Phytotoxic Activity of Sesquiterpene Lactones-Enriched Fractions from Cynara cardunculus L. Leaves on Pre-Emergent and Post-Emergent Weed Species and Putative Mode of Action
Abstract
:1. Introduction
2. Results and Discussion
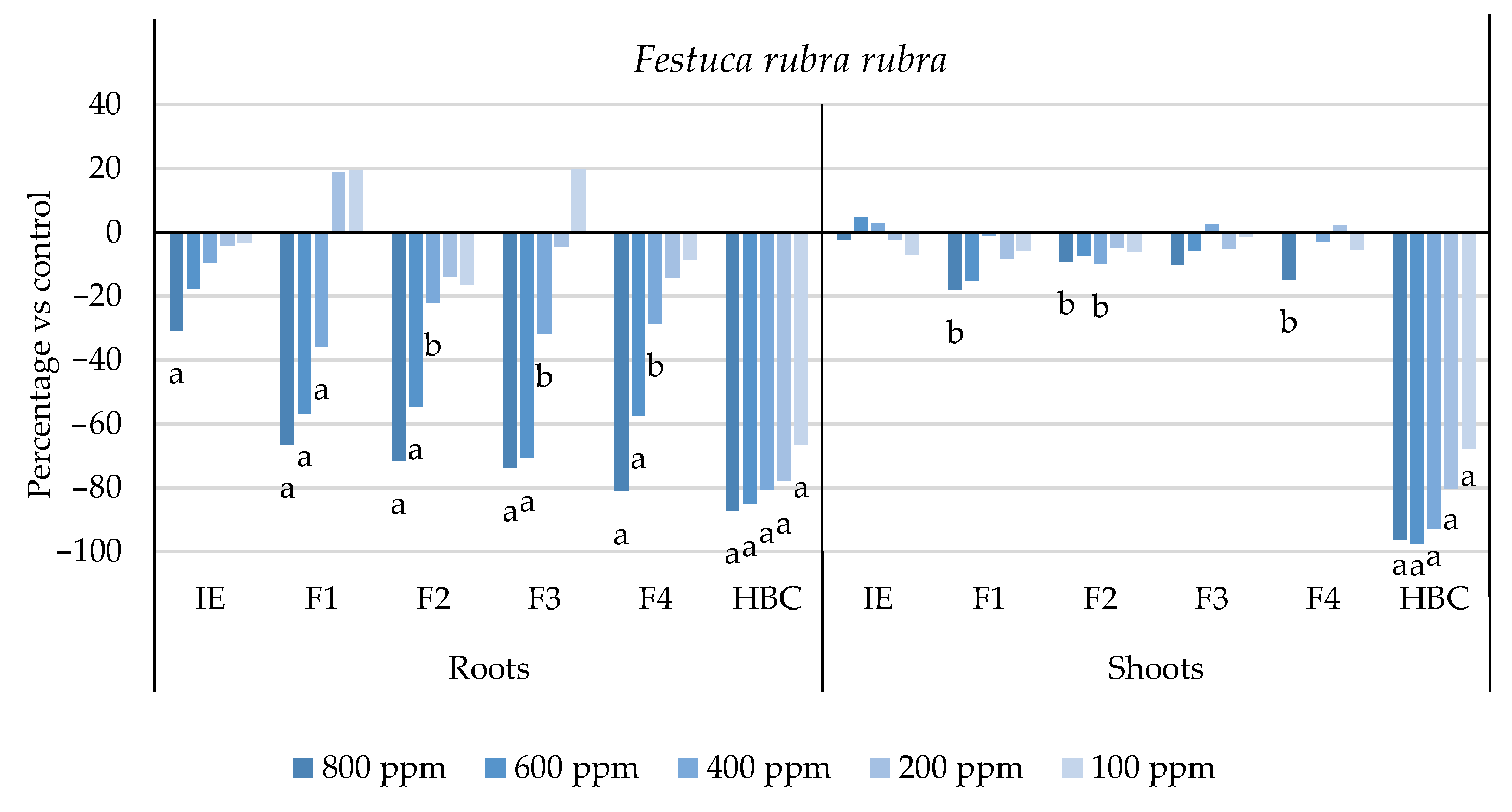
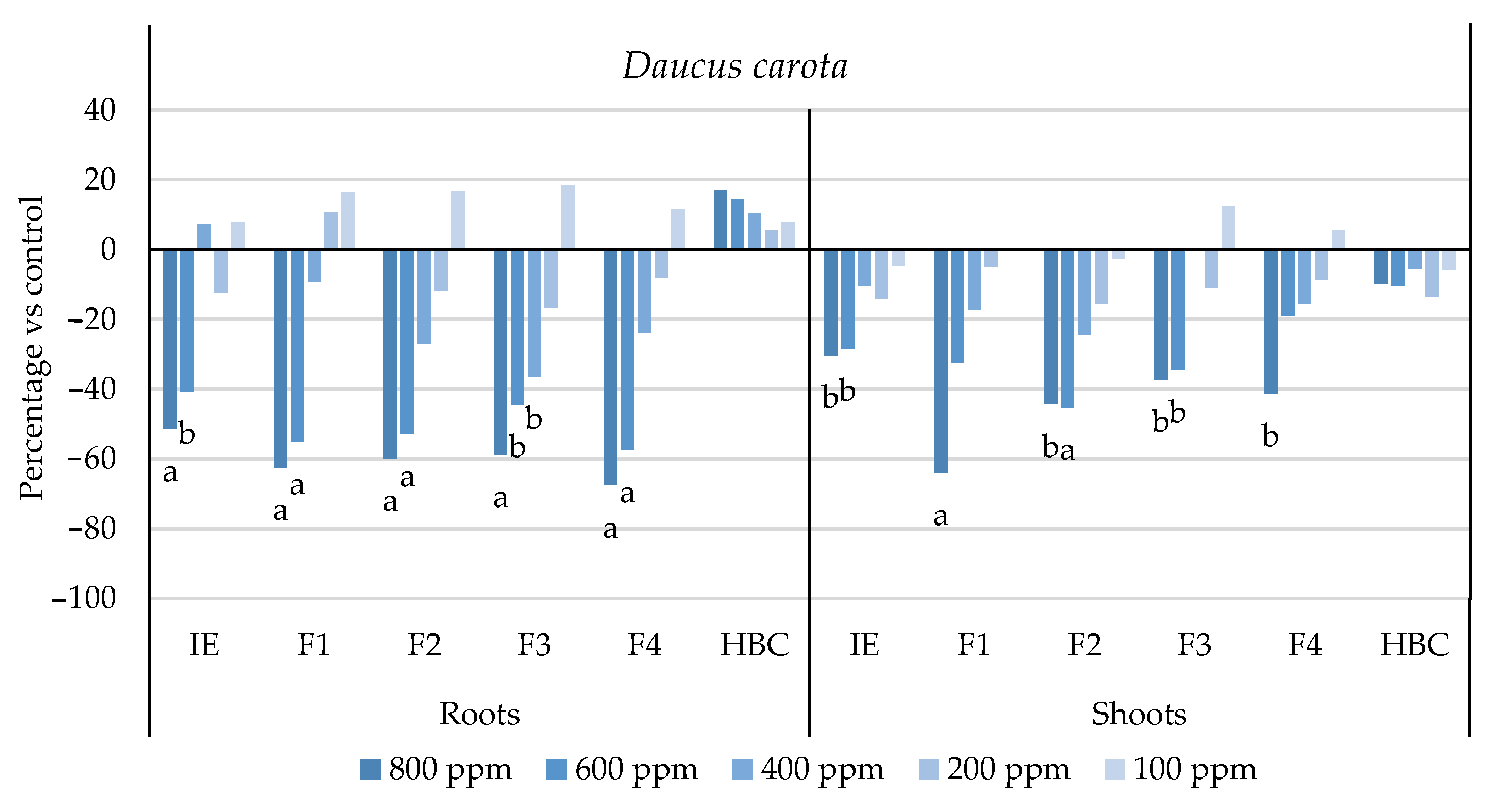
2.1. Phytotoxic Activity against a Panel of Weed Species
2.2. Phytotoxicity Bioassay on Portulaca oleracea on Post-Emergence Stage
2.2.1. Dry Weight vs. Control Ratio
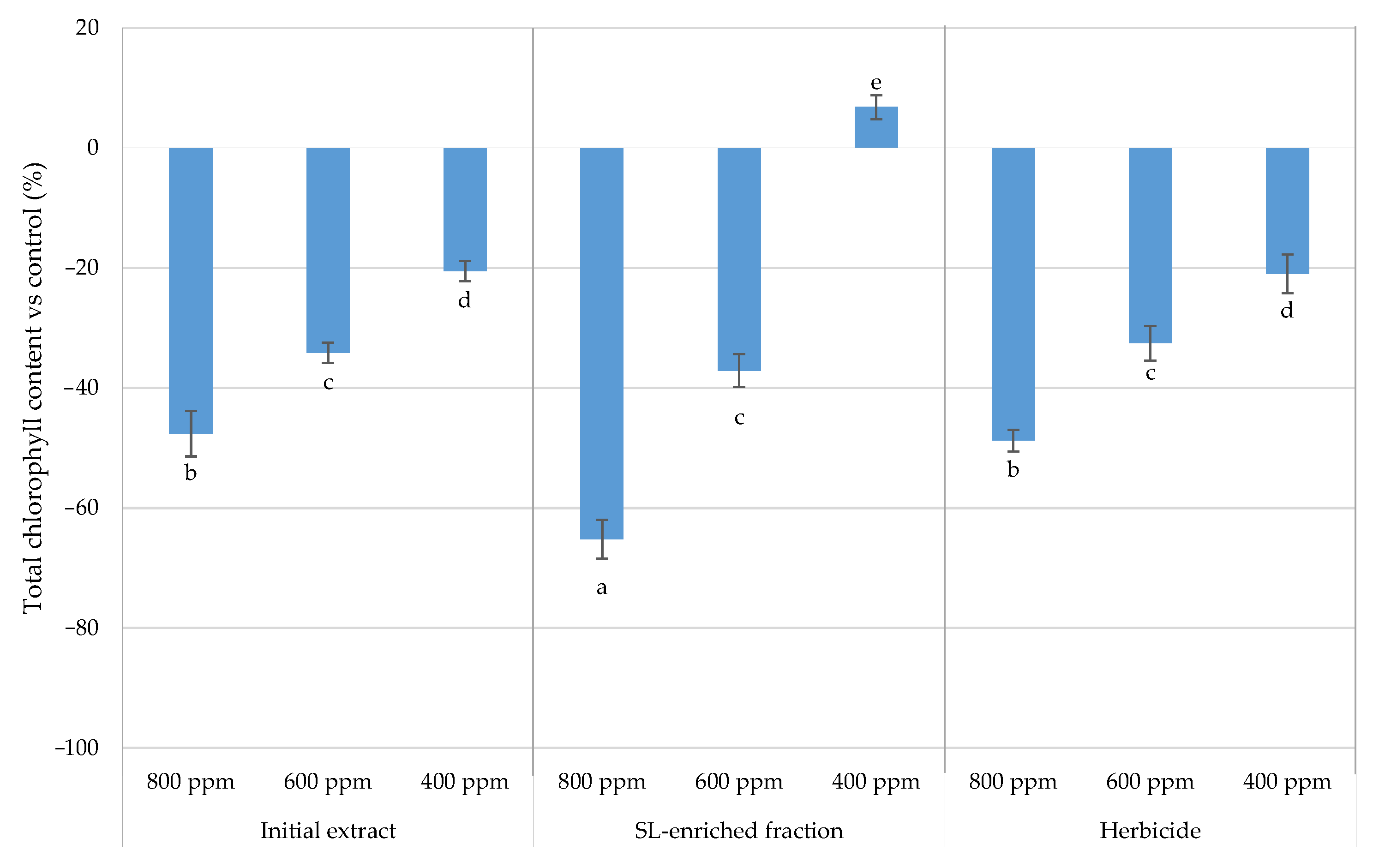
2.2.2. Total Chlorophyll Content
2.2.3. SOD Activity Measurement
2.2.4. Determination of Lipid Peroxidation
3. Material and Methods
3.1. Reagents
3.2. Cynara cardunculus L. SL-Enriched Fractions Obtention
3.3. Phytotoxic Activity Bioassay against a Panel of Weed Species
3.4. Calculation of IC50 Values and Cluster Analysis
3.5. Phytotoxicity Bioassay on the Metabolism of Adult Portulaca oleracea
3.5.1. Measurement of Dry Weight
3.5.2. Total Chlorophyll Content Determination
3.5.3. Enzymatic Extract Preparation for Biochemical Parameter Determination
3.5.4. Superoxide Dismutase (SOD)-Specific Activity Measurement
3.5.5. Malondialdehyde (MDA) Content Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scavo, A.; Mauromicale, G. Crop Allelopathy for Sustainable Weed Management in Agroecosystems: Knowing the Present with a View to the Future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Mohsin, S.M.; Borhannuddin Bhuyan, M.H.M.; Farha Bhuiyan, T.; Anee, T.I.; Awal, A.; Masud, C.; Nahar, K. Phytotoxicity, environmental and health hazards of herbicides: Challenges and ways forward. In Agrochemicals Detection, Treatment and Remediation. Pesticides and Chemical Fertilizers; Butterworth Heinemann: Hyderabad, India, 2020. [Google Scholar]
- Macías, F.A.; Molinillo, M.G.; Varela, R.M.; Galindo, J.C.G. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. Former. Pestic. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef] [PubMed]
- A Macías, F.; Mejías, F.J.R.; Molinillo, J.M.G. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Macías, F.A.; Galindo, J.C.G.; Castellano, D.; Velasco, R.F. Sesquiterpene Lactones with Potential Use as Natural Herbicide Models. 2. Guaianolides. J. Agric. Food Chem. 2000, 48, 5288–5296. [Google Scholar] [CrossRef] [PubMed]
- Rial, C.; Tomé, S.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Phytochemical Study of Safflower Roots (Carthamus tinctorius) on the Induction of Parasitic Plant Germination and Weed Control. J. Chem. Ecol. 2020, 46, 871–880. [Google Scholar] [CrossRef]
- Jmii, G.; Zorrilla, J.G.; Haouala, R. Allelochemicals from Thapsia garganica leaves for Lolium perenne L. control: The magic of mixtures. Chemoecology 2022, 32, 81–87. [Google Scholar] [CrossRef]
- El Marsni, Z.; Torres, A.; Varela, R.M.; Molinillo, J.M.G.; Casas, L.; Mantell, C.; de la Ossa, E.J.M.; Macias, F.A. Isolation of Bioactive Compounds from Sunflower Leaves (Helianthus annuus L.) Extracted with Supercritical Carbon Dioxide. J. Agric. Food Chem. 2015, 63, 6410–6421. [Google Scholar] [CrossRef]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Abdel-Razeek, N.; Soliman, S.A. Herbicidal Activity of Three Sesquiterpene Lactones on Wild Oat (Avena fatua) and Their Possible Mode of Action. Weed Sci. 2009, 57, 6–9. [Google Scholar] [CrossRef]
- Gominho, J.; Curt, M.D.; Lourenço, A.; Fernández, J.; Pereira, H. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenergy 2018, 109, 257–275. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Guerra, R.; Guerreiro, O.; Freire, C.S.R.; Silva, A.M.S.; Duarte, M.F.; Silvestre, A.J.D. Lipophilic Extracts of Cynara cardunculus L. var. altilis (DC): A Source of Valuable Bioactive Terpenic Compounds. J. Agric. Food Chem. 2013, 61, 8420–8429. [Google Scholar] [CrossRef] [PubMed]
- Scavo, A.; Rial, C.; Molinillo, J.M.; Varela, R.M.; Mauromicale, G.; Macias, F.A. The extraction procedure improves the allelopathic activity of cardoon (Cynara cardunculus var. altilis) leaf allelochemicals. Ind. Crop. Prod. 2019, 128, 479–487. [Google Scholar] [CrossRef]
- Rial, C.; Novaes, P.; Varela, R.M.; Molinillo, J.M.; Macias, F.A. Phytotoxicity of Cardoon (Cynara cardunculus) Allelochemicals on Standard Target Species and Weeds. J. Agric. Food Chem. 2014, 62, 6699–6706. [Google Scholar] [CrossRef] [PubMed]
- Brás, T.; Paulino, A.F.C.; Neves, L.A.; Crespo, J.G.; Duarte, M.F. Ultrasound assisted extraction of cynaropicrin from Cynara cardunculus leaves: Optimization using the response surface methodology and the effect of pulse mode. Ind. Crop. Prod. 2020, 150, 112395. [Google Scholar] [CrossRef]
- Rosa, D.; Brás, T.; Rial, C.; Varela, R.M.; Maçãs, B.; Macías, F.A.; Duarte, M.F. Sesquiterpene lactones enriched-fractions obtained from Cynara cardunculus extract diaultrafiltration. Ind. Crop. Prod. 2024, 218, 118926. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, A.; Lombardo, S.; Pesce, G.R.; Mauromicale, G. Allelopathic potential of leaf aqueous extracts from Cynara cardunculus L. on the seedling growth of two cosmopolitan weed species. Ital. J. Agron. 2019, 14, 78–83. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K.; Saxena, D.B.; Arora, V. Effect of Parthenin—A Sesquiterpene Lactone from Parthenium hysterophorus—On Early Growth and Physiology of Ageratum conyzoides. J. Chem. Ecol. 2002, 28, 2169–2179. [Google Scholar] [CrossRef]
- Rial, C.; García, B.F.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Macías, F.A. The Joint Action of Sesquiterpene Lactones from Leaves as an Explanation for the Activity of Cynara cardunculus. J. Agric. Food Chem. 2016, 64, 6416–6424. [Google Scholar] [CrossRef]
- Anese, S.; Rial, C.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Macías, F.A. Search of New Tools for Weed Control Using Piptocarpha rotundifolia, a Dominant Species in the Cerrado. J. Agric. Food Chem. 2021, 69, 8684–8694. [Google Scholar] [CrossRef]
- da Silva, B.P.; Nepomuceno, M.P.; Varela, R.M.; Torres, A.; Molinillo, J.M.G.; Alves, P.L.C.A.; Macías, F.A. Phytotoxicity Study on Bidens sulphurea Sch. Bip. as a Preliminary Approach for Weed Control. J. Agric. Food Chem. 2017, 65, 5161–5172. [Google Scholar] [CrossRef] [PubMed]
- Motmainna, M.; Juraimi, A.S.; Uddin, K.; Asib, N.B.; Islam, A.K.M.M.; Ahmad-Hamdani, M.S.; Berahim, Z.; Hasan, M. Physiological and biochemical responses of Ageratum conyzoides, Oryza sativa f. spontanea (weedy rice) and Cyperus iria to Parthenium hysterophorus methanol extract. Plants 2021, 10, 1205. [Google Scholar] [CrossRef]
- Batish, D.R.; Setia, N.; Singh, H.; Kohli, R. Phytotoxicity of lemon-scented eucalypt oil and its potential use as a bioherbicide. Crop. Prot. 2004, 23, 1209–1214. [Google Scholar] [CrossRef]
- Ladhari, A.; Zarrelli, A.; Di Meo, M.C.; Ghannem, M.; Ben Mimoun, M. Physiological mechanisms and adaptation strategies of Lactuca sativa L. in response to Olea europaea L. and Ficus carica L. allelochemicals. S. Afr. J. Bot. 2022, 147, 106–118. [Google Scholar] [CrossRef]
- Araniti, F.; Miras-Moreno, B.; Lucini, L.; Landi, M.; Abenavoli, M.R. Metabolomic, proteomic and physiological insights into the potential mode of action of thymol, a phytotoxic natural monoterpenoid phenol: The phytotoxic effect of thymol on adult plants of A. thaliana. Plant Physiol. Biochem. 2020, 153, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R.; Kapoor, A.; Zhu, J.-K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis Is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 2006, 18, 2051–2065. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Q. Effects of herbicide chlorimuron-ethyl on physiological mechanisms in wheat (Triticum aestivum). Ecotoxicol. Environ. Saf. 2006, 64, 190–197. [Google Scholar] [CrossRef]
- Mylona, P.V.; Polidoros, A.N.; Scandalios, J.G. Antioxidant gene responses to ROS-generating xenobiotics in developing and germinated scutella of maize. J. Exp. Bot. 2007, 58, 1301–1312. [Google Scholar] [CrossRef]
- Mahdavikia, F.; Saharkhiz, M.J.; Karami, A. Defensive response of radish seedlings to the oxidative stress arising from phenolic compounds in the extract of peppermint (Mentha × piperita L.). Sci. Hortic. 2017, 214, 133–140. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hegab, M.M.; Abdelgawad, H.; Abdelhamed, M.S.; Hammouda, O.; Pandey, R.; Kumar, V.; Zinta, G. Effects of tricin isolated from jungle rice (Echinochloa colona L.) on amylase activity and oxidative stress in wild oat (Avena fatua L.). Allelopath. J. 2013, 31, 345. [Google Scholar]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Rial, C.; Varela, R.M.; Molinillo, J.M.; Bautista, E.; Hernández, A.O.; Macías, F.A. Phytotoxicity evaluation of sesquiterpene lactones and diterpenes from species of the Decachaeta, Salvia and Podachaenium genera. Phytochem. Lett. 2016, 18, 68–76. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1938, 347, 39. [Google Scholar]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.K.; Nielsen, B.V.; Milledge, J.J. Effect of post-harvest conditions on antioxidant enzyme activity in Dunaliella tertiolecta biomass. Biocatal. Agric. Biotechnol. 2020, 27, 101661. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. Protein Protoc. Handb. 1996, 32, 15–20. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Scavo, A.; Restuccia, A.; Mauromicale, G. Allelopathy: General principles and basic aspects for agroecosystem control. In Sustainable Agriculture Reviews; Springer International Publishing: Cham, Switzerland, 2018; Volume 28, pp. 47–101. [Google Scholar] [CrossRef]
- Choudhary, C.S.; Behera, B.; Raza, B.; Mrunalini, K.; Bhoi, T.K.; Lal, M.K.; Nongmaithem, D.; Pradhan, S.; Song, B.; Das, T.K. Mechanisms of allelopathic interactions for sustainable weed management. Rhizosphere 2023, 25, 100667. [Google Scholar] [CrossRef]













| Fractions | Cynaropicrin (mg/g of Extract) | Aguerin B (mg/g of Extract) | Grosheimin (mg/g of Extract) | Total SL Concentration (mg/g of Extract) |
|---|---|---|---|---|
| Initial extract (IE) | 198.89 ± 2.03 c | 3.10 ± 0.02 d | 5.49 ± 0.02 c | 207.48 ± 2.07 c |
| Fraction 1 (F1) | 376.20 ± 3.86 a | 5.86 ± 0.09 b | 7.18 ± 0.17 b | 389.24 ± 4.12 a |
| Fraction 2 (F2) | 313.42 ± 8.46 b | 5.35 ± 0.05 c | 6.83 ± 0.68 b | 325.60 ± 9.19 b |
| Fraction 3 (F3) | 349.87 ± 6.82 a | 5.16 ± 0.10 c | 5.64 ± 0.13 c | 360.67 ± 7.05 a |
| Fraction 4 (F4) | 352.95 ± 19.88 a | 6.25 ± 0.08 a | 10.64 ± 0.29 a | 369.84 ± 20.25 a |
| IC50 (ppm) | ||||||
|---|---|---|---|---|---|---|
| IE | F1 | F2 | F3 | F4 | HBC | |
| T. repens | >800 a | 663.6 ± 1.2 b | >800 a | 527.9 ± 1.1 c | >800 a | >800 a |
| P. lanceolata | >800 a | 524.0 ± 1.1 e | 549.6 ± 1.1 d | 588.6 ± 1.1 b | 555.2 ± 1.1 c | 32.9 ± 1.3 f |
| D. glomerata | 583.8 ± 1.1 a | 323.7 ± 1.1 b | 375.1 ± 1.1 c | 356.0 ± 1.1 d | 597.2 ± 1.1 e | 321.3 ± 1.1 f |
| P. arundinacea | >800 a | 403.7 ± 1.1 c | 317.1 ± 1.2 e | 387.9 ± 1.1 d | 610.1 ± 1.1 b | 50.4 ± 1.2 f |
| L. rigidum | >800 a | 683.0 ± 1.1 d | 727.8 ± 1.1 c | >800 a | 749.8 ± 1.0 b | 91.4 ± 2.0 e |
| F. rubra rubra | >800 a | 529.6 ± 1.1 b | 623.0 ± 1.1 c | 485.8 ± 1.1 d | 559.8 ± 1.1 e | 28.9 ± 1.4 f |
| D. carota | 749.7 ± 1.1 b | 623.7 ± 1.1 c | 609.2 ± 1.1 d | 622.7 ± 1.2 c | 575.6 ± 1.1 e | >800 a |
| M. recutita | >800 a | 609.8 ± 1.1 c | 579.9 ± 1.1 d | 436.0 ± 1.2 f | 566.0 ± 1.2 e | 843.5 ± 2.1 b |
| T. incarnatum | >800 a | >800 a | >800 a | >800 a | >800 a | 1035.0 ± 1.9 b |
| P. oleracea | 394.5 ± 1.1 a | 306.0 ± 1.1 c | 300.2 ± 1.1 d | 293.4 ± 1.1 e | 308.7 ± 1.0 b | 13.5 ± 0.15 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, D.; Rial, C.; Brás, T.; Varela, R.M.; Macías, F.A.; Duarte, M.F. Phytotoxic Activity of Sesquiterpene Lactones-Enriched Fractions from Cynara cardunculus L. Leaves on Pre-Emergent and Post-Emergent Weed Species and Putative Mode of Action. Plants 2024, 13, 2758. https://doi.org/10.3390/plants13192758
Rosa D, Rial C, Brás T, Varela RM, Macías FA, Duarte MF. Phytotoxic Activity of Sesquiterpene Lactones-Enriched Fractions from Cynara cardunculus L. Leaves on Pre-Emergent and Post-Emergent Weed Species and Putative Mode of Action. Plants. 2024; 13(19):2758. https://doi.org/10.3390/plants13192758
Chicago/Turabian StyleRosa, Daniela, Carlos Rial, Teresa Brás, Rosa M. Varela, Francisco A. Macías, and Maria F. Duarte. 2024. "Phytotoxic Activity of Sesquiterpene Lactones-Enriched Fractions from Cynara cardunculus L. Leaves on Pre-Emergent and Post-Emergent Weed Species and Putative Mode of Action" Plants 13, no. 19: 2758. https://doi.org/10.3390/plants13192758







