Extraction and Concentration of Spirulina Water-Soluble Metabolites by Ultrafiltration
Abstract
1. Introduction
2. Results
2.1. Extraction
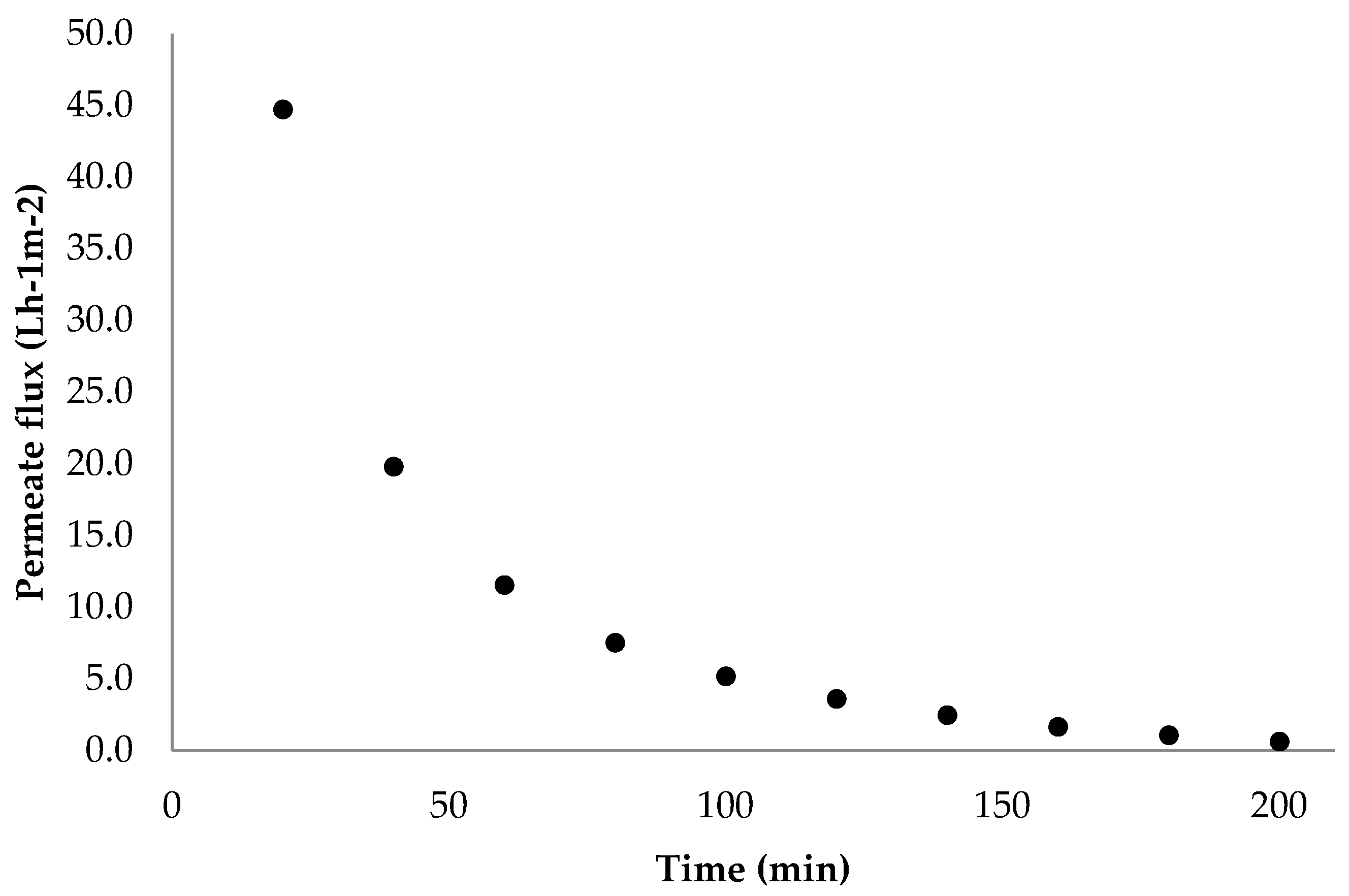
2.2. Ultrafiltration Process (UF)
3. Discussion
3.1. Extraction
3.1.1. Phycocyanin
3.1.2. Protein and Purity Index (PI)
3.1.3. Chlorophyll a (Cla) and Total Carotenoid Content (TCC)
3.2. Extraction Ultrafiltration Process (UF)
4. Materials and Methods
4.1. Chemicals and Biomaterials
4.2. Extraction Methods
4.2.1. Experimental Design
4.2.2. Conventional Extraction (CE)
4.2.3. Ultrasound-Assisted Extraction (UAE)
4.3. Concentration (Ultrafiltration)
4.4. Freeze-Drying
4.5. Quantification of Intracellular Compounds
4.5.1. Phycocyanin Content (PC)
4.5.2. Protein and Purity Index (PI)
4.5.3. Chlorophyll a (Cla) and Total Carotenoid Content (TCC)
4.5.4. Rejection
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bürck, M.; Ramos, S.d.P.; Braga, A.R.C. Enhancing the Biological Effects of Bioactive Compounds from Microalgae through Advanced Processing Techniques: Pioneering Ingredients for Next-Generation Food Production. Foods 2024, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef]
- Athiyappan, K.D.; Routray, W.; Paramasivan, B. Phycocyanin from Spirulina: A comprehensive review on cultivation, extraction, purification, and its application in food and allied industries. Food Humanit. 2024, 2, 100235. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- de Amarante, M.C.A.; Corrêa Júnior, L.C.S.; Sala, L.; Kalil, S.J. Analytical grade C-phycocyanin obtained by a single-step purification process. Process Biochem. 2020, 90, 215–222. [Google Scholar] [CrossRef]
- Balti, R.; Zayoud, N.; Hubert, F.; Beaulieu, L.; Massé, A. Fractionation of Arthrospira platensis (Spirulina) water soluble proteins by membrane diafiltration. Sep. Purif. Technol. 2021, 256, 117756. [Google Scholar] [CrossRef]
- Brião, V.B.; Sbeghen, A.L.; Colla, L.M.; Castoldi, V.; Seguenka, B.; de Oliveira Schimidt, G.; Costa, J.A.V. Is downstream ultrafiltration enough for production of food-grade phycocyanin from Arthrospira platensis? J. Appl. Phycol. 2020, 32, 1129–1140. [Google Scholar] [CrossRef]
- Nisticò, D.M.; Piro, A.; Oliva, D.; Osso, V.; Mazzuca, S.; Fagà, F.A.; Morelli, R.; Conidi, C.; Figoli, A.; Cassano, A. A Combination of Aqueous Extraction and Ultrafiltration for the Purification of Phycocyanin from Arthrospira maxima. Microorganisms 2022, 10, 308. [Google Scholar] [CrossRef]
- Martínez-Vega, J.E.; Villafaña-Estarrón, E.; Escalante, F.M.E. Comparative Study of the Efficiency of Additives in the Extraction of Phycocyanin-C from Arthrospira maxima Using Ultrasonication. Molecules 2023, 28, 334. [Google Scholar] [CrossRef]
- Hockey, J.T. Improving an Aqueous Two-Phase Process for C-Phycocyanin Extraction from Spirulina. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2022. [Google Scholar]
- Menegotto, A.L.L.; Fernandes, I.A.; Colla, L.M.; Duarte, J.; Andrade, M.Z.; Abirached, C.; Franceschi, E.; Steffens, J.; Valduga, E. Thermic and techno-functional properties of Arthrospira platensis protein fractions obtained by membrane separation process. J. Appl. Phycol. 2020, 32, 3885–3900. [Google Scholar] [CrossRef]
- Kurpan, D.; Idà, A.; Körner, F.; Lauceri, R.; Rocculi, P.; Phillips, R.; Schievano, A. Pilot-scale concentration and partial purification of food-grade phycocyanin from Arthrospira platensis via cross flow filtration: From biomass to final product. J. Appl. Phycol. 2023, 35, 2709–2718. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C. Chapter 15—An Energy-Based Approach to Scale Up Microwave-Assisted Extraction of Plant Bioactives. In Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M.B.T., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 561–597. [Google Scholar]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Rutkowska, M.; Namieśnik, J.; Konieczka, P. Chapter 10—Ultrasound-Assisted Extraction; Pena-Pereira, F., Tobiszewski, M.B.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 301–324. [Google Scholar]
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020, 60, 104726. [Google Scholar] [CrossRef]
- Park, W.S.; Kim, H.-J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.-S.; Kang, C.-M.; Ferruzzi, M.G.; Ahn, M.-J. Two Classes of Pigments, Carotenoids and C-Phycocyanin, in Spirulina Powder and Their Antioxidant Activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef]
- Ho, C.C.; Zydney, A.L. A Combined Pore Blockage and Cake Filtration Model for Protein Fouling during Microfiltration. J. Colloid Interface Sci. 2000, 232, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, L.; Grimi, N.; Jaffrin, M.Y.; Tang, B. Application of UF-RDM (Ultafiltration Rotating Disk Membrane) module for separation and concentration of leaf protein from alfalfa juice: Optimization of operation conditions. Sep. Purif. Technol. 2017, 175, 365–375. [Google Scholar] [CrossRef]
- Alotaiby, S.; Zhao, X.; Boesch, C.; Sergeeva, N.N. Sustainable approach towards isolation of photosynthetic pigments from Spirulina and the assessment of their prooxidant and antioxidant properties. Food Chem. 2024, 436, 137653. [Google Scholar] [CrossRef]
- Paes, J.; Da Cunha, C.R.; Viotto, L.A. Concentration of lycopene in the pulp of papaya (Carica papaya L.) by ultrafiltration on a pilot scale. Food Bioprod. Process. 2015, 96, 296–305. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Loha, V.; Tia, S.; Bunnag, B. Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Bioresour. Technol. 2011, 102, 7159–7164. [Google Scholar] [CrossRef] [PubMed]
- López Mejía, N.; Martínez Correa, H.A.; Lobatón García, H.F. Biostimulating activity of biomass extracts and supernatants from a culture of Arthrospira platensis enriched with L-tryptophan. J. Appl. Phycol. 2024, 36, 1875–1884. [Google Scholar] [CrossRef]
- Abalde, J.; Betancourt, L.; Torres, E.; Cid, A.; Barwell, C. Purification and characterization of phycocyanin from the marine cyanobacterium Synechococcus sp. IO9201. Plant Sci. 1998, 136, 109–120. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]

| Treatment | PC (mg/g) | Protein (mg/g) | PI | Cla (mg/g) | TCC (mg/g) |
|---|---|---|---|---|---|
| CE_H2O_1:10 | 11.9 d ± 1.6 | 648 ± 195 | 0.3 d ± 0.003 | 0.9 a ± 0.50 | 0.50 a ± 0.43 |
| T: 18 °C | |||||
| CE_H2O_1:10 | 23.4 c ± 3.0 | 557 ± 11 | 0.5 bcd ± 0.05 | 0.6 a ± 0.58 | 0.25 a ± 0.34 |
| CE_H2O_1:5 | 29.0 bc ± 2.3 | 479 ± 50 | 0.7 ab ± 0.003 | 0.7 a ± 0.11 | 0.18 a ± 0.007 |
| CE_H2O_1:5 | 38.3 a ± 1.4 | 531 ± 47 | 0.6 abc ± 0.2 | 1.5 a ± 0.72 | 0.36 a ± 0.12 |
| t: 18 h | |||||
| UAE_H2O_1:10 | 9.0 d ± 0.7 | 600 ± 115 | 0.2 d ± 0.02 | 0.5 a ± 0.12 | 0.18 a ± 0.10 |
| UAE_H2O_1:5 | 7.7 d ± 3.3 | 271 ± 27 | 0.3 cd ± 0.09 | 0.2 a ± 0.06 | 0.02 a ± 0.004 |
| CE_Buffer_1:5 | 35.4 ab ± 0.8 | 541 ± 4.5 | 0.8 ab ± 0.03 | 0.6 a ± 0.20 | 0.14 a ± 0.07 |
| UAE_Buffer_1:10 | 37.0 a ± 1.9 | 617 ± 15 | 0.6 ab ± 0.04 | 0.4 a ± 0.2 | 0.15 a ± 0.14 |
| UAE_Buffer_1:5 | 36.1 a ± 0.03 | 416 ± 33 | 0.9 a ± 0.06 | 0.3 a ± 0.15 | 0.10 a ± 0.08 |
| Buffer Extraction | Water Extraction | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude Extract | Retentate | Permeate | Rejection (%) | Crude Extract | Retentate | Permeate | Rejection (%) | |
| V (mL) | 341 | 32 | 305 | 333 | 35 | 280 | ||
| PC (g/L) | 0.21 | 2.0 | 0.21 | 1.8 | ||||
| PC (mg) | 71.6 | 65.2 | 0 | 91% | 68.6 | 64.5 | 0 | 94% |
| Protein (g/L) | 3.57 | 32.7 | 1.0 | 4.05 | 35.5 | 0.62 | ||
| Protein (mg) | 1217 | 1047 | 306 | 86% | 1350 | 1242 | 174 | 92% |
| PI | 0.6 | 0.6 | 0.6 | 0.6 | ||||
| Chla (mg/L) | 2.84 | 16 | 4.2 | 24.7 | ||||
| Chla (ug) | 971 | 511 | 0 | 52% | 1400 | 864 | 0 | 62% |
| TCC (mg/L) | 0.92 | 2.9 | 0.83 | 3.23 | ||||
| TCC (ug) | 313 | 95 | 0 | 30% | 277 | 113 | 6.3 | 41% |
| Salts (mg) | 7843 | 784 | 10% | - | - | |||
| RLio (mg) | 993 | 1023 | ||||||
| Extraction Method | Solvent | Ratio (w/v) |
|---|---|---|
| Conventional | Water | 1:5 |
| Conventional | Water | 1:10 |
| Ultrasound-assisted | Water | 1:5 |
| Ultrasound-assisted | Water | 1:10 |
| Conventional | Buffer | 1:10 |
| Ultrasound-assisted | Buffer | 1:5 |
| Ultrasound-assisted | Buffer | 1:10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-González, C.; Mendoza Ramos, C.; Martínez-Correa, H.A.; Lobatón García, H.F. Extraction and Concentration of Spirulina Water-Soluble Metabolites by Ultrafiltration. Plants 2024, 13, 2770. https://doi.org/10.3390/plants13192770
Salazar-González C, Mendoza Ramos C, Martínez-Correa HA, Lobatón García HF. Extraction and Concentration of Spirulina Water-Soluble Metabolites by Ultrafiltration. Plants. 2024; 13(19):2770. https://doi.org/10.3390/plants13192770
Chicago/Turabian StyleSalazar-González, Claudia, Carolina Mendoza Ramos, Hugo A. Martínez-Correa, and Hugo Fabián Lobatón García. 2024. "Extraction and Concentration of Spirulina Water-Soluble Metabolites by Ultrafiltration" Plants 13, no. 19: 2770. https://doi.org/10.3390/plants13192770
APA StyleSalazar-González, C., Mendoza Ramos, C., Martínez-Correa, H. A., & Lobatón García, H. F. (2024). Extraction and Concentration of Spirulina Water-Soluble Metabolites by Ultrafiltration. Plants, 13(19), 2770. https://doi.org/10.3390/plants13192770






