Differential Drought Responses of Soybean Genotypes in Relation to Photosynthesis and Growth-Yield Attributes
Abstract
:1. Introduction
2. Results
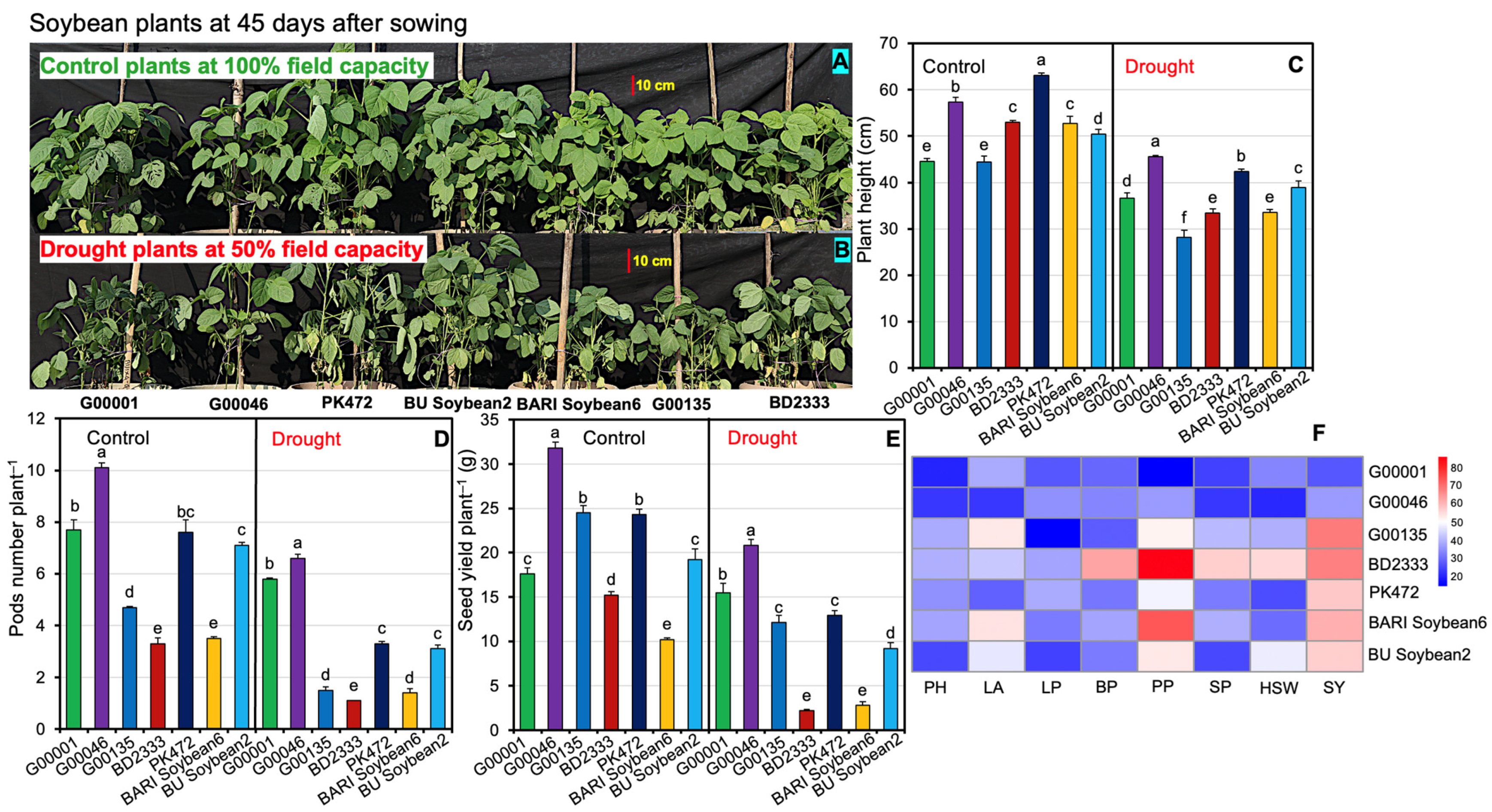
2.1. Phenotypic Responses of Soybeans Genotypes Exposed to Drought Stress
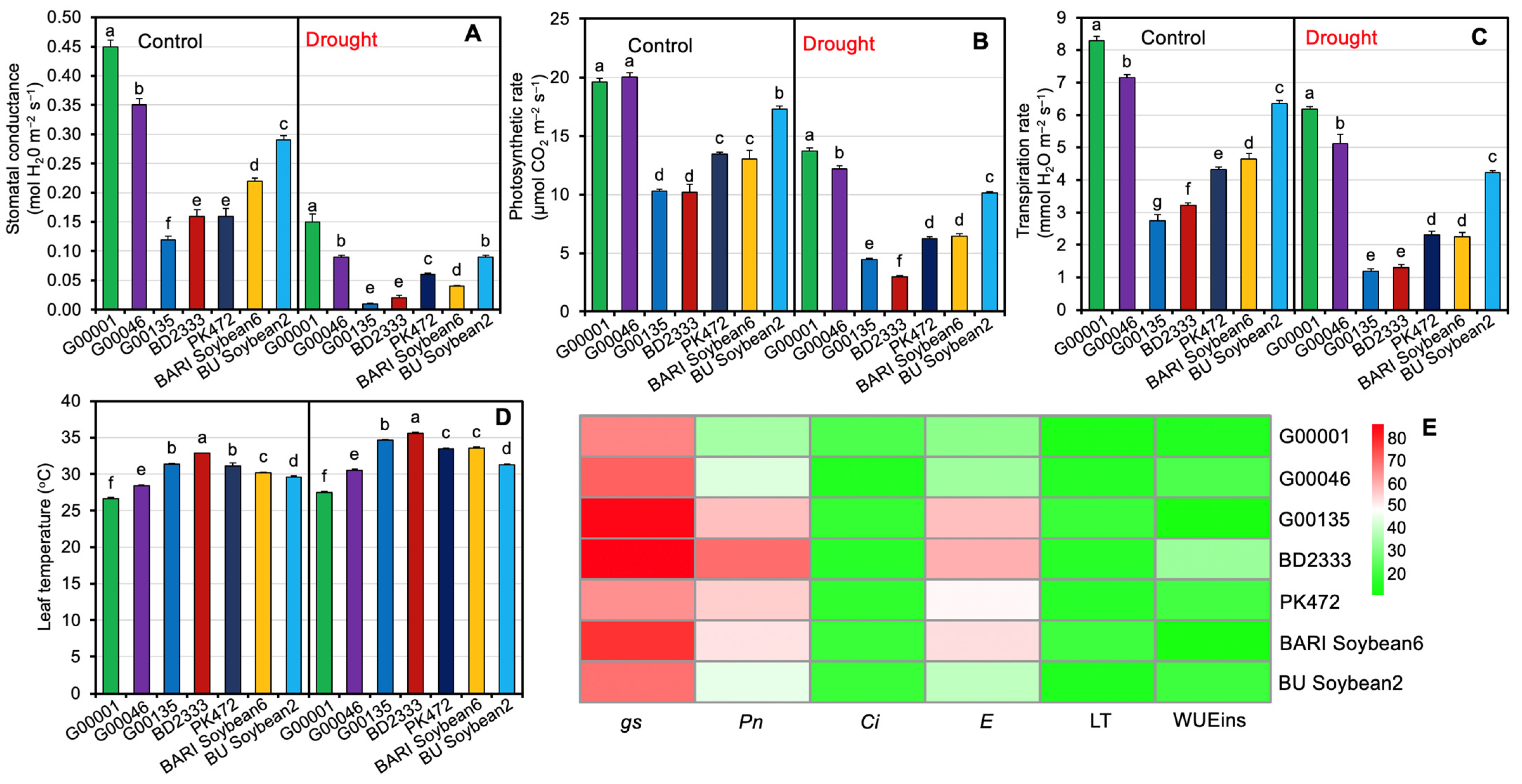
2.2. Photosynthetic Responses of Soybean Genotypes Exposed to Drought Stress
2.3. Plant Water Relations of Soybean Genotypes Exposed to Drought Stress
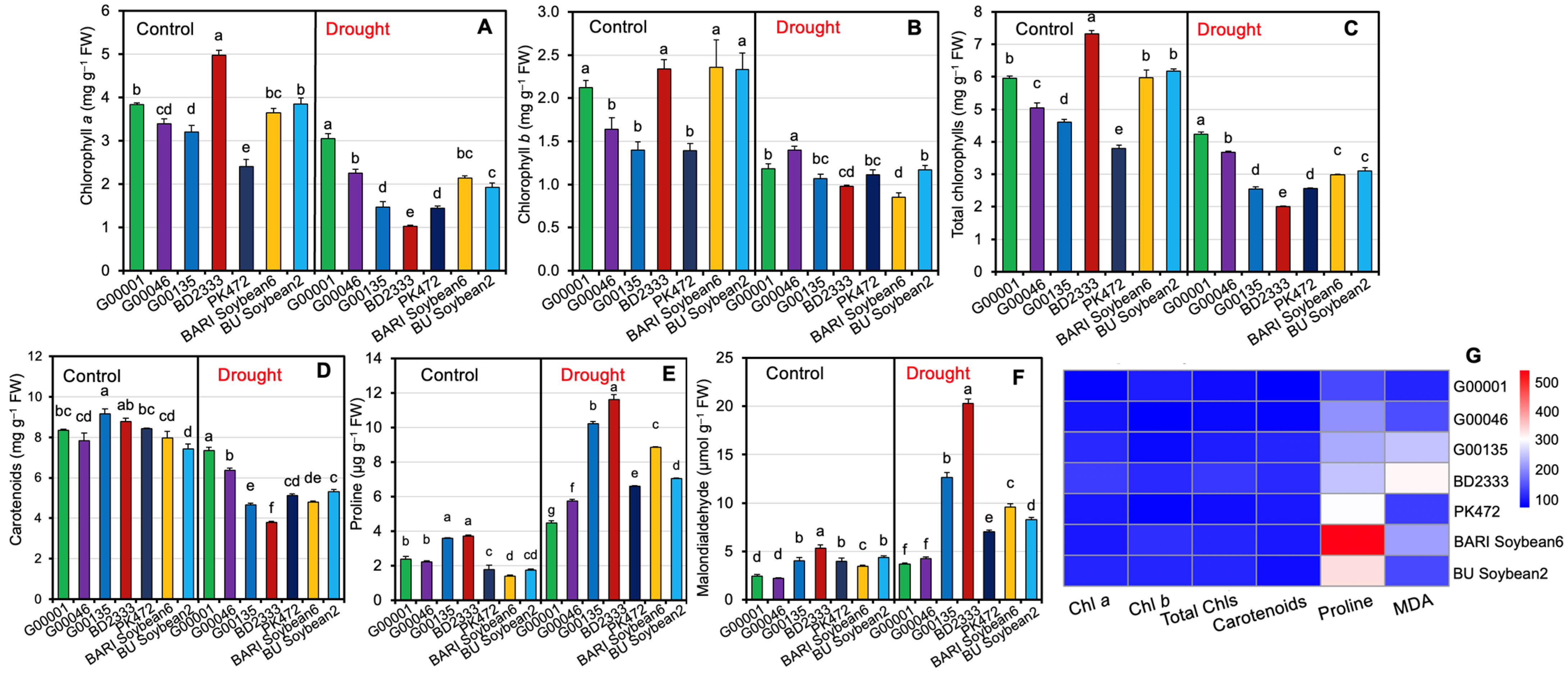
2.4. Levels of Photosynthetic Pigments, Proline, and Malondialdehyde in the Leaves of Soybean Genotypes Exposed to Drought Stress
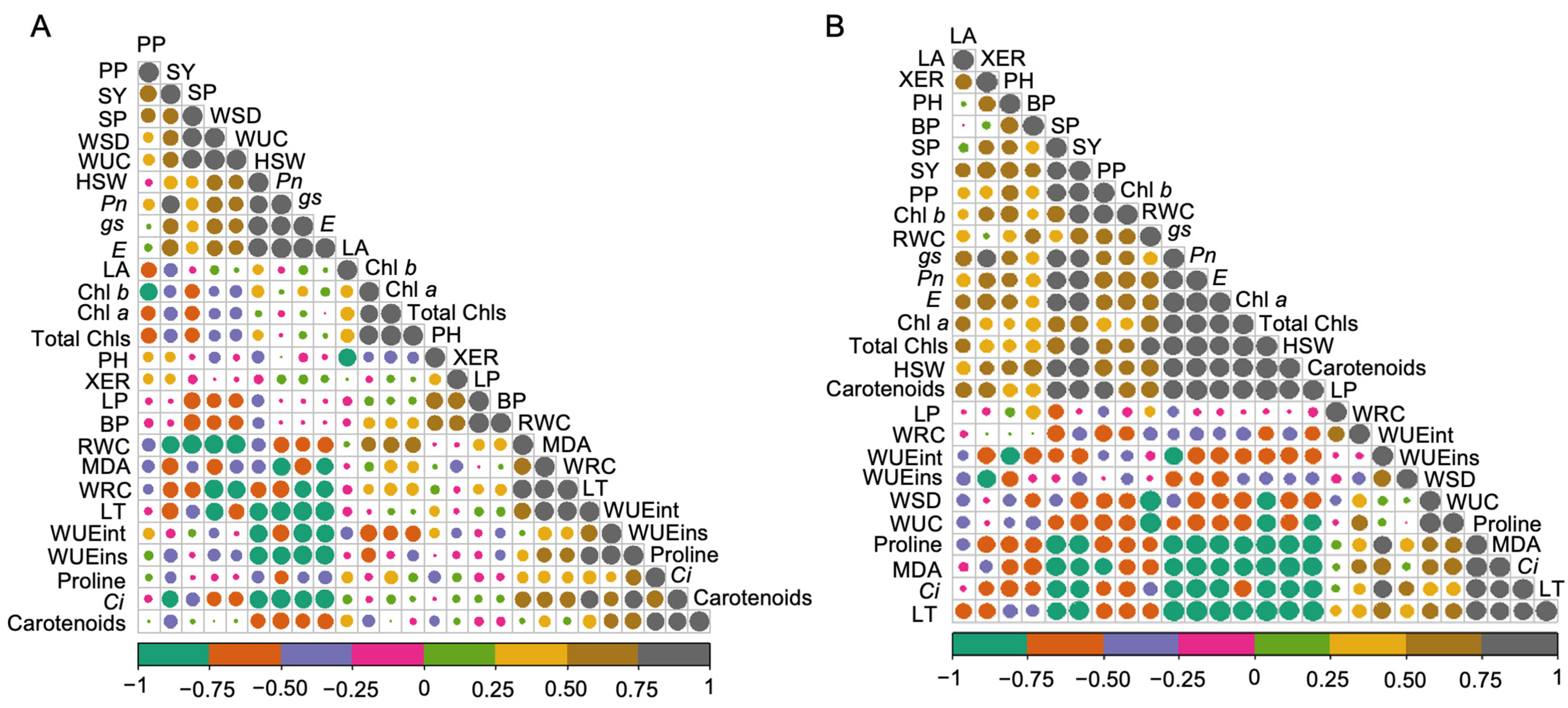
2.5. Correlation between Studied Traits under Control and Drought Stress Conditions
2.6. Stress Tolerance Indices Utilizing Seed Yield
2.7. Ranking of Soybean Genotypes Utilizing Stress Tolerance Indices
3. Discussion
4. Materials and Methods
4.1. Experimental Setup and Management
4.2. Assessment of Studied Traits
4.3. Quantification of Photosynthetic Pigments, Proline, and Malondialdehyde
4.4. Measurement of Agronomic Traits and Stress Tolerance Indices
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fatema, M.K.; Al Mamun, M.A.; Sarker, U.; Hossain, M.S.; Mia, M.A.B.; Roychowdhury, R.; Ercisli, S.; Marc, R.A.; Babalola, O.O.; Karim, M.A. Assessing morpho-physiological and biochemical markers of soybean for drought tolerance potential. Sustainability 2023, 15, 1427. [Google Scholar] [CrossRef]
- Bradford, J.B.; Schlaepfer, D.R.; Lauenroth, W.K.; Palmquist, K.A. Robust ecological drought projections for drylands in the 21st century. Glob. Chang. Biol. 2020, 26, 3906–3919. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Berwal, M.K.; Saroj, P.L. Morphological, physiological, biochemical and molecular facet of drought stress in horticultural crops. Int. J. Bio-Resour. Stress Manag. 2019, 10, 545–560. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Wijewardana, C.; Henry, W.B.; Reddy, K.R. Evaluation of drought tolerant maize germplasm to induced drought stress. J. Miss. Acad. Sci. 2017, 62, 316–329. [Google Scholar]
- Srivalli, B.; Sharma, G.; Khanna-Chopra, R. Antioxidative defense system in an upland rice cultivar subjected to increasing intensity of water stress followed by recovery. Physiol. Plant. 2003, 119, 503–512. [Google Scholar] [CrossRef]
- Athar, H.R.; Ashraf, H. Photosynthesis under Drought Stress. In Handbooks of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; Taylor and Francis, Inc.: New York, NY, USA, 2005; pp. 793–809. [Google Scholar]
- Jordan, W.R.; Ritchie, J.T. Influence of soil water stress on evaporation, root absorption, and internal water status of cotton. Plant Physiol. 1971, 48, 783–788. [Google Scholar] [CrossRef]
- Sharma, N. Effect of Brassinosteroid [Brassinolide] on Morpho-Physiological, Biochemical Attributes and Antioxidant Enzyme Activity in Tomato (Lycopersicon esculentum L.) under Drought Stress. Master’s Thesis, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi, India, 2016. Available online: https://krishikosh.egranth.ac.in/items/28607e11-fbcd-4188-83b2-1d96fe79694d (accessed on 25 June 2024).
- Moradi, A.B.; Conesa, H.M.; Robinson, B.H.; Lehmann, E.; Kaestner, A.; Schulin, R. Root responses to soil Ni heterogeneity in a hyperaccumulator and a non-accumulator species. Environ. Pollut. 2009, 157, 2189–2196. [Google Scholar] [CrossRef]
- Ilyas, M.; Nisar, M.; Khan, N.; Hazrat, A.; Khan, A.H.; Hayat, K.; Fahad, S.; Khan, A.; Ullah, A. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 2021, 40, 926–944. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Hunt, L.; Afsharinafar, M.; Al Meselmani, M.; Mitchell, A.; Howells, R.; Wallington, E.; Fleming, A.J.; Gray, J.E. Reduced stomatal density in bread wheat leads to increased water-use efficiency. J. Exp. Bot. 2019, 70, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Hossain, M.S.; Islam, M.N.; Khan, M.A.R. Role of tocopherol in conferring abiotic stress tolerance in plants. In Antioxidant Defense in Plants: Molecular Basis of Regulation; Springer Nature: Singapore, 2022; pp. 215–233. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Rahman, M.; Mostofa, M.G.; Keya, S.S.; Rahman, A.; Das, A.K.; Islam, R.; Abdelrahman, M.; Bhuiyan, S.U.; Naznin, T.; Ansary, M.U.; et al. Acetic acid improves drought acclimation in soybean: An integrative response of photosynthesis, osmoregulation, mineral uptake and antioxidant defense. Physiol. Plant. 2021, 172, 334–350. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Anik, T.R.; Mostofa, M.G.; Rahman, M.M.; Khan, M.A.R.; Ghosh, P.K.; Sultana, S.; Das, A.K.; Hossain, S.; Keya, S.S.; Rahman, A.; et al. Zn Supplementation mitigates Drought effects on Cotton by improving photosynthetic performance and antioxidant defense mechanisms. Antioxidants 2023, 12, 854. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Cao, D.; Li, Y.; Liu, B.; Kong, F.; Tran, L.S.P. Adaptive mechanisms of soybean grown on salt-affected soils. Land Degrad. Dev. 2018, 29, 1054–1064. [Google Scholar] [CrossRef]
- Padalkar, G.; Mandlik, R.; Sudhakaran, S.; Vats, S.; Kumawat, S.; Kumar, V.; Kumar, V.; Rani, A.; Ratnaparkhe, M.B.; Jadhav, P.; et al. Necessity and challenges for exploration of nutritional potential of staple-food grade soybean. J. Food Compos. Anal. 2023, 117, 105093. [Google Scholar] [CrossRef]
- Rahman, M.; Mostofa, M.G.; Das, A.K.; Anik, T.R.; Keya, S.S.; Ahsan, S.M.; Khan, A.R.; Ahmed, M.; Rahman, A.; Hossain, M.; et al. Ethanol positively modulates photosynthetic traits, antioxidant defense and osmoprotectant levels to enhance drought acclimatization in soybean. Antioxidants 2022, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Ruano, L.; Moreira, C.; Carvalho, O.P.; Barros, H. Prevalence and incidence of cognitive impairment in an elder Portuguese population (65–85 years old). BMC Geriatr. 2020, 20, 470. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-Y.; Thakur, K.; Feng, J.-Y.; Cai, J.-S.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. B-vitamin enriched fermented soymilk: A novel strategy for soy-based functional foods development. Trends Food Sci. Technol. 2020, 105, 43–55. [Google Scholar] [CrossRef]
- Hossain, M.S.; Mamun, M.A.A.; Akter, M.; Karim, A.J.M.S.; Khaliq, Q.A.; Khan, M.A.R.; Karim, M.A. Effect of waterlogging stress on yield and yield contributing trait of soybean. Bangladesh J. Ecol. 2019, 1, 83–89. [Google Scholar]
- Li, M.-W.; Xin, D.; Gao, Y.; Li, K.-P.; Fan, K.; Muñoz, N.B.; Yung, W.-S.; Lam, H.-M. Using genomic information to improve soybean adaptability to climate change. J. Exp. Bot. 2017, 68, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Abdelrahman, M.; Tran, L.-S.P. Carbon metabolic adjustment in soybean nodules in response to phosphate limitation: A metabolite perspective. Environ. Exp. Bot. 2022, 196, 104810. [Google Scholar] [CrossRef]
- Li, R.; Chen, H.; Yang, Z.; Yuan, S.; Zhou, X. Research status of soybean symbiosis nitrogen fixation. Oil Crop. Sci. 2020, 5, 6–10. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO). 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 June 2024).
- Pagano, M.C.; Miransari, M. The importance of soybean production worldwide. In Abiotic and Biotic Stresses in Soybean Production; Academic Press: Cambridge, MA, USA, 2016; pp. 1–26. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Babalola, O.O. Bioprospecting and challenges of plant microbiome research for sustainable agriculture, a review on soybean endophytic bacteria. Microb. Ecol. 2022, 85, 1113–1135. [Google Scholar] [CrossRef]
- Fibl.Statistics. 2020 Fibl.Statistics; FiBl: Frick, Switzerland, 2020. [Google Scholar]
- Satter, M.A.; Rahman, M.M.; Rashid, M.H.; Ali, M.S.; Alam, M.S. Krishi Projukti Hatboi (Handbook on Agro-Technology); Bangladesh Agricultural Research Institute: Gazipur, Bangladesh, 2005; Volume 25, pp. 144–151. [Google Scholar]
- BBS (Bangladesh Bureau of Statistics). Statistical Year of Bangladesh; Ministry of Planning, Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2020; pp. 20–105.
- Poudel, S.; Vennam, R.R.; Shrestha, A.; Reddy, K.R.; Wijewardane, N.K.; Reddy, K.N.; Bheemanahalli, R. Resilience of soybean cultivars to drought stress during flowering and early-seed setting stages. Sci. Rep. 2023, 13, 1277. [Google Scholar] [CrossRef]
- Luan, X.; Bommarco, R.; Scaini, A.; Vico, G. Combined heat and drought suppress rainfed maize and soybean yields and modify irrigation benefits in the USA. Environ. Res. Lett. 2021, 16, 064023. [Google Scholar] [CrossRef]
- Yan, C.; Song, S.; Wang, W.; Wang, C.; Li, H.; Wang, F.; Li, S.; Sun, X. Screening diverse soybean genotypes for drought tolerance by membership function value based on multiple traits and drought-tolerant coefficient of yield. BMC Plant Biol. 2020, 20, 321. [Google Scholar] [CrossRef] [PubMed]
- Matcham, E.; Conley, S.P. Soybean Irrigation during Reproductive Growth. Cool Bean. 2020. Available online: https://coolbean.info/2020/06/16/soybean-irrigation-reproductive-growth (accessed on 25 June 2024).
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relationsand photosynthesis. Emir. J. Food Agric. 2012, 24, 57–72. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, S.; Jin, J.; Ning, S.; Feng, P. Quantitative assessment of soybean drought loss sensitivity at different growth stages based on S-shaped damage curve. Agric. Water Manag. 2019, 213, 821–832. [Google Scholar] [CrossRef]
- Dubey, A.; Kumar, A.; Abd_Allah, E.F.; Hashem, A.; Khan, M.L. Growing more with less: Breeding and developing drought resilient soybean to improve food security. Ecol. Indic. 2019, 105, 425–437. [Google Scholar] [CrossRef]
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef]
- Ning, L.-H.; Du, W.-K.; Song, H.-N.; Shao, H.-B.; Qi, W.-C.; Sheteiwy, M.S.A.; Yu, D.-Y. Identification of responsive miRNAs involved in combination stresses of phosphate starvation and salt stress in soybean root. Environ. Exp. Bot. 2019, 167, 103823. [Google Scholar] [CrossRef]
- Qi, Y.; Weng, J.-K. Editorial overview: Advancing basic plant research and crop improvement through cutting-edge biotechnologies. Curr. Opin. Plant Biol. 2021, 60, 102069. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Zandalinas, S.I.; Fritschi, F.B.; Sengupta, S.; Fichman, Y.; Azad, R.K.; Mittler, R. The impact of water deficit and heat stress combination on the molecular response, physiology, and seed production of soybean. Physiol. Plant. 2021, 172, 41–52. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Xie, F. Effect of drought stress at reproductive stages on growth and nitrogen metabolism in soybean. Agronomy 2020, 10, 302. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, W.; Wang, G.; Hu, Y.; Zhong, X.; Tang, G. Physiological regulation of photosynthetic-related indices, antioxidant defense, and proline anabolism on drought tolerance of wild soybean (Glycine soja L.). Plants 2024, 13, 880. [Google Scholar] [CrossRef]
- Mutava, R.N.; Prince, S.J.K.; Syed, N.H.; Song, L.; Valliyodan, B.; Chen, W.; Nguyen, H.T. Understanding abiotic stress tolerance mechanisms in soybean: A comparative evaluation of soybean response to drought and flooding stress. Plant Physiol. Biochem. 2015, 86, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Terrones, M.; Park, C.R.; Mukherjee, R.; Monthioux, M.; Koratkar, N.; Kim, Y.S.; Hurt, R.; Frackowiak, E.; Enoki, T.; et al. Carbon science in 2016: Status, challenges and perspectives. Carbon 2016, 98, 708–732. [Google Scholar] [CrossRef]
- Gates, D.M. Transpiration and leaf temperature. Annu. Rev. Plant Physiol. 1968, 19, 211–238. [Google Scholar] [CrossRef]
- Ye, H.; Yuan, Z.; Zhang, S. The heat and mass transfer analysis of a leaf. J. Bionic Eng. 2013, 10, 170–176. [Google Scholar] [CrossRef]
- Chowdhury, J.A.; Karim, M.A.; Khaliq, Q.A.; Ahmed, A.U.; Mondol, A.M. Effect of drought stress on water relation traits of four soybean genotypes. SAARC J. Agric. 2017, 15, 163–175. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Lv, Y.; Li, T.; Tang, J.; Yang, X.; Bai, J.; Jin, X.; Zhou, H. Effects of drought stress during critical periods on the photosynthetic characteristics and production performance of naked oat (Avena nuda L.). Sci. Rep. 2022, 12, 11199. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Ramamoorthy, P.; Poudel, S.; Samiappan, S.; Wijewardane, N.; Reddy, K.R. Effects of drought and heat stresses during reproductive stage on pollen germination, yield, and leaf reflectance properties in maize (Zea mays L.). Plant Direct 2022, 6, e434. [Google Scholar] [CrossRef]
- Bazzer, S.K.; Purcell, L.C. Identification of quantitative trait loci associated with canopy temperature in soybean. Sci. Rep. 2020, 10, 17604. [Google Scholar] [CrossRef] [PubMed]
- Haworth, M.; Killi, D.; Materassi, A.; Raschi, A.; Centritto, M. Impaired stomatal control is associated with reduced photosynthetic physiology in crop species grown at elevated [CO2]. Front. Plant Sci. 2016, 7, 1568. [Google Scholar] [CrossRef]
- Imtiaz, A.A.; Shahriar, S.A.; Baque, M.A.; Eaty, M.N.K.; Falguni, M.R. Screening of mungbean genotypes under polyethylene glycol (PEG) induced drought stress condition. Annu. Res. Rev. Biol. 2020, 1–12. [Google Scholar] [CrossRef]
- Islam, M.R.; Kamal, M.M.; Alam, M.A.; Hossain, J.; Soufan, W.; Skalicky, M.; Brestic, M.; Habib-Ur-Rahman, M.; EL Sabagh, A.; Islam, M.S. Physiochemical changes of mung bean [Vigna radiata (L.) R. Wilczek] in responses to varying irrigation regimes. Horticulturae 2021, 7, 565. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Tripathi, M.; Tiwari, S.; Tripathi, N.; Gupta, N.; Sharma, A. Morphological and Physiological Performance of Indian Soybean [Glycine max (L.) Merrill] Genotypes in Respect to Drought. Legum. Res. Int. J. 2024, 47, 705–713. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, Z.-Q.; Zhao, J.; Zhang, G.; Wu, F. Physiological and biochemical responses to drought stress in cultivated and Tibetan wild barley. Plant Growth Regul. 2015, 75, 567–574. [Google Scholar] [CrossRef]
- Filek, M.; Łabanowska, M.; Kościelniak, J.; Biesaga-Kościelniak, J.; Kurdziel, M.; Szarejko, I.; Hartikainen, H. Characterization of barley leaf tolerance to drought stress by chlorophyll fluorescence and electron paramagnetic resonance studies. J. Agron. Crop. Sci. 2015, 201, 228–240. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef]
- Ahsan, K.; Al Mamun, A.; Ghosh, T.K.; Karim, M.A. Dissecting drought stress tolerance of soybean genotypes based on morphological and physiological attributes. J. Bangladesh Acad. Sci. 2023, 47, 223–240. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Hung, C.-M. Synthesis of magnetic biochar from bamboo biomass to activate persulfate for the removal of polycyclic aromatic hydrocarbons in marine sediments. Bioresour. Technol. 2017, 245, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kleyer, M.; Trinogga, J.; Cebrián-Piqueras, M.A.; Trenkamp, A.; Fløjgaard, C.; Ejrnæs, R.; Bouma, T.J.; Minden, V.; Maier, M.; Mantilla-Contreras, J.; et al. Trait correlation network analysis identifies biomass allocation traits and stem specific length as hub traits in herbaceous perennial plants. J. Ecol. 2019, 107, 829–842. [Google Scholar] [CrossRef]
- Ahmmed, S.; Jahiruddin, M. Fertilization Recommendation Guide-2018; Bangladesh Agricultural Research Council (BARC): Dhaka, Bangladesh, 2018; p. 223.
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Rosielle, A.A.; Hamblin, J. Theoretical aspects of selection for yield in stress and non-stress environment. Crop Sci. 1981, 21, 943–946. [Google Scholar] [CrossRef]
- Fernandez, G.C. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress, Tainan, Taiwan, 13–18 August 1992; pp. 257–270. Available online: https://worldveg.tind.io/record/72511?ln=en&v=pdf (accessed on 25 June 2024).
- Bidinger, F.; Mahalakshmi, V.; Rao, G. Assessment of drought resistance in pearl millet (Pennisetum americanum (L.) Leeke). II. Estimation of genotype response to stress. Aust. J. Agric. Res. 1987, 38, 49–59. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Gavuzzi, P.; Rizza, F.; Palumbo, M.; Campanile, R.G.; Ricciardi, G.L.; Borghi, B. Evaluation of field and laboratory predictors of drought and heat tolerance in winter cereals. Can. J. Plant Sci. 1997, 77, 523–531. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T. Stress tolerance in soybeans. I. Evaluation of three screening techniques for heat and drought tolerance. Crop. Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Fischer, R.; Wood, J. Drought resistance in spring wheat cultivars. III.* Yield associations with morpho-physiological traits. Aust. J. Agric. Res. 1979, 30, 1001–1020. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.M.; Yousefian, H.; Moradkhani, M.; Moghaddam Vahed, P.; Poczai, P.; Siddique, K.H.M. iPASTIC: An online toolkit to estimate plant abiotic stress indices. Appl. Plant Sci. 2019, 7, e11278. [Google Scholar] [CrossRef]

| Genotypes | TOL | MP | GMP | HM | SSI | STI | YI | YSI | RSI | PR |
|---|---|---|---|---|---|---|---|---|---|---|
| G00001 | 1.90 f | 6.75 b | 6.68 b | 6.62 b | 0.49 f | 1.14 b | 1.86 a | 0.75 a | 1.52 a | 24.68 g |
| G00046 | 3.50 c | 8.35 a | 8.16 a | 7.98 a | 0.69 e | 1.69 a | 2.12 a | 0.65 b | 1.32 b | 34.65 f |
| G00135 | 3.20 d | 3.10 d | 2.66 e | 2.27 e | 1.35 b | 0.18 e | 0.48 bc | 0.32 d | 0.64 f | 68.09 b |
| BD2333 | 3.20 d | 1.70 f | 0.57 g | 0.19 g | 1.93 a | 0.01 g | 0.03 c | 0.03 e | 0.06 g | 96.97 a |
| PK472 | 4.30 a | 5.45 c | 5.01 c | 4.60 c | 1.12 d | 0.64 c | 1.06 b | 0.43 c | 0.87 d | 56.58 d |
| BARI soybean6 | 2.10 e | 2.45 e | 2.21 f | 2.00 f | 1.19 c | 0.12 f | 0.45 bc | 0.40 c | 0.81 e | 60.00 c |
| BU soybean2 | 3.90 b | 5.05 c | 4.66 d | 4.30 d | 1.11 d | 0.55 d | 1.00 b | 0.44 c | 0.89 c | 55.71 e |
| SD | 0.88 | 2.41 | 2.65 | 2.73 | 0.46 | 0.61 | 0.77 | 0.23 | 0.47 | 23.36 |
| Genotypes | TOL | MP | GMP | HM | SSI | STI | YI | YSI | RSI | Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| G00001 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 1.55 |
| G00046 | 5 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 1.77 |
| G00135 | 4 | 5 | 5 | 5 | 6 | 5 | 5 | 6 | 6 | 5.22 |
| BD2333 | 3 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 6.55 |
| PK472 | 7 | 3 | 3 | 3 | 4 | 3 | 3 | 4 | 4 | 3.77 |
| BARI soybean6 | 2 | 6 | 6 | 6 | 5 | 6 | 6 | 5 | 5 | 5.22 |
| BU soybean2 | 6 | 4 | 4 | 4 | 3 | 4 | 4 | 3 | 3 | 3.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.S.; Khan, M.A.R.; Mahmud, A.; Ghosh, U.K.; Anik, T.R.; Mayer, D.; Das, A.K.; Mostofa, M.G. Differential Drought Responses of Soybean Genotypes in Relation to Photosynthesis and Growth-Yield Attributes. Plants 2024, 13, 2765. https://doi.org/10.3390/plants13192765
Hossain MS, Khan MAR, Mahmud A, Ghosh UK, Anik TR, Mayer D, Das AK, Mostofa MG. Differential Drought Responses of Soybean Genotypes in Relation to Photosynthesis and Growth-Yield Attributes. Plants. 2024; 13(19):2765. https://doi.org/10.3390/plants13192765
Chicago/Turabian StyleHossain, Md. Saddam, Md. Arifur Rahman Khan, Apple Mahmud, Uttam Kumar Ghosh, Touhidur Rahman Anik, Daniel Mayer, Ashim Kumar Das, and Mohammad Golam Mostofa. 2024. "Differential Drought Responses of Soybean Genotypes in Relation to Photosynthesis and Growth-Yield Attributes" Plants 13, no. 19: 2765. https://doi.org/10.3390/plants13192765







