Analysis of Main Components of Five Mulberry Varieties in Tropics
Abstract
1. Introduction
2. Results
2.1. Variation in Morphology and Brix of Different Mulberry Varieties and Ripeness Stages
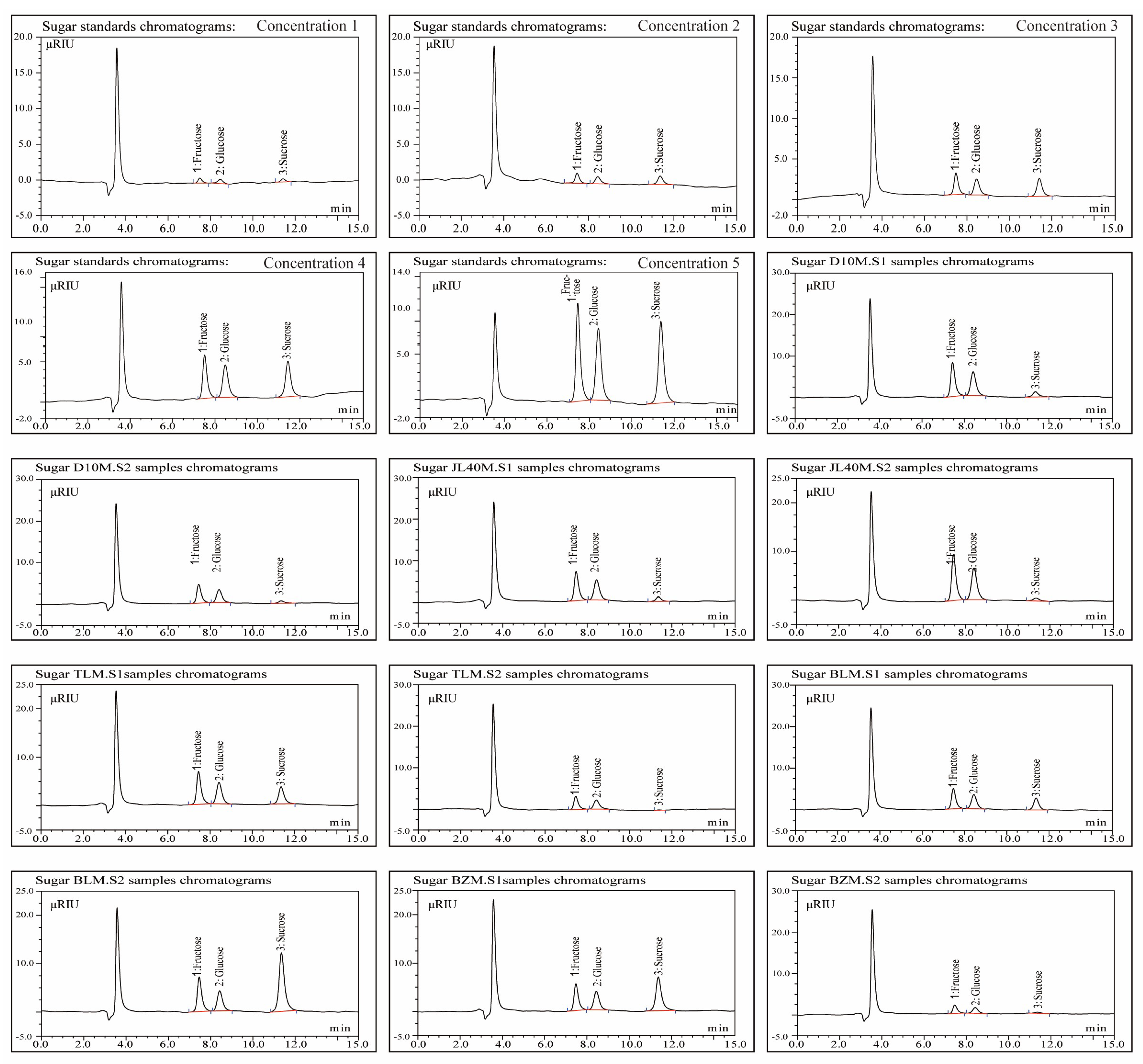
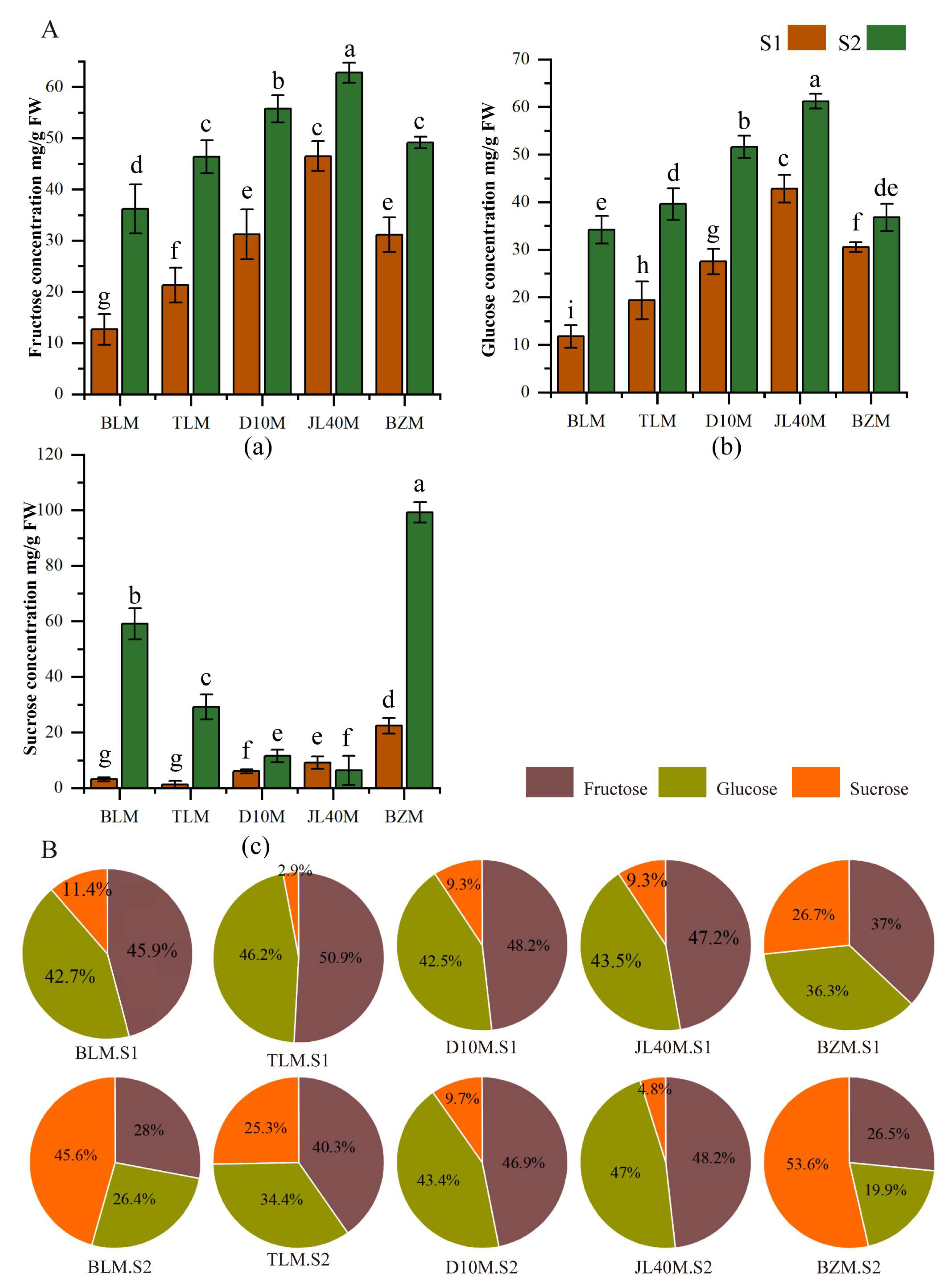
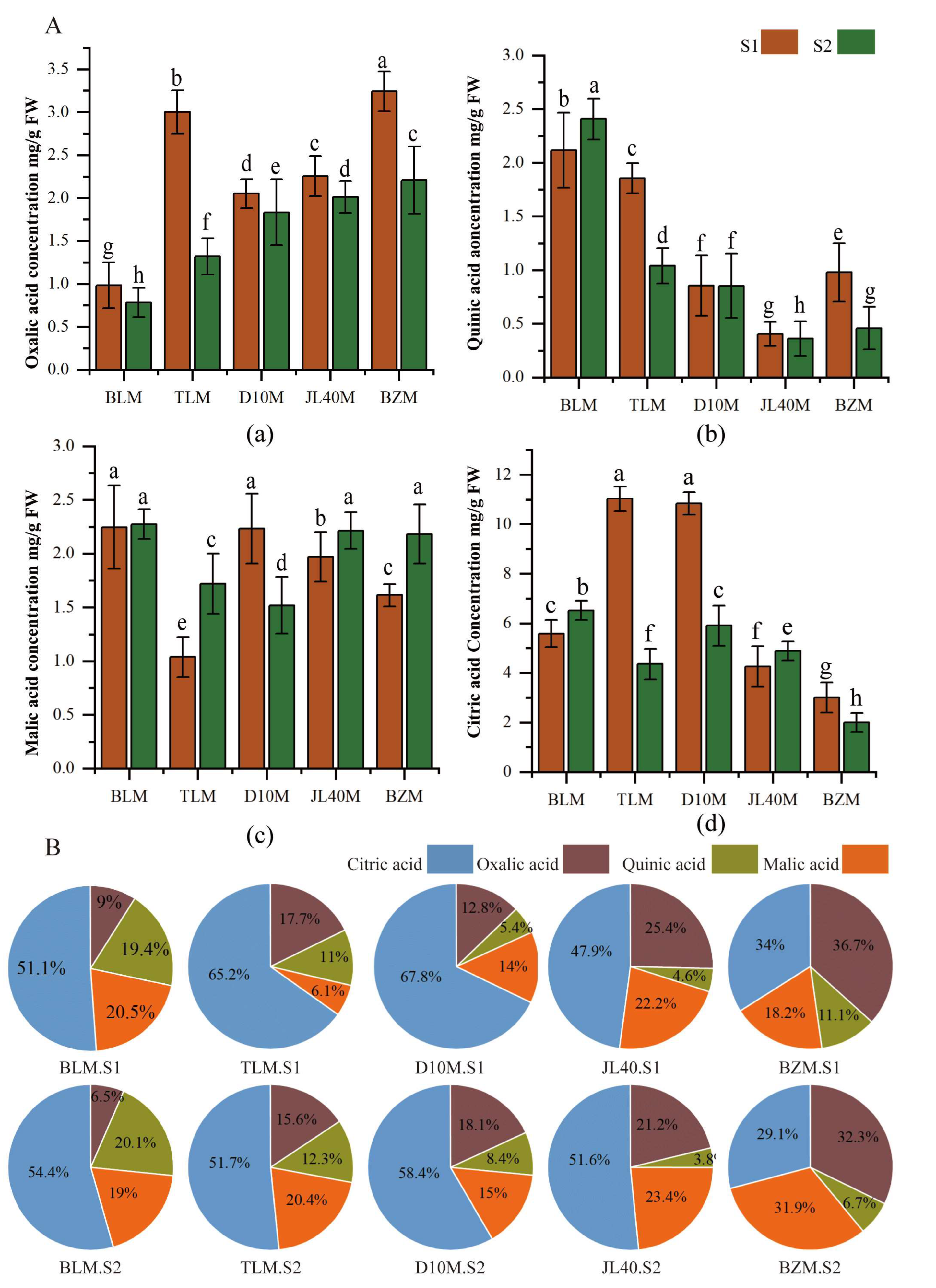
2.2. Sugar and Organic Acid Contents of Different Mulberry Varieties and Ripeness Stages
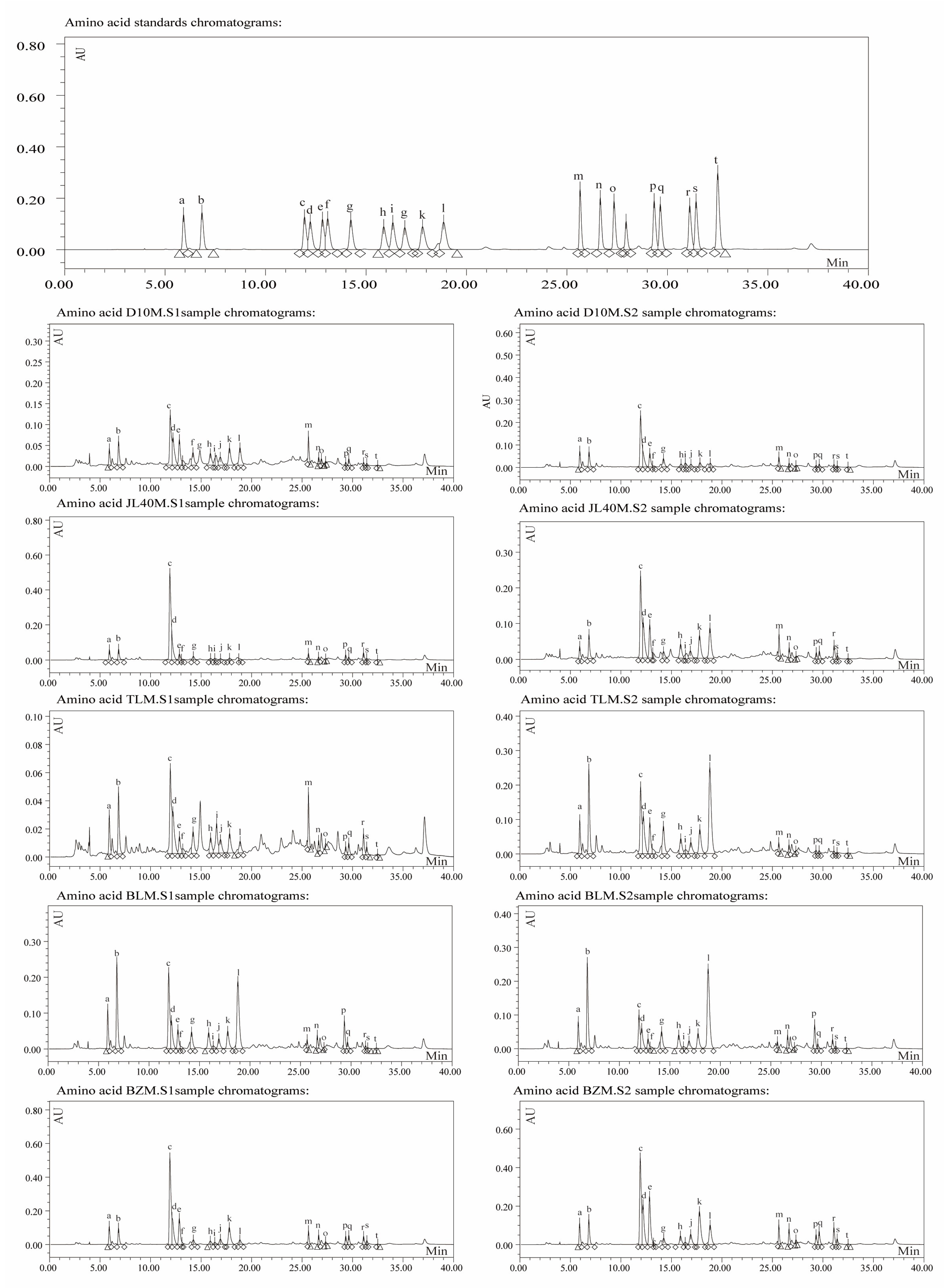
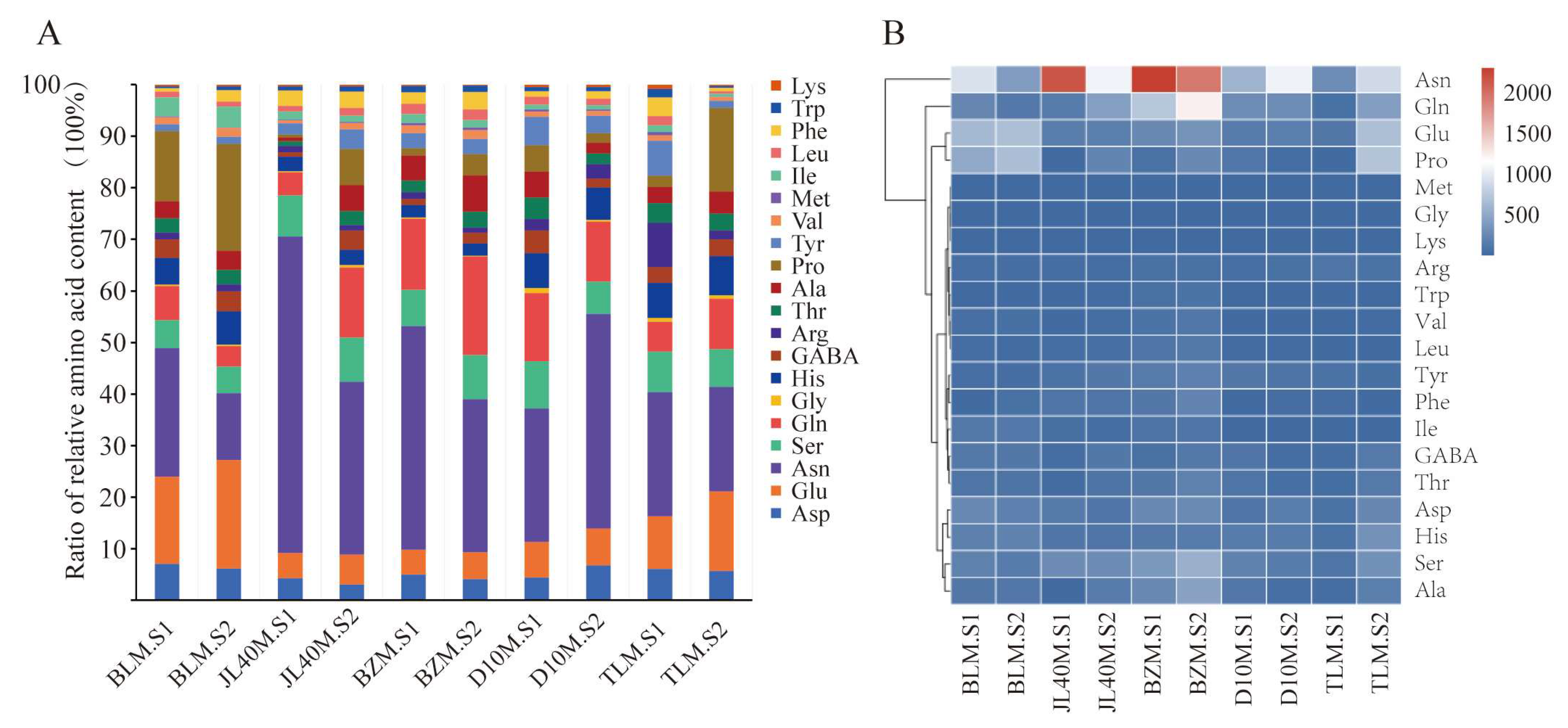
2.3. Free Amino Acid Content of Different Mulberry Varieties and Ripeness Stages
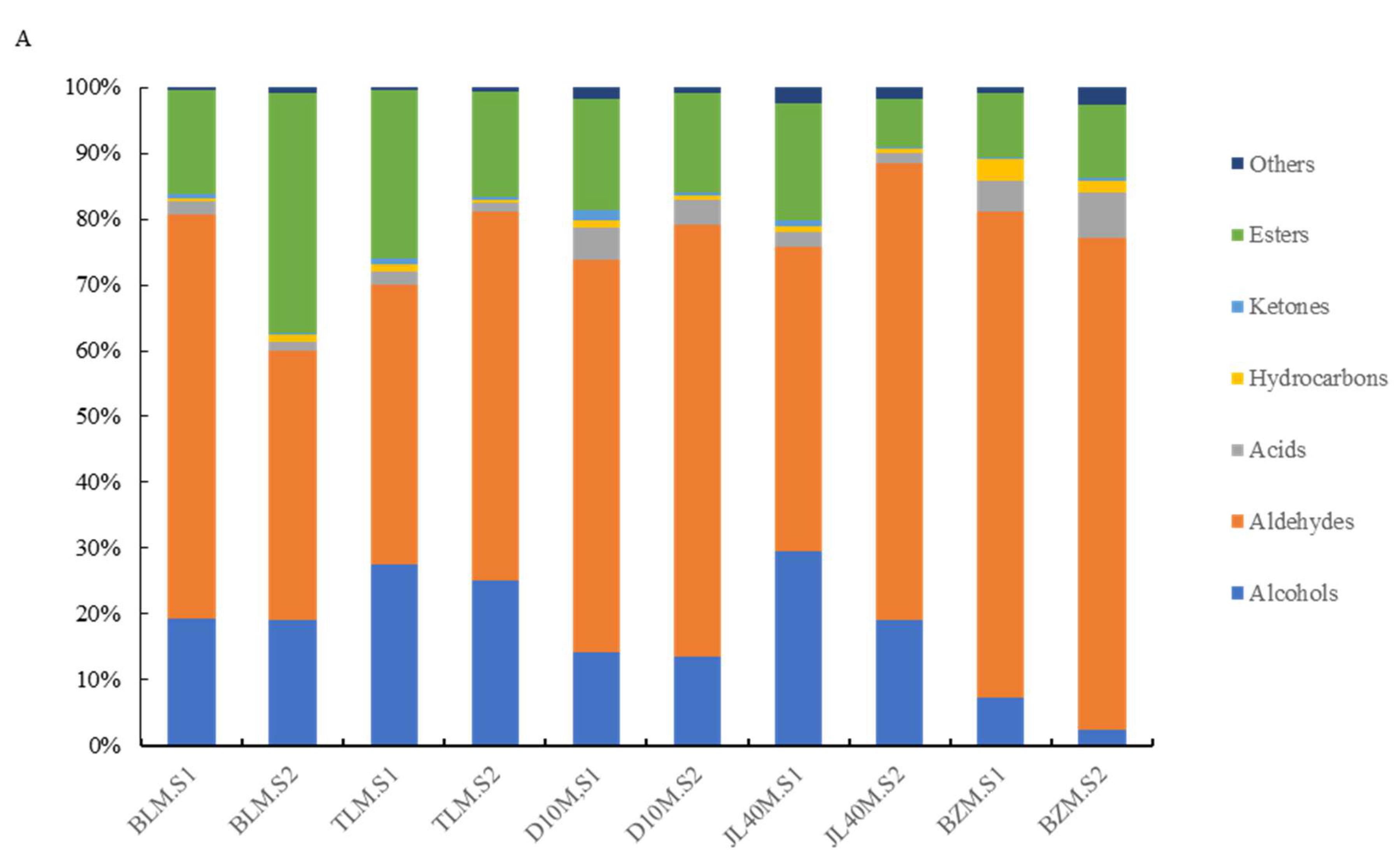
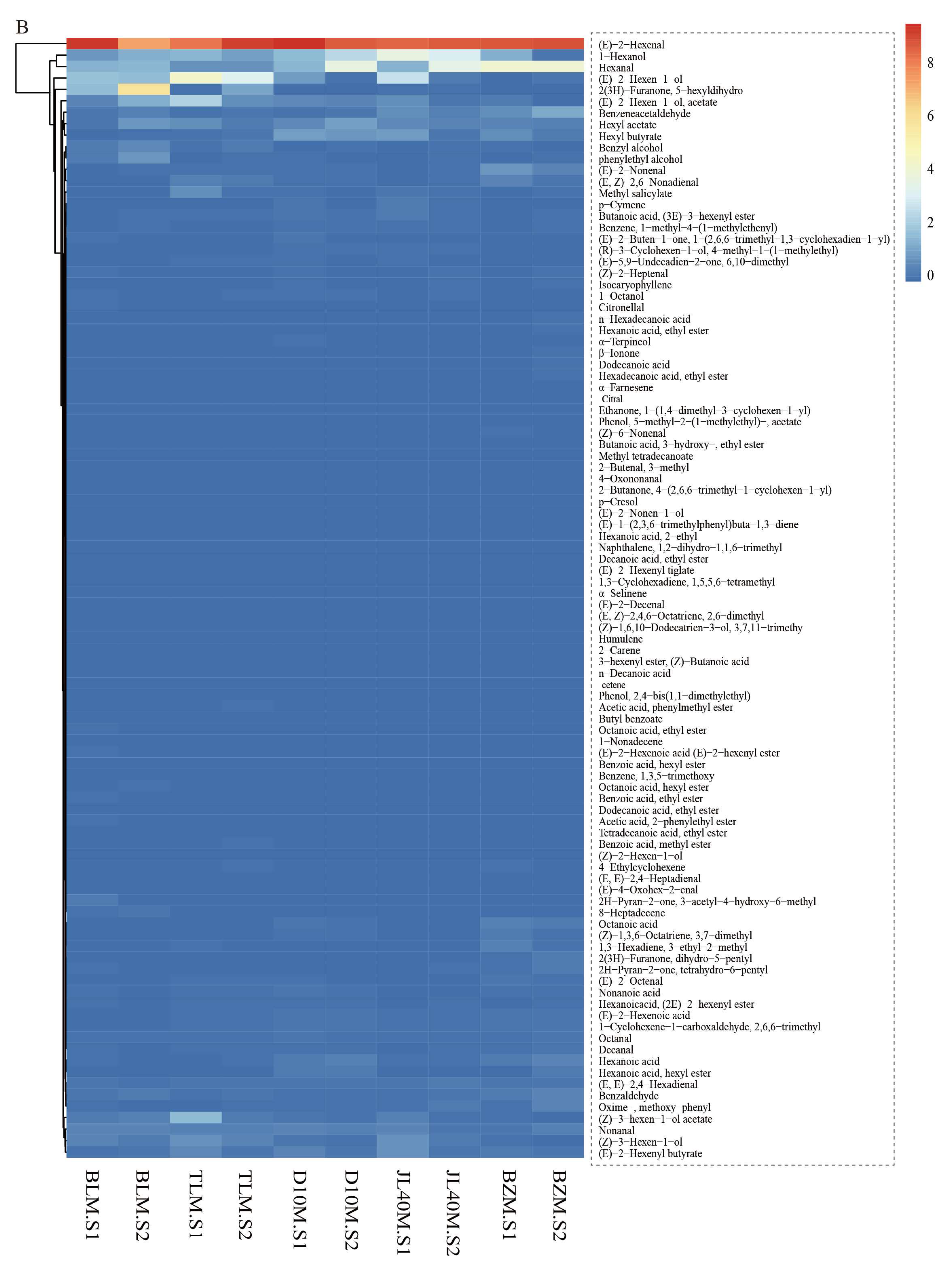
2.4. Aroma Components of Different Mulberry Varieties and Ripeness
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction, Purification, and Isolation of Sugars and Organic Acids
4.3. HPLC Analysis Method for Amino Acid Content
4.4. HS-SP ME-GCMS Analysis Method
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

| Independent Variable | Dependent Variable | df | F | p |
|---|---|---|---|---|
| Variety | Length | 4 | 858.266 | 0.000 |
| Transverse stem | 4 | 166.238 | 0.000 | |
| Weight | 4 | 3246.023 | 0.000 | |
| Brix | 4 | 171.833 | 0.000 | |
| Ripeness stages | Length | 1 | 2.972 | 0.100 |
| Transverse stem | 1 | 9.333 | 0.006 | |
| Weight | 1 | 36.364 | 0.000 | |
| Brix | 1 | 640.774 | 0.000 | |
| Variety × Ripeness | Length | 4 | 0.266 | 0.896 |
| Transverse stem | 4 | 0.286 | 0.884 | |
| Weight | 4 | 3.864 | 0.017 | |
| Brix | 4 | 25.183 | 0.000 |
| Independent Variable | Dependent Variable | df | F | p |
|---|---|---|---|---|
| Variety | fructose | 4 | 297.673 | 0.000 |
| glucose | 4 | 261.559 | 0.000 | |
| sucrose | 4 | 1373.434 | 0.000 | |
| oxalic acid | 4 | 444.463 | 0.000 | |
| quinic acid | 4 | 2701.547 | 0.000 | |
| malic acid | 4 | 106.343 | 0.000 | |
| citric acid | 4 | 1398.679 | 0.000 | |
| Ripeness stages | fructose | 1 | 1364.053 | 0.000 |
| glucose | 1 | 901.173 | 0.000 | |
| sucrose | 1 | 3710.514 | 0.000 | |
| oxalic acid | 1 | 83.962 | 0.000 | |
| quinic acid | 1 | 0.652 | 0.429 | |
| malic acid | 1 | 5.170 | 0.034 | |
| citric acid | 1 | 1006.504 | 0.000 | |
| Variety × Ripeness | fructose | 4 | 9.713 | 0.000 |
| glucose | 4 | 26.890 | 0.000 | |
| sucrose | 4 | 782.084 | 0.000 | |
| oxalic acid | 4 | 226.520 | 0.000 | |
| quinic acid | 4 | 315.748 | 0.000 | |
| malic acid | 4 | 80.894 | 0.000 | |
| citric acid | 4 | 846.141 | 0.000 |
| Amino Acid Name | Type | BLM.S1 | BLM.S2 | TLM.S1 | TLM.S2 | D10M.S1 | D10M.S2 | JL40M.S1 | JL40M.S2 | BZM.S1 | BZM.S2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp | NEAA | 266.12 ± 3.09 a | 195.43 ± 2.58 c | 73.25 ± 1.61 g | 248.55 ± 2.19 b | 100.07 ± 2.29 f | 174.18 ± 1.76 d | 151.35 ± 2.47 e | 97.75 ± 2.57 f | 265.73 ± 2.19 a | 269.74 ± 0.69 a |
| Glu | NEAA | 633.50 ± 14.89 b | 671.11 ± 8.33 a | 122.33 ± 1.96 f | 669.46 ± 8.66 a | 155.62 ± 1.70 e | 181.37 ± 0.75 d | 172.32 ± 1.20 de | 186.34 ± 1.73 d | 257.89 ± 2.26 d | 336.67 ± 12.41 c |
| Asn | NEAA | 934.50 ± 14.33 e | 411.41 ± 12.75 h | 290.04 ± 1.30 i | 882.40 ± 19.10 f | 583.96 ± 20.47 g | 1063.83 ± 20.84 d | 2174.22 ± 19.55 b | 1074.95 ± 14.66 d | 2313.01 ± 16.22 a | 1934.64 ± 18.64 c |
| Ser | NEAA | 201.44 ± 1.63 e | 164.87 ± 1.21 f | 94.57 ± 1.29 g | 318.74 ± 7.415 c | 206.34 ± 4.27 e | 160.82 ± 1.06 f | 282.18 ± 8.67 d | 274.76 ± 7.45 d | 378.10 ± 7.64 b | 555.14 ± 7.26 a |
| Gln | NEAA | 247.09 ± 3.53 d | 127.74 ± 6.01 ef | 70.17 ± 1.74 f | 425.99 ± 21.83 d | 298.47 ± 3.07 c | 297.22 ± 3.17 d | 158.20 ± 3.89 e | 436.29 ± 35.44 c | 735.46 ± 23.55 b | 1247.07 ± 39.95 a |
| Gly | NEAA | 11.13 ± 0.14 d | 8.61 ± 0.15 f | 8.71 ± 0.11 f | 26.66 ± 0.91 a | 23.03 ± 1.12 b | 9.15 ± 0.13 ef | 8.15 ± 0.26 f | 16.87 ± 0.90 c | 10.72 ± 0.09 de | 8.58 ± 0.18 f |
| His | EAA | 192.98 ± 5.09 b | 206.33 ± 4.01 b | 81.77 ± 2.26 e | 332.02 ± 20.69 a | 152.09 ± 2.71 cd | 160.55 ± 2.42 c | 98.96 ± 2.68 e | 93.52 ± 1.19 e | 131.77 ± 3.19 d | 156.30 ± 2.76 c |
| GABA | NEAA | 134.41 ± 4.46 a | 122.31 ± 3.60 b | 36.32 ± 1.71 ef | 140.98 ± 2.53 a | 99.42 ± 2.57 c | 42.65 ± 1.87 e | 30.15 ± 1.71 f | 120.89 ± 2.72 b | 62.86 ± 2.50 d | 132.77 ± 2.12 a |
| Arg | NEAA | 48.35 ± 1.88 de | 41.60 ± 2.32 e | 103.96 ± 1.51 a | 75.61 ± 2.73 b | 49.99 ± 2.83 d | 72.30 ± 2.07 bc | 42.75 ± 2.20 e | 31.06 ± 0.38 f | 67.18 ± 3.51 c | 66.19 ± 2.24 c |
| Thr | EAA | 106.91 ± 2.24 d | 91.41 ± 1.55 e | 46.11 ± 0.25 g | 144.05 ± 2.55 b | 94.52 ± 2.36 e | 53.60 ± 0.92 f | 32.22 ± 2.23 h | 91.28 ± 1.54 e | 125.14 ± 1.33 c | 204.04 ± 2.52 a |
| Ala | NEAA | 124.55 ± 2.03 e | 116.19 ± 2.39 e | 38.32 ± 1.26 fg | 185.23 ± 9.51 c | 113.86 ± 1.94 e | 53.01 ± 0.62 f | 26.48 ± 1.13 g | 161.75 ± 1.93 d | 255.60 ± 11.03 b | 459.25 ± 12.46 a |
| Pro | NEAA | 508.53 ± 16.70 c | 662.09 ± 14.30 b | 25.32 ± 1.06 g | 707.27 ± 10.66 a | 115.00 ± 1.84 e | 47.11 ± 1.07 g | 20.86 ± 1.42 g | 222.00 ± 10.49 d | 75.83 ± 1.79 f | 263.20 ± 14.10 c |
| Tyr | NEAA | 50.57 ± 3.48 fg | 43.48 ± 2.82 g | 81.62 ± 1.01 de | 56.40 ± 4.00 f | 125.07 ± 2.86 c | 86.57 ± 3.19 d | 75.31 ± 3.10 e | 122.06 ± 2.48 c | 159.33 ± 4.22 b | 195.87 ± 2.90 a |
| Val | EAA | 51.86 ± 1.57 c | 55.25 ± 1.66 c | 13.12 ± 0.17 g | 32.41 ± 1.79 e | 23.18 ± 1.22 g | 23.78 ± 0.54 g | 21.49 ± 0.72 g | 40.56 ± 1.79 d | 78.95 ± 1.20 b | 112.90 ± 1.68 a |
| Met | EAA | 3.79 ± 0.03 e | 3.74 ± 0.02 e | 7.28 ± 0.22 d | 4.06 ± 0.02 e | 8.65 ± 0.20 c | 8.11 ± 0.17 cd | 4.79 ± 0.04 e | 7.18 ± 0.17 d | 25.23 ± 0.23 b | 28.63 ± 0.99 a |
| Ile | EAA | 138.65 ± 2.19 a | 127.26 ± 2.27 b | 16.10 ± 0.17 i | 24.66 ± 0.19 g | 19.73 ± 0.22 hi | 20.53 ± 0.22 gh | 55.69 ± 1.57 e | 37.78 ± 1.31 f | 89.49 ± 1.44 d | 94.25 ± 1.94 c |
| Leu | EAA | 41.43 ± 0.23 d | 31.55 ± 0.93 f | 21.75 ± 0.28 f | 23.22 ± 0.28 f | 35.30 ± 1.24 ef | 32.24 ± 0.19 f | 36.89 ± 0.18 e | 48.95 ± 1.52 c | 106.86 ± 2.42 b | 136.53 ± 2.42 a |
| Phe | EAA | 26.40 ± 0.30 h | 70.72 ± 1.77 e | 43.32 ± 1.33 f | 27.13 ± 0.31 h | 25.30 ± 0.16 h | 37.58 ± 2.39 f | 107.14 ± 2.43 c | 100.79 ± 2.43 d | 121.91 ± 3.08 b | 219.29 ± 2.99 a |
| Trp | EAA | 13.53 ± 0.19 i | 22.67 ± 0.23 e | 19.28 ± 0.10 fg | 14.66 ± 0.21 hi | 17.14 ± 0.26 gh | 20.63 ± 0.18 ef | 28.83 ± 0.34 d | 32.59 ± 0.25 c | 64.99 ± 1.87 b | 80.28 ± 2.46 a |
| Lys | EAA | 10.55 ± 0.09 bc | 10.75 ± 0.09 b | 9.98 ± 0.08 d | 10.67 ± 0.07 bc | 10.41 ± 0.08 c | 10.64 ± 0.08 bc | 10.71 ± 0.07 b | 10.65 ± 0.09 bc | 11.18 ± 0.08 a | 11.27 ± 0.05 a |
| Independent Variable | Dependent Variable | df | F | p |
|---|---|---|---|---|
| Variety | Asp | 4 | 1548.195 | 0.000 |
| Glu | 4 | 1468.338 | 0.000 | |
| Asn | 4 | 3262.770 | 0.000 | |
| Ser | 4 | 905.216 | 0.000 | |
| Gln | 4 | 552.724 | 0.000 | |
| Gly | 4 | 85.436 | 0.000 | |
| His | 4 | 78.731 | 0.000 | |
| GABA | 4 | 141.719 | 0.000 | |
| Arg | 4 | 158.669 | 0.000 | |
| Thr | 4 | 881.795 | 0.000 | |
| Ala | 4 | 681.161 | 0.000 | |
| Pro | 4 | 962.102 | 0.000 | |
| Tyr | 4 | 504.431 | 0.000 | |
| Val | 4 | 1045.831 | 0.000 | |
| Met | 4 | 1525.118 | 0.000 | |
| Ile | 4 | 2392.687 | 0.000 | |
| Leu | 4 | 1767.010 | 0.000 | |
| Phe | 4 | 1756.072 | 0.000 | |
| Trp | 4 | 1121.752 | 0.000 | |
| Lys | 4 | 37.244 | 0.000 | |
| Ripeness | Asp | 1 | 333.838 | 0.000 |
| Glu | 1 | 921.029 | 0.000 | |
| Asn | 1 | 308.422 | 0.000 | |
| Ser | 1 | 305.611 | 0.000 | |
| Gln | 1 | 264.662 | 0.000 | |
| Gly | 1 | 21.409 | 0.000 | |
| His | 1 | 164.712 | 0.000 | |
| GABA | 1 | 526.298 | 0.000 | |
| Arg | 1 | 12.167 | 0.002 | |
| Thr | 1 | 898.011 | 0.000 | |
| Ala | 1 | 447.313 | 0.000 | |
| Pro | 1 | 1457.816 | 0.000 | |
| Tyr | 1 | 1.592 | 0.222 | |
| Val | 1 | 319.003 | 0.000 | |
| Met | 1 | 3.239 | 0.087 | |
| Ile | 1 | 11.431 | 0.003 | |
| Leu | 1 | 50.733 | 0.000 | |
| Phe | 1 | 429.898 | 0.000 | |
| Trp | 1 | 73.201 | 0.000 | |
| Lys | 1 | 21.512 | 0.000 | |
| Variety × Ripeness | Asp | 4 | 1020.393 | 0.000 |
| Glu | 4 | 486.274 | 0.000 | |
| Asn | 4 | 909.574 | 0.000 | |
| Ser | 4 | 258.062 | 0.000 | |
| Gln | 4 | 84.980 | 0.000 | |
| Gly | 4 | 238.734 | 0.000 | |
| His | 4 | 113.069 | 0.000 | |
| GABA | 4 | 336.324 | 0.000 | |
| Arg | 4 | 31.774 | 0.000 | |
| Thr | 4 | 514.866 | 0.000 | |
| Ala | 4 | 162.398 | 0.000 | |
| Pro | 4 | 411.016 | 0.000 | |
| Tyr | 4 | 72.625 | 0.000 | |
| Val | 4 | 50.364 | 0.000 | |
| Met | 4 | 28.549 | 0.000 | |
| Ile | 4 | 31.064 | 0.000 | |
| Leu | 4 | 65.881 | 0.000 | |
| Phe | 4 | 262.702 | 0.000 | |
| Trp | 4 | 27.330 | 0.000 | |
| Lys | 4 | 6.396 | 0.002 |
| Volatile Compounds | CAS | BLM | TLM | D10M | JL40M | BZM | Odor Descriptions | Kovats Index | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | ||||
| Alcohols (11) | |||||||||||||
| 1-Hexanol | 111-27-3 | 4.46% | 5.30% | 6.08% | 4.89% | 7.55% | 11.94% | 14.59% | 15.38% | 6.07% | 1.05% | light blue shoots breath, wine, fruit, fat flavor | 868 |
| (Z)-3-Hexen-1-ol | 928-96-1 | 2.25% | 1.09% | 3.25% | 2.08% | 1.00% | 0.41% | 3.12% | 1.44% | 0.56% | 0.11% | grassy green | 1009 |
| (E)-2-Hexen-1-ol | 928-95-0 | 9.27% | 6.73% | 17.11% | 16.38% | 4.30% | 0.29% | 10.27% | 1.35% | 0.15% | 0.71% | green fruity | 862 |
| (Z)-2-Hexen-1-ol | 928-94-9 | 0.09% | 0.08% | 0.19% | 0.08% | 0.00% | 0.11% | 0.00% | 0.00% | 0.00% | 0.00% | green | 862 |
| 1-Octanol | 111-87-5 | 0.43% | 0.23% | 0.21% | 0.26% | 0.27% | 0.20% | 0.26% | 0.17% | 0.13% | 0.12% | dry sweet, sharp fat wax aroma, grease and fruit | 998 |
| (R)-3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl) | 20126-76-5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.41% | 0.21% | 0.70% | 0.17% | 0.00% | 0.00% | spicy, earthy, green and woody | 1175 |
| (E)-2-Nonen-1-ol | 31502-14-4 | 0.01% | 0.03% | 0.09% | 0.02% | 0.02% | 0.00% | 0.01% | 0.01% | 0.00% | 0.00% | fat violet aroma | 1162 |
| α-Terpineol | 98-55-5 | 0.00% | 0.00% | 0.00% | 0.00% | 0.40% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | clove scent | 1197 |
| Benzyl alcohol | 100-51-6 | 1.52% | 2.39% | 0.44% | 1.11% | 0.11% | 0.20% | 0.32% | 0.18% | 0.25% | 0.17% | odorl | 1036 |
| phenylethyl alcohol | 60-12-8 | 1.32% | 3.30% | 0.09% | 0.29% | 0.22% | 0.09% | 0.33% | 0.32% | 0.17% | 0.23% | soft, pleasant and persistent rose fragrance | 1102 |
| (Z)-1,6,10-Dodecatrien-3-ol, 3,7,11-trimethy | 142-50-7 | 0.00% | 0.01% | 0.02% | 0.01% | 0.02% | 0.00% | 0.02% | 0.00% | 0.01% | 0.02% | sweet smell of fresh roses | 1542 |
| Total | 19.36% | 19.17% | 27.48% | 25.13% | 14.30% | 13.45% | 29.62% | 19.02% | 7.34% | 2.40% | |||
| Aldehydes (20) | |||||||||||||
| Hexanal | 66-25-1 | 7.06% | 5.85% | 3.07% | 3.68% | 7.25% | 17.87% | 5.60% | 18.12% | 19.14% | 18.39% | oil and grass smell and apple flavor | 801 |
| 2-Butenal, 3-methyl | 107-86-8 | 0.01% | 0.01% | 0.08% | 0.05% | 0.04% | 0.03% | 0.06% | 0.07% | 0.05% | 0.19% | sweet, fruity and green with a nutty and cherry background | 748 |
| (E)-2-Hexenal | 6728-26-3 | 47.35% | 27.69% | 31.58% | 44.61% | 44.72% | 41.63% | 32.64% | 44.25% | 39.95% | 39.77% | leafy, fruity, fatty, apple banana, strawberry | 854 |
| Octanal | 124-13-0 | 0.51% | 0.40% | 0.40% | 0.44% | 0.69% | 0.44% | 0.48% | 0.14% | 0.30% | 0.75% | aldehyde, green with a peely, citrus, orange | 985 |
| (Z)-2-Heptenal | 57266-86-1 | 0.16% | 0.18% | 0.32% | 0.00% | 0.32% | 0.14% | 0.20% | 0.59% | 0.12% | 0.34% | lipoid aroma | 964 |
| (E, E)-2,4-Hexadienal | 142-83-6 | 1.06% | 0.68% | 0.73% | 1.03% | 0.80% | 0.82% | 0.85% | 1.19% | 0.84% | 0.99% | sweet, green aroma | 909 |
| Benzaldehyde | 100-52-7 | 0.94% | 1.23% | 0.38% | 0.76% | 0.32% | 0.35% | 0.59% | 0.75% | 1.14% | 2.19% | odor of bitter almonds | 960 |
| Nonanal | 124-19-6 | 2.32% | 1.81% | 1.69% | 1.83% | 2.40% | 1.80% | 2.01% | 1.07% | 1.08% | 1.89% | strong odor resembling an essence of orange and rose | 1102 |
| (E)-2-Octenal | 2548-87-0 | 0.09% | 0.19% | 0.50% | 0.20% | 0.46% | 0.15% | 0.21% | 0.06% | 0.73% | 0.42% | fat and meat, cucumber and chicken | 1060 |
| (E, E)-2,4-Heptadienal | 4313-03-5 | 0.08% | 0.08% | 0.13% | 0.09% | 0.07% | 0.03% | 0.03% | 0.02% | 0.17% | 0.08% | fatty, green odor | 990 |
| (Z)-6-Nonenal | 2277-19-2 | 0.00% | 0.00% | 0.04% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.29% | 0.05% | citrus, melon odor | 1101 |
| Citronellal | 106-23-0 | 0.54% | 0.12% | 0.06% | 0.08% | 0.05% | 0.06% | 0.08% | 0.08% | 0.07% | 0.11% | intense, lemon-, citronella-, rose-type odor | 1153 |
| (E)-4-Oxohex-2-enal | 1000374-04-2 | 0.11% | 0.07% | 0.09% | 0.12% | 0.09% | 0.08% | 0.05% | 0.08% | 0.08% | 0.07% | 959 | |
| Decanal | 112-31-2 | 0.28% | 0.25% | 0.60% | 0.44% | 0.42% | 0.45% | 0.32% | 0.33% | 0.38% | 0.74% | penetrating, sweet, waxy, floral, citrus, pronounced fatty odor | 1205 |
| Benzeneacetaldehyde | 122-78-1 | 0.72% | 1.50% | 0.48% | 0.99% | 0.55% | 0.63% | 2.62% | 2.00% | 3.15% | 5.41% | honey-like sweet | 1048 |
| (E)-2-Nonenal | 18829-56-6 | 0.12% | 0.46% | 0.68% | 0.41% | 0.44% | 0.61% | 0.19% | 0.42% | 3.90% | 2.22% | grassy, cucumber smell | 1162 |
| (E, Z)-2,6-Nonadienal | 557-48-2 | 0.07% | 0.30% | 1.51% | 1.24% | 0.50% | 0.27% | 0.20% | 0.17% | 2.16% | 0.97% | green cucumber | 1156 |
| (E)-2-Decenal | 3913-81-3 | 0.01% | 0.01% | 0.00% | 0.00% | 0.05% | 0.02% | 0.02% | 0.01% | 0.00% | 0.00% | waxy, fatty, earthy, coriander, green, mushroom, fat | 1263 |
| Citral | 5392-40-5 | 0.00% | 0.00% | 0.05% | 0.02% | 0.10% | 0.14% | 0.07% | 0.08% | 0.02% | 0.03% | strong fatty, floral odor | 1273 |
| 4-Oxononanal | 1000314-10-4 | 0.00% | 0.00% | 0.05% | 0.01% | 0.09% | 0.05% | 0.02% | 0.03% | 0.13% | 0.14% | strong lemon flavor | 1200 |
| Total | 61.43% | 40.83% | 42.45% | 56.00% | 59.39% | 65.60% | 46.24% | 69.46% | 73.69% | 74.75% | |||
| Acids (10) | |||||||||||||
| Hexanoic acid | 142-62-1 | 0.13% | 0.21% | 0.33% | 0.30% | 1.52% | 1.85% | 0.50% | 0.49% | 1.16% | 2.26% | odor that is fatty, cheesy, waxy, and like that of goats | 983 |
| (E)-2-Hexenoic acid | 13419-69-7 | 0.10% | 0.18% | 0.54% | 0.29% | 0.72% | 0.64% | 0.61% | 0.40% | 0.66% | 1.04% | pleasant fatty characteristic | 1942 |
| Hexanoic acid, 2-ethyl | 149-57-5 | 0.00% | 0.00% | 0.02% | 0.00% | 0.10% | 0.01% | 0.03% | 0.02% | 0.01% | 0.00% | mild odour | 1121 |
| 1-Cyclohexene-1-carboxaldehyde, 2,6,6-trimethyl | 432-25-7 | 0.09% | 0.18% | 0.54% | 0.29% | 0.72% | 0.64% | 0.61% | 0.40% | 0.66% | 1.04% | camphoraceous odor | 1220 |
| Octanoic acid | 124-07-2 | 0.03% | 0.05% | 0.03% | 0.03% | 0.89% | 0.27% | 0.04% | 0.02% | 1.80% | 1.11% | a faint, fruity–acid odor and slightly sour taste | 1192 |
| Nonanoic acid | 112-05-0 | 0.16% | 0.24% | 0.46% | 0.30% | 0.89% | 0.21% | 0.16% | 0.08% | 0.26% | 0.40% | fatty, characteristic odor and a corresponding unpleasant taste | 1275 |
| n-Decanoic acid | 334-48-5 | 0.06% | 0.04% | 0.01% | 0.01% | 0.04% | 0.06% | 0.05% | 0.03% | 0.03% | 0.02% | 1368 | |
| 2H-Pyran-2-one, 3-acetyl-4-hydroxy-6-methyl | 771-03-9 | 1.13% | 0.02% | 0.04% | 0.02% | 0.02% | 0.03% | 0.01% | 0.01% | 0.15% | 0.15% | 1673 | |
| Dodecanoic acid | 143-07-7 | 0.07% | 0.19% | 0.03% | 0.08% | 0.02% | 0.02% | 0.02% | 0.01% | 0.02% | 0.23% | slightly fragrant with laurel oil | 1570 |
| n-Hexadecanoic acid | 57-10-3 | 0.02% | 0.30% | 0.02% | 0.02% | 0.09% | 0.03% | 0.04% | 0.03% | 0.05% | 0.55% | 1965 | |
| Total | 1.79% | 1.42% | 2.03% | 1.35% | 5.01% | 3.76% | 2.08% | 1.49% | 4.82% | 6.81% | |||
| Hydrocarbons (14) | |||||||||||||
| (Z)-1,3,6-Octatriene, 3,7-dimethyl | 3338-55-4 | 0.00% | 0.00% | 0.18% | 0.03% | 0.41% | 0.41% | 0.15% | 0.02% | 1.31% | 0.55% | straw and flower fragrance with smell of orange blossom oil | 1035 |
| 2-Carene | 554-61-0 | 0.00% | 0.00% | 0.00% | 0.00% | 0.05% | 0.01% | 0.07% | 0.00% | 0.06% | 0.03% | 1015 | |
| 1,3-Cyclohexadiene, 1,5,5,6-tetramethyl | 514-94-3 | 0.00% | 0.00% | 0.01% | 0.02% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 1360 | |
| (E, Z)-2,4,6-Octatriene, 2,6-dimethyl | 7216-56-0 | 0.02% | 0.02% | 0.00% | 0.00% | 0.03% | 0.03% | 0.03% | 0.02% | 0.00% | 0.00% | sweet, floral, nut, skin, peppery, herbal, tropical | 1125 |
| 1,3-Hexadiene, 3-ethyl-2-methyl | 61142-36-7 | 0.03% | 0.14% | 0.60% | 0.12% | 0.15% | 0.08% | 0.05% | 0.03% | 1.57% | 0.49% | nutty | 1025 |
| 4-Ethylcyclohexene | 3742-42-5 | 0.06% | 0.07% | 0.21% | 0.16% | 0.10% | 0.08% | 0.06% | 0.06% | 0.24% | 0.12% | 876 | |
| Isocaryophyllene | 118-65-0 | 0.00% | 0.00% | 0.01% | 0.01% | 0.29% | 0.02% | 0.30% | 0.51% | 0.09% | 0.61% | 1416 | |
| Humulene | 6753-98-6 | 0.01% | 0.00% | 0.00% | 0.00% | 0.02% | 0.00% | 0.03% | 0.02% | 0.03% | 0.03% | clove like fragrance | 1445 |
| α-Selinene | 473-13-2 | 0.00% | 0.00% | 0.00% | 0.00% | 0.02% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 1495 | |
| α-Farnesene | 502-61-4 | 0.00% | 0.00% | 0.19% | 0.03% | 0.04% | 0.02% | 0.19% | 0.00% | 0.03% | 0.01% | citrus, herbal, lavender, bergamot, myrrh, neroli, green | 1506 |
| 8-Heptadecene | 2579-04-6 | 0.38% | 0.84% | 0.03% | 0.06% | 0.00% | 0.00% | 0.04% | 0.02% | 0.00% | 0.00% | ||
| 1-Nonadecene | 18435-45-5 | 0.08% | 0.04% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 1895 | |
| cetene | 629-73-2 | 0.04% | 0.00% | 0.02% | 0.03% | 0.03% | 0.03% | 0.03% | 0.03% | 0.03% | 0.06% | 1587 | |
| Total | 0.63% | 1.11% | 1.24% | 0.47% | 1.15% | 0.69% | 0.96% | 0.72% | 3.37% | 1.91% | |||
| Ketones (6) | |||||||||||||
| Ethanone, 1-(1,4-dimethyl-3-cyclohexen-1-yl) | 43219-68-7 | 0.06% | 0.00% | 0.00% | 0.00% | 0.09% | 0.03% | 0.14% | 0.02% | 0.02% | 0.00% | fruity | 1128 |
| (E)-1-(2,3,6-trimethylphenyl)buta-1,3-diene | 1000357-25-7 | 0.04% | 0.02% | 0.06% | 0.04% | 0.03% | 0.02% | 0.03% | 0.01% | 0.02% | 0.01% | 1832 | |
| (E)-2-Buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl) | 23726-93-4 | 0.21% | 0.04% | 0.11% | 0.02% | 0.94% | 0.11% | 0.21% | 0.00% | 0.01% | 0.01% | 1382 | |
| 2-Butanone, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl) | 17283-81-7 | 0.01% | 0.01% | 0.10% | 0.12% | 0.02% | 0.02% | 0.04% | 0.01% | 0.01% | 0.03% | 1432 | |
| (E)-5,9-Undecadien-2-one, 6,10-dimethyl | 3796-70-1 | 0.09% | 0.04% | 0.34% | 0.20% | 0.36% | 0.15% | 0.35% | 0.09% | 0.05% | 0.07% | 1459 | |
| β-Ionone | 79-77-6 | 0.08% | 0.05% | 0.13% | 0.12% | 0.14% | 0.08% | 0.22% | 0.09% | 0.05% | 0.24% | 1478 | |
| Total | 0.50% | 0.17% | 0.75% | 0.51% | 1.58% | 0.42% | 0.99% | 0.22% | 0.17% | 0.37% | |||
| Esters (31) | |||||||||||||
| Hexyl acetate | 142-92-7 | 0.93% | 3.49% | 2.57% | 1.49% | 2.80% | 4.83% | 2.50% | 2.46% | 2.31% | 1.83% | pear, apple like aroma | 997 |
| (Z)-3-hexen-1-ol acetate | 3681-71-8 | 1.51% | 1.67% | 6.33% | 1.49% | 0.93% | 0.42% | 1.44% | 0.49% | 0.31% | 0.10% | strong smell of grass | 998 |
| (E)-2-Hexen-1-ol, acetate | 2497-18-9 | 2.28% | 5.14% | 8.94% | 3.37% | 2.67% | 2.23% | 2.60% | 0.78% | 1.22% | 0.11% | green grass fragrance | 995 |
| Butanoic acid, 3-hydroxy-, ethyl ester | 5405-41-4 | 0.03% | 0.02% | 0.01% | 0.00% | 0.01% | 0.03% | 0.00% | 0.00% | 0.03% | 0.16% | fruit, grape, green and white wine like aroma | 947 |
| Hexyl butyrate | 2639-63-6 | 0.12% | 0.53% | 0.51% | 0.68% | 4.76% | 4.16% | 3.84% | 1.07% | 2.83% | 1.32% | green, fruity, ester, vegetable and strong mixed fruit aroma and pineapple aroma | 1185 |
| Octanoic acid, ethyl ester | 106-32-1 | 0.13% | 0.01% | 0.00% | 0.06% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | pineapple fragrance with sweet taste | 1126 |
| 3-hexenyl ester, (Z)-Butanoic acid | 16491-36-4 | 0.00% | 0.00% | 0.01% | 0.00% | 0.00% | 0.00% | 0.07% | 0.02% | 0.06% | 0.04% | green aroma of fruit is slightly creamy | 1172 |
| Butanoic acid, (3E)-3-hexenyl ester | 53398-84-8 | 0.11% | 0.48% | 0.58% | 0.27% | 0.65% | 0.25% | 1.08% | 0.25% | 0.21% | 0.31% | green aroma of fruit is slightly creamy | 1152 |
| (E)-2-Hexenyl butyrate | 53398-83-7 | 0.45% | 0.78% | 2.44% | 1.04% | 2.22% | 0.88% | 3.11% | 0.74% | 1.32% | 0.75% | green aroma of fruit is slightly creamy | 1182 |
| Benzoic acid, methyl ester | 93-58-3 | 0.08% | 0.09% | 0.30% | 0.25% | 0.00% | 0.00% | 0.19% | 0.05% | 0.00% | 0.00% | floral and fruity aroma, with the fragrance of ylang ylang ylang and tuberose | 1065 |
| Benzoic acid, ethyl ester | 93-89-0 | 0.16% | 0.23% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | fruity, slightly like ylang oil | 1165 |
| Acetic acid, phenylmethyl ester | 140-11-4 | 0.07% | 0.02% | 0.02% | 0.19% | 0.01% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | special fragrance of jasmine | 1159 |
| Methyl salicylate | 119-36-8 | 0.03% | 0.02% | 2.59% | 0.60% | 0.19% | 0.03% | 0.97% | 0.15% | 0.11% | 0.64% | holly leaf fragrance | 1182 |
| Hexanoic acid, hexyl ester | 6378-65-0 | 0.05% | 0.18% | 0.05% | 0.07% | 1.45% | 1.48% | 0.26% | 0.09% | 0.18% | 1.13% | green, waxy, herbal and tropical fruits and berries | 1385 |
| Decanoic acid, ethyl ester | 110-38-3 | 0.02% | 0.04% | 0.01% | 0.02% | 0.02% | 0.02% | 0.02% | 0.02% | 0.01% | 0.02% | coconut aroma | 1389 |
| (E)-2-Hexenyl tiglate | 1000131-83-6 | 0.00% | 0.05% | 0.02% | 0.01% | 0.03% | 0.00% | 0.02% | 0.01% | 0.01% | 0.00% | green, mushroom-like | 1345 |
| Hexanoicacid, (2E)-2-hexenyl ester | 53398-86-0 | 0.16% | 0.28% | 0.40% | 0.30% | 0.58% | 0.38% | 0.33% | 0.26% | 0.19% | 0.38% | 1385 | |
| Acetic acid, 2-phenylethyl ester | 103-45-7 | 0.15% | 0.16% | 0.03% | 0.07% | 0.00% | 0.00% | 0.03% | 0.05% | 0.02% | 0.05% | floral aroma of rose with honey like base fragrance | 1225 |
| Butyl benzoate | 136-60-7 | 0.02% | 0.03% | 0.02% | 0.12% | 0.03% | 0.04% | 0.02% | 0.02% | 0.03% | 0.02% | Sweet, soft and astringent fragrance of flowers and creams | 1362 |
| (E)-2-Hexenoic acid (E)-2-hexenyl ester | 54845-28-2 | 0.13% | 0.04% | 0.01% | 0.00% | 0.04% | 0.01% | 0.01% | 0.02% | 0.01% | 0.01% | 1890 | |
| Octanoic acid, hexyl ester | 1117-55-1 | 0.05% | 0.39% | 0.03% | 0.03% | 0.04% | 0.04% | 0.06% | 0.06% | 0.02% | 0.03% | fresh vegetables and light fruits, sweet and green fruits | 1562 |
| Dodecanoic acid, ethyl ester | 106-33-2 | 0.10% | 0.21% | 0.09% | 0.08% | 0.04% | 0.02% | 0.07% | 0.07% | 0.02% | 0.07% | Special fruity smell | 1845 |
| Phenol, 5-methyl-2-(1-methylethyl)-, acetate | 528-79-0 | 0.00% | 0.00% | 0.00% | 0.00% | 0.05% | 0.03% | 0.15% | 0.06% | 0.04% | 0.06% | 1345 | |
| Benzoic acid, hexyl ester | 6789-88-4 | 0.08% | 0.08% | 0.02% | 0.06% | 0.04% | 0.03% | 0.09% | 0.05% | 0.04% | 0.01% | wood and balsam with fruity notes | 1562 |
| Methyl tetradecanoate | 124-10-7 | 0.00% | 0.00% | 0.01% | 0.01% | 0.00% | 0.00% | 0.01% | 0.01% | 0.03% | 0.22% | 1715 | |
| 2(3H)-Furanone, dihydro-5-pentyl | 104-61-0 | 0.04% | 0.08% | 0.05% | 0.05% | 0.03% | 0.05% | 0.04% | 0.02% | 0.22% | 1.41% | coconut fragrance, with peach like and apricot like | 1332 |
| Tetradecanoic acid, ethyl ester | 124-06-1 | 0.08% | 0.18% | 0.03% | 0.04% | 0.02% | 0.02% | 0.05% | 0.05% | 0.03% | 0.14% | sweet beeswax like flavor | 1772 |
| 2(3H)-Furanone, 5-hexyldihydro | 706-14-9 | 8.78% | 21.80% | 0.38% | 5.36% | 0.03% | 0.03% | 0.60% | 0.39% | 0.07% | 0.22% | coconut and peach aromas | 1425 |
| 2H-Pyran-2-one, tetrahydro-6-pentyl | 705-86-2 | 0.27% | 0.24% | 0.04% | 0.09% | 0.03% | 0.06% | 0.02% | 0.14% | 0.31% | 1.27% | coconut, cream aroma | 1487 |
| Hexadecanoic acid, ethyl ester | 628-97-7 | 0.05% | 0.06% | 0.05% | 0.04% | 0.05% | 0.07% | 0.06% | 0.07% | 0.05% | 0.32% | wax, fruity, and creamy aromas | 1975 |
| Hexanoic acid, ethyl ester | 123-66-0 | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.51% | strong fruity odor, reminiscent of pineapples | 985 |
| Total | 15.91% | 36.32% | 25.56% | 15.82% | 16.75% | 15.12% | 17.64% | 7.41% | 9.69% | 11.14% | |||
| Others (8) | |||||||||||||
| p-Cymene | 99-87-6 | 0.03% | 0.00% | 0.00% | 0.00% | 0.68% | 0.10% | 1.18% | 0.25% | 0.14% | 0.00% | 1019 | |
| Benzene, 1-methyl-4-(1-methylethenyl) | 1195-32-0 | 0.00% | 0.55% | 0.08% | 0.05% | 0.62% | 0.24% | 0.63% | 0.19% | 0.15% | 0.55% | 1084 | |
| Oxime-, methoxy-phenyl | 67160-14-9 | 0.29% | 0.23% | 0.31% | 0.57% | 0.41% | 0.53% | 0.56% | 1.15% | 0.55% | 1.98% | 2085 | |
| Naphthalene, 1,2-dihydro-1,1,6-trimethyl | 30364-38-6 | 0.00% | 0.00% | 0.00% | 0.00% | 0.06% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 1718 | |
| p-Cresol | 106-44-5 | 0.00% | 0.00% | 0.06% | 0.02% | 0.00% | 0.00% | 0.04% | 0.01% | 0.00% | 0.00% | 1037 | |
| Benzene, 1,3,5-trimethoxy | 621-23-8 | 0.04% | 0.19% | 0.01% | 0.07% | 0.02% | 0.01% | 0.00% | 0.00% | 0.02% | 0.02% | 1409 | |
| Phenol, 2,4-bis(1,1-dimethylethyl) | 96-76-4 | 0.02% | 0.02% | 0.03% | 0.01% | 0.04% | 0.07% | 0.06% | 0.07% | 0.06% | 0.06% | 1516 | |
| Total | 0.38% | 0.98% | 0.50% | 0.73% | 1.83% | 0.95% | 2.47% | 1.68% | 0.92% | 2.62% | |||
| Component | Curve Equation | Range Linearity (ppm) | R2 | LOD/(μmol/L) | LOQ/(μmol/L) |
|---|---|---|---|---|---|
| Fructose | y = 0.0027x − 0.0012 | 50~1000 | 0.9998 | 18.369 | 48.985 |
| Glucose | y = 0.0024x + 0.0224 | 50~1000 | 0.9992 | 29.236 | 77.962 |
| Sucrose | y = 0.0028x − 0.0303 | 50~1000 | 0.9990 | 30.981 | 82.616 |
| Oxalic acid | y = 2708x + 50774 | 50~1000 | 0.9959 | 0.12 | 0.38 |
| Quinic acid | y = 320.7x − 1837.1 | 50~1000 | 0.9961 | 8.9 | 29.34 |
| Malic acid | y = 474.98x + 1876.3 | 50~1000 | 0.9996 | 2.17 | 7.25 |
| Citric acid | y = 551.86x − 3397.8 | 50~1000 | 0.9996 | 3.77 | 12.57 |
| Aspartate | y = 4,498,075.699x − 25,997.083 | 5~100 | 0.9982 | 0.09 | 0.31 |
| Glutamate | y = 5,199,804.473x − 25,974.458 | 5~100 | 0.9988 | 0.13 | 0.45 |
| Asparagine | y = 26,726.711x − 30,237.833 | 5~100 | 0.9983 | 0,05 | 0.23 |
| Serine | y = 5,229,290.495x − 18,395.917 | 5~100 | 0.9994 | 0.02 | 0.1 |
| Glutamine | y = 24,803.972x − 28,174.167 | 5~100 | 0.9981 | 0.05 | 0.16 |
| Glycine | y = 6,027,587.613x − 9337.750 | 5~100 | 1.0000 | 0.41 | 1.37 |
| Histidine | y = 5,542,889.720x − 57,670.042 | 5~100 | 0.9929 | 0.1 | 0.54 |
| γ- aminobutyric acid | y = 48,592.782x − 12,001.375 | 5~100 | 0.9998 | 0.08 | 0.32 |
| Arginine | y = 5,570,750.796x − 25,065.083 | 5~100 | 0.9990 | 0.24 | 1.57 |
| Threonine | y = 4,972,276.129x − 19,294.250 | 5~100 | 0.9994 | 0.21 | 1.28 |
| Alanine | y = 5,393,247.742x − 17,293.875 | 5~100 | 0.9997 | 0.25 | 0.83 |
| Proline | y = 6,902,924.989x − 20,802.458 | 5~100 | 0.9997 | 0.32 | 1.28 |
| Tyrosine | y = 6,415,341.419x − 25,994.000 | 5~100 | 0.9996 | 0.06 | 0.18 |
| Valine | y = 6,317,776.086x − 13,418.958 | 5~100 | 0.9998 | 0.3 | 0.98 |
| Methionine | y = 6,319,528.516x − 15,133.125 | 5~100 | 0.9992 | 0.23 | 1.15 |
| Isoleucine | y = 6,728,787.527x − 31,162.292 | 5~100 | 0.9994 | 0.14 | 0.47 |
| Leucine | y = 6,398,705.892x − 29,049.833 | 5~100 | 0.9994 | 0.15 | 0.51 |
| Phenylalanine | y = 6,410,138.065x − 30,038.125 | 5~100 | 0.9997 | 0.18 | 0.6 |
| Tryptophan | y = 35,277.749x − 17,456.583 | 5~100 | 0.9998 | 0.07 | 0.25 |
| Lysine | y = 11,504,741.680x − 69,850.250 | 5~100 | 0.9995 | 0.05 | 0.16 |
References
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, T.; Xiao, L.; Yang, L.; Chen, R. Two new chalcones from leaves of Morus alba L. Fitoterapia 2010, 81, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Kusano, G.; Orihara, S.; Tsukamoto, D.; Shibano, M.; Coskun, M.; Guvenc, A.; Erdurak, C.S. Five new nortropane alkaloids and six new amino acids from the fruit of Morus alba Linne growing in Turkey. Chem. Pharm. Bull. 2002, 50, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, L.; Zheng, H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010, 48, 2374–2379. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Suhl, H.J.; Noh, D.O.; Kang, C.S.; Kim, J.M.; Lee, S.W. Thermal kinetics of color degradation of mulberry fruit extract. Die Nahr. 2003, 47, 132–135. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, G.; Chen, W.; Xu, Y.; Wu, J. Quantification and purification of mulberry anthocyanins with macroporous resins. J. Biomed. Biotechnol. 2004, 5, 326–331. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Aly, H.F.; Abd-Alla, H.I.; Biomy, D.F. Therapeutic impact of berries (Morus alba and Morus rubra) fruit extract in the regression of high-fat diet-induced cardiac dysfunction in rats. Asian J. Pharm. Clin. Res. 2018, 11, 314–320. [Google Scholar] [CrossRef]
- Kim, S.B.; Chang, B.Y.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Pyrrole alkaloids from the fruits of Morus alba. Bioorg. Med. Chem. Lett. 2014, 24, 5656–5659. [Google Scholar] [CrossRef]
- Kim, S.B.; Chang, B.Y.; Jo, Y.H.; Lee, S.H.; Han, S.-B.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Macrophage activating activity of pyrrole alkaloids from Morus alba fruits. J. Ethnopharmacol. 2013, 145, 393–396. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Zhang, X.; Jin, Q.; Li, J. Phenolic profiles, antioxidant activities, and neuroprotective properties of mulberry (Morus atropurpurea Roxb.) fruit extracts from different ripening stages. J. Food Sci. 2016, 81, C2439–C2446. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Hur, J.Y.; Kim, H.B.; Ryu, J.H.; Kim, S.Y. Neuroprotective effects of the cyanidin-3-O-beta-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neurosci. Lett. 2006, 391, 122–126. [Google Scholar] [CrossRef]
- Qin, C.; Li, Y.; Niu, W.; Ding, Y.; Zhang, R.; Shang, X. Analysis and characterisation of anthocyanins in mulberry fruit. Czech J. Food Sci. 2010, 28, 117–126. [Google Scholar] [CrossRef]
- Du, Q.; Zheng, J.; Xu, Y. Composition of anthocyanins in mulberry and their antioxidant activity. J. Food Comp. Anal. 2008, 21, 390–395. [Google Scholar] [CrossRef]
- Jiang, Y.; Nie, W.-J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef]
- Chang, J.C.; Chang, M.W. ‘Elongated fruit No. 1′ mulberry: An elite cultivar for fresh consumption. J. Am. Pomol. Soc. 2010, 64, 101–105. [Google Scholar]
- Chang, J.C.; Chang, M.W. Elongated Fruit No. 1′ Mulberry: An Elite Cultivar for Fresh Consumption. J. Am. Pomol. Soc. 2010, 64, 101. [Google Scholar]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Martínez, J.J.; Hernández, F. Phytochemical evaluation of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits, a starting point for the assessment of their beneficial properties. J. Funct. Foods 2015, 12, 399–408. [Google Scholar] [CrossRef]
- Eyduran, S.P.; Ercisli, S.; Akin, M.; Beyhan, O.; Gecer, M.; Eyduran, E.; Erturk, Y.E. Organic acids, sugars, vitamin C, antioxidant capacity, and phenolic compounds in fruits of white (Morus alba L.) and black (Morus nigra L.) mulberry genotypes. J. Appl. Bot. Food Qual. 2015, 88, 134–138. [Google Scholar] [CrossRef]
- Gundogdu, M.; Muradoglu, F.; Sensoy, R.G.; Yilmaz, H. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci. Hortic. 2011, 132, 37–41. [Google Scholar] [CrossRef]
- Roussos, P.A.; Denaxa, N.-K.; Ntanos, E.; Tsafouros, A.; Mavrikou, S.; Kintzios, S. Organoleptic, Nutritional and Anti-carcinogenic Characteristics of the Fruit and Rooting Performance of Cuttings of Black Mulberry (Morus nigra L.) Genotypes. J. Berry Res. 2020, 10, 77–93. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Özgen, M.; Serçe, S.; Kaya, C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci. Hortic. 2009, 119, 275–279. [Google Scholar] [CrossRef]
- Donno, D.; Cerutti, A.; Prgomet, I.; Mellano, M.; Beccaro, G. Foodomics for mulberry fruit (Morus spp.): Analytical fingerprint as antioxidants’and health properties’ determination tool. Food Res. Intern. 2015, 69, 179–188. [Google Scholar] [CrossRef]
- Sánchez, E.M.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Melgarejo, P.; Hernández, F.; Martínez-Nicolás, J.J. Physicochemical characterisation of eight S panish mulberry clones: Processing and fresh market aptitudes. Intern. J. Food Sci. Techn. 2014, 49, 477–483. [Google Scholar] [CrossRef]
- Akin, M.; Eyduran, S.P.; Ercisli, S.; Yilmaz, I.; Cakir, O. Phytochemical profiles of wild grown blackberry and mulberry in Turkey. Acta. Sci. Pol. Hort. Cultus. 2016, 15, 3–12. [Google Scholar]
- Koyuncu, F. Organic acid composition of native black mulberry fruit. Chem Nat Comp. 2004, 40, 367–369. [Google Scholar] [CrossRef]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguiée-A-Mbeguiée, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef]
- Yang, N.C.; Jhou, K.Y.; Tseng, C.Y. Antihypertensive effect of mulberry leaf aqueous extract containing γ-aminobutyric acid in spontaneously hypertensive rats. Food Chem. 2012, 132, 1796–1801. [Google Scholar] [CrossRef]
- Tu, J.; Liu, G.; Jin, Y.; Tang, C.; Yao, T.; Zhuo, J.; Li, Q.; Liu, L.; Wang, J. Enrichment of γ-aminobutyric acid in mulberry leaves and the inhibitory effects of the water extract on ACE and α-glucosidase activity. Ind. Crops Prod. 2022, 177, 114485. [Google Scholar] [CrossRef]
- Jin, Y.; Tu, J.; Han, X.; Zhuo, J.; Liu, G.; Han, Y.; Du, H.; Wang, J.; Xiao, H. Characteristics of Mulberry Leaf Powder Enriched With γ-Aminobutyric Acid and Its Antioxidant Capacity as a Potential Functional Food Ingredient. Front. Nutr. 2022, 9, 900718. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Sun, S.; Lao, F.; Liao, X.; Wu, J. Gas chromatography mass spectrometry combined with multivariate data analysis as a tool for differentiating between processed orange juice samples on the basis of their volatile markers. Food Chem. 2020, 311, 125913. [Google Scholar] [CrossRef] [PubMed]
- Young, H.; Gilbert, J.M.; Murray, S.H.; Ball, R.D. Causal Effects of Aroma Compounds on Royal Gala Apple Flavours. J. Sci. Food Agric. 2015, 71, 329–336. [Google Scholar] [CrossRef]
- Ma, D.; Zhao, H.; Liu, Z.; Liu, M.; Qi, P.; Di, S.; Zhang, S.; Wang, X. Recent advances on mulberry volatile flavor: A review. J. Food Compos. Anal. 2023, 124, 105665. [Google Scholar] [CrossRef]
- Waldmann, D.; Winterhalter, P. Identification of a novel vitispirane precursor in Riesling wine. Vitis 2015, 31, 169–174. [Google Scholar] [CrossRef]

| Variety | Length (cm) | Transverse Stem (mm) | Weight (g) | Brix |
|---|---|---|---|---|
| D10M.S1 | 49.00 ± 0.58 b | 19.00 ± 0.58 a | 9.30 ± 0.15 b | 11.10 ± 0.55 f |
| D10M.S2 | 49.33 ± 0.33 b | 20.00 ± 0.57 a | 9.63 ± 0.03 a | 14.80 ± 0.15 d |
| JL40M.S1 | 30.33 ± 1.86 c | 13.33 ± 0.67 bc | 4.50 ± 0.06 f | 10.07 ± 0.09 f |
| JL40M.S2 | 31.33 ± 1.20 c | 14.33 ± 0.33 b | 5.07 ± 0.09 e | 13.57 ± 0.58 de |
| TLM.S1 | 82.00 ± 1.73 a | 10.00 ± 0.58 d | 6.90 ± 0.06 d | 16.97 ± 0.55 c |
| TLM.S2 | 83.67 ± 0.88 a | 10.33 ± 0.33 d | 7.13 ± 0.03 c | 25.90 ± 0.53 a |
| BLM.S1 | 46.00 ± 1.15 b | 7.33 ± 0.33 e | 3.70 ± 0.06 g | 12.57 ± 0.33 e |
| BLM.S2 | 48.33 ± 1.20 b | 8.33 ± 0.33 e | 3.80 ± 0.06 g | 22.47 ± 0.37 b |
| BZM.S1 | 23.67 ± 0.33 d | 12.00 ± 0.58 c | 2.27 ± 0.03 h | 14.27 ± 0.15 d |
| BZM.S2 | 24.33 ± 0.33 d | 13.33 ± 0.33 bc | 2.37 ± 0.03 h | 22.77 ± 0.58 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, D.; Wu, H.; Wei, H.; Lu, F.; Geng, T.; Lin, P.; Wang, S. Analysis of Main Components of Five Mulberry Varieties in Tropics. Plants 2024, 13, 2763. https://doi.org/10.3390/plants13192763
Lou D, Wu H, Wei H, Lu F, Geng T, Lin P, Wang S. Analysis of Main Components of Five Mulberry Varieties in Tropics. Plants. 2024; 13(19):2763. https://doi.org/10.3390/plants13192763
Chicago/Turabian StyleLou, Dezhao, Huazhou Wu, Hongxian Wei, Fuping Lu, Tao Geng, Peiqun Lin, and Shuchang Wang. 2024. "Analysis of Main Components of Five Mulberry Varieties in Tropics" Plants 13, no. 19: 2763. https://doi.org/10.3390/plants13192763
APA StyleLou, D., Wu, H., Wei, H., Lu, F., Geng, T., Lin, P., & Wang, S. (2024). Analysis of Main Components of Five Mulberry Varieties in Tropics. Plants, 13(19), 2763. https://doi.org/10.3390/plants13192763






