New Chloroplast Microsatellites in Helichrysum italicum (Roth) G. Don: Their Characterization and Application for the Evaluation of Genetic Resources
Abstract
:1. Introduction
2. Results
2.1. Chloroplast Microsatellites Characterization
2.2. Primer Design and Marker Validation
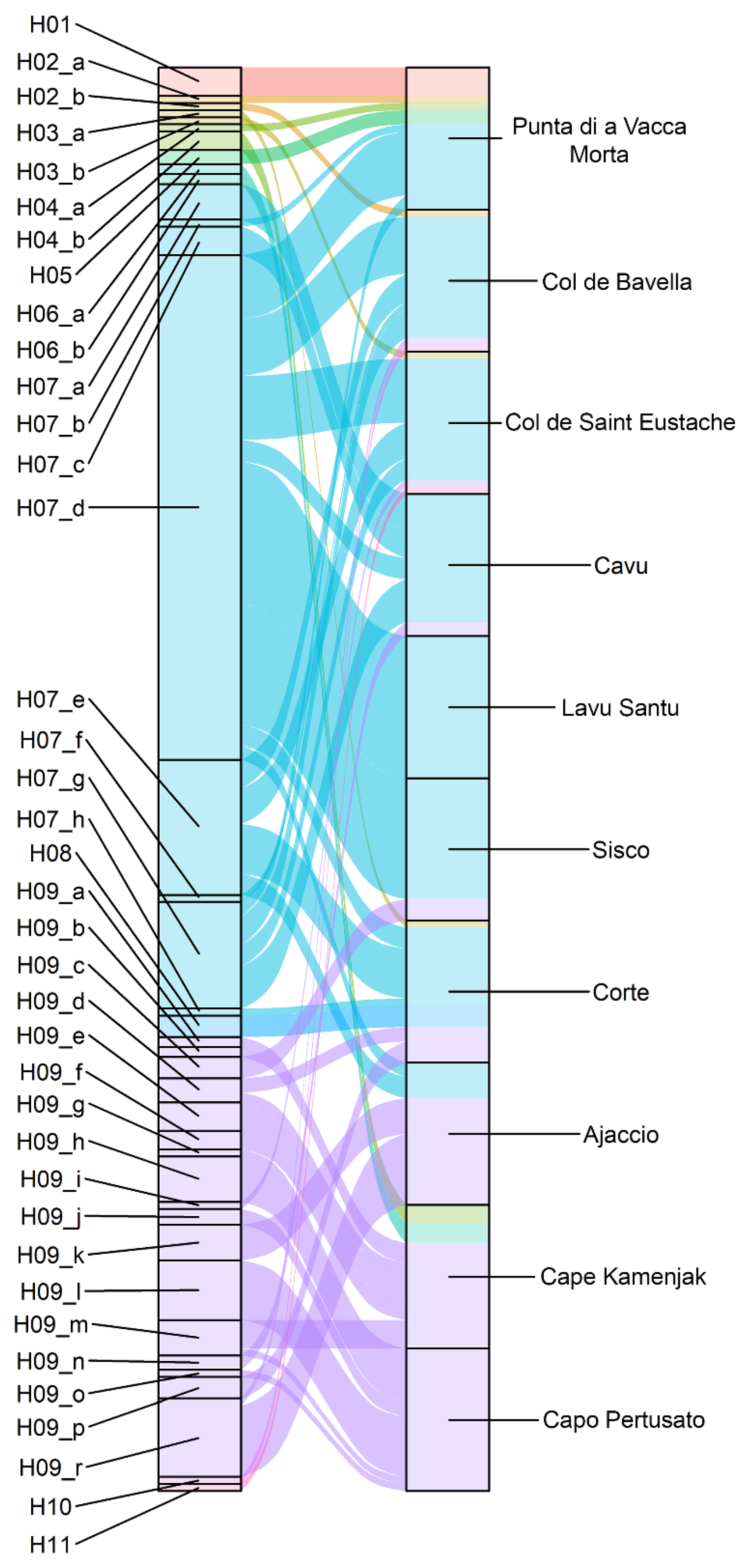
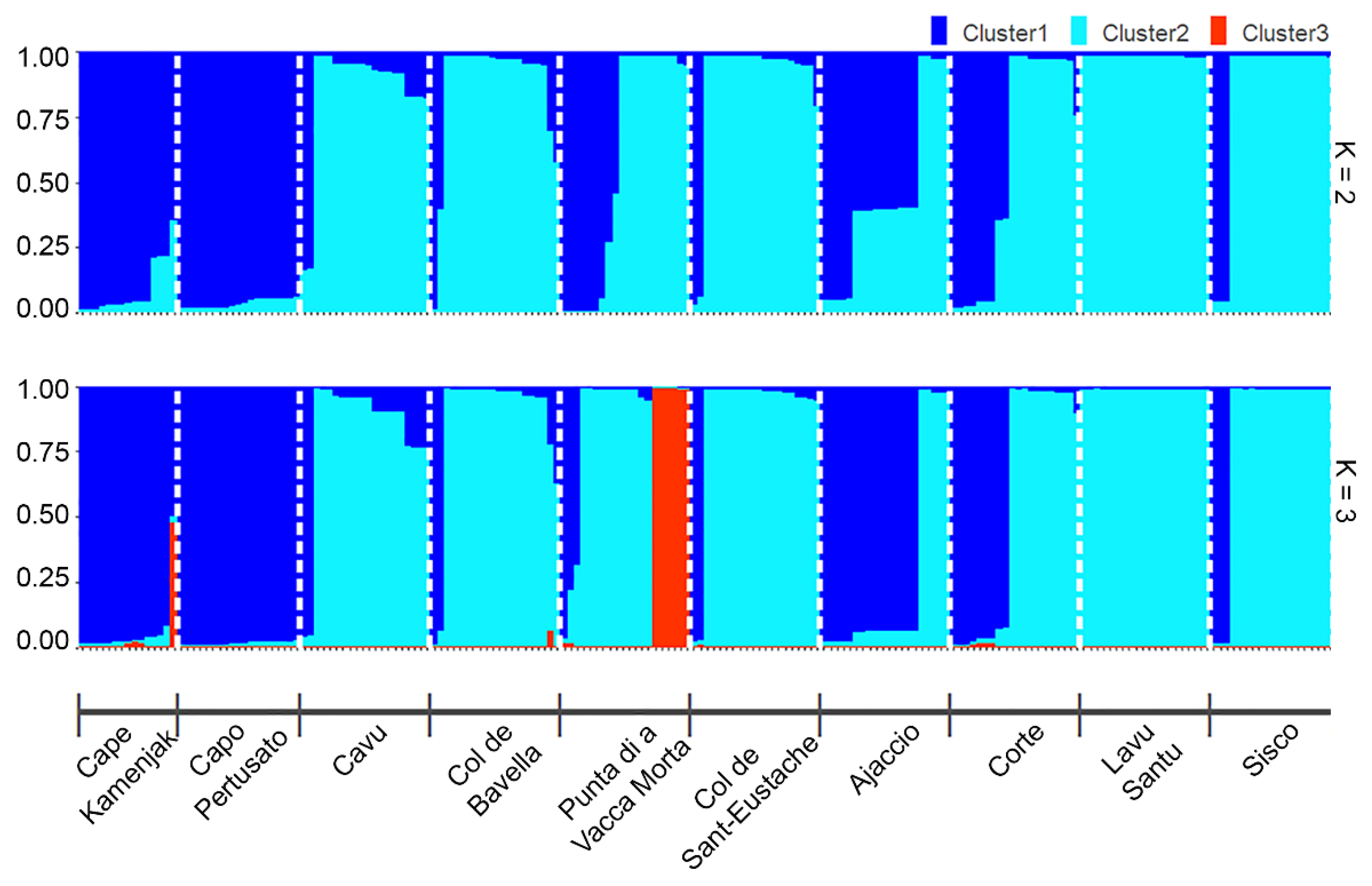
2.3. Genetic Structure of H. italicum Populations
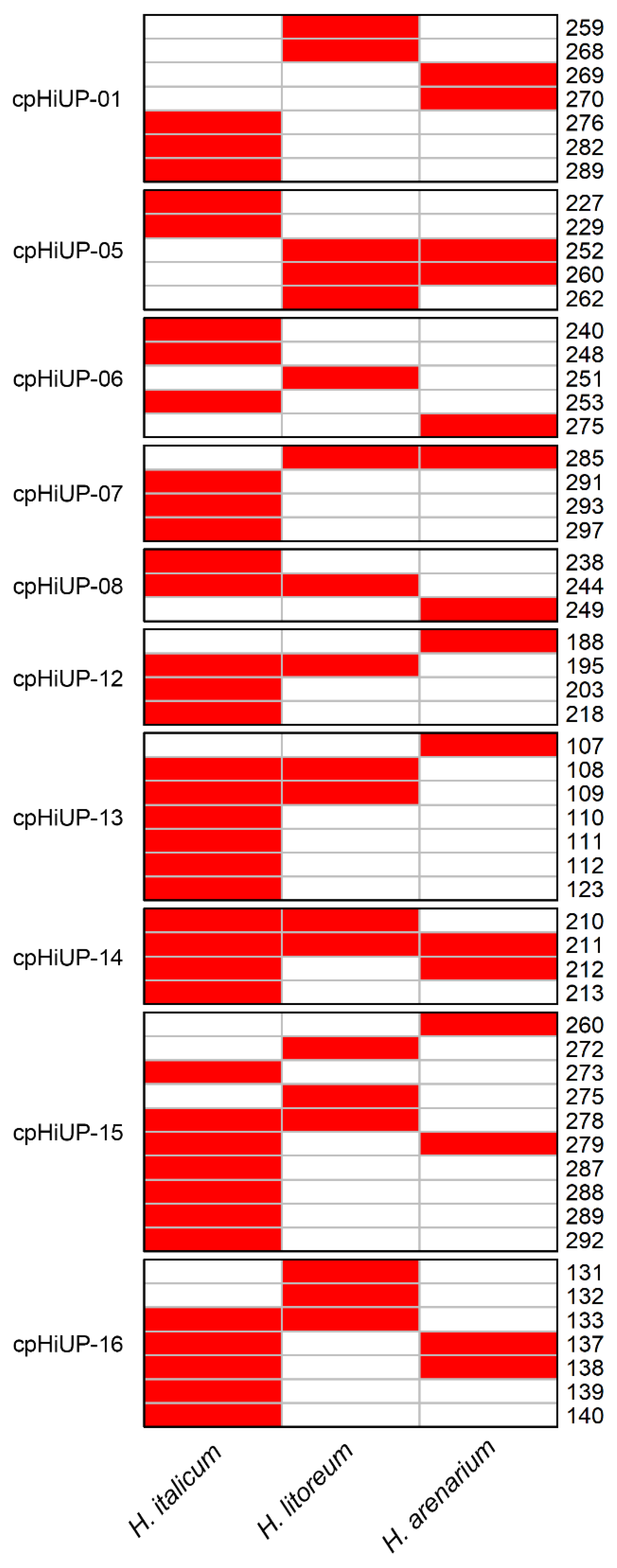
2.4. Transferability of cpSSR Markers in Helichrysum Species
3. Discussion
4. Materials and Methods
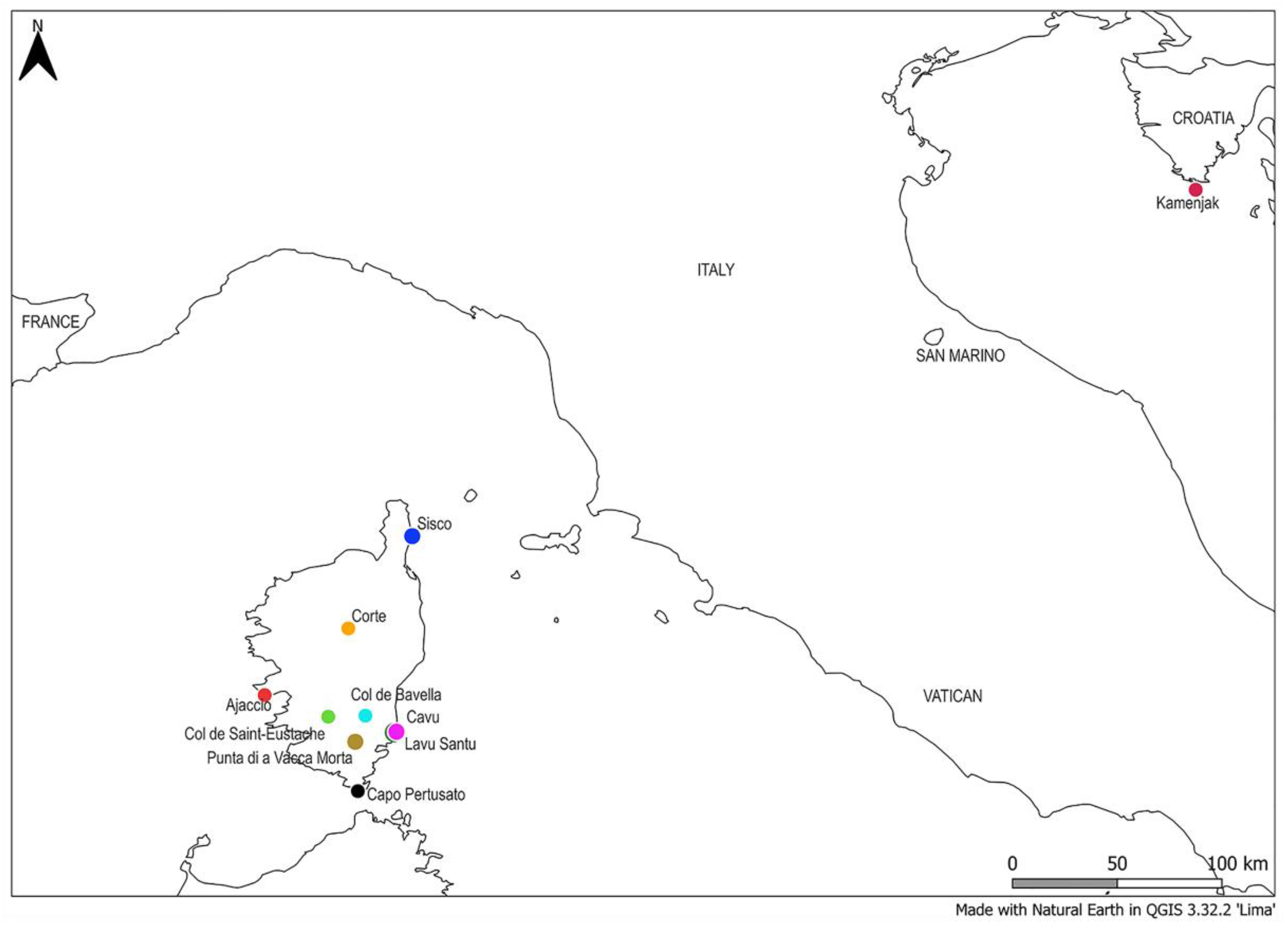
4.1. Plant Material and DNA Extraction
4.2. Chloroplast SSR Marker Identification and Primer Development
4.3. Chloroplast SSR Marker Preliminary Testing and Genotyping
4.4. Chloroplast SSR Marker Sequencing
4.5. Chloroplast SSR Markers Diversity Parameters, Haplotype Identification and Population Analysis
4.6. Cross-Species Transferability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antunes Viegas, D.; Palmeira-De-Oliveira, A.; Salgueiro, L.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Helichrysum italicum: From Traditional Use to Scientific Data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Taglialatela-Scafati, O.; Minassi, A.; Pollastro, F.; Ballero, M.; Maxia, A.; Sanna, C. Helichrysum italicum: The Sleeping Giant of Mediterranean Herbal Medicine. HerbalGram 2015, 105, 34–45. [Google Scholar]
- Kramberger, K.; Kenig, S.; Jenko Pražnikar, Z.; Kočevar Glavač, N.; Barlič-Maganja, D. A Review and Evaluation of the Data Supporting Internal Use of Helichrysum italicum. Plants 2021, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, S.; Tadic, V.; Skala, D.; Zizovic, I. Separation of Phytochemicals from Helichrysum italicum: An Analysis of Different Isolation Techniques and Biological Activity of Prepared Extracts. Phytochemistry 2017, 138, 9–28. [Google Scholar] [CrossRef]
- Ninčević, T.; Grdiša, M.; Šatović, Z.; Jug-Dujaković, M. Helichrysum italicum (Roth) G. Don: Taxonomy, Biological Activity, Biochemical and Genetic Diversity. Ind. Crop. Prod. 2019, 138, 111487. [Google Scholar] [CrossRef]
- Galbany-Casals, M.; Blanco-Moreno, J.M.; Garcia-Jacas, N.; Breitwieser, I.; Smissen, R.D. Genetic Variation in Mediterranean Helichrysum italicum (Asteraceae; Gnaphalieae): Do Disjunct Populations of Subsp. Microphyllum Have a Common Origin? Plant Biol. 2011, 13, 678–687. [Google Scholar] [CrossRef]
- Galbany-Casals, M.; Sáez, L.; Benedí, C. A Taxonomic Revision of Helichrysum Sect. Stoechadina (Asteraceae, Gnaphalieae). Can. J. Bot. 2006, 84, 1203–1232. [Google Scholar] [CrossRef]
- Herrando-Moraira, S.; Blanco-Moreno, J.M.; Sáez, L.; Galbany-Casals, M. Re-Evaluation of the Helichrysum italicum Complex (Compositae: Gnaphalieae): A New Species from Majorca (Balearic Islands). Collect. Bot. 2016, 35, e009. [Google Scholar] [CrossRef]
- Tomičić, M.; Magdić, M.; Peršić, M.; Zec Vojinović, M.; Dudaš, S. Morphometric Characteristics of Helichrysum italicum (Roth.) G. Don from Northwestern Coast of Istria. J. Polytech. Rij. 2022, 10, 453–466. [Google Scholar] [CrossRef]
- Bianchini, A.; Santoni, F.; Paolini, J.; Bernardini, A.-F.; Mouillot, D.; Costa, J. Partitioning the Relative Contributions of Inorganic Plant Composition and Soil Characteristics to the Quality of Helichrysum italicum subsp. italicum (Roth) G. Don Fil. Essential Oil. Chem. Biodiversity 2009, 6, 1014–1033. [Google Scholar] [CrossRef]
- Aghababyan, M.; Greuter, W.; Mazzola, P.; Raimondo, F.M. On the Taxonomy and Nomenclature of Gnaphalium angustifolium Lam. and Helichrysum litoreum Guss. (Compositae). Bocconea 2009, 23, 157–163. [Google Scholar]
- Baruca Arbeiter, A.; Hladnik, M.; Jakše, J.; Bandelj, D. First Set of Microsatellite Markers for Immortelle (Helichrysum italicum (Roth) G. Don): A Step towards the Selection of the Most Promising Genotypes for Cultivation. Ind. Crop. Prod. 2021, 162, 113298. [Google Scholar] [CrossRef]
- Hladnik, M.; Baruca Arbeiter, A.; Bandelj, D. Sequence Characterization of ITS Regions of Immortelle Helichrysum italicum (Roth) G. Don from the East Adriatic Coast. Genes 2023, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Morone-Fortunato, I.; Montemurro, C.; Ruta, C.; Perrini, R.; Sabetta, W.; Blanco, A.; Lorusso, E.; Avato, P. Essential Oils, Genetic Relationships and in Vitro Establishment of Helichrysum italicum (Roth) G. Don ssp. italicum from Wild Mediterranean Germplasm. Ind. Crop. Prod. 2010, 32, 639–649. [Google Scholar] [CrossRef]
- Hladnik, M.; Baruca Arbeiter, A.; Knap, T.; Jakše, J.; Bandelj, D. The Complete Chloroplast Genome of Helichrysum italicum (Roth) G. Don (Asteraceae). Mitochondrial DNA Part. B 2019, 4, 1036–1037. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast Genomes: Diversity, Evolution, and Applications in Genetic Engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef]
- Kan, J.; Nie, L.; Mi, Z.; Liu, X.; Xu, D.; Tembrock, L.R.; Wu, Z.; Hong, Z.; Kan, J.; Nie, L.; et al. Insights into Aquilaria Phylogenetics through Comparative Plastomic Resources. For. Res. 2024, 4, e030. [Google Scholar] [CrossRef]
- Wang, J.; Kan, S.; Liao, X.; Zhou, J.; Tembrock, L.R.; Daniell, H.; Jin, S.; Wu, Z. Plant Organellar Genomes: Much Done, Much More to Do. Trends Plant Sci. 2024, 29, 754–769. [Google Scholar] [CrossRef]
- CBOL Plant Working Group; Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; et al. A DNA Barcode for Land Plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef]
- Nazar, N.; Howard, C.; Slater, A.; Sgamma, T. Challenges in Medicinal and Aromatic Plants DNA Barcoding—Lessons from the Lamiaceae. Plants 2022, 11, 137. [Google Scholar] [CrossRef]
- Provan, J.; Powell, W.; Hollingsworth, P.M. Chloroplast Microsatellites: New Tools for Studies in Plant Ecology and Evolution. Trends Ecol. Evol. 2001, 16, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Peakall, R. Chloroplast Simple Sequence Repeats (cpSSRs): Technical Resources and Recommendations for Expanding cpSSR Discovery and Applications to a Wide Array of Plant Species. Mol. Ecol. Resour. 2009, 9, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the Number of Clusters of Individuals Using the Software Structure: A Simulation Study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, S.; Erdoğan, Ü.; Mutlucan, M. The Scent Compounds of Immortelle Ecotypes (Helichrysum italicum (Roth) G. Don.) Grown in Türkiye and Its New Products (Absolute and Concrete). S. Afr. J. Bot. 2023, 158, 301–311. [Google Scholar] [CrossRef]
- Cheng, Y.; De Vicente, M.C.; Meng, H.; Guo, W.; Tao, N.; Deng, X. A Set of Primers for Analyzing Chloroplast DNA Diversity in Citrus and Related Genera. Tree Physiol. 2005, 25, 661–672. [Google Scholar] [CrossRef]
- Islam, M.A.; Shivaraj, S.M.; Kumar, V.; Phad, D.S.; Sonah, H.; Tripathi, S.B.; Deshmukh, R.K. Development of Chloroplast Microsatellite Markers in Capsicum: Insight into Evolution of Bhut Jolokia—A Clad of Ghost Chilli Landraces. Indian J. Genet. Plant Breed. 2021, 81, 93–100. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, L.; Li, Y.; Yang, H.; Hu, X.; Song, C.; Hou, X. Development and Characterization of Microsatellite Markers Based on the Chloroplast Genome of Tree Peony. Genes 2022, 13, 1543. [Google Scholar] [CrossRef]
- Liu, F.; Movahedi, A.; Yang, W.; Xu, D.; Jiang, C. The Complete Plastid Genome and Characteristics Analysis of Achillea millefolium. Funct. Integr. Genom. 2023, 23, 192. [Google Scholar] [CrossRef]
- Shi, X.; Xu, W.; Wan, M.; Sun, Q.; Chen, Q.; Zhao, C.; Sun, K.; Shu, Y. Comparative Analysis of Chloroplast Genomes of Three Medicinal Carpesium Species: Genome Structures and Phylogenetic Relationships. PLoS ONE 2022, 17, e0272563. [Google Scholar] [CrossRef]
- Lukas, B.; Novak, J. The Complete Chloroplast Genome of Origanum vulgare L. (Lamiaceae). Gene 2013, 528, 163–169. [Google Scholar] [CrossRef]
- Zhang, D.; Tu, J.; Ding, X.; Guan, W.; Gong, L.; Qiu, X.; Huang, Z.; Su, H. Analysis of the Chloroplast Genome and Phylogenetic Evolution of Bidens pilosa. BMC Genom. 2023, 24, 113. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-Y.; Zhang, X.-S.; Zhang, D.-G.; Wang, Y.; Deng, T.; Huang, X.-H.; Kuang, T.-H.; Zhou, Q. Newly Reported Chloroplast Genome of Sinosenecio albonervius Y. Liu & Q. E. Yang and Comparative Analyses with Other Sinosenecio Species. BMC Genom. 2022, 23, 639. [Google Scholar] [CrossRef]
- Vieira, M.L.C.; Santini, L.; Diniz, A.L.; Munhoz, C.D.F. Microsatellite Markers: What They Mean and Why They Are so Useful. Genet. Mol. Biol. 2016, 39, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.L.; Borland, A.M.; Gustafsson, M.H.G.; Wolff, K. Causes of Size Homoplasy Among Chloroplast Microsatellites in Closely Related Clusia Species. J. Mol. Evol. 2004, 58, 182–190. [Google Scholar] [CrossRef]
- Peakall, R.; Gilmore, S.; Keys, W.; Morgante, M.; Rafalski, A. Cross-Species Amplification of Soybean (Glycine max) Simple Sequence Repeats (SSRs) within the Genus and Other Legume Genera: Implications for the Transferability of SSRs in Plants. Mol. Biol. Evol. 1998, 15, 1275–1287. [Google Scholar] [CrossRef]
- Carbonell-Caballero, J.; Alonso, R.; Ibañez, V.; Terol, J.; Talon, M.; Dopazo, J. A Phylogenetic Analysis of 34 Chloroplast Genomes Elucidates the Relationships between Wild and Domestic Species within the Genus Citrus. Mol. Biol. Evol. 2015, 32, 2015–2035. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Xu, Y.; Yu, B.; Wang, L.; Qu, X. Comparative and Phylogenetic Analysis of Asparagus meioclados Levl. and Asparagus munitus Wang et S. C. Chen Plastomes and Utility of Plastomes Mutational Hotspots. Sci. Rep. 2023, 13, 15622. [Google Scholar] [CrossRef]
- Lācis, G.; Kārkliņa, K.; Bartulsons, T.; Stalažs, A.; Jundzis, M.; Baļķe, I.; Ruņģis, D.; Strautiņa, S. Genetic Structure of a Ribes Genetic Resource Collection: Inter- and Intra- Specific Diversity Revealed by Chloroplast DNA Simple Sequence Repeats (cpSSRs). Sci. Hortic. 2022, 304, 111285. [Google Scholar] [CrossRef]
- Ninčević, T.; Jug-Dujaković, M.; Grdiša, M.; Liber, Z.; Varga, F.; Pljevljakušić, D.; Šatović, Z. Population Structure and Adaptive Variation of Helichrysum italicum (Roth) G. Don along Eastern Adriatic Temperature and Precipitation Gradient. Sci. Rep. 2021, 11, 24333. [Google Scholar] [CrossRef]
- Marini, L.; Bini, L.; Gori, M.; Biricolti, S.; Galbany-Casals, M.; Foggi, B.; Palchetti, E.; Bruschi, P. Genetic and Morphological Assessment of Helichrysum Mill. from Tuscan Archipelago (Italy). Sci. Hortic. 2023, 321, 112360. [Google Scholar] [CrossRef]
- Ljubičić, I.; Paulik, H.; Bogdanović, S. Habitat Mapping of Protected Landscape of Donji Kamenjak, Istria (Croatia). J. Cent. Eur. Agric. 2020, 21, 676–685. [Google Scholar] [CrossRef]
- Rogstad, S.H. Saturated NaCl-CTAB Solution as a Means of Field Preservation of Leaves for DNA Analyses. Taxon 1992, 41, 701–708. [Google Scholar] [CrossRef]
- Japelaghi, R.H.; Haddad, R.; Garoosi, G.-A. Rapid and Efficient Isolation of High Quality Nucleic Acids from Plant Tissues Rich in Polyphenols and Polysaccharides. Mol. Biotechnol. 2011, 49, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-Web: A Web Server for Microsatellite Prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef] [PubMed]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST Databases for the Development and Characterization of Gene-Derived SSR-Markers in Barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and Modifications of Primer Design Program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Schuelke, M. An Economic Method for the Fluorescent Labeling of PCR Fragments. Nat. Biotechnol. 2000, 18, 233–234. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Eliades, N.-G.; Eliades, D.G. HAPLOTYPE ANALYSIS: Software for Analysis of Haplotype Data. Distributed by the Authors. Forest Genetics and Forest Tree Breeding, Georg-August University Goettingen, Germany. 2009. Available online: http://www.uni-goettingen.de/en/134935.html (accessed on 29 August 2024).
- Bruvo, R.; Michiels, N.K.; D’souza, T.G.; Schulenburg, H. A Simple Method for the Calculation of Microsatellite Genotype Distances Irrespective of Ploidy Level. Mol. Ecol. 2004, 13, 2101–2106. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R Package for Genetic Analysis of Populations with Clonal, Partially Clonal, and/or Sexual Reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M. Pophelper: An R Package and Web App to Analyse and Visualize Population Structure. Mol. Ecol. Resour. 2017, 17, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-Joining Networks for Inferring Intraspecific Phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
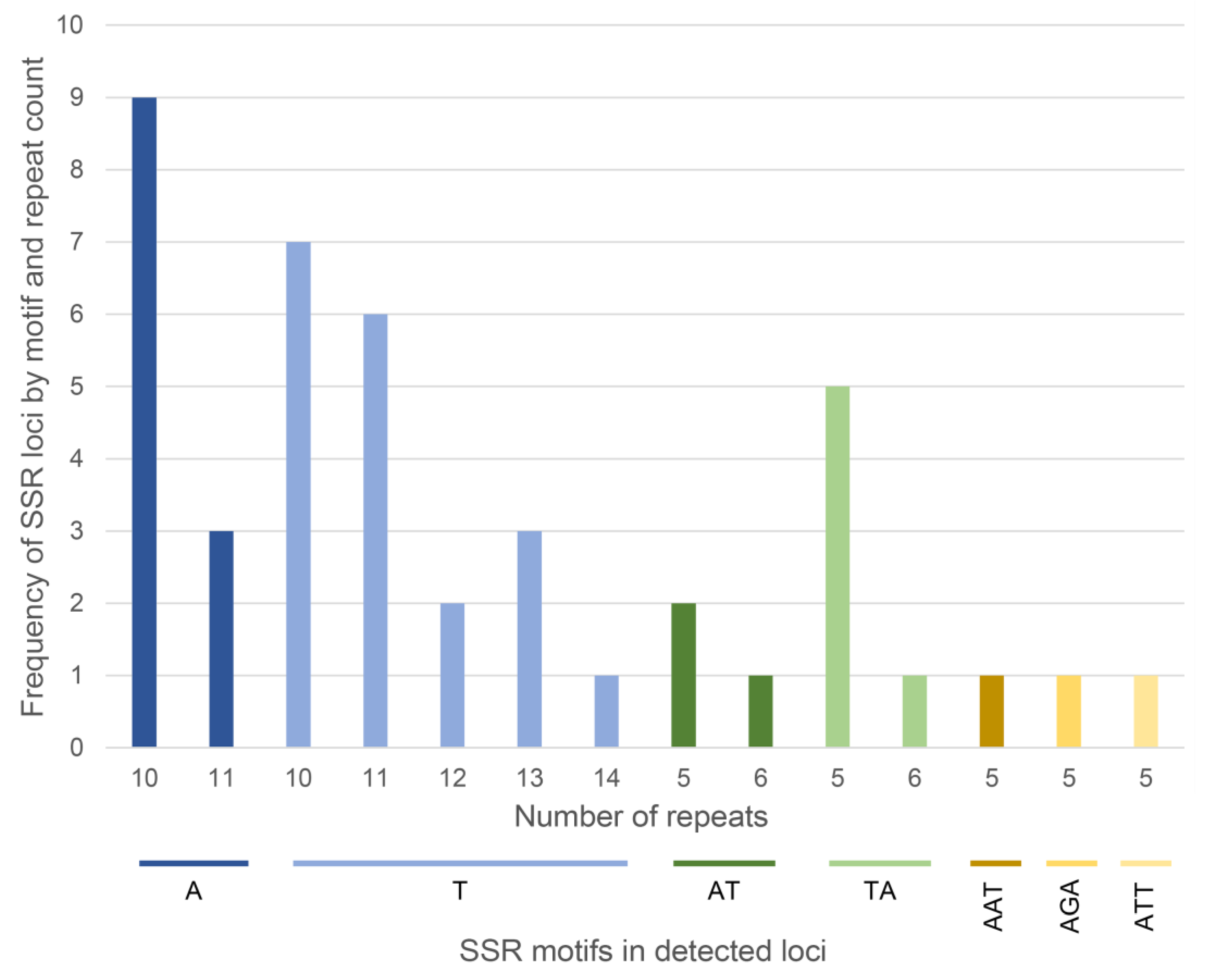

| Locus | Repeat Motif | Primer Sequences (5′-3′) | Location/ Region | SSR Position | Expected Allele Size (bp) | Allele Size Range (bp) | GenBank Accession Number |
|---|---|---|---|---|---|---|---|
| cpHiUP-01 | (ATT)5 | F: *TTCATTGCACACGGCTTTCC R: TGATTTGGCCAATCATGAATGAT | tRNK-UUU intron | 2063–2077 | 283 | 276–289 | PP747094 |
| cpHiUP-02 | (TA)5 | F: *AGGTAGTCCTTTGTGGCTGC R: TGAGACTCGAATTCCCGCTG | RpoC1 | 18669–18678 | 271 | 271 | PP747095 |
| cpHiUP-03 | (AT)5 | F: *GCCCGTAAAGGAGTTGTGGA R: TTGACAAGCCCAACCCCAAT | RpoC2 | 19665–19674 | 266 | 266 | PP747096 |
| cpHiUP-04 | (AT)5 | F: *ACCCGCCCAAAATGAAGTCA R: AGGCAAGGAGGGGAAGGATA | intergenic | 36185–36194 | 275 | 253–293 | PP747101 |
| cpHiUP-05 | (TA)6 | F: *TACTTCTGGTTCCGGCGAAC R: TAGATCCGAACACTTGCCCC | intergenic | 41628–41639 | 228 | 227–229 | PP747102 |
| cpHiUP-06 | (AT)6 | F: *ATTGCAAATCACGCGATCGG R: TCCCTTCTAAGCGTTCTATTTCA | intergenic | 42072–42083 | 247 | 240–253 | PP747103 |
| cpHiUP-07 | (TA)5 | F: *ACGCGACATAAAGACTCCTTCT R: TCCTCTTTTCGCAAAAGCCA | intergenic | 46438–46447 | 294 | 291–297 | PP747104 |
| cpHiUP-08 | (TA)5 | F: *AGATCGAAGCGAGTACCTGC R: GGCAAACGCCTACGAAAAGA | intergenic | 67942–67951 | 244 | 238–244 | PP747105 |
| cpHiUP-09 | (TA)5 | F: *CTGAGACGACCCAGAAAGCA R: TTTCATCATCCGGCTCGAGC | petD intron | 77234–77243 | 260 | 260–264 | PP747106 |
| cpHiUP-10 | (AGA)5 | F: *AGAAACTGAACAACCTCGACA R: TCGTAATGGCCTATTCCATCGG | Ycf1 | 109291–109305 | 215 | 207–213 | PP747107 |
| cpHiUP-11 | (AAT)5 | F: *TGGACAAATTATTCACAGGCCG R: TTGTAGAGTGATTCTGCGCA | Ycf1 | 111479–111493 | 279 | 274 | PP747108 |
| cpHiUP-12 | (TA)5 | F: *GAGCAGCGTGTCTACCGATT R: TCGCGAGAATTCTCTAGTTGCA | intergenic | 123336–123345 | 202 | 195–218 | PP747109 |
| cpHiUP-13 | (T)13 | F: *AAGGGCTGATTTGCGGATGA R: TCCTTGCTTGCAACCCTTCT | intergenic | 26467–26479 | 112 | 108–123 | PP747097 |
| cpHiUP-14 | (T)11 | F: *TTGGGGGCGATGAAACAACT R: ACGAGCAATGCCATCACCTA | intergenic | 27974–27984 | 212 | 210–213 | PP747098 |
| cpHiUP-15 | (A)10 | F: *TGGACGCCTTTCATTGTGAT R: ACCCCTAGCCTTCCAAGCTA | intergenic | 29779–29788 | 287 | 273–292 | PP747099 |
| cpHiUP-16 | (T)10 | F: *AGTCACTTTGGTTCCCG R: AAAGAAAGGGAAGGGGCTCGCAT | intergenic | 34914–34923 | 134 | 133–140 | PP747100 |
| Locus | Na | Ne | I | h |
|---|---|---|---|---|
| cpHiUP-01 | 3 | 1.098 | 0.204 | 0.089 |
| cpHiUP-05 | 2 | 1.097 | 0.188 | 0.088 |
| cpHiUP-06 | 3 | 1.087 | 0.195 | 0.080 |
| cpHiUP-07 | 3 | 1.975 | 0.772 | 0.494 |
| cpHiUP-08 | 2 | 1.031 | 0.080 | 0.030 |
| cpHiUP-12 | 3 | 1.075 | 0.170 | 0.070 |
| cpHiUP-13 | 6 | 1.762 | 0.863 | 0.432 |
| cpHiUP-14 | 4 | 2.053 | 0.913 | 0.513 |
| cpHiUP-15 | 7 | 1.883 | 0.974 | 0.469 |
| cpHiUP-16 | 5 | 1.813 | 0.804 | 0.448 |
| Average | 3.8 | 1.487 | 0.516 | 0.271 |
| Population | N | No. of alleles | No. of Private Alleles | I | A | P | Ne | Rh | He | D2sh |
|---|---|---|---|---|---|---|---|---|---|---|
| Kamenjak | 15 | 25 | 2 | 0.571 | 9 | 7 | 7.258 | 8.000 | 0.924 | 13.914 |
| Pertusato | 19 | 15 | 2 | 0.213 | 6 | 5 | 3.374 | 4.333 | 0.743 | 1.553 |
| Cavu | 20 | 15 | / | 0.239 | 5 | 3 | 4.444 | 3.938 | 0.816 | 0.524 |
| Col de Bavella | 20 | 19 | 1 | 0.268 | 7 | 3 | 4.000 | 4.998 | 0.789 | 7.319 |
| Punta di a Vacca Morta | 20 | 23 | 1 | 0.665 | 7 | 5 | 3.704 | 5.144 | 0.768 | 86.404 |
| Col de Saint-Eustache | 20 | 17 | 1 | 0.229 | 6 | 3 | 3.390 | 4.241 | 0.742 | 1.947 |
| Ajaccio | 20 | 15 | 1 | 0.280 | 4 | 1 | 2.899 | 2.939 | 0.689 | 30.806 |
| Corte | 20 | 22 | 2 | 0.457 | 7 | 4 | 4.878 | 5.421 | 0.837 | 9.962 |
| Lavu Santu | 20 | 10 | / | 0.000 | 1 | 0 | 1.000 | 0.000 | 0.000 | 0.000 |
| Sisco | 20 | 14 | / | 0.169 | 2 | 1 | 1.342 | 0.991 | 0.268 | 0.671 |
| Mean | / | / | / | 0.309 | 5.400 | 3.200 | 3.629 | 4.000 | 0.658 | 15.310 |
| Population | Locus | Allele | Frequency |
|---|---|---|---|
| Kamenjak | cpHiUP-06 | 240 | 0.133 |
| Kamenjak | cpHiUP-16 | 140 | 0.067 |
| Pertusato | cpHiUP-15 | 279 | 0.053 |
| Pertusato | cpHiUP-15 | 292 | 0.053 |
| Col de Bavella | cpHiUP-12 | 218 | 0.050 |
| Punta di a Vacca Morta | cpHiUP-07 | 297 | 0.300 |
| Col de Saint-Eustache | cpHiUP-01 | 289 | 0.050 |
| Ajaccio | cpHiUP-15 | 273 | 0.250 |
| Corte | cpHiUP-08 | 238 | 0.150 |
| Corte | cpHiUP-13 | 112 | 0.050 |
| Population | N | c | Hs | Dst | Ht | Fst |
|---|---|---|---|---|---|---|
| Kamenjak | 15 | 0.077 | 0.067 | 0.019 | 0.086 | 0.224 |
| Pertusato | 19 | 0.098 | 0.069 | 0.030 | 0.099 | 0.306 |
| Cavu | 20 | 0.103 | 0.080 | 0.022 | 0.102 | 0.217 |
| Col de Bavella | 20 | 0.103 | 0.077 | 0.014 | 0.091 | 0.149 |
| Punta di a Vacca Morta | 20 | 0.103 | 0.075 | 0.015 | 0.091 | 0.169 |
| Col de Saint-Eustache | 20 | 0.103 | 0.073 | 0.014 | 0.087 | 0.163 |
| Ajaccio | 20 | 0.103 | 0.068 | 0.029 | 0.096 | 0.298 |
| Corte | 20 | 0.103 | 0.082 | 0.020 | 0.102 | 0.200 |
| Lavu Santu | 20 | 0.103 | 0.000 | 0.034 | 0.034 | 1.000 |
| Sisco | 20 | 0.103 | 0.026 | 0.026 | 0.052 | 0.496 |
| Total | 194 | 1.000 | 0.617 | 0.223 | 0.840 | 0.266 |
| Sampling Location | Species | Number of Samples |
|---|---|---|
| Croatia, Črišnjeva | H. italicum | 1 * |
| Croatia, Cape Kamenjak | H. italicum | 15 |
| France, Corsica, Capo Pertusato | H. italicum | 19 |
| France, Corsica, Cavu | H. italicum | 20 |
| France, Corsica, Col de Bavella | H. italicum | 20 |
| France, Corsica, Punta di a Vacca Morta | H. italicum | 20 |
| France, Corsica, Col de Saint-Eustache | H. italicum | 20 |
| France, Corsica, Ajaccio | H. italicum | 20 |
| France, Corsica, Corte | H. italicum | 20 |
| France, Corsica, Lavu Santu | H. italicum | 20 |
| France, Corsica, Sisco | H. italicum | 20 |
| Plants from purchased certified seeds, grown on experimental collection of the University of Primorska (Slovenia) (45°34′19″ N 13°46′32″ E) | H. litoreum | 8 |
| Slovenia, commercially available H. arenarium tea (Flora Ltd., Rogatec, Slovenia) | H. arenarium | 4 bulk samples |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hladnik, M.; Baruca Arbeiter, A.; Gabrovšek, P.; Tomi, F.; Gibernau, M.; Brana, S.; Bandelj, D. New Chloroplast Microsatellites in Helichrysum italicum (Roth) G. Don: Their Characterization and Application for the Evaluation of Genetic Resources. Plants 2024, 13, 2740. https://doi.org/10.3390/plants13192740
Hladnik M, Baruca Arbeiter A, Gabrovšek P, Tomi F, Gibernau M, Brana S, Bandelj D. New Chloroplast Microsatellites in Helichrysum italicum (Roth) G. Don: Their Characterization and Application for the Evaluation of Genetic Resources. Plants. 2024; 13(19):2740. https://doi.org/10.3390/plants13192740
Chicago/Turabian StyleHladnik, Matjaž, Alenka Baruca Arbeiter, Petra Gabrovšek, Félix Tomi, Marc Gibernau, Slavko Brana, and Dunja Bandelj. 2024. "New Chloroplast Microsatellites in Helichrysum italicum (Roth) G. Don: Their Characterization and Application for the Evaluation of Genetic Resources" Plants 13, no. 19: 2740. https://doi.org/10.3390/plants13192740









