Gamma-Aminobutyric Acid (GABA) as a Defense Booster for Wheat against Leaf Rust Pathogen (Puccinia triticina)
Abstract
:1. Introduction
2. Results
2.1. GABA Enhanced Wheat Resistance to Leaf Rust Fungal Infection
2.2. GABA-Mediated Stomatal Closure in Wheat Leaves under Dark Conditions
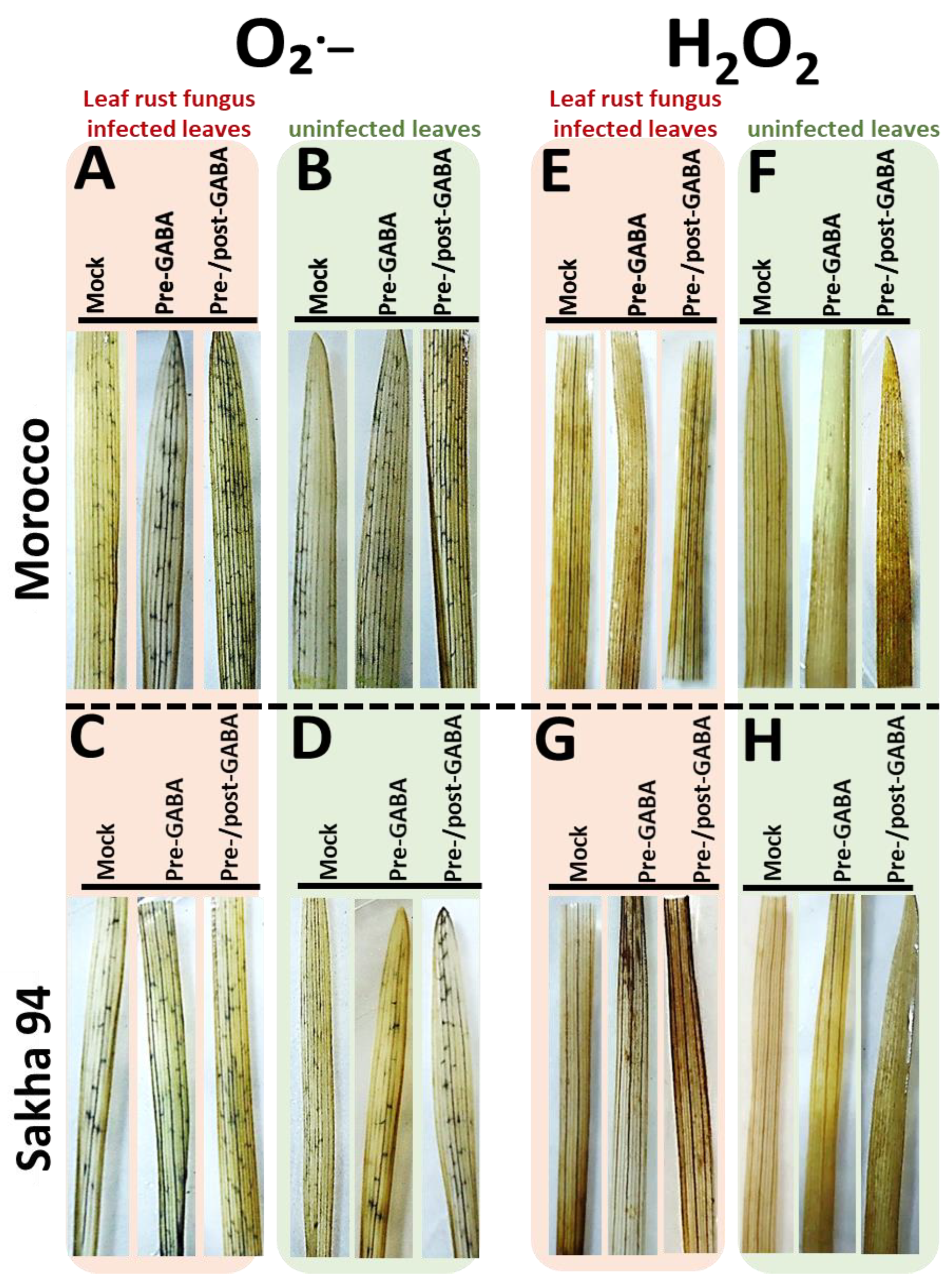
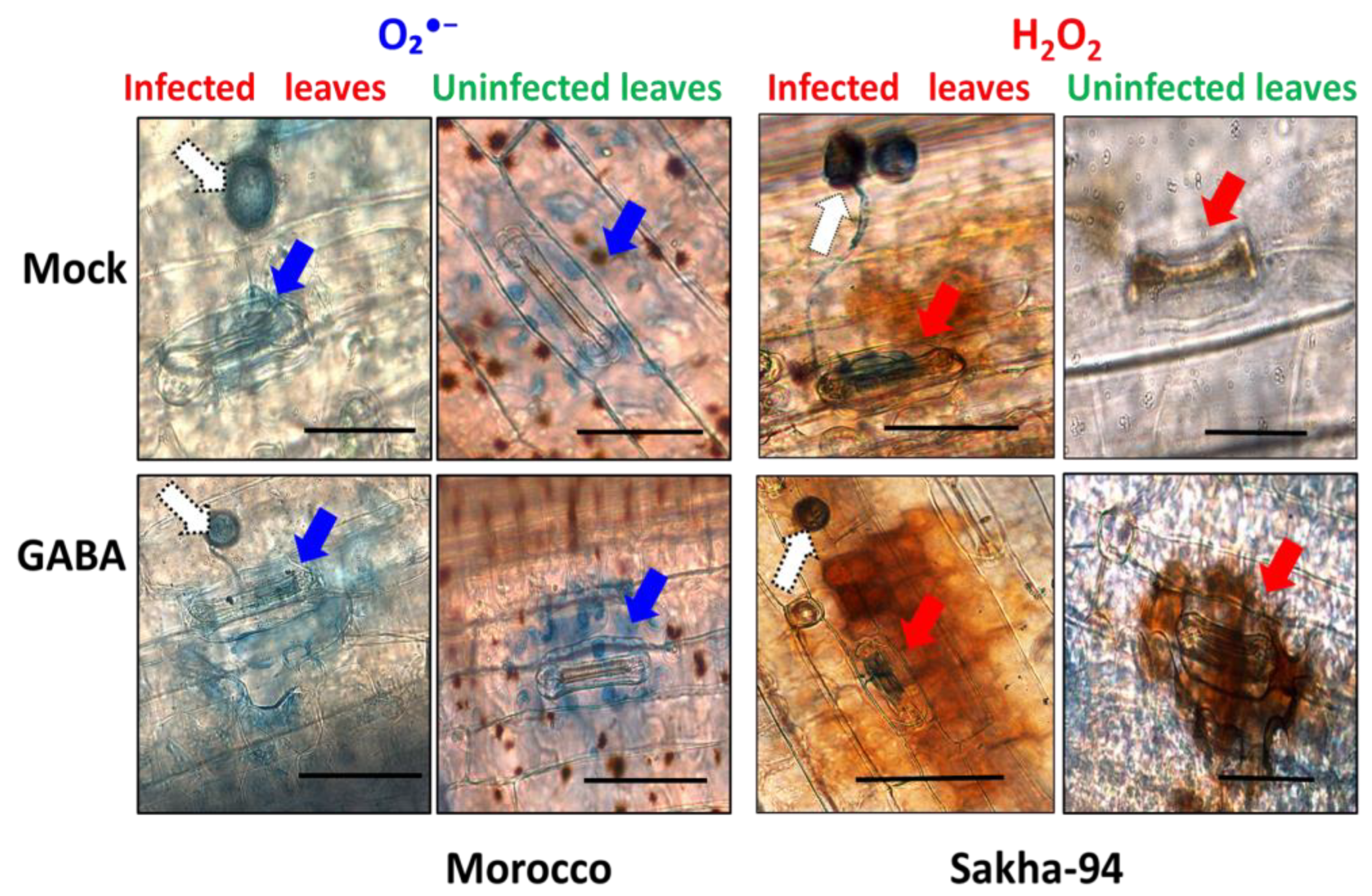
2.3. Accumulation of ROS Enhanced by GABA Treatment
2.4. GABA Enhancing Phenolic Accumulation in Plants
2.5. Antioxidant Activation as a Response to GABA Treatment
2.5.1. Catalase (CAT)
2.5.2. Peroxidase (POX)
3. Discussion
4. Materials and Methods
4.1. Leaf Rust Fungal Race
4.2. Selected Wheat Cultivars
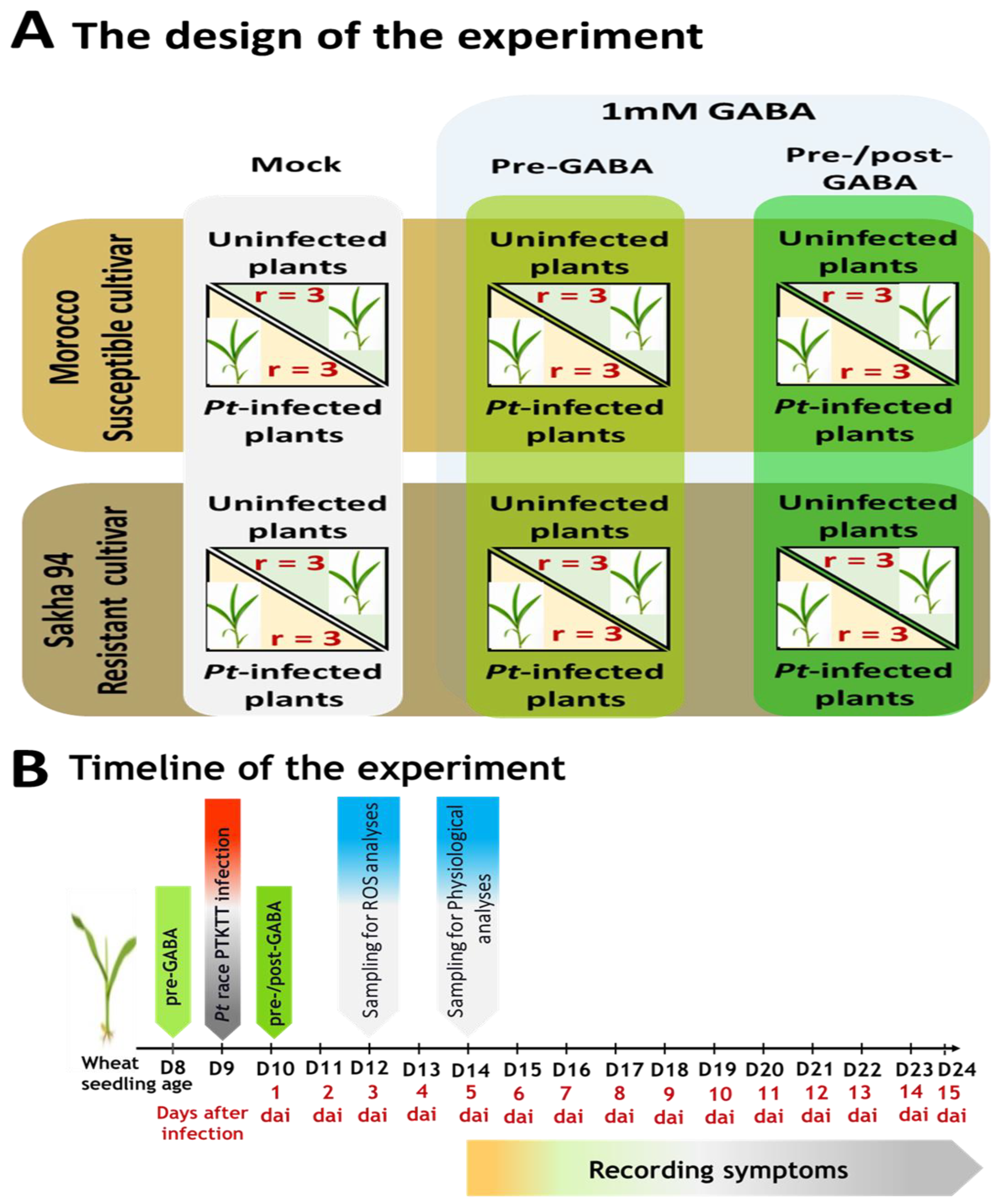
4.3. Experimental Design and GABA Treatment
4.4. Rust Inoculation
4.5. Sampling
4.6. Symptom Records
4.7. Incubation and Latent Periods
4.8. Pustule Size Measurements
4.9. Stomatal Closure Assessment
4.10. Histochemical Analysis of Reactive Oxygen Species (ROS)
4.11. Assay of Total Phenols
4.12. Assay of Total Soluble Protein Content
4.13. Enzyme Analyses
4.14. Statistical Analysis
5. Conclusions
6. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awika, J.M. Major cereal grains production and use around the world. ACS Symp. Ser. 2011, 1089, 1–13. [Google Scholar]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases—A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Hovmøller, M.S.; Thach, T.; Justesen, A.F. Global dispersal and diversity of rust fungi in the context of plant health. Curr. Opin. Microbiol. 2023, 71, 102243. [Google Scholar] [CrossRef]
- Soko, T.; Bender, C.M.; Prins, R.; Pretorius, Z.A. Yield loss associated with different levels of stem rust resistance in bread wheat. Plant Dis. 2018, 102, 2531–2538. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Mustafa, M.; Charles, K.; Wagara, I.W.; Kappel, N. Selected emerging and reemerging plant pathogens affecting the food basket: A threat to food security. J. Agric. Food Res. 2023, 14, 100827. [Google Scholar] [CrossRef]
- Bolton, M.D.; Kolmer, J.A.; Garvin, D.F. Wheat leaf rust caused by Puccinia triticina. Mol. Plant Pathol. 2008, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, K.M.A.; Thabet, M.; Negm, S.S.; El-Deeb, S.H.; Fahim, M.A. Early warning of wheat leaf rust disease and prediction disease status based on the modeling weather. J. Appl. Plant Protect. 2021, 10, 87–95. [Google Scholar]
- Thabet, M.; Najeeb, K.M.A. Impact of wheat leaf rust severity on grain yield losses in relation to host resistance for some Egyptian wheat cultivars. Middle East J. Agric. Res. 2017, 6, 1501–1509. [Google Scholar]
- Cuomo, C.A.; Bakkeren, G.; Khalil, H.B.; Panwar, V.; Joly, D.; Linning, R.; Sakthikumar, S.; Song, X.; Adiconis, X.; Fan, L.; et al. Comparative analysis highlights variable genome content of wheat rusts and divergence of the mating loci. G3 2017, 7, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Atia, M.A.M.; El-Khateeb, E.A.; Abd El-Maksoud, R.M.; Abou-Zeid, M.A.; Salah, A.; Abdel-Hamid, A.M.E. Mining of leaf rust resistance genes content in Egyptian bread wheat collection. Plants 2021, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
- Omara, R.I.; Nehela, Y.; Mabrouk, O.I.; Elsharkawy, M.M. The emergence of new aggressive leaf rust races with the potential to supplant the resistance of wheat cultivars. Biology 2021, 10, 925. [Google Scholar] [CrossRef]
- Negm, S.S.; Boulot, O.A.; Gamalat, A.H. Virulence dynamics and diversity in wheat leaf rust (Puccinia triticina) populations in Egypt during 2009/2010 and 2010/2012 growing seasons. Egypt. J. Appl. Sci. 2013, 28, 183–212. [Google Scholar]
- Boulot, O.A.; Aly, A.A. Partial resistance of wheat (Triticum aestivum) to leaf rust (Puccinia triticina) in Egypt. A. Evaluation of seven Egyptian wheat cultivars for partial resistance against leaf rust, under field conditions. Egypt. J. Agric. Res. 2014, 92, 835–850. [Google Scholar] [CrossRef]
- Mapuranga, J.; Zhang, N.; Zhang, L.; Liu, W.; Chang, J.; Yang, W. Harnessing genetic resistance to rusts in wheat and integrated rust management methods to develop more durable resistant cultivars. Front. Plant Sci. 2022, 13, 951095. [Google Scholar] [CrossRef] [PubMed]
- Mapuranga, J.; Chang, J.; Zhao, J.; Liang, M.; Li, R.; Wu, Y.; Zhang, N.; Zhang, L.; Yang, W. The underexplored mechanisms of wheat resistance to leaf rust. Plants 2023, 12, 3996. [Google Scholar] [CrossRef]
- Najeeb, K.M.A.; Thabet, M.; Negm, S.S.; El-Deeb, S.H. Monitoring of Puccinia triticina Erikss. physiologic races and effectiveness of Lr-genes in Egyptian wheat during 2014-2016 growing seasons. Int. J. Agric. Technol. 2019, 15, 35–54. [Google Scholar]
- Kolmer, J.A.; Long, D.L.; Hughes, M.E. Physiological specialization of Puccinia triticina on wheat in the United States in 2003. Plant Dis. 2005, 89, 1201–1206. [Google Scholar] [CrossRef]
- Moricca, S.; Ragazzi, A. Biological and integrated means to control rust diseases. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Ciancio, A., Mukerji, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; p. 3. [Google Scholar]
- Thabet, M.S.; Gado, E.A.M.; Najeeb, M.A.A.; El-Deeb, S.H. The role of reactive oxygen species and β-1,3 glucanase in the mechanism of resistance induction in wheat plants against leaf rust. J. Biol. Chem. Environ. Sci. 2012, 7, 155–175. [Google Scholar]
- Guo, Z.; Gong, J.; Luo, S.; Zuo, Y.; Shen, Y. Role of gamma-aminobutyric acid in plant defense response. Metabolites 2023, 13, 741. [Google Scholar] [CrossRef] [PubMed]
- Nehela, Y.; Killiny, N. Gamma-aminobutyric acid supplementation boosts the phytohormonal profile in ’Candidatus Liberibacter asiaticus’-infected citrus. Plants 2023, 12, 3647. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-Aminobutyrate (GABA) regulated plant defense: Mechanisms and opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Kamran, M.; Sullivan, W.; Chirkova, L.; Okamoto, M.; Degryse, F.; McLaughlin, M.; Gilliham, M.; Tyerman, S.D. Aluminum-activated malate transporters can facilitate GABA transport. Plant Cell 2018, 30, 1147–1164. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, X.; Ding, Z.J.; Zhang, X.; Luo, Y.; Xu, X.; Xie, Y.; Li, X.; Yuan, T.; Zheng, S.J.; et al. Structural basis of ALMT1-mediated aluminum resistance in Arabidopsis. Cell Res. 2022, 32, 89–98. [Google Scholar] [CrossRef]
- Zafar, S.; Jabeen, I. Structure, function, and modulation of γ-aminobutyric acid transporter 1 (GAT1) in neurological disorders: A pharmacoinformatic prospective. Front. Chem. 2018, 6, 397. [Google Scholar] [CrossRef]
- Ahmad, S.; Fariduddin, Q. Deciphering the enigmatic role of gamma-aminobutyric acid (GABA) in plants: Synthesis, transport, regulation, signaling, and biological roles in interaction with growth regulators and abiotic stresses. Plant Physiol. Biochem. 2024, 208, 108502. [Google Scholar] [CrossRef]
- Khan, Z.; Jan, R.; Asif, S.; Farooq, M.; Kim, K.M. Exogenous GABA enhances copper stress resilience in rice plants via antioxidant defense mechanisms, gene regulation, mineral uptake, and copper homeostasis. Antioxidants 2024, 13, 700. [Google Scholar] [CrossRef]
- Thrall, P.H.; Laine, A.L.; Ravensdale, M.; Nemri, A.; Dodds, P.N.; Barrett, L.G.; Burdon, J.J. Rapid genetic change underpins antagonistic coevolution in a natural host-pathogen metapopulation. Ecol. Lett. 2012, 15, 425–435. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bown, A.W.; Faure, D. Extracellular γ-aminobutyrate mediates communication between plants and other organisms. Plant Physiol. 2006, 142, 1350–1352. [Google Scholar] [CrossRef]
- Tarkowski, K.P.; Signorelli, S.; Höfte, M. γ-Aminobutyric acid and related amino acids in plant immune responses: Emerging mechanisms of action. Plant Cell Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Abd Elbar, O.H.; Elkelish, A.; Niedbała, G.; Farag, R.; Wojciechowski, T.; Mukherjee, S.; Abou-Hadid, A.F.; El-Hennawy, H.M.; Abou El-Yazied, A.; Abd El-Gawad, H.G.; et al. Protective effect of γ-aminobutyric acid against chilling stress during reproductive stage in tomato plants through modulation of sugar metabolism, chloroplast integrity, and antioxidative defense systems. Front. Plant Sci. 2021, 12, 663750. [Google Scholar] [CrossRef] [PubMed]
- Mead, O.; Thynne, E.; Winterberg, B.; Solomon, P.S. Characterising the role of GABA and its metabolism in the wheat pathogen Stagonospora nodorum. PLoS ONE 2013, 8, e78368. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Zhawar, V.K.; Pannu, P.P. Effect of gamma-aminobutyric acid on resistance against stripe rust (caused by Puccinia striiformis f. sp. tritici) in wheat cultivars. J. Environ. Biol. 2023, 44, 159–166. [Google Scholar]
- Mekonnen, D.W.; Flügge, U.-I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, J.E.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Xu, B.; Feng, X.; Piechatzek, A.; Zhang, S.; Konrad, K.R.; Kromdijk, J.; Hedrich, R.; Gilliham, M. The GABA shunt contributes to ROS homeostasis in guard cells of Arabidopsis. New Phytol. 2024, 241, 73–81. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Ashraf, U.; Li, G.; Li, Y.; Lu, W.; Gao, L.; Han, F.; Hu, J. Exogenous γ-aminobutyric acid (GABA) application improved early growth, net photosynthesis, and associated physio-biochemical events in maize. Front. Plant Sci. 2016, 7, 919. [Google Scholar] [CrossRef]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Xu, B.; Sai, N.; Gilliham, M. The emerging role of GABA as a transport regulator and physiological signal. Plant Physiol. 2021, 187, 2005–2016. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.G.; Mukherjee, S.; Farag, R.; Abd Elbar, O.H.; Hikal, M.; Abou El-Yazied, A.; Abd Elhady, S.A.; Helal, N.; ElKelish, A.; El Nahhas, N.; et al. Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal. Behav. 2021, 16, 1853384. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asif, S.; Asaf, S.; Lubna; Khan, Z.; Khan, W.; Kim, K.M. Gamma-aminobutyric acid treatment promotes resistance against Sogatella furcifera in rice. Front. Plant Sci. 2024, 15, 1419999. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Che, M.Z.; Khalil, H.B.; McCallum, B.D.; Bakkeren, G.; Rampitsch, C.; Saville, B.J. The role of reactive oxygen species in the virulence of wheat leaf rust fungus Puccinia triticina. Environ. Microbiol. 2020, 22, 2956–2967. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xu, X.; Wang, H.; Wang, H.; Tao, Y. Exogenous gamma-aminobutyric acid alleviates oxidative damage caused by aluminium and proton stresses on barley seedlings. J. Sci. Food Agric. 2010, 90, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, Y.; Liu, B.; Xu, R.; Shi, Y.; Lv, L.; Wang, H.; Shang, Y.; Liang, W.; Ma, F.; et al. γ-Aminobutyric acid plays a key role in alleviating Glomerella leaf spot in apples. Mol. Plant Pathol. 2023, 24, 588–601. [Google Scholar]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef]
- Aljuaid, B.S.; Ashour, H. Exogenous γ-aminobutyric acid (GABA) application mitigates salinity stress in maize plants. Life 2022, 12, 1860. [Google Scholar] [CrossRef]
- Rasheed, Z. Therapeutic potentials of catalase: Mechanisms, applications, and future perspectives. Int. J. Health Sci. 2024, 18, 1–6. [Google Scholar]
- Yang, J.; Sun, C.; Zhang, Y.; Fu, D.; Zheng, X.; Yu, T. Induced resistance in tomato fruit by γ-aminobutyric acid for the control of alternaria rot caused by Alternaria alternata. Food Chem. 2017, 221, 1014–1020. [Google Scholar] [CrossRef]
- Soleiman, N.H.; Solis, I.; Sillero, J.C.; Herrera-Foesel, S.A.; Ammar, K.; Martínez, F. Evaluation of macroscopic and microscopic components of partial resistance to leaf rust in durum wheat. J. Phytopathol. 2014, 162, 359–366. [Google Scholar] [CrossRef]
- Johnston, C.O.; Browder, L.E. Seventh revision of the international register of physiologic races of Puccinia recondita f. sp. tritici. Plant Dis. 1966, 50, 756–760. [Google Scholar]
- Menzies, J.G.; Bélanger, R.R. Recent advances in cultural management of diseases of greenhouse crops. Can. J. Plant Pathol. 1996, 18, 186–193. [Google Scholar] [CrossRef]
- Parlevliet, J.E.; Kuiper, H.J. Partial resistance of barley to leaf rust, Puccinia hordei. IV. Effect of cultivar and development stage on infection frequency. Euphytica 1977, 26, 249–255. [Google Scholar] [CrossRef]
- Kemen, E.; Hahn, M.; Mendgen, K.; Struck, C. Different resistance mechanisms of Medicago truncatula ecotypes against the rust fungus Uromyces striatus. Phytopathology 2005, 95, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1974; pp. 673–684. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Ashraf, U.; Anjum, S.A.; Naseer, S.; Abbas, A.; Abrar, M.; Nawaz, M.; Luo, K. Gamma amino butyric acid (GABA) application modulated the morpho-physiological and yield traits of fragrant rice under well-watered and drought conditions. BMC Plant Biol. 2024, 24, 569. [Google Scholar] [CrossRef]
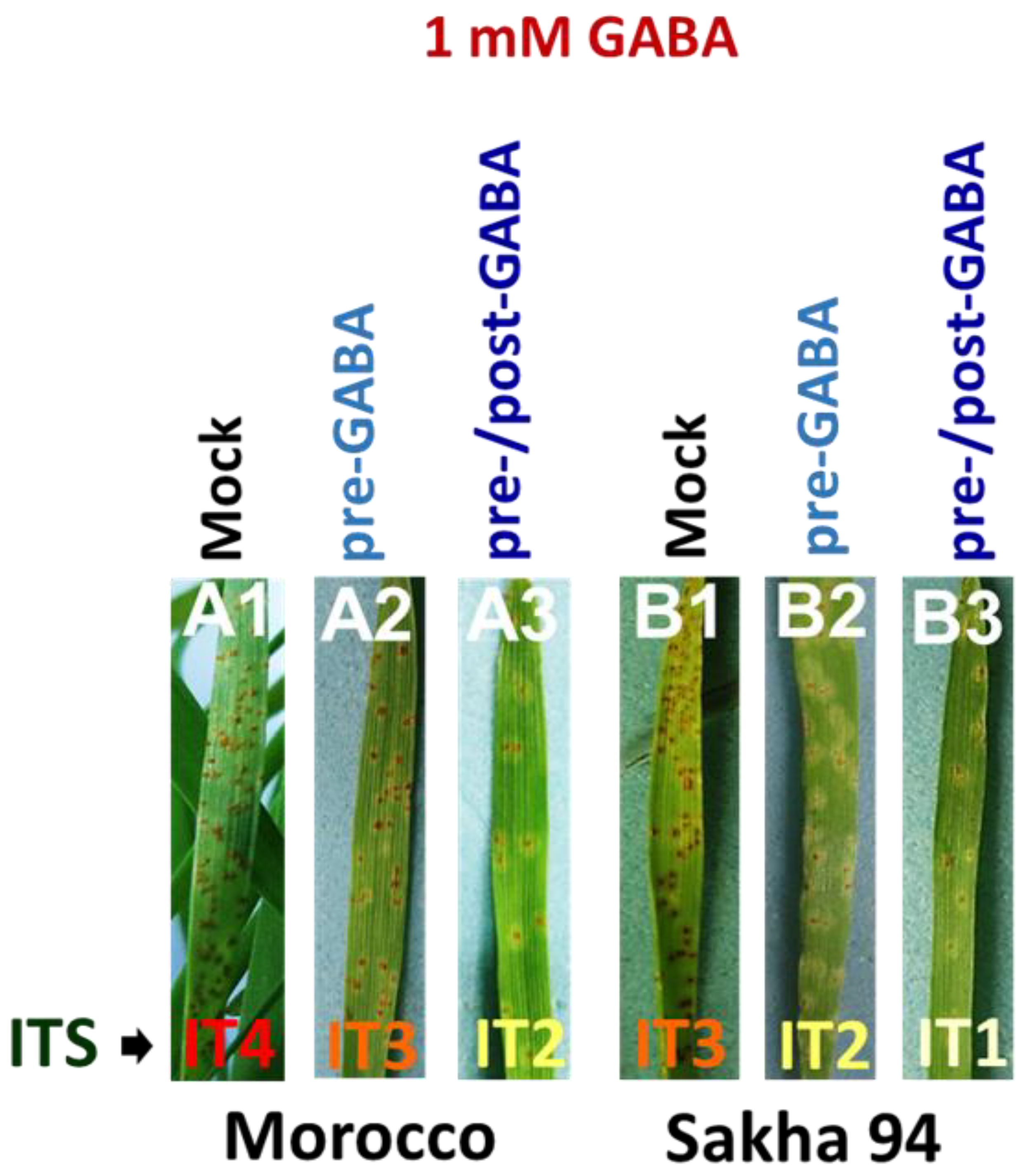


| Symptom Measurements | Mock | Pre-GABA Application | Pre-/Post-GABA Application | |||
|---|---|---|---|---|---|---|
| Morocco | Sakha 94 | Morocco | Sakha 94 | Morocco | Sakha 94 | |
| Infection type | 4 | 3 | 3 | 2 | 2 | 1 |
| No. of pustules/ (cm2) | 32.88 a | 23.42 b | 24 b | 17.83 c | 12.06 d | 9.16 d |
| Pustules size (mm2) | 0.947 a | 0.743 b | 0.586 c | 0.393 d | 0.356 d | 0.253 e |
| Incubation period (IP) | 6.25 d | 8.58 c | 7.78 c | 11.18 b | 11.18 b | 13.2 a |
| Latent period (IP) | 11.27 e | 14.22 cd | 13.15 d | 15.3 bc | 15.95 b | 20.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, H.B.; Lutfi, A.M.; Sayed, A.R.; Mahmoud, M.T.; Mostafa, S.A.; Ibrahim, Z.A.; Sharf-Eldin, A.A.; Abou-Zeid, M.A.; Ibrahim, M.F.M.; Thabet, M. Gamma-Aminobutyric Acid (GABA) as a Defense Booster for Wheat against Leaf Rust Pathogen (Puccinia triticina). Plants 2024, 13, 2792. https://doi.org/10.3390/plants13192792
Khalil HB, Lutfi AM, Sayed AR, Mahmoud MT, Mostafa SA, Ibrahim ZA, Sharf-Eldin AA, Abou-Zeid MA, Ibrahim MFM, Thabet M. Gamma-Aminobutyric Acid (GABA) as a Defense Booster for Wheat against Leaf Rust Pathogen (Puccinia triticina). Plants. 2024; 13(19):2792. https://doi.org/10.3390/plants13192792
Chicago/Turabian StyleKhalil, Hala Badr, Abdullah Mohsen Lutfi, Ahmed Reyad Sayed, Mohamed Tharwat Mahmoud, Salah Abdelfatah Mostafa, Zeyad Ahmed Ibrahim, Asmaa A. Sharf-Eldin, Mohamed A. Abou-Zeid, Mohamed F. M. Ibrahim, and Marian Thabet. 2024. "Gamma-Aminobutyric Acid (GABA) as a Defense Booster for Wheat against Leaf Rust Pathogen (Puccinia triticina)" Plants 13, no. 19: 2792. https://doi.org/10.3390/plants13192792
APA StyleKhalil, H. B., Lutfi, A. M., Sayed, A. R., Mahmoud, M. T., Mostafa, S. A., Ibrahim, Z. A., Sharf-Eldin, A. A., Abou-Zeid, M. A., Ibrahim, M. F. M., & Thabet, M. (2024). Gamma-Aminobutyric Acid (GABA) as a Defense Booster for Wheat against Leaf Rust Pathogen (Puccinia triticina). Plants, 13(19), 2792. https://doi.org/10.3390/plants13192792











