disposal of unused or expired medications [134]. Fungicides used as plant protection agents have the potential to contaminate soil, groundwater, and surfaces through runoff from agricultural fields and fruit and vegetable processing facilities. They have become serious contaminants in several environmental compartments, including soil, fruits and vegetables, drinking water, municipal sewage, groundwater, and sewage effluent sludge. These drugs and their byproducts may pose potential hazards to humans and other living things in the environment [134]. Additionally, antifungal metals (such as copper) sprayed on plants may lead to the evolution of aquatic microorganisms and the emergence of metal-tolerant microbes [135].
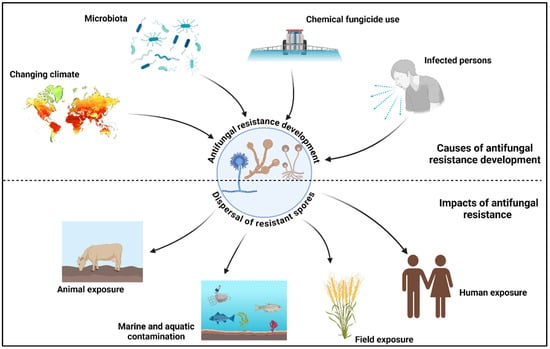
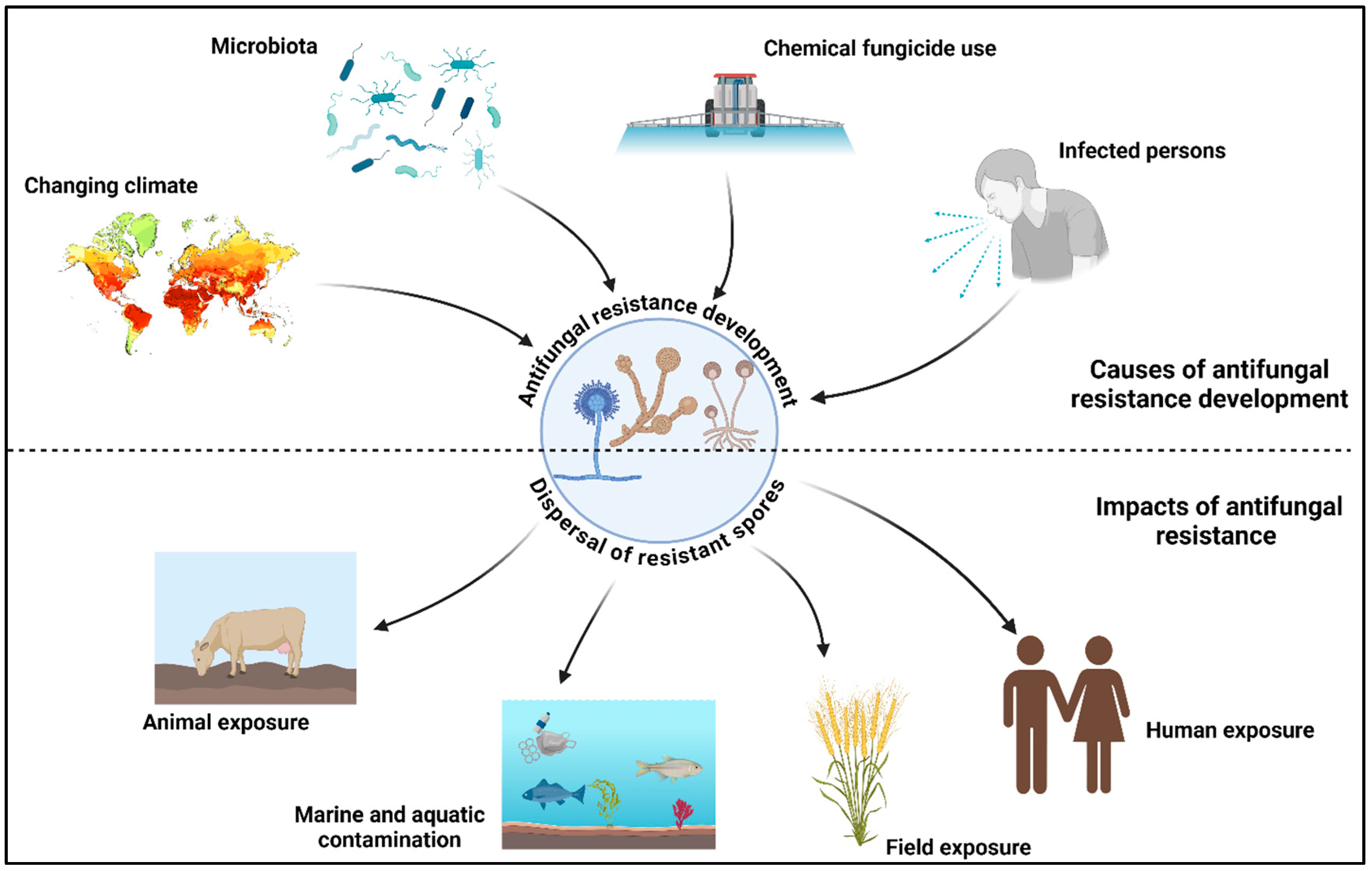
Figure 1.
Antifungal resistance development and dispersal of resistant isolates. This figure was created using BioRender software v. 04.
Antifungal medications have been associated with various negative impacts on marine life, including endocrine disruption, reduced larval growth and development, and reproductive abnormalities [136,137,138]. Azole fungicides, in particular, have been linked to disruptions in hormonal balance and effects on the reproductive and physiological systems of aquatic organisms [136].
5. BCAs and Nanotechnologies in Controlling PPF
5.1. Use of Antagonistic Microorganisms against PPF
Global trends are shifting away from the usage of agrochemicals to treat plant diseases. Presently, extensive research is underway to discover safe, environmentally acceptable, and efficient substitutes for synthetic chemical fungicides. The aim is to combat crop diseases in the field, which cause significant economic losses, and reduce decay loss in harvested commodities. Biological control, also known as biocontrol, emerges as the most suitable non-chemical approach to managing pests and pathogens in organic farming. It is highly specialized, environmentally safe, sustainable, and profitable [2]. Any reduction in the quantity or impact of pathogens (disease-producing activity) achieved by triggering biological mechanisms or the action of naturally occurring or introduced antagonists and modifications to the microenvironment favoring antagonist activity are considered biological control strategies for plant diseases [2,139].
Microbial BCAs (Biological Control Agents), typically derived from fungal or bacterial strains isolated from the phyllosphere, endosphere, or rhizosphere, play a vital role in controlling plant-harming microorganisms [2]. These agents preclude pathogens from infecting or establishing themselves in host plants. It is widely acknowledged that effective control methods primarily target pathogens. Antagonists employ various direct and indirect modes of action to manage biological diseases (Table 4) [2].

Table 4.
Different types of mechanisms of action of beneficial microbes as BCAs.
However, BCAs can encompass bacterial, fungal, or viral agents. Among bacterial BCAs, Bacillus and Pseudomonas are the most commonly used [139,140,141,142,143,144]. Within fungal BCAs, Trichoderma stands out as the most extensively studied genus, showing the greatest potential against PPF, with 25 BCAs identified [1].
5.1.1. Use of Antagonistic Bacteria
The contribution of beneficial bacteria to crop disease resistance and plant growth has been the subject of numerous studies. Many bacteria from different genera have been identified as BCAs to manage a variety of diseases in major crops, including Bacillus, Paenibacillus, Agrobacterium, Bradyrhizobium, Acinetobacter, Azospirillum, Azotobacter, Pseudomonas, Rhizobium, and Streptomyces [2,18,139]. The application of beneficial bacteria can enhance plant development and manage a range of bacterial, fungal, and nematode diseases without endangering the environment. Through the formation of plant–microbial interactions in the rhizosphere, beneficial bacteria produce biofilms and secondary compounds such as fengycin, iturin, bacillomycin, and surfactin, which reduce the population of plant pathogens [2,139]. Lipopeptides like fengycin, iturin, pumilacidin, mixirin, and surfactin exhibit antifungal properties and act against pathogenic fungi in rhizospheres [16,139,144]. It has been observed that biocontrol bacteria, such as Bacillus spp. and Pseudomonas spp., are effective against a variety of phytopathogens in major crops. B. velezensis isolates have been found to contain numerous biosynthetic gene clusters involved in the production of secondary metabolites [139,144]. Furthermore, B. subtilis has been shown to play a significant role in the management of many agricultural diseases [142,145]. A potential BCA for Fusarium wilt of watermelon is B. subtilis IBFCBF-4 [142]. Erysiphe cichoracearum-caused powdery mildew severely reduces tobacco leaf yield and quality, often resulting in significant financial losses upon its appearance. Lipopeptides, particularly bacillomycin D and fengycin, produced by B. amyloliquefaciens YN201732, exhibit excellent biocontrol activity against the fungal disease E. cichoracearum [146]. When accompanied by other Bacillus isolates, seed treatment comprising B. subtilis BY-2 effectively suppresses S. sclerotiorum on oilseed rape [141]. Additionally, various rhizobacteria have shown the potential to control plant pathogens by inducing the production of different volatile organic compounds in plants [147]. Stenotrophomonas maltophilia UPMKH2, a rhizobacterium, effectively suppresses blast disease, enhances yield attributes, and induces systemic resistance against blast disease in rice [147]. Table 5 provides details of different bacterial BCAs found to be effective against various PPF.

Table 5.
Various bacterial BCAs found to be effective against PPF.
5.1.2. Use of Antagonistic Fungi
Fungal antagonists play a crucial role in managing plant diseases and infections [153]. Fungi are cited as effective biocontrol methods, tactics, and approaches employed in plant disease management, especially as modern agricultural practices aim to reduce the use of chemically manufactured pesticides [1,8]. Due to fungi’s short generation time, high rates of sexual and asexual reproduction, and target specificity, the potential for using fungal BCAs against plant infections has significantly increased. Moreover, they can persist in the environment without a host by switching from parasitism to saprotrophism, thus promoting sustainability [1,153]. The genus that has shown the most promise in treating a variety of plant fungal infections is Trichoderma. Other fungal taxa proven to be effective against various PPF include Alternaria, Aspergillus, Candida, Fusarium, Penicillium, Pichia, Pythium, Talaromyces, and Verticillium [1,153]. It has been discovered that Penicillium linzhiense, a novel fungal species, is efficient against Pyricularia oryzae, an economically significant or emerging fungal pathogen [154]. When the biocontrol agent and the fungicide are compatible, using a BCA in conjunction with a synthetic fungicide or physical additive, either concurrently or successively, should enhance disease suppression [153,155]. Table 6 lists the numerous fungal BCAs proven to be successful in combating various PPF.

Table 6.
Various fungal BCAs found to be effective against PPF.
5.1.3. Use of Genetically Modified (GM) Organisms
With the advent of different molecular tools, like genomics, genetic engineering, and recombinant DNA techniques, the biological control of plant diseases using fungal BCAs has significantly advanced in recent years. These precise methods have been employed to enhance fungal strains for agro-industrial operations. The creation of novel plant pathogen-resistant crop varieties or clones presents a generally accepted and perhaps long-term control strategy. Furthermore, extensive research has been conducted to identify the genetic characteristics of fungal antagonists and determine their potential for improving biocontrol performance [153,164,165]. For example, the genome of T. virens was modified to incorporate multiple lytic enzyme-encoding genes. This resulted in a strain that secretes a mixture of chitinases, proteases, and β-1,3- and β-1,6-glucanases, leading to significantly increased inhibition of the pathogens Pythium ultimum, R. solani, and Rhizopus oryzae [166]. Through insertional mutagenesis via restriction enzyme-mediated integration transformation, a reduced-pathogenic mutant of the avocado fruit pathogen Colletotrichum gloeosporioides was developed. This mutant can be used to biologically control anthracnose, which is caused by C. gloeosporioides [167]. Natural interactions between plants and pathogens, or between pathogens and BCAs at the molecular level, help to elucidate the disease progression and the antagonistic mechanisms of BCAs [168]. This understanding further facilitates the development and utilization of genetically modified (GM) BCAs for specific and effective applications (Table 7).

Table 7.
Some fungal genes and their contributions investigated in plant–fungus interaction studies. This table was prepared by a modification from Raman et al. 2020 [168].
Many R genes have been cloned and inserted into different types of plants. Transgenic potatoes expressing the wild potato R gene RB or Rpi-vnt1.1 demonstrate strong field resistance to P. infestans, the causative agent of potato late blight [179,180,181]. Molecular stacking is another genetic manipulation technique wherein researchers assemble multiple R gene cassettes on a single plasmid and then deliver this R gene cluster en bloc at a single genetic locus through plant transformation [182]. In an experiment using agrobacterium-based transformation of a susceptible cultivar, three broad-spectrum potato late blight R genes—Rpi-sto1, Rpi-vnt1.1, and Rpi-blb3—were molecularly stacked. Under greenhouse conditions, the resulting triple gene transformants demonstrated broad-spectrum resistance to the causal agent of potato late blight, P. infestans, equivalent to the cumulative strain-specific resistance imparted by each of the three unique Rpi genes [183]. It is thought that the correct placing and timing of late blight R gene stacks in potatoes could reduce the crop’s fungicide usage by more than 80% [184].
The guided development of a precisely defined window of genomic DNA in vivo has recently become possible through the development of the CRISPR-based mutagenesis platform called EvolvR [185]. EvolvR utilizes the mutagenesis activity of a fusion of the Cas9 nickase and an error-prone DNA polymerase to introduce nucleotide diversification at particular genomic areas defined by guide RNAs [185]. Recently, the CRISPR/Cas9 system has been applied to improve disease resistance in various crops, such as tomato, rice, cocoa, wheat, and grape. Modifying the genomes of fungi and oomycete pathogens can offer novel approaches to managing plant diseases, in addition to crop genome editing [186].
Using the CRISPR/Cas9 system, several methods are available for studying plant disease resistance. These methods include deleting, modifying, or introducing cis-elements in promoters; introducing specific mutations in coding regions via homology-directed repair (HDR); changing the amino acids in plant surface receptor proteins to evade secreted pathogen effectors (e.g., AtBAK1); deleting negative regulators of plant defense responses (e.g., TcNPR3); or modifying central regulators of defense response (e.g., BnWRKY70) [187,188,189,190,191]. The most extensively researched gene for resistance to fungal infections is the susceptibility gene, also known as the mildew resistance locus O (MLO) [186,191]. For instance, the SlMlo1 gene in tomato plants has been knocked out using CRISPR/Cas9. The resulting knock-out mutants provided resistance to the powdery mildew fungus Oidium neolycopersici without further undesirable phenotypic effects [192]. Additionally, one of the three MLO homoalleles, TaMLO-A1, demonstrated enhanced resistance to B. graminis f. sp. tritici infection in wheat plants modified using CRISPR/Cas9 [193]. In grapes, biallelic mutation mutant lines were produced by CRISPR/Cas9-mediated targeted mutagenesis of the transcription factor VvWRKY52, and the knock-out of VvWRKY52 increased resistance to gray mold disease caused by B. cinerea [194].
Utilizing CRISPR/Cas9 to edit the genomes of oomycete and fungal organisms can yield novel approaches to managing plant diseases. An example is the filamentous PPF Claviceps purpurea. The CRISPR/Cas9 technique was used for the targeted mutagenesis of genes (pyr4 and TrpE) involved in tryptophan biosynthesis and pyrimidine biosynthesis in C. purpurea. This led to a decrease in auxin, a plant hormone normally generated by C. purpurea, preventing TrpE mutants from infecting rye plants and hindering the fungus’s capacity to colonize rye plants [195]. Another instance involves the use of the CRISPR/Cas9 system to knock out the pectin acetylesterases (PlPAE4 and PlPAE5) from Peronophythora litchi, the causative agent of downy blossom blight. Knock-out mutants of PLPAE5 were less able to invade hosts compared to the wild-type strain, indicating PlPAE5’s involvement in oomycete pathogenicity [196]. Homozygous PpalEPIC8 mutants were also created in the papaya pathogen P. palmivorausing using CRISPR/Cas9 technology. These mutants showed decreased pathogenicity on papaya fruits because the cysteine protease inhibitor PpalEPC8 was knocked out, leading to less inhibition of papain, a cysteine protease that provides protection against plant pathogens [196].
5.1.4. Use of Viral Vectors/Phages
S. sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1) infects S. sclerotiorum DT-8, its host, resulting in hypovirulence, a decreased growth rate, and other morphological alterations to the colony. Still unknown, though, are the mechanisms underlying this [197]. One of the best examples of multitrophic interactions is mycovirus-mediated hypovirulence, which has gained interest because of its potential for biocontrol. By preventing the fungal manufacture of the phytotoxin Altersolanol A, a mycovirus known as Stemphylium lycopersici alternavirus 1 (SlAV1) from the plant disease Stemphylium lycopersici causes hypovirulence and pigmentation loss. Enhancing plant resistance to virulent strains, the pathogen becomes a biocontrol agent by genomic integration and the expression of a critical SlAV1 gene in the fungal host. This creates a viable and low-risk avenue for biocontrol to contain plant diseases [198]. Similarly, a replication initiation protein (Rep) and a coat protein (CP) are encoded by the S. sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1), which was identified from the PPF S. sclerotiorum [199]. The potential to investigate fungal viruses as useful tools for the molecular manipulation of fungi and the control of plant diseases is increased by many PPF, such as those that can transport mycovirus from one plant to another [200,201]. To counteract PPF, phages have been employed as BCAs. Peptidoglycan hydrolases and lysins from the phages Atu_ph02 and Atu_ph03 can lyse Agrobacterium tumefaciens (which causes crown gall disease) by preventing cell division [202].
5.2. Use of AMPs
As a part of their defense mechanism, bacteria, fungi, and plants all produce various AMPs that are effective against various kinds of PPF (Figure 2). Beneficial bacteria generate a wide variety of natural chemicals to effectively treat different plant diseases. It has been discovered that certain Bacillus species produce mycosubtilin, subtilisin, sublancin, bacilysin, chlorotetain, mycobacillin, rhizocticins, bacillaene, difficidin, iturin A, bacillomycin, fengycin, bacilysin, and mersacidin, which are helpful in managing different PPF [139,142,144,203]. Numerous natural products, including extracellular enzymes like cellulase, chitinase, proteases, and beta-glucanase, as well as other antimicrobial substances like cyanide, phenazines, siderophores, and 2,4-diacetyl phloroglucinol, which inhibit numerous PPF, have also been reported to be produced by Pseudomonas species [151,152,153]. The majority of fungal plant diseases are managed by members of the fengycin and iturin families. These lipopeptides have been reported to cause harm to the hyphae and conidia of a variety of fungal pathogens, such as F. graminearum and M. fructicola [151,152,204]. Furthermore, several volatile organic compounds (VOCs) produced by bacteria, such as alcohol, aldehydes, ketones, hydrocarbons, acids, and terpenes, are responsible for regulating numerous PPF [16,18]. Numerous investigations have been conducted on the antibacterial qualities of volatile organic compounds (VOCs) against S. sclerotiorum, M. fructicola, M. fructigena, B. cinerea, and so on [16,18,152].
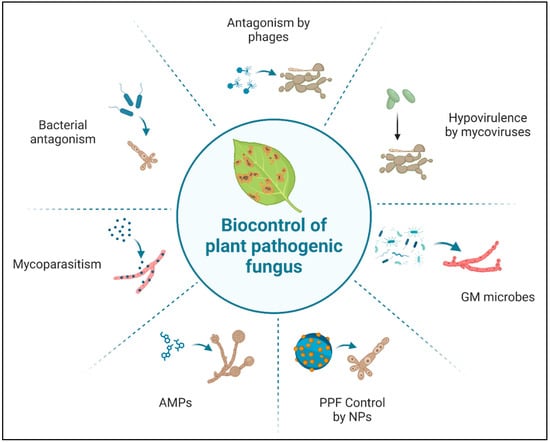
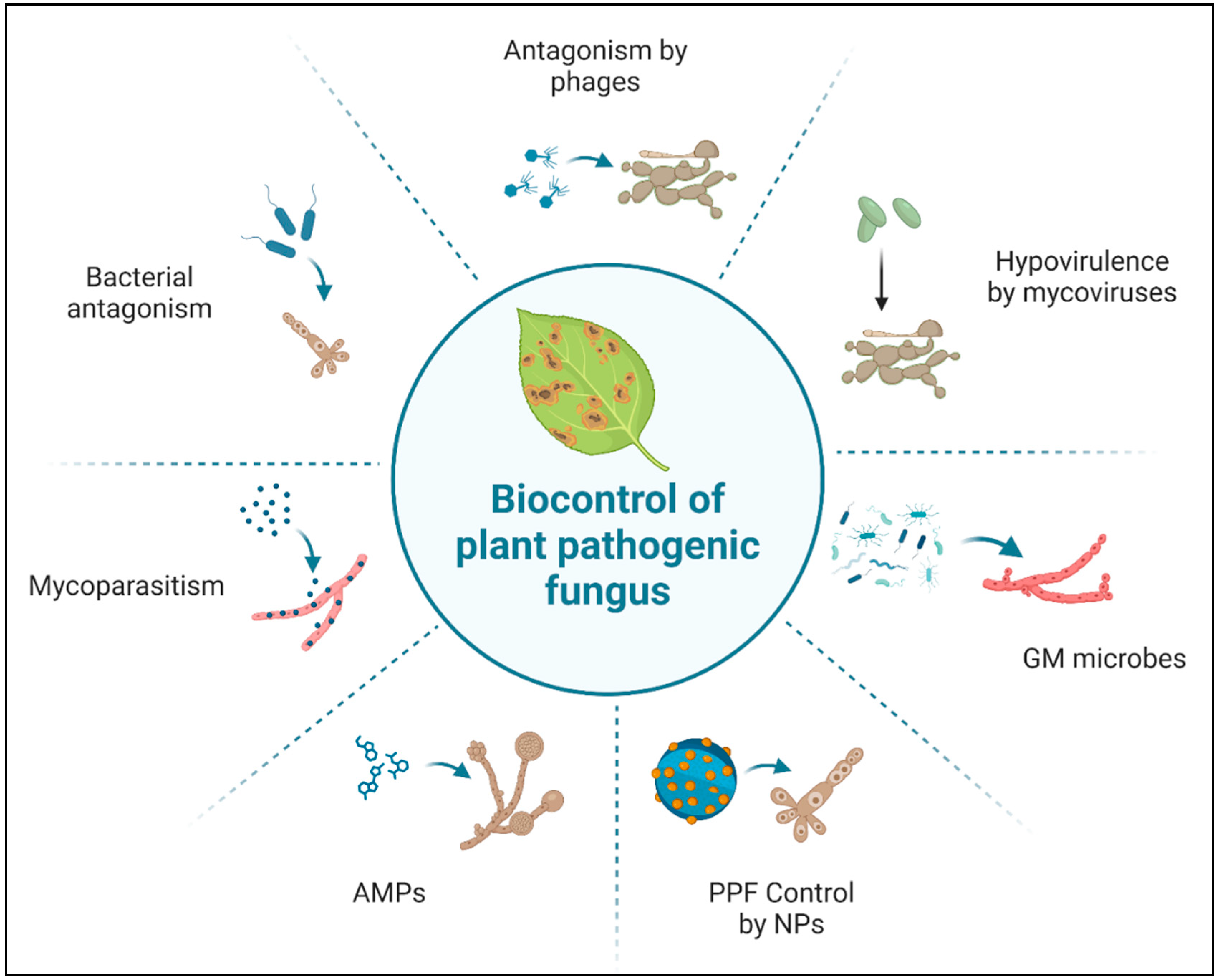
Figure 2.
Mode of action of different biopesticides. AMPs, antimicrobial peptides; NPs, nanoparticles; PPF, plant pathogenic fungus; GM, genetically modified. This figure was created using BioRender software.
A variety of natural chemicals are produced by fungi, such as polyketides, aromatic compounds, non-ribosomal peptides, antibiotics, and heterocyclic metabolites. Trichoderma species generate several volatile organic compounds (VOCs) and AMPs, which are utilized as BCAs to manage various agricultural diseases [157,160,163,165]. Trichoderma spp. and Gliocladium virens are known to produce numerous antifungal chemicals, including gliovirin, viridiol, valinotrocin, viridin, gliotoxin, and heptelidic acid [205]. Studies have reported that T. harzianum strains T22 and TC39 produce antibiotics such as azaphilone, harzianopyridone, harzianolide, and 1-hydroxy-3-methylanthraquinone. These natural compounds inhibit the growth of plant pathogens such as B. cinerea, R. solani, Leptosphaeria maculans, P. ultimum, and P. cinnamomin [206]. Chaetomium globosum, in its culture filtrate, produces chaetoglobosin, an antibiotic used to control late blight disease in potatoes and reduce post-harvest illnesses in various fruits [207].
Various defense mechanisms, such as soluble proteins and volatile organic compounds (VOCs) released by plants possessing antifungal and antibacterial properties, have evolved in their innate immune system to protect against phytopathogens [208,209,210,211]. These proteins have broad functions beyond their antifungal activity; they can limit fungal growth and infection through various methods [208]. Pathogenesis-related (PR) proteins, AMPs, and polygalacturonase-inhibiting (PI) proteins are among the antifungal proteins [208]. Plant proteins known as PR proteins play a dual role in biotic and abiotic stress mitigation. They frequently induce systemic acquired resistance in plants. Chitinase is one of the most well-known and researched plant antifungal proteins [212]. By lysing hyphal tips in fungi and dissolving chitin into its oligomers, it prevents fungal growth [212]. R. solani and B. cinerea are just two examples of the many phytopathogenic fungi against which chitinases exhibit potent antifungal activity [22,213]. Additionally, chitinase derived from microorganisms exhibits strong antifungal properties. Chitinase from Trichosanthes dioica was able to efficiently control Trichoderma spp. and A. niger. The majority of vegetative and reproductive plant tissues express plant defensins, another extensively researched PR protein, constitutively in the extracellular space [214]. These plant defense compounds are effective against several PPFs. For example, Phaseolus vulgaris beans’ defensin-like protein NRBAP maintained its antifungal action against Mycosphaerella arachidicola at temperatures as high as 100 °C and in the pH range of 1–13 [215]. Another class of PR proteins active against various PPFs is called plant protease inhibitors (PIs). In vitro, Rhizoctania solani and Candida ablicans are inhibited by potato peptide-G [216]. Similarly, the growth of fungi such as B. cinerea, P. infestans, R. solani, F. solani, and F. oxysporum can be inhibited by potato protease inhibitors I and II (PPI–I and PPI–II) [217,218]. A different study found that broad beans contain a Bowman–Birk-type trypsin–chymotrypsin inhibitor that can stop the growth of B. cinerea, F. oxysporum, and M. arachidicola with as little as 60 μg per plate [219]. Another protein of interest are AMPs. AMPs are involved in the innate immune system of plants. Different antifungal peptides (AFPs) are produced by plants [208]. Among them is tomato systemin. Research indicates that systemin travels through the phloem of plants, assisting in signaling amplification and enabling distal leaves to respond to wounds [220]. Furthermore, the late blight-causing pathogen P. infestans induces at least 50% fewer lesions in transgenic plants expressing prosystemin [221]. B. cinerea infection was successfully managed by spraying 100 pM of systemin onto grapevine and eggplant plants [222]. It has been demonstrated that the potato antimicrobial peptide Snakin-2 is effective in vitro against B. cinerea and several Fusarium species [223].
5.3. Use of Nano-Based Technologies
In addition to being essential micronutrients for plants, NPs can be utilized to directly reduce pathogen infections, thereby enhancing crop growth and yield, and potentially leading to further growth enhancement through nutritional benefits (Figure 2) [224,225]. Through their antifungal activities, NPs such as Ag, ZnO, Mg, Si, and TiO2 can directly inhibit crop diseases [224,225,226,227]. Late blight in potatoes caused by P. infestans was greatly suppressed by Ag2O and Ag2O/TiO2 nanostructures [224]. Potato plants treated with Ag2O and Ag2O/TiO2 NPs showed improved expression of defense genes and decreased disease severity in both greenhouse and field conditions [224]. One extensively researched NP known for its antifungal efficacy against various PPF is silver NP, sourced from various origins [225,227]. The synthesis of AgNPs (30 μg) induced by peanut shell extract demonstrated a 5 to 6 mm zone of inhibition against P. infestans and P. capsici, two agricultural fungal diseases [227]. AgNPs derived from rice leaf extract exhibited fungicidal action against R. solani, the rice pathogen responsible for sheath blight [228]. Treatment with AgNPs of 16.5 nm (20 μg/mL) improved the seedling vigor index in rice cultivars by 10 μg/mL and completely prevented the spread of illness caused by R. solani isolates [228]. According to a separate study, Ag-NPs, in conjunction with thiophanate methyl and fluazinam, were effective against isolates of M. fructicola, whether susceptible or resistant to benzimidazole. The mode of action of Ag-NPs is likely related to ATP metabolism rather than silver ion release [229]. Furthermore, the accumulation of Fusarium mycotoxin was significantly reduced by AgNPs (30 nm) [230]. Thus, AgNPs can be used to manage and treat various fungal infections affecting crops. ZnO-NPs could effectively and dose-dependently inhibit the proliferation of mycelial cells in isolates of Alternaria alternative that were resistant (BOSC-R) and sensitive (BOSC-S) to boscalid (BOSC) [226]. The fungitoxic activity of ZnO-NPs is attributed to either ROS generation or Zn+2 release. The enhanced toxic action of ZnO-NPs is likely related to ion homeostasis [226]. 2-Substituted-sulfanyl-5-amino/methyl-1,3,4-thiadiazoles were synthesized and evaluated against two phytopathogenic fungi, specifically, R. bataticola and R. solani. Nano-thiadiazole derivatives exhibited 2–4 times greater fungicidal efficacy against the test PPF, according to the study [231]. Rice pathogen P. grisea growth was inhibited by chitosan guar NPs (CGNPs) [232]. These studies collectively demonstrate NPs as promising alternatives to chemical fungicides for sustainable agriculture systems.
6. Conclusions and Future Perspectives
It is imperative to closely monitor the antibiotic resistance (AR) status of PPF. However, there is a lack of information on this subject, necessitating routine surveillance. Regulatory bodies should implement routine AR surveillance programs to provide direction, control antibiotic use, and monitor antibiotic resistance in the field. Additionally, the excessive use of fungicides can lead to the development of antifungal resistance in non-target fungal groups. Horizontal gene transfer (HGT) can contribute to resistance development against antifungal chemicals, allowing non-target fungal pathogens to spread resistance to target groups with prolonged or repeated exposure to antifungal chemicals. Therefore, monitoring antifungal resistance development among non-pathogenic and facultative pathogenic fungal populations is crucial. BCAs are rapidly emerging as excellent alternatives to chemical fungicides. However, certain obstacles hinder their widespread use. Ensuring that the selected biocontrol agent is specific to the target pathogen and does not harm beneficial organisms is a primary concern. Additionally, consideration must be given to the nutritional requirements of BCAs for their survival, as inadequate provision may compromise their biocontrol activity [233]. The stability and growth rate of BCAs under field conditions are also critical factors, as pathogens may outgrow BCAs, reducing their effectiveness. Thus, extensive research is needed to ascertain the specificity and efficacy of BCAs before field trials. Although there are limitations to using living microbes such as BCAs, antimicrobial peptides (AMPs) and nanoparticles (NPs) offer several advantages. They are less prone to resistance, highly specific, fast-acting, and do not require external support such as food or specific growth conditions. Combining the application of AMPs with NPs may yield revolutionary results in PPF biocontrol.
Author Contributions
Conceptualization, T.I.; Methodology, D., N.T.T. and M.N.M.; Software, T.I., D. and M.A.H.; Validation, N.T.T., M.N.M., H.R.B. and M.A.H.; Formal analysis, T.I. and D.; Investigation, N.T.T., M.N.M., H.R.B. and M.A.H.; Resources, T.I. and M.A.H.; Data curation, T.I.; Writing—original draft preparation, T.I., D. and M.A.H.; Writing—review and editing, T.I., D., N.T.T., M.N.M., H.R.B. and M.A.H.; Visualization, T.I.; Supervision, M.A.H.; Project administration, H.R.B. and M.A.H.; Funding acquisition, H.R.B. and M.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Islam, T.; Haque, M.A.; Barai, H.R.; Istiaq, A.; Kim, J.-J. Antibiotic Resistance in Plant Pathogenic Bacteria: Recent Data and Environmental Impact of Unchecked Use and the Potential of Biocontrol Agents as an Eco-Friendly Alternative. Plants 2024, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.T.; Gifford, H.; Rhodes, J. Emerging Antifungal Resistance in Fungal Pathogens. Curr. Clin. Microbiol. Rep. 2024, 11, 43–50. [Google Scholar] [CrossRef]
- Boyce, K.J. The Microevolution of Antifungal Drug Resistance in Pathogenic Fungi. Microorganisms 2023, 11, 2757. [Google Scholar] [CrossRef]
- Lee, Y.; Robbins, N.; Cowen, L.E. Molecular Mechanisms Governing Antifungal Drug Resistance. NPJ Antimicrob. Resist. 2023, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.L.; Thangaraj, K.; Deng, C.; Deng, W.W.; Zhang, Z.Z. Phyllosticta capitalensis Causes Leaf Spot on Tea Plant (Camellia sinensis) in China. Plant Dis. 2019, 103, 2964. [Google Scholar] [CrossRef]
- Chen, F.; Ma, R.; Chen, X.L. Advances of Metabolomics in Fungal Pathogen–Plant Interactions. Metabolites 2019, 9, 169. [Google Scholar] [CrossRef]
- Al-Sadi, A.M. Bipolaris Sorokiniana-Induced Black Point, Common Root Rot, and Spot Blotch Diseases of Wheat: A Review. Front. Cell. Infect. Microbiol. 2021, 11, 584899. [Google Scholar] [CrossRef] [PubMed]
- Tibpromma, S.; Dong, Y.; Ranjitkar, S.; Schaefer, D.A.; Karunarathna, S.C.; Hyde, K.D.; Jayawardena, R.S.; Manawasinghe, I.S.; Bebber, D.P.; Promputtha, I.; et al. Climate-Fungal Pathogen Modeling Predicts Loss of Up to One-Third of Tea Growing Areas. Front. Cell. Infect. Microbiol. 2021, 11, 610567. [Google Scholar] [CrossRef]
- Karunarathna, S.C.; Maharachchikumbura, S.S.N.; Ariyawansa, H.A.; Shenoy, B.D.; Jeewon, R. Editorial: Emerging Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2021, 11, 765549. [Google Scholar] [CrossRef]
- McLean, M.A.; Angilletta, M.J.; Williams, K.S. If You Can’t Stand the Heat, Stay out of the City: Thermal Reaction Norms of Chitinolytic Fungi in an Urban Heat Island. J. Therm. Biol. 2005, 30, 384–391. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Climatic Changes and Their Role in Emergence and Re-Emergence of Diseases. Environ. Sci. Pollut. Res. 2020, 27, 22336–22352. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 284486. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus Atrophaeus Gbsc56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne Incognita. Int. J. Mol. Sci. 2021, 22, 5049. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir, H.A.S.; Sheikh, T.M.M.; Ayaz, M.; Gao, X. Suppression of Sclerotinia sclerotiorum by the Induction of Systemic Resistance and Regulation of Antioxidant Pathways in Tomato Using Fengycin Produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 9, 613. [Google Scholar] [CrossRef]
- Gao, H.; Qi, G.; Yin, R.; Zhang, H.; Li, C.; Zhao, X. Bacillus cereus Strain S2 Shows High Nematicidal Activity against Meloidogyne Incognita by Producing Sphingosine. Sci. Rep. 2016, 6, 28756. [Google Scholar] [CrossRef]
- Massawe, V.C.; Hanif, A.; Farzand, A.; Mburu, D.K.; Ochola, S.O.; Wu, L.; Tahir, H.A.S.; Gu, Q.; Wu, H.; Gao, X. Volatile Compounds of Endophytic bacillus Spp. Have Biocontrol Activity against Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1373–1385. [Google Scholar] [CrossRef]
- Zubair, M.; Farzand, A.; Mumtaz, F.; Khan, A.R.; Sheikh, T.M.M.; Haider, M.S.; Yu, C.; Wang, Y.; Ayaz, M.; Gu, Q.; et al. Novel Genetic Dysregulations and Oxidative Damage in Fusarium graminearum Induced by Plant Defense Eliciting Psychrophilic Bacillus atrophaeus TS1. Int. J. Mol. Sci. 2021, 22, 12094. [Google Scholar] [CrossRef] [PubMed]
- Schoina, C.; Stringlis, I.A.; Pantelides, I.S.; Tjamos, S.E.; Paplomatas, E.J. Evaluation of Application Methods and Biocontrol Efficacy of Paenibacillus Alvei Strain K-165, against the Cotton Black Root Rot Pathogen Thielaviopsis Basicola. Biol. Control 2011, 58, 68–73. [Google Scholar] [CrossRef]
- Zaker, M. Natural Plant Products as Eco-Friendly Fungicides for Plant Diseases Control—A Review. Agriculturists 2016, 14, 134–141. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Gunasinghe, N.; Barbetti, M.J.; You, M.P.; Burrell, D.; Neate, S. White Leaf Spot Caused by Neopseudocercosporella capsellae: A Re-Emerging Disease of Brassicaceae. Front. Cell. Infect. Microbiol. 2020, 10, 588090. [Google Scholar] [CrossRef] [PubMed]
- Gunasinghe, N.; Barbetti, M.J.; You, M.P.; Dehigaspitiya, P.; Neate, S. Dimorphism in Neopseudocercosporella capsellae, an Emerging Pathogen Causing White Leaf Spot Disease of Brassicas. Front. Cell. Infect. Microbiol. 2021, 11, 678231. [Google Scholar] [CrossRef]
- Gao, H.; Pan, M.; Tian, C.; Fan, X. Cytospora and Diaporthe Species Associated with Hazelnut Canker and Dieback in Beijing, China. Front. Cell. Infect. Microbiol. 2021, 11, 664366. [Google Scholar] [CrossRef]
- Karunarathna, A.; Tibpromma, S.; Jayawardena, R.S.; Nanayakkara, C.; Asad, S.; Xu, J.; Hyde, K.D.; Karunarathna, S.C.; Stephenson, S.L.; Lumyong, S.; et al. Fungal Pathogens in Grasslands. Front. Cell. Infect. Microbiol. 2021, 11, 695087. [Google Scholar] [CrossRef]
- Wilson, S. Grasses and Grassland Ecology. Ann. Bot. 2009, 104, ix. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging Fungal Threats to Animal, Plant and Ecosystem Health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Saha, O.; Rahman Sid, A.; Saha, A.; Hussain, M.S.; Islam, T. Molecular Detection of Multidrug Resistance Pathogenic Bacteria from Protective Materials Used By Healthcare Workers (HCW); Bangladesh Scenario. J. Appl. Sci. 2018, 18, 48–55. [Google Scholar] [CrossRef]
- Sagor, M.S.; Hossain, M.S.; Islam, T.; Mahmud, M.A.; Miah, M.S.; Karim, M.R.; Giasuddin, M.; Samad, M.A. Phenotypic and Genotypic Antibiotic Resistance and Virulence Profiling of Enterococcus Faecalis Isolated from Poultry at Two Major Districts in Bangladesh. Pak. Vet. J. 2022, 42, 153–160. [Google Scholar] [CrossRef]
- Islam, T.; Kubra, K.; Chowdhury, M.M.H. Prevalence of Methicillin-Resistant Staphylococcus Aureus in Hospitals in Chittagong, Bangladesh: A Threat of Nosocomial Infection. J. Microsc. Ultrastruct. 2018, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Saha, O.; Sultana, S.; Hridoy, M.; Hasan, M.; Marzan, S.; Musfiqur Rahman, M. Comparison between Reduced Susceptibility to Disinfectants and Multidrug Resistance among Hospital Isolates of Pseudomonas aeruginosa and Staphylococcus aureus in Bangladesh. Bagcilar Med. Bull. 2017, 2, 88–97. [Google Scholar] [CrossRef]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to Global Food Security from Emerging Fungal and Oomycete Crop Pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.N.; Eyles, C.J.; Kay, W.; Cowper, J.; Gurr, S.J. A Role for Random, Humidity-Dependent Epiphytic Growth Prior to Invasion of Wheat by Zymoseptoria tritici. Fungal Genet. Biol. 2017, 106, 51–60. [Google Scholar] [CrossRef]
- Suffert, F.; Ravigné, V.; Sachec, I. Seasonal Changes Drive Short-Term Selection for Fitness Traits in the Wheat Pathogen Zymoseptoria tritici. Appl. Environ. Microbiol. 2015, 81, 6367. [Google Scholar] [CrossRef] [PubMed]
- Renato Echeveste da Rosa, C. Asian Soybean Rust Resistance: An Overview. J. Plant Pathol. Microbiol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Li, X.; Esker, P.D.; Pan, Z.; Dias, A.P.; Xue, L.; Yang, X.B. The Uniqueness of the Soybean Rust Pathosystem: An Improved Understanding of the Risk in Different Regions of the World. Plant Dis. 2010, 94, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.N.; Fisher, M.C.; Gurr, S.J. Emerging Fungal Threats to Plants and Animals Challenge Agriculture and Ecosystem Resilience. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Brasier, C.M.; Kirk, S.A. Rapid Emergence of Hybrids between the Two Subspecies of Ophiostoma Novo-Ulmi with a High Level of Pathogenic Fitness. Plant Pathol. 2010, 59, 186–199. [Google Scholar] [CrossRef]
- Mottaleb, K.A.; Singh, P.K.; Sonder, K.; Kruseman, G.; Tiwari, T.P.; Barma, N.C.D.; Malaker, P.K.; Braun, H.J.; Erenstein, O. Threat of Wheat Blast to South Asia’s Food Security: An Ex-Ante Analysis. PLoS ONE 2018, 13, e0197555. [Google Scholar] [CrossRef] [PubMed]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Ribas e Ribas, A.D.; Spolti, P.; Del Ponte, E.M.; Donato, K.Z.; Schrekker, H.; Fuentefria, A.M. Is the Emergence of Fungal Resistance to Medical Triazoles Related to Their Use in the Agroecosystems? A Mini Review. Braz. J. Microbiol. 2016, 47, 793–799. [Google Scholar] [CrossRef]
- Mazu, T.K.; Bricker, B.A.; Flores-Rozas, H.; Ablordeppey, S.Y. The Mechanistic Targets of Antifungal Agents: An Overview. Mini-Rev. Med. Chem. 2016, 16, 555–578. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Pasquali, M.; Pallez-Barthel, M.; Beyer, M. Searching Molecular Determinants of Sensitivity Differences towards Four Demethylase Inhibitors in Fusarium Graminearum Field Strains. Pestic. Biochem. Physiol. 2020, 164, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Yadav, A.; Wadhwa, K.; Jain, K.; Ghorai, S.M. Azoles Used in Agriculture as Possible Cause of Azole-Resistance in Clinical Candida Isolates. Biosci. Biotechnol. Res. Asia 2021, 17, 789–799. [Google Scholar] [CrossRef]
- Cappelletty, D.; Eiselstein-McKitrick, K. The Echinocandins. Pharmacotherapy 2007, 27, 369–388. [Google Scholar] [CrossRef]
- Klittich, C.J. Milestones in Fungicide Discovery: Chemistry That Changed Agriculture. Plant Health Prog. 2018, 9, 31. [Google Scholar] [CrossRef]
- Bartholomäus, A.; Mittler, S.; Märländer, B.; Varrelmann, M. Control of Rhizoctonia Solani in Sugar Beet and Effect of Fungicide Application and Plant Cultivar on Inoculum Potential in the Soil. Plant Dis. 2017, 101, 941–947. [Google Scholar] [CrossRef]
- Buysens, C.; Dupré de Boulois, H.; Declerck, S. Do Fungicides Used to Control Rhizoctonia Solani Impact the Non-Target Arbuscular Mycorrhizal Fungus Rhizophagus Irregularis? Mycorrhiza 2015, 25, 277–288. [Google Scholar] [CrossRef] [PubMed]
- IDM for Important Diseases of Maize—ICAR-Indian Institute of Maize Research. Available online: https://iimr.icar.gov.in/?page_id=2134 (accessed on 25 May 2024).
- Ayed, F.; Daami-Remadi, M.; Jabnoun-Khiareddine, H.; Hibar, K.; El Mahjoub, M. Evaluation of Fungicides for Control of Fusarium Wilt of Potato. Plant Pathol. J. 2006, 5, 239–243. [Google Scholar] [CrossRef]
- Ben Naim, Y.; Cohen, Y. Replacing Mancozeb with Alternative Fungicides for the Control of Late Blight in Potato. J. Fungi 2023, 9, 1046. [Google Scholar] [CrossRef]
- Pethybridge, S.J.; Sharma, S.; Hansen, Z.R.; Vaghefi, N.; Hanson, L.E.; Kikkert, J.R. Improving Fungicide-Based Management of Cercospora Leaf Spot in Table Beet in New York, USA. Can. J. Plant Pathol. 2020, 42, 353–366. [Google Scholar] [CrossRef]
- Amini, J.; Fevzi Sidovich, D. The Effects of Fungicides on Fusarium oxysporum f. sp. lycopersici Associated with Fusarium Wilt of Tomato. J. Plant Prot. Res. 2010, 50, 172–178. [Google Scholar]
- Ahmad, S.; Yousaf, M.; Anjum, R.; Raza, W.; Ali, Y.; Rehman, M.A. Evaluation of Fungicides against Fusarium oxysporum f.Sp. lycopersici the Cause of Fusarium Wilt of Tomato. J. Plant Environ. 2021, 3, 125–135. [Google Scholar] [CrossRef]
- Veloukas, T.; Bardas, G.A.; Karaoglanidis, G.S.; Tzavella-Klonari, K. Management of Tomato Leaf Mould Caused by Cladosporium Fulvum with Trifloxystrobin. Crop Prot. 2007, 26, 845–851. [Google Scholar] [CrossRef]
- Ishaq, H.; Khan, M.A.; Ahmed, I.; Shah, S.M.A.; Ali, U.; Jatoi, G.H.; Sultan, A.; Akhtar, A.; Usmani, M.M.; Iftikhar, S. Antifungal Exploitation of Fungicides and Plant Extracts against Fusarium oxysporum f.sp. melongenae causing fusarium wilt of eggplant. Pakistan J. Biotechnol. 2023, 20, 59–67. [Google Scholar] [CrossRef]
- Acosta-Gonz Alez, U.; Silva-Rojas, H.V.; Fuentes-Arag, D.; Us Hern Andez-Castrej, J.; Romero-Bautista, A.; Rebollar-Alviter, A. Comparative Performance of Fungicides and Biocontrol Products in the Management of Fusarium Wilt of Blackberry. Plant Dis. 2022, 106, 1419–1427. [Google Scholar] [CrossRef]
- Steentjes, M.B.F.; Scholten, O.E.; van Kan, J.A.L. Peeling the Onion: Towards a Better Understanding of Botrytis Diseases of Onion. Phytopathology 2021, 111, 464–473. [Google Scholar] [CrossRef]
- Sanglard, D.; Coste, A.; Ferrari, S. Antifungal Drug Resistance Mechanisms in Fungal Pathogens from the Perspective of Transcriptional Gene Regulation. FEMS Yeast Res. 2009, 9, 1029–1050. [Google Scholar] [CrossRef] [PubMed]
- Seblani, R.; Keinath, A.P.; Munkvold, G. Gummy Stem Blight: One Disease, Three Pathogens. Mol. Plant Pathol. 2023, 24, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Benigni, M.; Bompeix, G. Chemical and Biological Control of Sclerotinia sclerotiorum in Witloof Chicory Culture. Pest Manag. Sci. 2010, 66, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.P.; Song, Y.F.; Li, B.X.; Mu, W.; Liu, F. Baseline Sensitivity of Isopyrazam against Sclerotinia Sclerotiorum and Its Efficacy for the Control of Sclerotinia Stem Rot in Vegetables. Crop Prot. 2019, 122, 42–48. [Google Scholar] [CrossRef]
- Rajput, M.A.; Rajput, N.A.; Syed, R.N.; Lodhi, A.M.; Que, Y. Sugarcane Smut: Current Knowledge and the Way Forward for Management. J. Fungi 2021, 7, 1095. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Matzen, N.; Hansen, J.G.; Semaskiene, R.; Korbas, M.; Danielewicz, J.; Glazek, M.; Maumene, C.; Rodemann, B.; Weigand, S.; et al. Four Azoles’ Profile in the Control of Septoria, Yellow Rust and Brown Rust in Wheat across Europe. Crop Prot. 2018, 105, 16–27. [Google Scholar] [CrossRef]
- Ramanauskienė, J.; Gaurilčikienė, I.; Supronienė, S. Effects of Fungicides on the Occurrence of Winter Wheat Eyespot Caused by Fungi Oculimacula acuformis and O. yallundae. Crop Prot. 2016, 90, 90–95. [Google Scholar] [CrossRef]
- Xu, S.; Wang, B.; Li, L.; Zhou, Q.; Tian, M.; Zhao, X.; Peng, J.; Liu, F.; Chen, Y.; Xu, Y.; et al. Effects of Camptothecin on the Rice Blast Fungus Magnaporthe Oryzae. Pestic. Biochem. Physiol. 2020, 163, 108–116. [Google Scholar] [CrossRef]
- Juliatti, F.C.; Azevedo, L.A.; Juliatti, F.C. Strategies of Chemical Protection for Controlling Soybean Rust. In Soybean: The Basis of Yield, Biomass and Productivity; Books on Demand: Norderstedt, Germany, 2017. [Google Scholar] [CrossRef]
- Baginski, M.; Czub, J. Amphotericin B and Its New Derivatives—Mode of Action. Curr. Drug Metab. 2009, 10, 459–469. [Google Scholar] [CrossRef]
- Chapeland, F.; Fritz, R.; Lanen, C.; Gredt, M.; Leroux, P. Inheritance and Mechanisms of Resistance to Anilinopyrimidine Fungicides in Botrytis Cinerea (Botryotinia fuckeliana). Pestic. Biochem. Physiol. 1999, 64, 85–100. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Progress in Understanding Molecular Mechanisms and Evolution of Resistance to Succinate Dehydrogenase Inhibiting (SDHI) Fungicides in Phytopathogenic Fungi. Crop. Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Davidse, L.C. Benzimidazole Fungicides: Mechanism of Action and Biological Impact. Annu. Rev. Phytopathol. 1986, 24, 43–65. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Tores, J.A.; Pérez-García, A. Mechanisms of Resistance to QoI Fungicides in Phytopathogenic Fungi. Int. Microbiol. 2008, 11, 1–9. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Ma, Z.; Michailides, T.J. Advances in Understanding Molecular Mechanisms of Fungicide Resistance and Molecular Detection of Resistant Genotypes in Phytopathogenic Fungi. Crop Prot. 2005, 24, 853–863. [Google Scholar] [CrossRef]
- Leroux, P.; Chapeland, F.; Desbrosses, D.; Gredt, M. Patterns of Cross-Resistance to Fungicides in Botryotinia Fuckeliana (Botrytis cinerea) Isolates from French Vineyards. Crop Prot. 1999, 18, 687–697. [Google Scholar] [CrossRef]
- Schoustra, S.E.; Debets, A.J.M.; Rijs, A.J.M.M.; Zhang, J.; Snelders, E.; Leendertse, P.C.; Melchers, W.J.G.; Rietveld, A.G.; Zwaan, B.J.; Verweij, P.E. Environmental Hotspots for Azole Resistance Selection of Aspergillus Fumigatus, the Netherlands. Emerg. Infect. Dis. 2019, 25, 1347–1353. [Google Scholar] [CrossRef]
- Hurst, S.F.; Berkow, E.L.; Stevenson, K.L.; Litvintseva, A.P.; Lockhart, S.R. Isolation of Azole-Resistant Aspergillus Fumigatus from the Environment in the South-Eastern USA. J. Antimicrob. Chemother. 2017, 72, 2443–2446. [Google Scholar] [CrossRef]
- Sewell, T.R.; Zhu, J.; Rhodes, J.; Hagen, F.; Meis, J.F.; Fisher, M.C.; Jombart, T. Nonrandom Distribution of Azole Resistance across the Global Population of Aspergillus fumigatus. mBio 2019, 10, e00392-19. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.; Lavergne, R.A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Azole-Resistant Aspergillus fumigatus Harboring TR34/L98H, TR46/Y121F/T289A and TR53 Mutations Related to Flower Fields in Colombia. Sci. Rep. 2017, 7, 45631. [Google Scholar] [CrossRef]
- Etienne, K.A.; Berkow, E.L.; Gade, L.; Nunnally, N.; Lockhart, S.R.; Beer, K.; King Jordan, I.; Rishishwar, L.; Litvintseva, A.P. Genomic Diversity of Azole-Resistant Aspergillus Fumigatus in the United States. mBio 2021, 12, e0180321. [Google Scholar] [CrossRef] [PubMed]
- Willger, S.D.; Puttikamonkul, S.; Kim, K.H.; Burritt, J.B.; Grahl, N.; Metzler, L.J.; Barbuch, R.; Bard, M.; Lawrence, C.B.; Cramer, R.A. A Sterol-Regulatory Element Binding Protein Is Required for Cell Polarity, Hypoxia Adaptation, Azole Drug Resistance, and Virulence in Aspergillus fumigatus. PLoS Pathog. 2008, 4. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Bien, C.M.; Lee, H.; Espenshade, P.J.; Kwon-Chung, K.J. Sre1p, a Regulator of Oxygen Sensing and Sterol Homeostasis, Is Required for Virulence in Cryptococcus neoformans. Mol. Microbiol. 2007, 64, 614–629. [Google Scholar] [CrossRef]
- Hagiwara, D.; Miura, D.; Shimizu, K.; Paul, S.; Ohba, A.; Gonoi, T.; Watanabe, A.; Kamei, K.; Shintani, T.; Moye-Rowley, W.S.; et al. A Novel Zn2-Cys6 Transcription Factor AtrR Plays a Key Role in an Azole Resistance Mechanism of Aspergillus fumigatus by Co-Regulating Cyp51A and Cdr1B Expressions. PLoS Pathog. 2017, 13, e1006096. [Google Scholar] [CrossRef]
- Paul, S.; Stamnes, M.; Thomas, G.H.; Liu, H.; Hagiwara, D.; Gomi, K.; Filler, S.G.; Moye-Rowley, W.S. AtrR Is an Essential Determinant of Azole Resistance in Aspergillus fumigatus. mBio 2019, 10, e02563-18. [Google Scholar] [CrossRef]
- Du, W.; Zhai, P.; Wang, T.; Bromley, M.J.; Zhang, Y.; Lu, L. The C2H2 Transcription Factor SltA Contributes to Azole Resistance by Coregulating the Expression of the Drug Target Erg11A and the Drug Efflux Pump Mdr1 in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2021, 65, e01839-20. [Google Scholar] [CrossRef]
- Park, S.; Kelly, R.; Kahn, J.N.; Robles, J.; Hsu, M.J.; Register, E.; Li, W.; Vyas, V.; Fan, H.; Abruzzo, G.; et al. Specific Substitutions in the Echinocandin Target Fks1p Account for Reduced Susceptibility of Rare Laboratory and Clinical Candida Sp. Isolates. Antimicrob. Agents Chemother. 2005, 49, 3264–3273. [Google Scholar] [CrossRef]
- Garcia-Effron, G.; Lee, S.; Park, S.; Cleary, J.D.; Perlin, D.S. Effect of Candida Glabrata FKS1 and FKS2 Mutations on Echinocandin Sensitivity and Kinetics of 1,3-Beta-D-Glucan Synthase: Implication for the Existing Susceptibility Breakpoint. Antimicrob. Agents Chemother. 2009, 53, 3690–3699. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, P.; Tronchin, G.; Larcher, G.; Ernoult, E.; Bergès, T.; Chabasse, D.; Bouchara, J.P. A Nonsense Mutation in the ERG6 Gene Leads to Reduced Susceptibility to Polyenes in a Clinical Isolate of Candida Glabrata. Antimicrob. Agents Chemother. 2008, 52, 3701–3709. [Google Scholar] [CrossRef]
- Vincent, B.M.; Lancaster, A.K.; Scherz-Shouval, R.; Whitesell, L.; Lindquist, S. Fitness Trade-Offs Restrict the Evolution of Resistance to Amphotericin B. PLoS Biol. 2013, 11, e1001692. [Google Scholar] [CrossRef]
- Rybak, J.M.; Barker, K.S.; Muñoz, J.F.; Parker, J.E.; Ahmad, S.; Mokaddas, E.; Abdullah, A.; Elhagracy, R.S.; Kelly, S.L.; Cuomo, C.A.; et al. In Vivo Emergence of High-Level Resistance during Treatment Reveals the First Identified Mechanism of Amphotericin B Resistance in Candida Auris. Clin. Microbiol. Infect. 2022, 28, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-Mediated Antifungal Drug Resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Turner, V.; Ischer, F.; Morschhäuser, J.; Forche, A.; Selmecki, A.; Berman, J.; Bille, J.; Sanglard, D. A Mutation in Tac1p, a Transcription Factor Regulating CDR1 and CDR2, Is Coupled with Loss of Heterozygosity at Chromosome 5 to Mediate Antifungal Resistance in Candida albicans. Genetics 2006, 172, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, N.; Blaß, J.; Rogers, P.D.; Morschhäuser, J. Mutations in the Multi-Drug Resistance Regulator MRR1, Followed by Loss of Heterozygosity, Are the Main Cause of MDR1 Overexpression in Fluconazole-Resistant Candida Albicans Strains. Mol. Microbiol. 2008, 69, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Coste, A.T.; Bachmann, D.; Sanglard, D.; Lamoth, F. Deciphering the Mrr1/Mdr1 Pathway in Azole Resistance of Candida Auris. Antimicrob. Agents Chemother. 2022, 66, e00067-22. [Google Scholar] [CrossRef]
- Rybak, J.M.; Muñoz, J.F.; Barker, K.S.; Parker, J.E.; Esquivel, B.D.; Berkow, E.L.; Lockhart, S.R.; Gade, L.; Palmer, G.E.; White, T.C.; et al. Mutations in TAC1B: A Novel Genetic Determinant of Clinical Fluconazole Resistance in Candida Auris. mBio 2020, 11, e00365-20. [Google Scholar] [CrossRef]
- Selmecki, A.; Forche, A.; Berman, J. Aneuploidy and Isochromosome Formation in Drug-Resistant Candida Albicans. Science 2006, 313, 367–370. [Google Scholar] [CrossRef]
- Selmecki, A.; Gerami-Nejad, M.; Paulson, C.; Forche, A.; Berman, J. An Isochromosome Confers Drug Resistance in Vivo by Amplification of Two Genes, ERG11 and TAC1. Mol. Microbiol. 2008, 68, 624–641. [Google Scholar] [CrossRef]
- Yang, F.; Gritsenko, V.; Futterman, Y.S.; Gao, L.; Zhen, C.; Lu, H.; Jiang, Y.Y.; Berman, J. Tunicamycin Potentiates Antifungal Drug Tolerance via Aneuploidy in Candida Albicans. mBio 2021, 12, e02272-21. [Google Scholar] [CrossRef]
- Yang, F.; Teoh, F.; Tan, A.S.M.; Cao, Y.; Pavelka, N.; Berman, J.; Malik, H. Aneuploidy Enables Cross-Adaptation to Unrelated Drugs. Mol. Biol. Evol. 2019, 36, 1768–1782. [Google Scholar] [CrossRef]
- Poláková, S.; Blume, C.; Zárate, J.Á.; Mentel, M.; Jørck-Ramberg, D.; Stenderup, J.; Piškur, J. Formation of New Chromosomes as a Virulence Mechanism in Yeast Candida glabrata. Proc. Natl. Acad. Sci. USA 2009, 106, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Sionov, E.; Lee, H.; Chang, Y.C.; Kwon-Chung, K.J. Cryptococcus neoformans Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes. PLoS Pathog. 2010, 6, e1000848. [Google Scholar] [CrossRef] [PubMed]
- Morogovsky, A.; Handelman, M.; Abou Kandil, A.; Shadkchan, Y.; Osherov, N. Horizontal Gene Transfer of Triazole Resistance in Aspergillus fumigatus. Microbiol. Spectr. 2022, 10, e01112-22. [Google Scholar] [CrossRef] [PubMed]
- Koenraadt, H. Characterization of Mutations in the Beta-Tubulin Gene of Benomyl-Resistant Field Strains of Venturia inaequalis and Other Plant Pathogenic Fungi. Phytopathology 1992, 82, 1348. [Google Scholar] [CrossRef]
- Sierotzki, H.; Scalliet, G. A Review of Current Knowledge of Resistance Aspects for the Next-Generation Succinate Dehydrogenase Inhibitor Fungicides. Phytopathology 2013, 103, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Bardas, G.A.; Veloukas, T.; Koutita, O.; Karaoglanidis, G.S. Multiple Resistance of Botrytis Cinerea from Kiwifruit to SDHIs, QoIs and Fungicides of Other Chemical Groups. Pest Manag. Sci. 2010, 66, 967–973. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Resistance to Boscalid Fungicide in Alternaria Alternata Isolates from Pistachio in California. Plant Dis. 2007, 91, 1345–1350. [Google Scholar] [CrossRef]
- McKay, A.H.; Hagerty, G.C.; Follas, G.B.; Moore, M.S.; Christie, M.S.; Beresford, R.M. Succinate Dehydrogenase Inhibitor (SDHI) Fungicide Resistance Prevention Strategy. N. Z. Plant Prot. 2011, 64, 119–124. [Google Scholar] [CrossRef]
- Müller, M.A.; Stammler, G.; May De Mio, L.L. Multiple Resistance to DMI, QoI and SDHI Fungicides in Field Isolates of Phakopsora pachyrhizi. Crop Prot. 2021, 145, 105618. [Google Scholar] [CrossRef]
- Masiello, M.; Somma, S.; Haidukowski, M.; Logrieco, A.F.; Moretti, A. Genetic Polymorphisms Associated to SDHI Fungicides Resistance in Selected Aspergillus Flavus Strains and Relation with Aflatoxin Production. Int. J. Food Microbiol. 2020, 334, 108799. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide Emergence of Resistance to Antifungal Drugs Challenges Human Health and Food Security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide Resistance in Powdery Mildew Fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef] [PubMed]
- Latorre, B.A.; Spadaro, I.; Rioja, M.E. Occurrence of Resistant Strains of Botrytis Cinerea to Anilinopyrimidine Fungicides in Table Grapes in Chile. Crop Prot. 2002, 21, 957–961. [Google Scholar] [CrossRef]
- Mosbach, A.; Edel, D.; Farmer, A.D.; Widdison, S.; Barchietto, T.; Dietrich, R.A.; Corran, A.; Scalliet, G. Anilinopyrimidine Resistance in Botrytis Cinerea Is Linked to Mitochondrial Function. Front. Microbiol. 2017, 8, 304532. [Google Scholar] [CrossRef]
- Fiaccadori, R.; Collina, M.; Brunelli, A. Study on the Sensitivity of Venturia inaequalis to Anilinopyrimidine Fungicides in Italy. Commun. Agric. Appl. Biol. Sci. 2007, 71, 997–1001. [Google Scholar]
- Cools, H.J.; Hawkins, N.J.; Fraaije, B.A. Constraints on the Evolution of Azole Resistance in Plant Pathogenic Fungi. Plant Pathol. 2013, 62, 36–42. [Google Scholar] [CrossRef]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Azole Fungicides—Understanding Resistance Mechanisms in Agricultural Fungal Pathogens. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Hokken, M.W.J.; Zwaan, B.J.; Melchers, W.J.G.; Verweij, P.E. Facilitators of Adaptation and Antifungal Resistance Mechanisms in Clinically Relevant Fungi. Fungal Genet. Biol. 2019, 132, 103254. [Google Scholar] [CrossRef]
- Nuwamanya, A.M.; Runo, S.; Mwangi, M. In-Vitro Sensitivity of Alternaria Solani Isolates to Azoxystrobin and Difenoconazole Fungicides in Kenya and Detection of Cyt b Mutations Associated with Azoxystrobin Resistance. Crop Prot. 2022, 158, 106010. [Google Scholar] [CrossRef]
- Chen, F.; Tsuji, S.S.; Li, Y.; Hu, M.; Bandeira, M.A.; Câmara, M.P.S.; Michereff, S.J.; Schnabel, G. Reduced Sensitivity of Azoxystrobin and Thiophanate-Methyl Resistance in Lasiodiplodia Theobromae from Papaya. Pestic. Biochem. Physiol. 2020, 162, 60–68. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, F.; Zhao, J.; Fan, H.; Qin, C.; Li, R.; Verweij, P.E.; Zheng, Y.; Han, L. High Azole Resistance in Aspergillus Fumigatus Isolates from Strawberry Fields, China, 2018—Volume 26, Number 1—January 2020—Emerging Infectious Diseases Journal—CDC. Emerg. Infect. Dis. 2020, 26, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Beer, K.D.; Kuivila, K.M.; Chiller, T.M.; Jackson, B.R. Trends in Agricultural Triazole Fungicide Use in the United States, 1992–2016 and Possible Implications for Antifungal-Resistant Fungi in Human Disease. Environ. Health Perspect. 2021, 129, 055001. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the Emerging Threat of Antifungal Resistance to Human Health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A.; Puri, G.D.; Chakrabarti, A.; Agarwal, R. Coronavirus Disease (COVID-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia 2021, 186, 289–298. [Google Scholar] [CrossRef]
- Armstrong-James, D.; Youngs, J.; Bicanic, T.; Abdolrasouli, A.; Denning, D.W.; Johnson, E.; Mehra, V.; Pagliuca, T.; Patel, B.; Rhodes, J.; et al. Confronting and Mitigating the Risk of COVID-19 Associated Pulmonary Aspergillosis. Eur. Respir. J. 2020, 56, 2002554. [Google Scholar] [CrossRef] [PubMed]
- Wauters, J.; Baar, I.; Meersseman, P.; Meersseman, W.; Dams, K.; De Paep, R.; Lagrou, K.; Wilmer, A.; Jorens, P.; Hermans, G. Invasive Pulmonary Aspergillosis Is a Frequent Complication of Critically Ill H1N1 Patients: A Retrospective Study. Intensive Care Med. 2012, 38, 1761–1768. [Google Scholar] [CrossRef]
- Fisher, M.C.; Rannala, B.; Chaturvedi, V.; Taylor, J.W. Disease Surveillance in Recombining Pathogens: Multilocus Genotypes Identify Sources of Human Coccidioides Infections. Proc. Natl. Acad. Sci. USA 2002, 99, 9067–9071. [Google Scholar] [CrossRef]
- Farrer, R.A.; Chang, M.; Davis, M.J.; van Dorp, L.; Yang, D.H.; Shea, T.; Sewell, T.R.; Meyer, W.; Balloux, F.; Edwards, H.M.; et al. A New Lineage of Cryptococcus Gattii (VGV) Discovered in the Central Zambezian Miombo Woodlands. mBio 2019, 10, e02306-19. [Google Scholar] [CrossRef]
- Ashu, E.E.; Hagen, F.; Chowdhary, A.; Meis, J.F.; Xu, J. Global Population Genetic Analysis of Aspergillus fumigatus. mSphere 2017, 2, e00019-17. [Google Scholar] [CrossRef]
- Van Rhijn, N.; Bromley, M. The Consequences of Our Changing Environment on Life Threatening and Debilitating Fungal Diseases in Humans. J. Fungi 2021, 7, 367. [Google Scholar] [CrossRef]
- Carneiro, H.C.S.; Bastos, R.W.; Ribeiro, N.Q.; Gouveia-Eufrasio, L.; Costa, M.C.; Magalhães, T.F.F.; Oliveira, L.V.N.; Paixão, T.A.; Joffe, L.S.; Rodrigues, M.L.; et al. Hypervirulence and Cross-Resistance to a Clinical Antifungal Are Induced by an Environmental Fungicide in Cryptococcus gattii. Sci. Total Environ. 2020, 740, 140135. [Google Scholar] [CrossRef] [PubMed]
- Wroński, M.; Trawiński, J.; Skibiński, R. Antifungal Drugs in the Aquatic Environment: A Review on Sources, Occurrence, Toxicity, Health Effects, Removal Strategies and Future Challenges. J. Hazard. Mater. 2024, 465, 133167. [Google Scholar] [CrossRef] [PubMed]
- Islam, T. Heavy Metal Tolerance Pattern of Textile Dye Degrading Native Bacteria: A Bioremediation Viewpoint. Ann. Med. Health Sci. Res. 2017, 7, 67–73. [Google Scholar]
- Juksu, K.; Zhao, J.L.; Liu, Y.S.; Yao, L.; Sarin, C.; Sreesai, S.; Klomjek, P.; Jiang, Y.X.; Ying, G.G. Occurrence, Fate and Risk Assessment of Biocides in Wastewater Treatment Plants and Aquatic Environments in Thailand. Sci. Total Environ. 2019, 690, 1110–1119. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, P.; Yu, J.; Jiang, Z.; Guo, X. Experimental and Molecular Docking Study on Graphene/Fe3O4 Composites as a Sorbent for Magnetic Solid-Phase Extraction of Seven Imidazole Antifungals in Environmental Water Samples Prior to LC-MS/MS for Enantiomeric Analysis. Microchem. J. 2018, 140, 222–231. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zhu, Q.; Sun, S.; Peng, Y.; Shuai, Q. Suspect Screening and Target Quantification of Human Pharmaceutical Residues in the Surface Water of Wuhan, China, Using UHPLC-Q-Orbitrap HRMS. Sci. Total Environ. 2018, 635, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Rabbee, M.F.; Choi, J.; Baek, K.H. Biosynthesis, Molecular Regulation, and Application of Bacilysin Produced by Bacillus Species. Metabolites 2022, 12, 397. [Google Scholar] [CrossRef]
- Patel, A.; Kumar, A.; Sheoran, N.; Kumar, M.; Sahu, K.P.; Ganeshan, P.; Ashajyothi, M.; Gopalakrishnan, S.; Gogoi, R. Antifungal and Defense Elicitor Activities of Pyrazines Identified in Endophytic Pseudomonas Putida BP25 against Fungal Blast Incited by Magnaporthe Oryzae in Rice. J. Plant Dis. Prot. 2021, 128, 261–272. [Google Scholar] [CrossRef]
- Hu, X.; Roberts, D.P.; Xie, L.; Qin, L.; Li, Y.; Liao, X.; Han, P.; Yu, C.; Liao, X. Seed Treatment Containing Bacillus Subtilis BY-2 in Combination with Other Bacillus Isolates for Control of Sclerotinia sclerotiorum on Oilseed Rape. Biol. Control 2019, 133, 50–57. [Google Scholar] [CrossRef]
- Zhu, J.; Tan, T.; Shen, A.; Yang, X.; Yu, Y.; Gao, C.; Li, Z.; Cheng, Y.; Chen, J.; Guo, L.; et al. Biocontrol Potential of Bacillus Subtilis IBFCBF-4 against Fusarium Wilt of Watermelon. J. Plant Pathol. 2020, 102, 433–441. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Rashid Khan, A.; Hanif, A.; Tahir, H.A.S.; Gao, X. Marker Assisted Detection and LC-MS Analysis of Antimicrobial Compounds in Different Bacillus Strains and Their Antifungal Effect on Sclerotinia sclerotiorum. Biol. Control 2019, 133, 91–102. [Google Scholar] [CrossRef]
- Fazle Rabbee, M.; Baek, K.H. Antimicrobial Activities of Lipopeptides and Polyketides of Bacillus velezensis for Agricultural Applications. Molecules 2020, 25, 4973. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, P.; Zheng, L.; Li, Y.; Chen, Y.; Wen, S.; Yu, G. Comparative Genomics Analysis of Two Banana Fusarium Wilt Biocontrol Endophytes Bacillus Subtilis R31 and TR21 Provides Insights into Their Differences on Phytobeneficial Trait. Genomics 2021, 113, 900–909. [Google Scholar] [CrossRef]
- Jiao, R.; Cai, Y.; He, P.; Munir, S.; Li, X.; Wu, Y.; Wang, J.; Xia, M.; He, P.; Wang, G.; et al. Bacillus Amyloliquefaciens YN201732 Produces Lipopeptides with Promising Biocontrol Activity Against Fungal Pathogen Erysiphe Cichoracearum. Front. Cell. Infect. Microbiol. 2021, 11, 598999. [Google Scholar] [CrossRef]
- Badri Fariman, A.; Abbasiliasi, S.; Akmar Abdullah, S.N.; Mohd Saud, H.; Wong, M.Y. Stenotrophomonas Maltophilia Isolate UPMKH2 with the Abilities to Suppress Rice Blast Disease and Increase Yield a Promising Biocontrol Agent. Physiol. Mol. Plant Pathol. 2022, 121, 101872. [Google Scholar] [CrossRef]
- Azeem, S.; Agha, S.I.; Jamil, N.; Tabassum, B.; Ahmed, S.; Raheem, A.; Jahan, N.; Ali, N.; Khan, A. Characterization and Survival of Broad-Spectrum Biocontrol Agents against Phytopathogenic Fungi. Rev. Argent. Microbiol. 2022, 54, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Geng, L.; Sun, X.; Shu, C.; Song, F.; Zhang, J. Screening of Bacillus Thuringiensis Strains to Identify New Potential Biocontrol Agents against Sclerotinia Sclerotiorum and Plutella Xylostella in Brassica campestris L. Biol. Control 2020, 145, 104262. [Google Scholar] [CrossRef]
- Chet, I.; Ordentlich, A.; Shapira, R.; Oppenheim, A. Mechanisms of Biocontrol of Soil-Borne Plant Pathogens by Rhizobacteria. Plant Soil 1990, 129, 85–92. [Google Scholar] [CrossRef]
- Gu, Q.; Qiao, J.; Wang, R.; Lu, J.; Wang, Z.; Li, P.; Zhang, L.; Ali, Q.; Khan, A.R.; Gao, X.; et al. The Role of Pyoluteorin from Pseudomonas Protegens Pf-5 in Suppressing the Growth and Pathogenicity of Pantoea Ananatis on Maize. Int. J. Mol. Sci. 2022, 23, 6431. [Google Scholar] [CrossRef]
- Aiello, D.; Restuccia, C.; Stefani, E.; Vitale, A.; Cirvilleri, G. Postharvest Biocontrol Ability of Pseudomonas Synxantha against Monilinia Fructicola and Monilinia Fructigena on Stone Fruit. Postharvest Biol. Technol. 2019, 149, 83–89. [Google Scholar] [CrossRef]
- Ayaz, M.; Li, C.H.; Ali, Q.; Zhao, W.; Chi, Y.K.; Shafiq, M.; Ali, F.; Yu, X.Y.; Yu, Q.; Zhao, J.T.; et al. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Liang, L.J.; Jeewon, R.; Dhandevi, P.; Durairajan, S.S.K.; Li, H.; Lin, F.C.; Wang, H.K. A Novel Species of Penicillium With Inhibitory Effects Against Pyricularia Oryzae and Fungal Pathogens Inducing Citrus Diseases. Front. Cell. Infect. Microbiol. 2021, 10, 604504. [Google Scholar] [CrossRef]
- Eshel, D.; Regev, R.; Orenstein, J.; Droby, S.; Gan-Mor, S. Combining Physical, Chemical and Biological Methods for Synergistic Control of Postharvest Diseases: A Case Study of Black Root Rot of Carrot. Postharvest Biol. Technol. 2009, 54, 48–52. [Google Scholar] [CrossRef]
- Rivera-Méndez, W.; Obregón, M.; Morán-Diez, M.E.; Hermosa, R.; Monte, E. Trichoderma Asperellum Biocontrol Activity and Induction of Systemic Defenses against Sclerotium Cepivorum in Onion Plants under Tropical Climate Conditions. Biol. Control 2020, 141, 104145. [Google Scholar] [CrossRef]
- de Azevedo Silva, F.; de Oliveira Vieira, V.; Correia da Silva, R.; Guariz Pinheiro, D.; Antônio Soares, M. Introduction of Trichoderma spp. Biocontrol Strains against Sclerotinia Sclerotiorum (Lib.) de Bary Change Soil Microbial Community Composition in Common Bean (Phaseolus vulgaris L.) Cultivation. Biol. Control 2021, 163, 104755. [Google Scholar] [CrossRef]
- Wonglom, P.; Daengsuwan, W.; Ito, S.; Sunpapao, A. Biological Control of Sclerotium Fruit Rot of Snake Fruit and Stem Rot of Lettuce by Trichoderma Sp. T76-12/2 and the Mechanisms Involved. Physiol. Mol. Plant Pathol. 2019, 107, 1–7. [Google Scholar] [CrossRef]
- He, A.; Sun, J.; Wang, X.; Zou, L.; Fu, B.; Chen, J. Reprogrammed Endophytic Microbial Community in Maize Stalk Induced by Trichoderma Asperellum Biocontrol Agent against Fusarium Diseases and Mycotoxin Accumulation. Fungal Biol. 2019, 123, 448–455. [Google Scholar] [CrossRef]
- Ji, S.; An, Y.B.; Zhang, H.; Wang, Y.; Liu, Z. Trichoderma Biofertilizer (MixTroTha) Mediates Malus Sieversii Resistance to Alternaria Alternata. Biol. Control 2021, 156, 104539. [Google Scholar] [CrossRef]
- Cavalcanti, V.P.; Araújo, N.A.F.; Machado, N.B.; Costa Júnior, P.S.P.; Pasqual, M.; Alves, E.; Schwan-Estrada, K.R.F.; Dória, J. Yeasts and Bacillus Spp. as Potential Biocontrol Agents of Sclerotinia Sclerotiorum in Garlic. Sci. Hortic. 2020, 261, 108931. [Google Scholar] [CrossRef]
- Gajera, H.P.; Katakpara, Z.A.; Patel, S.V.; Golakiya, B.A. Antioxidant Defense Response Induced by Trichoderma Viride against Aspergillus Niger Van Tieghem Causing Collar Rot in Groundnut (Arachis hypogaea L.). Microb. Pathog. 2016, 91, 26–34. [Google Scholar] [CrossRef]
- de los Santos-Villalobos, S.; Guzmán-Ortiz, D.A.; Gómez-Lim, M.A.; Délano-Frier, J.P.; de Folter, S.; Sánchez-García, P.; Peña-Cabriales, J.J. Potential Use of Trichoderma Asperellum (Samuels, Liechfeldt et Nirenberg) T8a as a Biological Control Agent against Anthracnose in Mango (Mangifera indica L.). Biol. Control 2013, 64, 37–44. [Google Scholar] [CrossRef]
- O’Brien, P.A. Biological Control of Plant Diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Korsten, L. Biological Control of Postharvest Diseases of Fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef]
- Djonović, S.; Vittone, G.; Mendoza-Herrera, A.; Kenerley, C.M. Enhanced Biocontrol Activity of Trichoderma Virens Transformants Constitutively Coexpressing β-1,3- and β-1,6-Glucanase Genes. Mol. Plant Pathol. 2007, 8, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Yakoby, N.; Zhou, R.; Kobiler, I.; Dinoor, A.; Prusky, D. Development of Colletotrichum Gloeosporioides Restriction Enzyme-Mediated Integration Mutants as Biocontrol Agents Against Anthracnose Disease in Avocado Fruits. Phytopathology 2007, 91, 143–148. [Google Scholar] [CrossRef]
- Raman, N.M.; Easwaran, M.; Kaul, R.; Bharti, J.; Motelb, K.F.A.; Kaul, T.; Raman, N.M.; Easwaran, M.; Kaul, R.; Bharti, J.; et al. Antimicrobial Resistance with Special Emphasis on Pathogens in Agriculture. In Antimicrobial Resistance—A One Health Perspect; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Wubben, M.J.E.; Su, H.; Rodermel, S.R.; Baum, T.J. Susceptibility to the Sugar Beet Cyst Nematode Is Modulated by Ethylene Signal Transduction in Arabidopsis Thaliana. Mol. Plant-Microbe Interact. 2001, 14, 1206–1212. [Google Scholar] [CrossRef]
- Lumbreras, V.; Vilela, B.; Irar, S.; Solé, M.; Capellades, M.; Valls, M.; Coca, M.; Pagès, M. MAPK Phosphatase MKP2 Mediates Disease Responses in Arabidopsis and Functionally Interacts with MPK3 and MPK6. Plant J. 2010, 63, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Browse, J. Altered Rates of Protein Transport in Arabidopsis Mutants Deficient in Chloroplast Membrane Unsaturation. Phytochemistry 2006, 67, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Charles, S.; Levings, I. The Texas Cytoplasm of Maize: Cytoplasmic Male Sterility and Disease Susceptibility. Science 1990, 250, 942–947. [Google Scholar] [CrossRef]
- Humphry, M.; Reinstädler, A.; Ivanov, S.; Bisseling, T.; Panstruga, R. Durable Broad-Spectrum Powdery Mildew Resistance in Pea Er1 Plants Is Conferred by Natural Loss-of-Function Mutations in PsMLO1. Mol. Plant Pathol. 2011, 12, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.A.; Arévalo-Soliz, L.M.; Jia, L.; Navarre, D.A.; Chen, Z.; Howe, G.A.; Meng, Q.W.; Smith, J.E.; Goggin, F.L. Loss of Function of FATTY ACID DESATURASE7 in Tomato Enhances Basal Aphid Resistance in a Salicylate-Dependent Manner. Plant Physiol. 2012, 158, 2028–2041. [Google Scholar] [CrossRef]
- Krasikov, V.; Dekker, H.L.; Rep, M.; Takken, F.L.W. The Tomato Xylem Sap Protein XSP10 Is Required for Full Susceptibility to Fusarium Wilt Disease. J. Exp. Bot. 2011, 62, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T.; Miyamoto, K.; Shimogawa, T.; Shimizu, T.; Otake, Y.; Yokotani, N.; Nishizawa, Y.; Shibuya, N.; Nojiri, H.; Yamane, H.; et al. OsWRKY28, a PAMP-Responsive Transrepressor, Negatively Regulates Innate Immune Responses in Rice against Rice Blast Fungus. Plant Mol. Biol. 2013, 82, 23–37. [Google Scholar] [CrossRef]
- Yoshii, M.; Shimizu, T.; Yamazaki, M.; Higashi, T.; Miyao, A.; Hirochika, H.; Omura, T. Disruption of a Novel Gene for a NAC-Domain Protein in Rice Confers Resistance to Rice Dwarf Virus. Plant J. 2009, 57, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, M.; Yamazaki, M.; Rakwal, R.; Kishi-Kaboshi, M.; Miyao, A.; Hirochika, H. The NAC Transcription Factor RIM1 of Rice Is a New Regulator of Jasmonate Signaling. Plant J. 2010, 61, 804–815. [Google Scholar] [CrossRef]
- Halterman, D.A.; Kramer, L.C.; Wielgus, S.; Jiang, J. Performance of Transgenic Potato Containing the Late Blight Resistance Gene RB. Plant Dis. 2008, 92, 339–343. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Witek, K.; Verweij, W.; Jupe, F.; Cooke, D.; Dorling, S.; Tomlinson, L.; Smoker, M.; Perkins, S.; Foster, S. Elevating Crop Disease Resistance with Cloned Genes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130087. [Google Scholar] [CrossRef]
- Bradeen, J.M.; Iorizzo, M.; Mollov, D.S.; Raasch, J.; Kramer, L.C.; Millett, B.P.; Austin-Phillips, S.; Jiang, J.; Carputo, D. Higher Copy Numbers of the Potato RB Transgene Correspond to Enhanced Transcript and Late Blight Resistance Levels. Mol. Plant. Microbe Interact. 2009, 22, 437–446. [Google Scholar] [CrossRef]
- Dong, O.X.; Ronald, P.C. Genetic Engineering for Disease Resistance in Plants: Recent Progress and Future Perspectives. Plant Physiol. 2019, 180, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, Y.; Vossen, J.H.; Visser, R.G.F.; Jacobsen, E. Functional Stacking of Three Resistance Genes against Phytophthora Infestans in Potato. Transgenic Res. 2012, 21, 89. [Google Scholar] [CrossRef]
- Haverkort, A.J.; Boonekamp, P.M.; Hutten, R.; Jacobsen, E.; Lotz, L.A.P.; Kessel, G.J.T.; Vossen, J.H.; Visser, R.G.F. Durable Late Blight Resistance in Potato Through Dynamic Varieties Obtained by Cisgenesis: Scientific and Societal Advances in the DuRPh Project. Potato Res. 2016, 59, 35–66. [Google Scholar] [CrossRef]
- Halperin, S.O.; Tou, C.J.; Wong, E.B.; Modavi, C.; Schaffer, D.V.; Dueber, J.E. CRISPR-Guided DNA Polymerases Enable Diversification of All Nucleotides in a Tunable Window. Nature 2018, 560, 248–252. [Google Scholar] [CrossRef]
- Paul, N.C.; Park, S.W.; Liu, H.; Choi, S.; Ma, J.; MacCready, J.S.; Chilvers, M.I.; Sang, H. Plant and Fungal Genome Editing to Enhance Plant Disease Resistance Using the CRISPR/Cas9 System. Front. Plant Sci. 2021, 12, 700925. [Google Scholar] [CrossRef]
- Sun, Q.; Lin, L.; Liu, D.; Wu, D.; Fang, Y.; Wu, J.; Wang, Y. CRISPR/Cas9-Mediated Multiplex Genome Editing of the BnWRKY11 and BnWRKY70 Genes in Brassica napus L. Int. J. Mol. Sci. 2018, 19, 2716. [Google Scholar] [CrossRef]
- Fister, A.S.; Landherr, L.; Maximova, S.N.; Guiltinan, M.J. Transient Expression of CRISPR/Cas9 Machinery Targeting TcNPR3 Enhances Defense Response in Theobroma Cacao. Front. Plant Sci. 2018, 9, 329023. [Google Scholar] [CrossRef]
- Li, L.; Kim, P.; Yu, L.; Cai, G.; Chen, S.; Alfano, J.R.; Zhou, J.M. Activation-Dependent Destruction of a Co-Receptor by a Pseudomonas Syringae Effector Dampens Plant Immunity. Cell Host Microbe 2016, 20, 504–514. [Google Scholar] [CrossRef]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-Spectrum Resistance to Bacterial Blight in Rice Using Genome Editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid Generation of a Transgene-Free Powdery Mildew Resistant Tomato by Genome Deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef]
- Santillán Martínez, M.I.; Bracuto, V.; Koseoglou, E.; Appiano, M.; Jacobsen, E.; Visser, R.G.F.; Wolters, A.M.A.; Bai, Y. CRISPR/Cas9-Targeted Mutagenesis of the Tomato Susceptibility Gene PMR4 for Resistance against Powdery Mildew. BMC Plant Biol. 2020, 20, 284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous Editing of Three Homoeoalleles in Hexaploid Bread Wheat Confers Heritable Resistance to Powdery Mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis in Grape in the First Generation. Plant Biotechnol. J. 2018, 16, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Králová, M.; Bergougnoux, V.; Frébort, I. CRISPR/Cas9 Genome Editing in Ergot Fungus Claviceps Purpurea. J. Biotechnol. 2021, 325, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Wan, L.; Deng, Y.Z.; Yang, W.; Li, W.; Jiang, L.; Situ, J.; Xi, P.; Li, M.; Jiang, Z. Pectin Acetylesterase PAE5 Is Associated with the Virulence of Plant Pathogenic Oomycete Peronophythora Litchii. Physiol. Mol. Plant Pathol. 2019, 106, 16–22. [Google Scholar] [CrossRef]
- Qu, Z.; Fu, Y.; Lin, Y.; Zhao, Z.; Zhang, X.; Cheng, J.; Xie, J.; Chen, T.; Li, B.; Jiang, D. Transcriptional Responses of Sclerotinia Sclerotiorum to the Infection by SsHADV-1. J. Fungi 2021, 7, 493. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Liao, X.L.; Gao, B.; Lu, X.; Sun, D.; Gong, W.; Zhong, J.; Zhu, H.; Pan, X.; et al. Mycoviral Gene Integration Converts a Plant Pathogenic Fungus into a Biocontrol Agent. Proc. Natl. Acad. Sci. USA 2022, 119, e2214096119. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, B.; Fu, Y.; Jiang, D.; Ghabrial, S.A.; Li, G.; Peng, Y.; Xie, J.; Cheng, J.; Huang, J.; et al. A Geminivirus-Related DNA Mycovirus That Confers Hypovirulence to a Plant Pathogenic Fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 8387–8392. [Google Scholar] [CrossRef]
- Andika, I.B.; Wei, S.; Cao, C.; Salaipeth, L.; Kondo, H.; Sun, L. Phytopathogenic Fungus Hosts a Plant Virus: A Naturally Occurring Cross-Kingdom Viral Infection. Proc. Natl. Acad. Sci. USA 2017, 114, 12267–12272. [Google Scholar] [CrossRef]
- Bian, R.; Andika, I.B.; Pang, T.; Lian, Z.; Wei, S.; Niu, E.; Wu, Y.; Kondo, H.; Liu, X.; Sun, L. Facilitative and Synergistic Interactions between Fungal and Plant Viruses. Proc. Natl. Acad. Sci. USA 2020, 117, 3779–3788. [Google Scholar] [CrossRef]
- Attai, H.; Rimbey, J.; Smith, G.P.; Brown, P.J.B. Expression of a Peptidoglycan Hydrolase from Lytic Bacteriophages Atu_ph02 and Atu_ph03 Triggers Lysis of Agrobacterium Tumefaciens. Appl. Environ. Microbiol. 2017, 83, e01498-17. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Sarafat Ali, M.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K. Bacillus Velezensis: A Valuable Member of Bioactive Molecules within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Li, H.P.; Yuan, Q.S.; Song, X.S.; Yao, W.; He, W.J.; Zhang, J.B.; Liao, Y.C. Antagonistic Mechanism of Iturin A and Plipastatin A from Bacillus Amyloliquefaciens S76-3 from Wheat Spikes against Fusarium Graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Marra, R.; Scala, F.; Ghisalberti, E.L.; Lorito, M.; Sivasithamparam, K. Major Secondary Metabolites Produced by Two Commercial Trichoderma Strains Active against Different Phytopathogens. Lett. Appl. Microbiol. 2006, 43, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Shanthiyaa, V.; Saravanakumar, D.; Rajendran, L.; Karthikeyan, G.; Prabakar, K.; Raguchander, T. Use of Chaetomium Globosum for Biocontrol of Potato Late Blight Disease. Crop Prot. 2013, 52, 33–38. [Google Scholar] [CrossRef]
- Chiu, T.; Poucet, T.; Li, Y. The Potential of Plant Proteins as Antifungal Agents for Agricultural Applications. Synth. Syst. Biotechnol. 2022, 7, 1075. [Google Scholar] [CrossRef]
- Rahman, M.; Wadud, M.; Islam, T.; Hussain, M.; Bristy, E.; Tuhin, A. Evaluation of Antibacterial Activity of Piper Betel Leaves and Nigella Sativa Seeds against Multidrug Resistant Food and Water Borne Pathogenic Bacteria: An in Vitro Study Model. Microbiol. Res. J. Int. 2018, 22, 1–11. [Google Scholar] [CrossRef]
- Shalahuddin Millat, M.; Islam, S.; Hussain, M.S.; Rahman Moghal, M.M.; Islam, T. Anti-Bacterial Profiling of Launaea Sarmentosa (Willd.) and Bruguiera cylindrical (L.): Two Distinct Ethno Medicinal Plants of Bangladesh. Eur. J. Exp. Biol. 2017, 7, 1–5. [Google Scholar] [CrossRef]
- Debnath, T.; Bhowmik, S.; Islam, T.; Chowdhury, M.M.H. Presence of Multidrug-Resistant Bacteria on Mobile Phones of Healthcare Workers Accelerates the Spread of Nosocomial Infection and Regarded as a Threat to Public Health in Bangladesh. J. Microsc. Ultrastruct. 2018, 6, 165. [Google Scholar] [CrossRef]
- Malik, A. Preety Purification and Properties of Plant Chitinases: A Review. J. Food Biochem. 2019, 43, e12762. [Google Scholar] [CrossRef]
- Shrestha, C.L.; Oña, I.; Muthukrishnan, S.; Mew, T.W. Chitinase Levels in Rice Cultivars Correlate with Resistance to the Sheath Blight Pathogen Rhizoctonia Solani. Eur. J. Plant Pathol. 2008, 120, 69–77. [Google Scholar] [CrossRef]
- Lay, F.T.; Anderson, M.A. Defensins–Components of the Innate Immune System in Plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Ng, T.B. Northeast Red Beans Produce a Thermostable and PH-Stable Defensin-like Peptide with Potent Antifungal Activity. Cell Biochem. Biophys. 2013, 66, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Park, S.C.; Kim, J.Y.; Lee, S.Y.; Lim, H.T.; Cheong, H.; Hahm, K.S.; Park, Y. Purification and Characterization of a Heat-Stable Serine Protease Inhibitor from the Tubers of New Potato Variety “Golden Valley”. Biochem. Biophys. Res. Commun. 2006, 346, 681–686. [Google Scholar] [CrossRef]
- Hermosa, M.R.; Turrà, D.; Fogliano, V.; Monte, E.; Lorito, M. Identification and Characterization of Potato Protease Inhibitors Able to Inhibit Pathogenicity and Growth of Botrytis Cinerea. Physiol. Mol. Plant Pathol. 2006, 68, 138–148. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J.; Jarošová, M. Antifungal and Antimicrobial Proteins and Peptides of Potato (Solanum tuberosum L.) Tubers and Their Applications. Appl. Microbiol. Biotechnol. 2019, 103, 5533–5547. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B.; Rao, P.F. A Bowman-Birk-Type Trypsin-Chymotrypsin Inhibitor from Broad Beans. Biochem. Biophys. Res. Commun. 2001, 289, 91–96. [Google Scholar] [CrossRef]
- Narváez-Vásquez, J.; Ryan, C.A. The Cellular Localization of Prosystemin: A Functional Role for Phloem Parenchyma in Systemic Wound Signaling. Planta 2004, 218, 360–369. [Google Scholar] [CrossRef]
- Jones, R.W.; Ospina-Giraldo, M.; Clemente, T. Prosystemin-Antimicrobial-Peptide Fusion Reduces Tomato Late Blight Lesion Expansion. Mol. Breed. 2004, 14, 83–89. [Google Scholar] [CrossRef]
- Molisso, D.; Coppola, M.; Aprile, A.M.; Avitabile, C.; Natale, R.; Romanelli, A.; Chiaiese, P.; Rao, R. Colonization of Solanum Melongena and Vitis Vinifera Plants by Botrytis Cinerea Is Strongly Reduced by the Exogenous Application of Tomato Systemin. J. Fungi 2020, 7, 15. [Google Scholar] [CrossRef]
- Berrocal-Lobo, M.; Segura, A.; Moreno, M.; López, G.; García-Olmedo, F.; Molina, A. Snakin-2, an Antimicrobial Peptide from Potato Whose Gene Is Locally Induced by Wounding and Responds to Pathogen Infection. Plant Physiol. 2002, 128, 951–961. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Arafa, R.A.; Omara, R.I.; Kamel, S.M.; Ismail, W.; Ismail, S.; Derbalah, A. Developing Ag2O and Ag2O/TiO2 Nanostructures as a New Strategy for Control Late Blight of Potato Caused by Phytophthora Infestans. Physiol. Mol. Plant Pathol. 2022, 120, 101856. [Google Scholar] [CrossRef]
- Soleimani, P.; Mehrvar, A.; Michaud, J.P.; Vaez, N. Optimization of Silver Nanoparticle Biosynthesis by Entomopathogenic Fungi and Assays of Their Antimicrobial and Antifungal Properties. J. Invertebr. Pathol. 2022, 190, 107749. [Google Scholar] [CrossRef] [PubMed]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Zinc Nanoparticles: Mode of Action and Efficacy against Boscalid-Resistant Alternaria Alternata Isolates. Sci. Total Environ. 2022, 829, 154638. [Google Scholar] [CrossRef]
- Velmurugan, P.; Sivakumar, S.; Young-Chae, S.; Seong-Ho, J.; Pyoung-In, Y.; Jeong-Min, S.; Sung-Chul, H. Synthesis and Characterization Comparison of Peanut Shell Extract Silver Nanoparticles with Commercial Silver Nanoparticles and Their Antifungal Activity. J. Ind. Eng. Chem. 2015, 31, 51–54. [Google Scholar] [CrossRef]
- Kora, A.J.; Mounika, J.; Jagadeeshwar, R. Rice Leaf Extract Synthesized Silver Nanoparticles: An in Vitro Fungicidal Evaluation against Rhizoctonia Solani, the Causative Agent of Sheath Blight Disease in Rice. Fungal Biol. 2020, 124, 671–681. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of Silver Nanoparticles to Counter Fungicide-Resistance in Monilinia Fructicola. Sci. Total Environ. 2020, 747, 141287. [Google Scholar] [CrossRef]
- Tarazona, A.; Gómez, J.V.; Mateo, E.M.; Jiménez, M.; Mateo, F. Antifungal Effect of Engineered Silver Nanoparticles on Phytopathogenic and Toxigenic Fusarium Spp. and Their Impact on Mycotoxin Accumulation. Int. J. Food Microbiol. 2019, 306, 108259. [Google Scholar] [CrossRef]
- Pal, S.; Singh, V.; Kumar, R.; Gogoi, R. Design and Development of 1,3,4-Thiadiazole Based Potent New Nano-Fungicides. J. Mol. Struct. 2020, 1219, 128507. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Muthukumar, S. Chitosan Guar Nanoparticle Preparation and Its in Vitro Antimicrobial Activity towards Phytopathogens of Rice. Int. J. Biol. Macromol. 2020, 153, 297–304. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Hwang, B.S.; Baek, K.H. Bacillus Velezensis: A Beneficial Biocontrol Agent or Facultative Phytopathogen for Sustainable Agriculture. Agronomy 2023, 13, 840. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).