Callose and Salicylic Acid Are Key Determinants of Strigolactone-Mediated Disease Resistance in Arabidopsis
Abstract
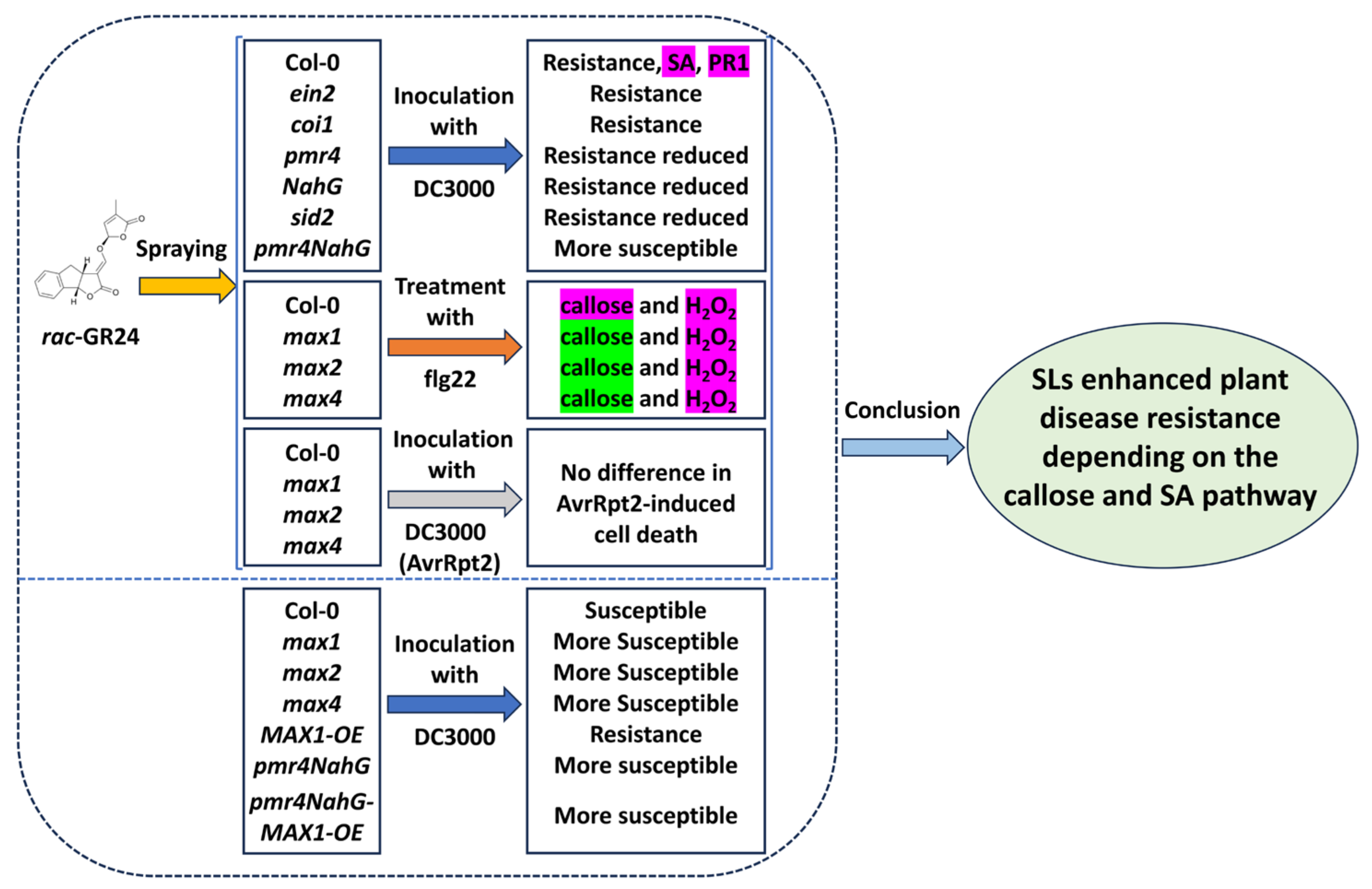
1. Introduction
2. Results
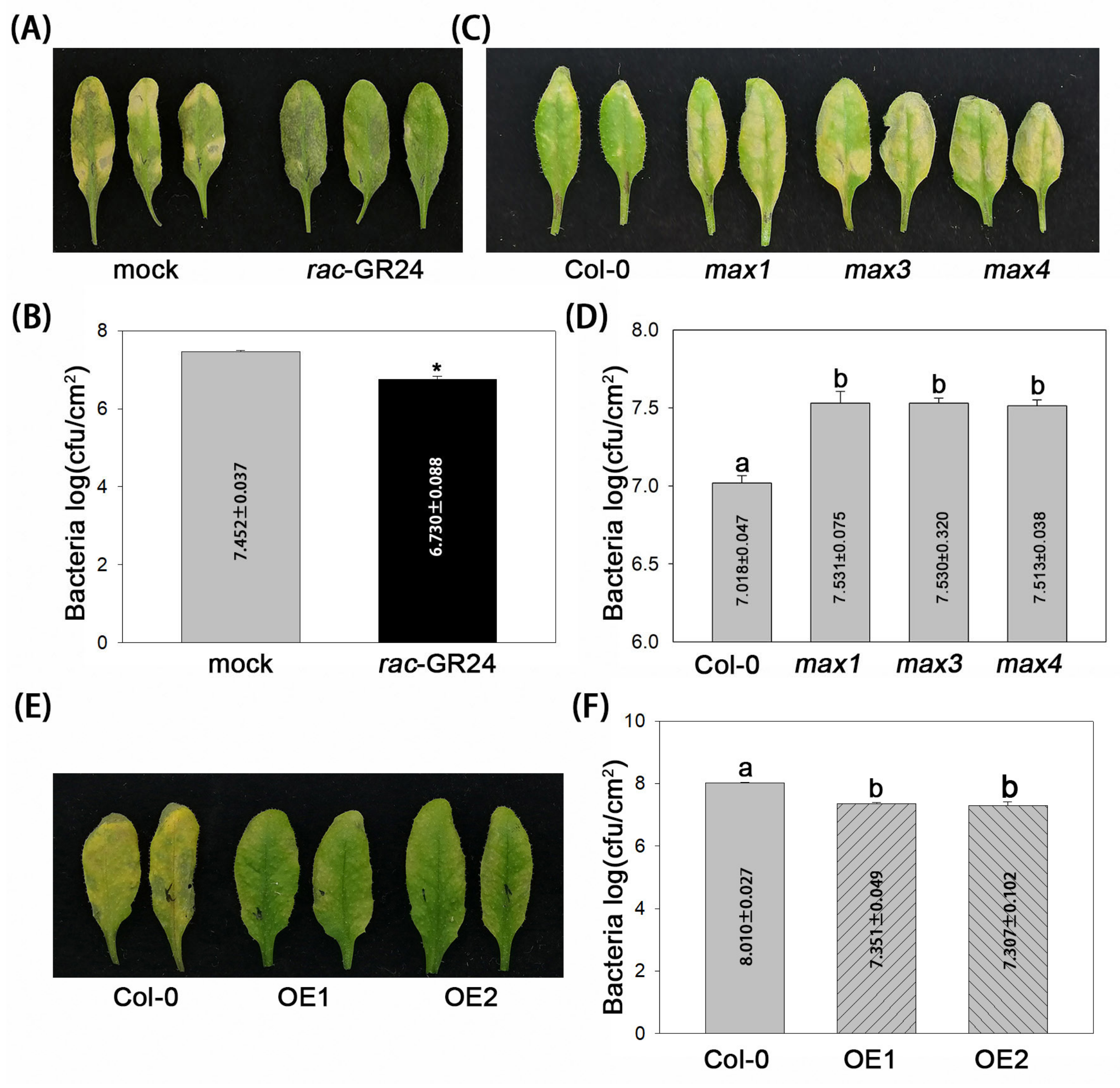
2.1. SLs Contribute to A. thaliana Resistance to P. syringae
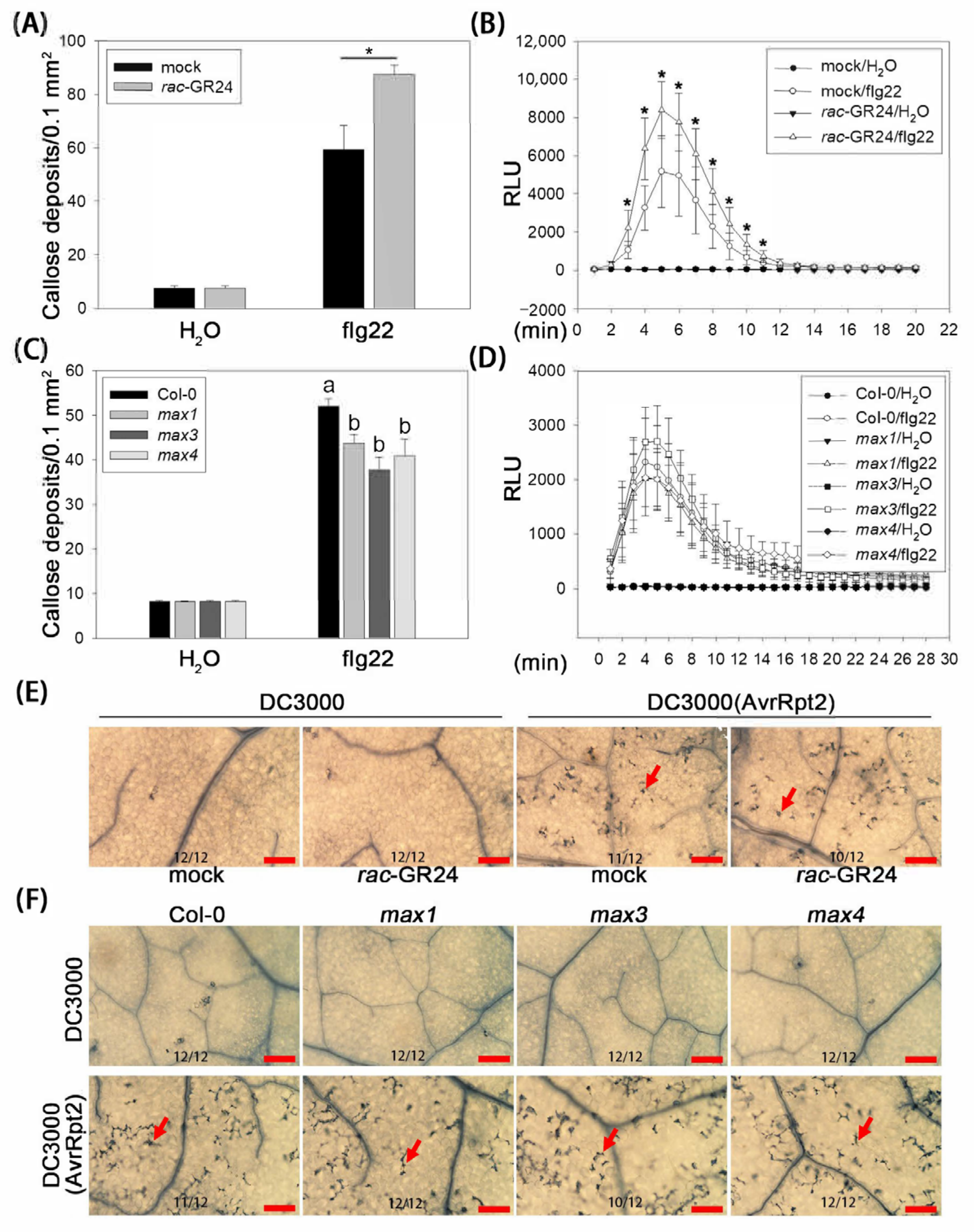
2.2. SLs Enhance Bacterial Pattern-Induced Callose Deposition
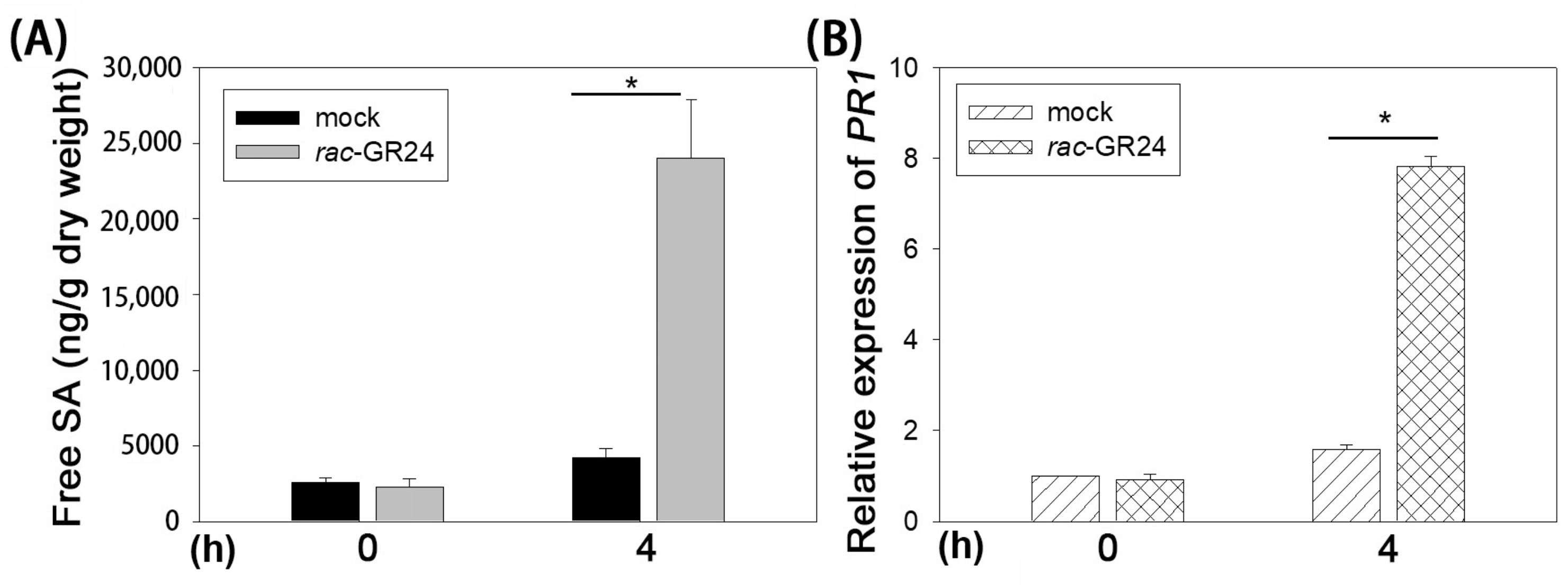
2.3. rac-GR24 Increases Salicylic Acid Signaling
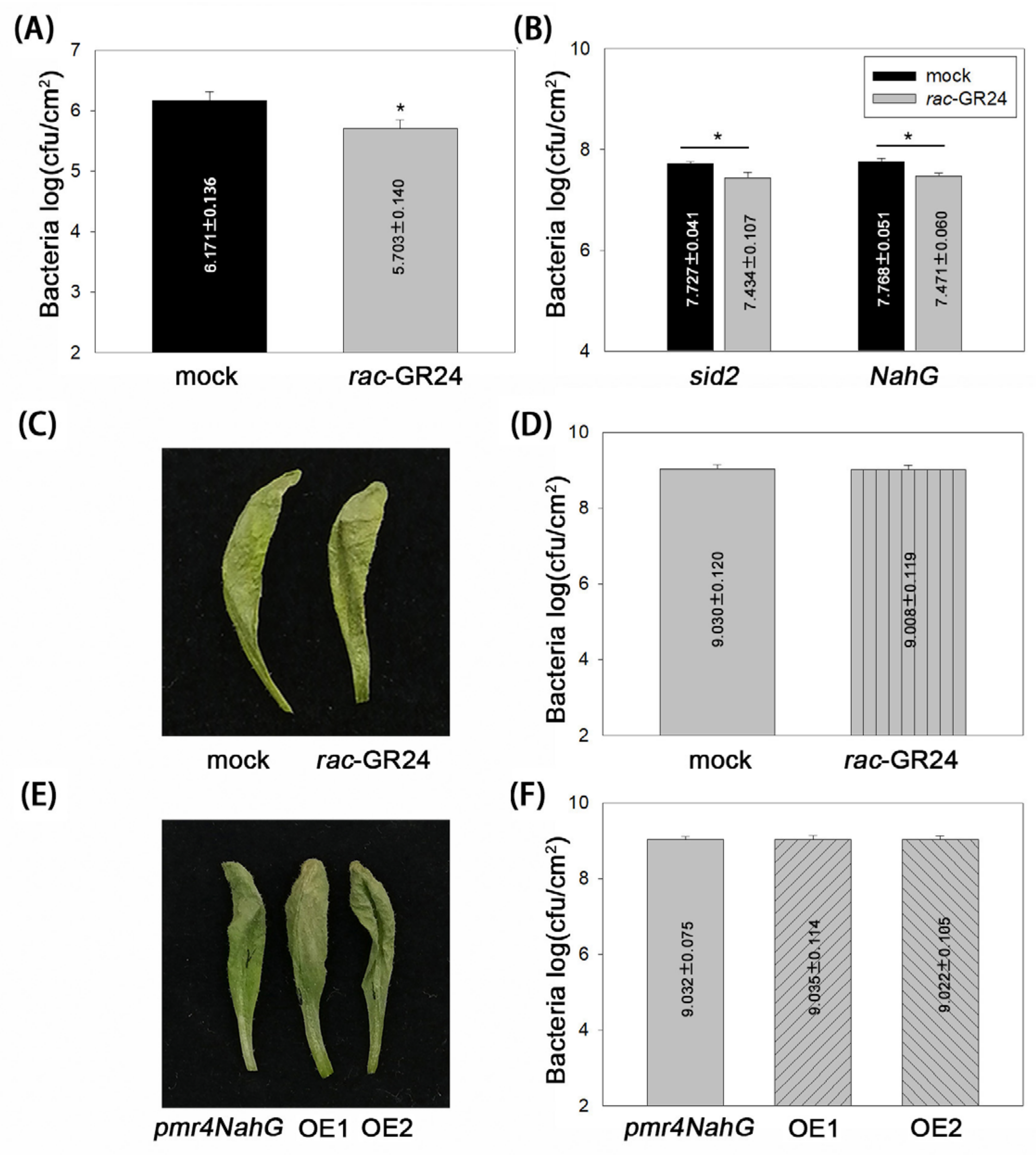
2.4. Callose and SA Are Required for SL-Induced Resistance to P. syringae
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plant Materials, and Growth Conditions
4.2. Hormone Treatment and Pathogenicity Analysis
4.3. Bacterial Growth under rac-GR24 Treatment
4.4. Vector Construction
4.5. Generation of Transgenic Plants and Western Blotting
4.6. Aniline Blue Staining and Fluorescence Observation
4.7. Detection of the H2O2
4.8. Trypan-Blue Staining and Microscopy
4.9. SA Measurements
4.10. Quantitative Real-Time Reverse Transcription PCR (qRT-PCR) Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H.; Kapulnik, Y. Strigolactones as mediators of plant growth responses to environmental conditions. Plant Signal. Behav. 2011, 6, 37–41. [Google Scholar] [CrossRef]
- Booker, J.; Auldridge, M.; Wills, S.; McCarty, D.; Klee, H.; Leyser, O. MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 2004, 14, 1232–1238. [Google Scholar] [CrossRef]
- Auldridge, M.E.; Block, A.; Vogel, J.T.; Dabney-Smith, C.; Mila, I.; Bouzayen, M.; Magallanes-Lundback, M.; DellaPenna, D.; McCarty, D.R.; Klee, H.J. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006, 45, 982–993. [Google Scholar] [CrossRef]
- Lazar, G.; Goodman, H.M. MAX1, a regulator of the flavonoid pathway, controls vegetative axillary bud outgrowth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 472–476. [Google Scholar] [CrossRef]
- Stirnberg, P.; van de Sande, K.; Leyser, H.M.O. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 2002, 129, 1131–1141. [Google Scholar] [CrossRef]
- Chevalier, F.; Nieminen, K.; Sánchez-Ferrero, J.C.; Rodríguez, M.L.; Chagoyen, M.; Hardtke, C.S.; Cubas, P. Strigolactone promotes degradation of DWARF14, an α/β hydrolase essential for strigolactone signaling in Arabidopsis. Plant Cell 2014, 26, 1134–1150. [Google Scholar] [CrossRef]
- Dong, X. SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Plant Biol. 1998, 1, 316–323. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.H.J.; Eggermont, K.; Penninckx, I.A.M.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.P.A.; Broekaert, W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef]
- Marzec, M. Strigolactones as part of the plant defence system. Trends Plant Sci. 2016, 21, 900–903. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Shirasu, K.; Foo, E. Strigolactones in plant interactions with beneficial and detrimental organisms: The Yin and Yang. Trends Plant Sci. 2017, 22, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vera, R.; García, J.M.; Pozo, M.J.; López-Ráez, J.A. Do strigolactones contribute to plant defence? New Phytol. 2014, 15, 211–216. [Google Scholar] [CrossRef]
- Stes, E.; Depuydt, S.; de Keyser, A.; Matthys, C.; Audenaert, K.; Yoneyama, K.; Werbrouck, S.; Goormachtig, S.; Vereecke, D. Strigolactones as an auxiliary hormonal defence mechanism against leafy gall syndrome in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 5123–5134. [Google Scholar] [CrossRef]
- Piisilä, M.; Keceli, M.A.; Brader, G.; Jakobson, L.; Jõesaar, I.; Sipari, N.; Kollist, H.; Palva, E.T.; Kariola, T. The F-box protein MAX2 contributes to resistance to bacterial phytopathogens in Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 53. [Google Scholar] [CrossRef][Green Version]
- Decker, E.L.; Alder, A.; Hunn, S.; Ferguson, J.; Lehtonen, M.T.; Scheler, B.; Kerres, K.L.; Wiedemann, G.; Safavi-Rizi, V.; Nordzieke, S.; et al. Strigolactone biosynthesis is evolutionarily conserved, regulated by phosphate starvation and contributes to resistance against phytopathogenic fungi in a moss, Physcomitrella patens. New Phytol. 2017, 216, 455–468. [Google Scholar] [CrossRef]
- Nasir, F.; Tian, L.; Shi, S.; Chang, C.; Ma, L.; Gao, Y.; Tian, C. Strigolactones positively regulate defense against Magnaporthe oryzae in rice (Oryza sativa). Plant Physiol. Biochem. 2019, 142, 106–116. [Google Scholar] [CrossRef]
- Kusajima, M.; Fujita, M.; Soudthedlath, K.; Nakamura, H.; Yoneyama, K.; Nomura, T.; Akiyama, K.; Maruyama-Nakashita, A.; Asami, T.; Nakashita, H. Strigolactones modulate salicylic acid-mediated disease resistance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 5246. [Google Scholar] [CrossRef] [PubMed]
- Kalliola, M.; Jakobson, L.; Davidsson, P.; Pennanen, V.; Waszczak, C.; Yarmolinsky, D.; Zamora, O.; Palva, E.T.; Kariola, T.; Kollist, H.; et al. Differential role of MAX2 and strigolactones in pathogen, ozone, and stomatal responses. Plant Direct 2020, 4, e00206. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.C.; Scaffidi, A.; Dun, E.A.; Waters, M.T.; Flematti, G.R.; Dixon, K.W.; Beveridge, C.A.; Ghisalberti, E.L.; Smith, S.M. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2011, 108, 8897–8902. [Google Scholar] [CrossRef]
- Wen, S.; Tu, Z.; Wei, L.; Li, H. Liriodendron chinense LcMAX1 regulates primary root growth and shoot branching in Arabidopsis thaliana. Plant Physiol. Biochem. 2022, 190, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Changenet, V.; Macadré, C.; Boutet-Mercey, S.; Magne, K.; Januario, M.; Dalmais, M.; Bendahmane, A.; Mouille, G.; Dufresne, M. Overexpression of a cytochrome P450 monooxygenase involved in orobanchol biosynthesis increases susceptibility to Fusarium Head Blight. Front. Plant Sci. 2021, 12, 662025. [Google Scholar] [CrossRef]
- Keinath, N.F.; Kierszniowska, S.; Lorek, J.; Bourdais, G.; Kessler, S.A.; Shimosato-Asano, H.; Grossniklaus, U.; Schulze, W.X.; Robatzek, S.; Panstruga, R. PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J. Biol. Chem. 2010, 285, 39140–39149. [Google Scholar] [CrossRef]
- Rozpądek, P.; Domka, A.M.; Nosek, M.; Ważny, R.; Jędrzejczyk, R.J.; Wiciarz, M.; Turnau, K. The role of strigolactone in the cross-talk between Arabidopsis thaliana and the endophytic fungus Mucor sp. Front. Microbiol. 2018, 9, 441. [Google Scholar] [CrossRef]
- King, E.; Ward, M.; Raney, D. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Chen, J.; Yang, H.; Ma, S.; Yao, R.; Huang, X.; Yan, J.; Xie, D. HbCOI1 perceives jasmonate to trigger signal transduction in Hevea brasiliensis. Tree Physiol. 2020, 41, 460–471. [Google Scholar] [CrossRef]
- Liu, Q.; Atta, U.R.; Wang, R.; Liu, K.; Ma, X.; Weng, Q. Defense-related hormone signaling coordinately controls the role of melatonin during Arabidopsis thaliana-Pseudomonas syringae interaction. Eur. J. Plant Pathol. 2021, 160, 707–716. [Google Scholar] [CrossRef]
- Mei, S.; Hou, S.; Cui, H.; Feng, F.; Rong, W. Characterization of the interaction between Oidium heveae and Arabidopsis thaliana. Mol. Plant Pathol. 2016, 17, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.T.; Stein, M.; Hou, B.-H.; Vogel, J.P.; Edwards, H.; Somerville, S.C. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 2003, 301, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Mayzlish-Gati, E.; De-Cuyper, C.; Goormachtig, S.; Beeckman, T.; Vuylsteke, M.; Brewer, P.B.; Beveridge, C.A.; Yermiyahu, U.; Kaplan, Y.; Enzer, Y.; et al. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol. 2012, 160, 1329–1341. [Google Scholar] [CrossRef]
- Zipfel, C.; Robatzek, S.; Navarro, L.; Oakeley, E.J.; Jones, J.D.G.; Felix, G.; Boller, T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 2004, 428, 764–767. [Google Scholar] [CrossRef]
- Hauck, P.; Thilmony, R.; He, S.Y. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc. Natl. Acad. Sci. USA 2003, 100, 8577–8582. [Google Scholar] [CrossRef]
- Navarro, L.; Bari, R.; Achard, P.; Lisón, P.; Nemri, A.; Harberd, N.P.; Jones, J.D. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr. Biol. 2008, 18, 650–655. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Liu, Q.; Tan, L. Callose and Salicylic Acid Are Key Determinants of Strigolactone-Mediated Disease Resistance in Arabidopsis. Plants 2024, 13, 2766. https://doi.org/10.3390/plants13192766
Zhao X, Liu Q, Tan L. Callose and Salicylic Acid Are Key Determinants of Strigolactone-Mediated Disease Resistance in Arabidopsis. Plants. 2024; 13(19):2766. https://doi.org/10.3390/plants13192766
Chicago/Turabian StyleZhao, Xiaosheng, Qiuping Liu, and Leitao Tan. 2024. "Callose and Salicylic Acid Are Key Determinants of Strigolactone-Mediated Disease Resistance in Arabidopsis" Plants 13, no. 19: 2766. https://doi.org/10.3390/plants13192766
APA StyleZhao, X., Liu, Q., & Tan, L. (2024). Callose and Salicylic Acid Are Key Determinants of Strigolactone-Mediated Disease Resistance in Arabidopsis. Plants, 13(19), 2766. https://doi.org/10.3390/plants13192766





