Assessing the Role of AtGRP7 Arginine 141, a Target of Dimethylation by PRMT5, in Flowering Time Control and Stress Response
Abstract
1. Introduction
2. Results
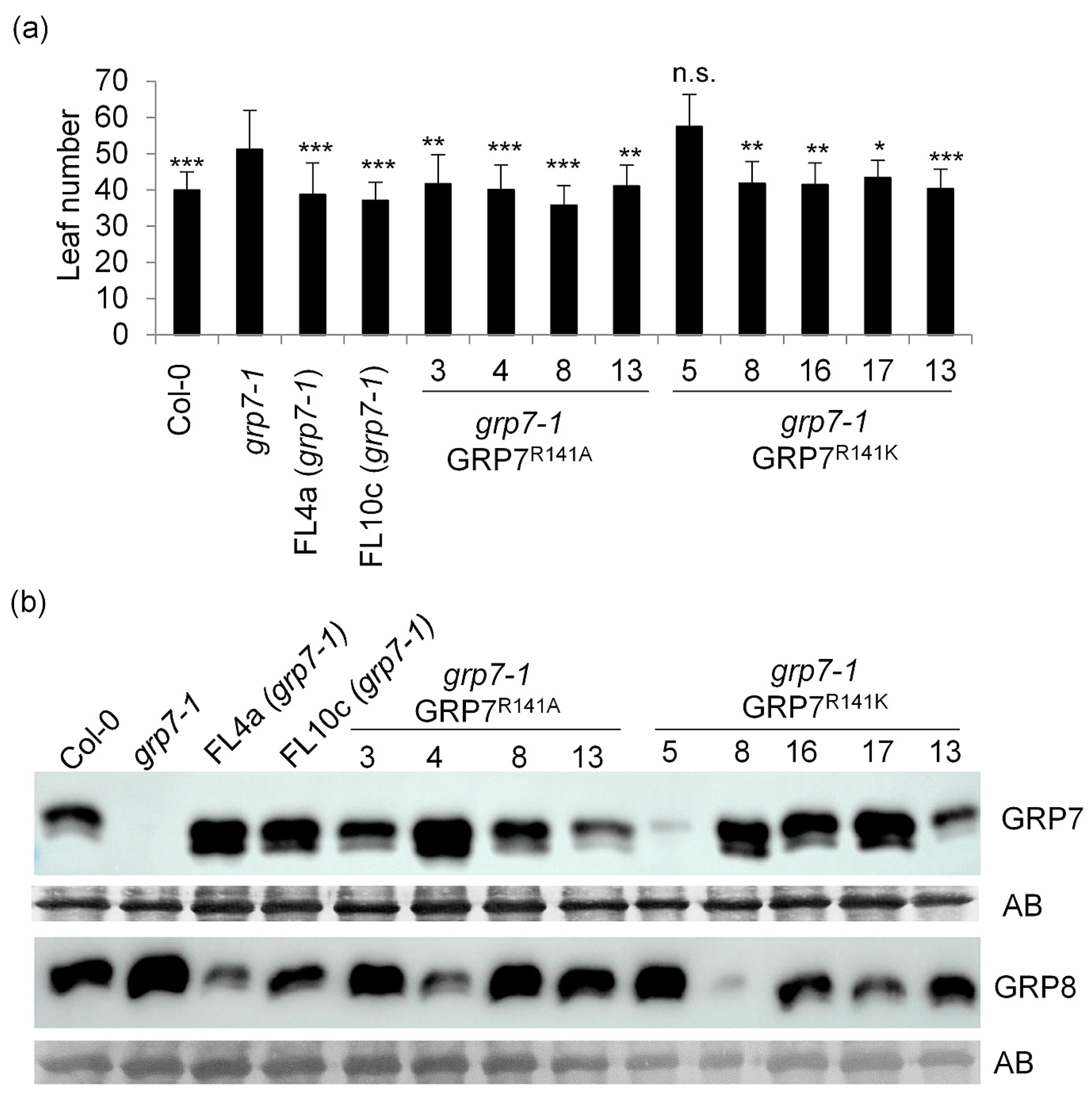
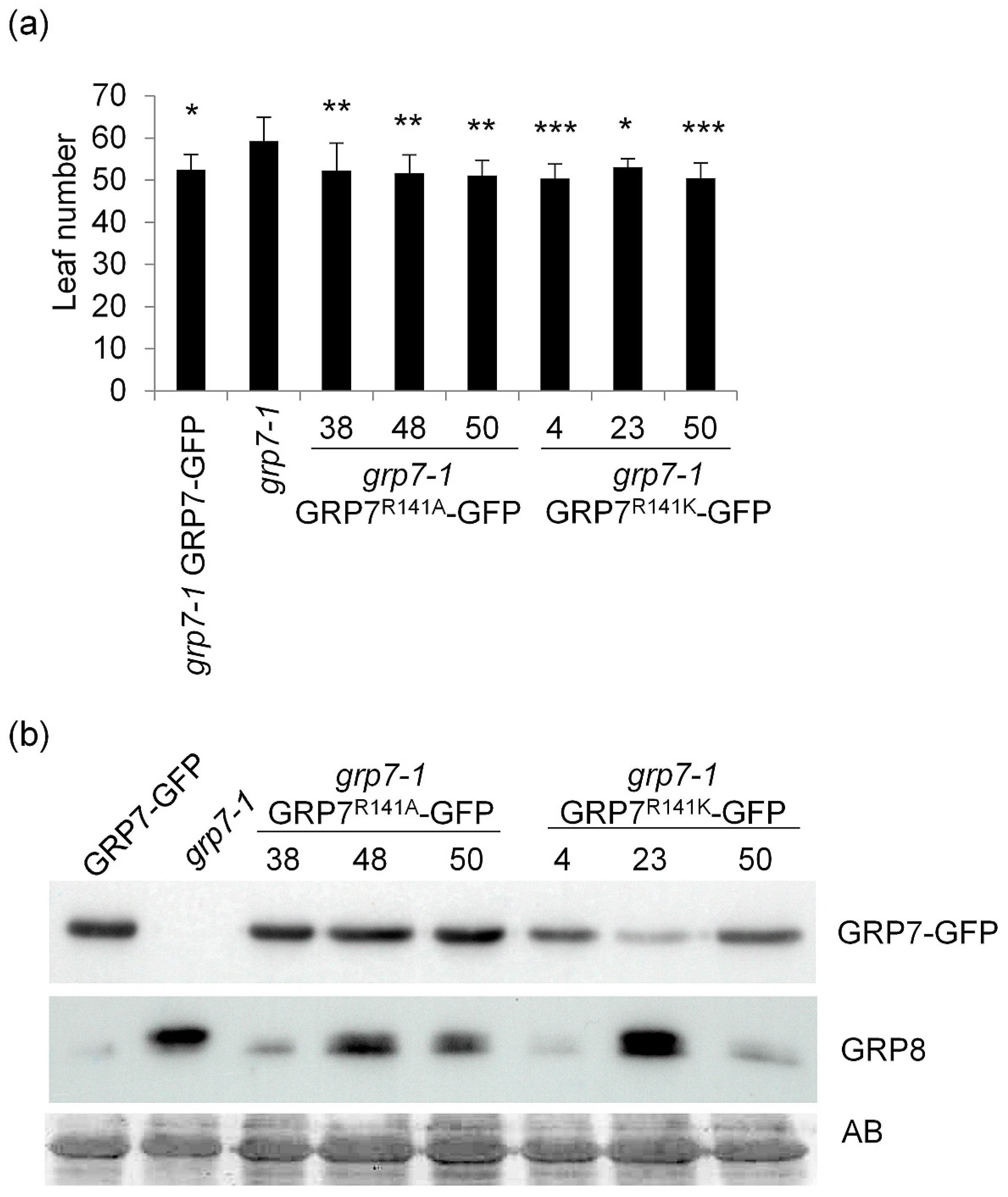
2.1. Genomic AtGRP7 R141 Variants Complement Late Flowering of grp7-1
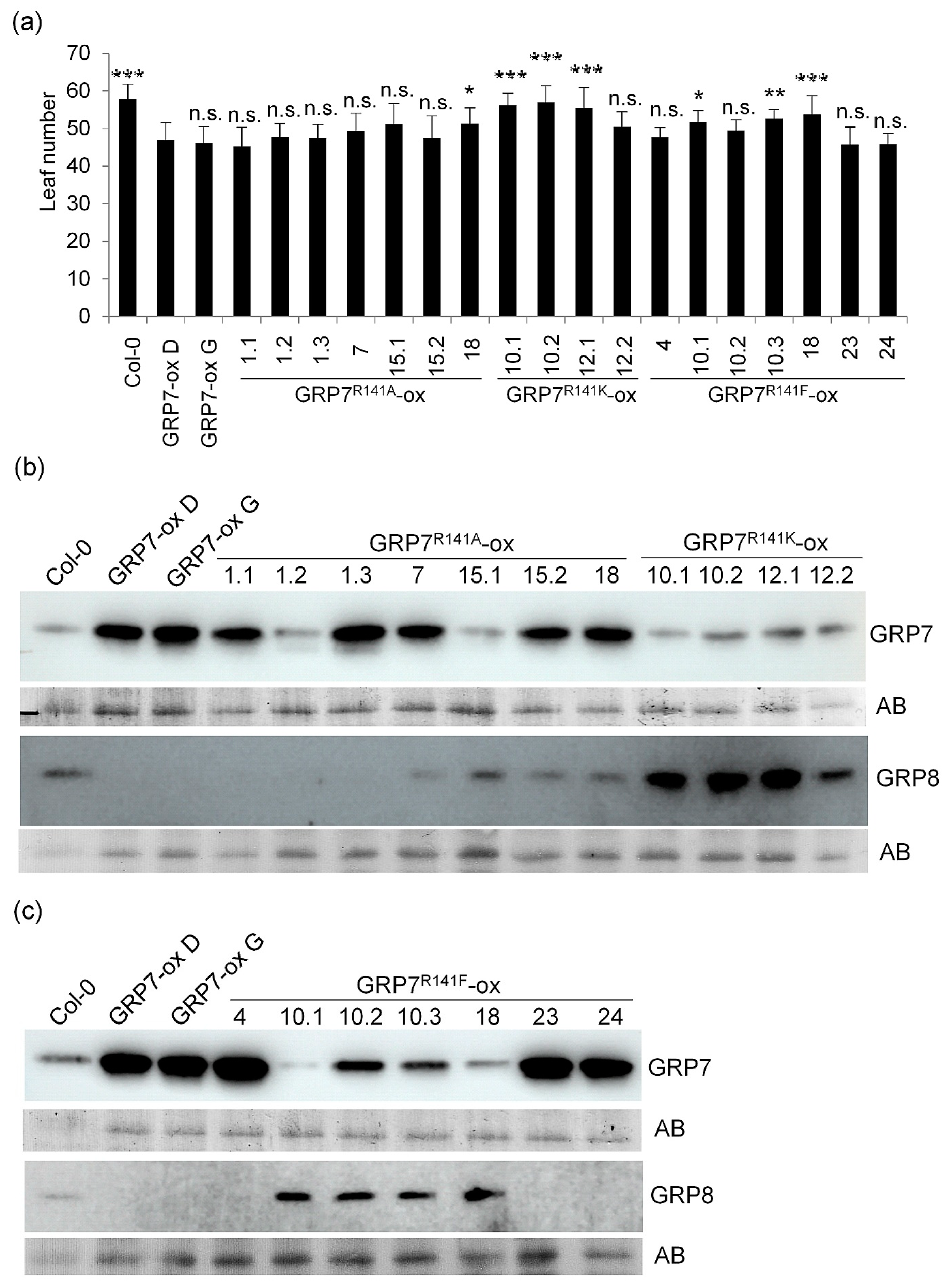
2.2. Overexpression of AtGRP7 Leads to Dose-Dependent Early Flowering Independent of R141 Mutations
2.3. AtGRP7 R141A Attenuates the Response to Abscisic Acid
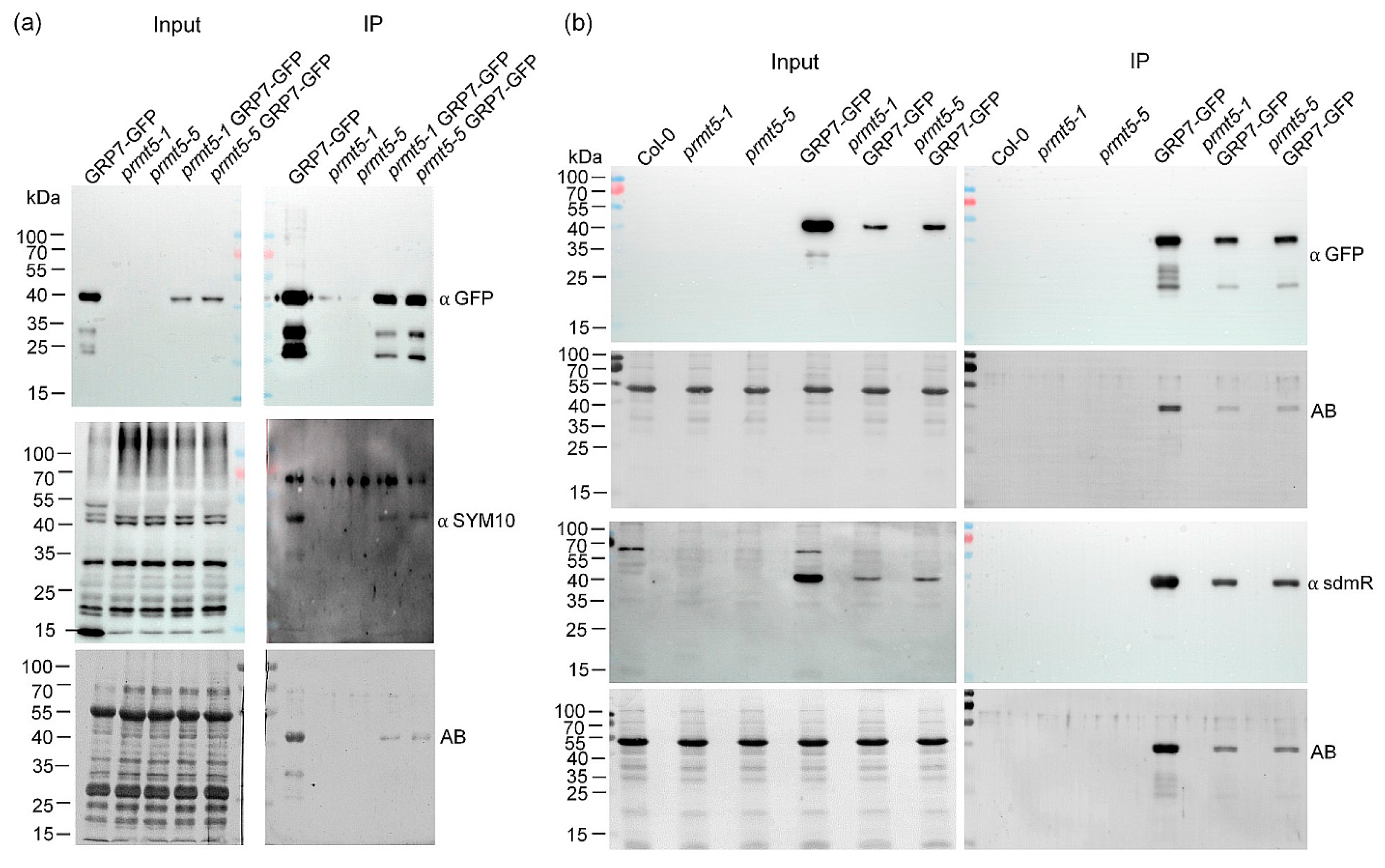
2.4. In Vivo Dimethylation Status of AtGRP7
3. Discussion
4. Materials and Methods
4.1. Constructs and Transgenic Plants
4.2. Determination of Flowering Time
4.3. Physiological Response to ABA
4.4. Immunoblot Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McBride, A.E.; Silver, P.A. State of the Arg: Protein methylation at arginine comes of age. Cell 2001, 106, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.T.; Richard, S. Arginine methylation an emerging regulator of protein function. Mol. Cell 2005, 18, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Cao, X. Plant PRMTs Broaden the Scope of Arginine Methylation. J. Genet. Genom. 2012, 39, 195–208. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Ma, Q.; Zhang, Z.; Xue, Y.; Bao, S.; Chong, K. SKB1-mediated symmetric dimethylation of histone H4R3 controls flowering time in Arabidopsis. EMBO J. 2007, 26, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Song, H.R.; Lutz, K.; Kerstetter, R.A.; Michael, T.P.; McClung, C.R. Type II protein arginine methyltransferase 5 (PRMT5) is required for circadian period determination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 21211–21216. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Petrillo, E.; Beckwith, E.J.; Zhang, X.; Rugnone, M.L.; Hernando, C.E.; Cuevas, J.C.; Godoy Herz, M.A.; Depetris-Chauvin, A.; Simpson, C.G.; et al. A methyl transferase links the circadian clock to the regulation of alternative splicing. Nature 2010, 468, 112–116. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Zhang, Y.; Wang, X.; Li, D.; Li, Q.; Yue, M.; Li, Q.; Zhang, Y.E.; Xu, Y.; et al. Arabidopsis Floral Initiator SKB1 Confers High Salt Tolerance by Regulating Transcription and Pre-mRNA Splicing through Altering Histone H4R3 and Small Nuclear Ribonucleoprotein LSM4 Methylation. Plant Cell 2011, 23, 396–411. [Google Scholar] [CrossRef]
- Cao, H.; Liang, Y.; Zhang, L.; Liu, Z.; Liu, D.; Cao, X.; Deng, X.; Jin, Z.; Pei, Y. AtPRMT5-mediated AtLCD methylation improves Cd2+ tolerance via increased H2S production in Arabidopsis. Plant Physiol. 2022, 190, 2637–2650. [Google Scholar] [CrossRef]
- Pei, Y.; Niu, L.; Lu, F.; Liu, C.; Zhai, J.; Kong, X.; Cao, X. Mutations in the Type II protein arginine methyltransferase AtPRMT5 result in pleiotropic developmental defects in Arabidopsis. Plant Physiol. 2007, 144, 1913–1923. [Google Scholar] [CrossRef]
- Deng, X.; Gu, L.; Liu, C.; Lu, T.; Lu, F.; Lu, Z.; Cui, P.; Pei, Y.; Wang, B.; Hu, S.; et al. Arginine methylation mediated by the Arabidopsis homolog of PRMT5 is essential for proper pre-mRNA splicing. Proc. Natl. Acad. Sci. USA 2010, 107, 19114–19119. [Google Scholar] [CrossRef]
- Wachter, A.; Rühl, C.; Stauffer, E. The role of polypyrimidine tract-binding proteins and other hnRNP proteins in plant splicing regulation. Front. Plant Sci. 2012, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Eggert, C.; Buhler, D.; Brahms, H.; Kambach, C.; Fischer, U. Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr. Biol. 2001, 11, 1990–1994. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, F.M.; Cote, J.; Boulanger, M.C.; Cleroux, P.; Bachand, F.; Autexier, C.; Richard, S. Symmetrical dimethylarginine methylation is required for the localization of SMN in Cajal bodies and pre-mRNA splicing. J. Cell Biol. 2002, 159, 957–969. [Google Scholar] [CrossRef]
- Deng, X.; Lu, T.; Wang, L.; Gu, L.; Sun, J.; Kong, X.; Liu, C.; Cao, X. Recruitment of the NineTeen Complex to the activated spliceosome requires AtPRMT5. Proc. Natl. Acad. Sci. USA 2016, 113, 5447–5452. [Google Scholar] [CrossRef]
- Agrofoglio, Y.C.; Iglesias, M.J.; Perez-Santangelo, S.; de Leone, M.J.; Koester, T.; Catala, R.; Salinas, J.; Yanovsky, M.J.; Staiger, D.; Mateos, J.L. Arginine methylation of SM-LIKE PROTEIN 4 antagonistically affects alternative splicing during Arabidopsis stress responses. Plant Cell 2024, 36, 2219–2237. [Google Scholar] [CrossRef]
- Hernando, C.E.; Sanchez, S.E.; Mancini, E.; Yanovsky, M.J. Genome wide comparative analysis of the effects of PRMT5 and PRMT4/CARM1 arginine methyltransferases on the Arabidopsis thaliana transcriptome. BMC Genom. 2015, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Park, S.J.; Kwak, K.J.; Kim, Y.O.; Kim, J.Y.; Song, J.; Jang, B.; Jung, C.H.; Kang, H. Cold shock domain proteins and glycine-rich RNA-binding proteins from Arabidopsis thaliana can promote the cold adaptation process in Escherichia coli. Nucleic Acids Res. 2007, 35, 506–516. [Google Scholar] [CrossRef]
- Kim, J.S.; Jung, H.J.; Lee, H.J.; Kim, K.A.; Goh, C.H.; Woo, Y.; Oh, S.H.; Han, Y.S.; Kang, H. Glycine-rich RNA-binding protein7 affects abiotic stress responses by regulating stomata opening and closing in Arabidopsis thaliana. Plant J. 2008, 55, 455–466. [Google Scholar] [CrossRef]
- Meyer, K.; Köster, T.; Nolte, C.; Weinholdt, C.; Lewinski, M.; Grosse, I.; Staiger, D. Adaptation of iCLIP to plants determines the binding landscape of the clock-regulated RNA-binding protein AtGRP7. Genome Biol. 2017, 18, 204. [Google Scholar] [CrossRef]
- Lewinski, M.; Steffen, A.; Kachariya, N.; Elgner, M.; Schmal, C.; Messini, N.; Koster, T.; Reichel, M.; Sattler, M.; Zarnack, K.; et al. Arabidopsis thaliana GLYCINE RICH RNA-BINDING PROTEIN 7 interaction with its iCLIP target LHCB1.1 correlates with changes in RNA stability and circadian oscillation. Plant J. 2024, 118, 203–224. [Google Scholar] [CrossRef]
- Köster, T.; Meyer, K.; Weinholdt, C.; Smith, L.M.; Lummer, M.; Speth, C.; Grosse, I.; Weigel, D.; Staiger, D. Regulation of pri-miRNA processing by the hnRNP-like protein AtGRP7 in Arabidopsis. Nucleic Acids Res. 2014, 42, 9925–9936. [Google Scholar] [CrossRef] [PubMed]
- Leder, V.; Lummer, M.; Tegeler, K.; Humpert, F.; Lewinski, M.; Schüttpelz, M.; Staiger, D. Mutational definition of binding requirements of an hnRNP-like protein in Arabidopsis using fluorescence correlation spectroscopy. Biochem. Biophys. Res. Commun. 2014, 453, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lühmann, K.L.; Seemann, S.; Martinek, N.; Ostendorp, S.; Kehr, J. The glycine-rich domain of GRP7 plays a crucial role in binding long RNAs and facilitating phase separation. Sci. Rep. 2024, 14, 16018. [Google Scholar] [CrossRef]
- Streitner, C.; Danisman, S.; Wehrle, F.; Schöning, J.C.; Alfano, J.R.; Staiger, D. The small glycine-rich RNA-binding protein AtGRP7 promotes floral transition in Arabidopsis thaliana. Plant J. 2008, 56, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Elgner, M.; Staiger, D. Regulation of Flowering Time by the RNA-Binding Proteins AtGRP7 and AtGRP8. Plant Cell Physiol. 2019, 60, 2040–2050. [Google Scholar] [CrossRef]
- Schöning, J.C.; Streitner, C.; Meyer, I.M.; Gao, Y.; Staiger, D. Reciprocal regulation of glycine-rich RNA-binding proteins via an interlocked feedback loop coupling alternative splicing to nonsense-mediated decay in Arabidopsis. Nucleic Acids Res. 2008, 36, 6977–6987. [Google Scholar] [CrossRef]
- Mateos, J.L.; Sanchez, S.E.; Legris, M.; Esteve-Bruna, D.; Torchio, J.C.; Petrillo, E.; Goretti, D.; Blanco-Touriñán, N.; Seymour, D.K.; Schmid, M.; et al. PICLN modulates alternative splicing and light/temperature responses in plants. Plant Physiol. 2022, 191, 1036–1051. [Google Scholar] [CrossRef]
- Liang, Q.; Geng, Q.; Jiang, L.; Liang, M.; Li, L.; Zhang, C.; Wang, W. Protein methylome analysis in Arabidopsis reveals regulation in RNA-related processes. J. Proteom. 2020, 213, 103601. [Google Scholar] [CrossRef]
- Willems, P.; Sterck, L.; Dard, A.; Huang, J.; De Smet, I.; Gevaert, K.; Van Breusegem, F. The Plant PTM Viewer 2.0: In-depth exploration of plant protein modification landscapes. J. Exp. Bot. 2024, 75, 4611–4624. [Google Scholar] [CrossRef]
- Martin-Merchan, A.; Lavatelli, A.; Engler, C.; Gonzalez-Miguel, V.M.; Moro, B.; Rosano, G.L.; Bologna, N.G. Arabidopsis AGO1 N-terminal extension acts as an essential hub for PRMT5 interaction and post-translational modifications. Nucleic Acids Res. 2024, 52, 8466–8482. [Google Scholar] [CrossRef]
- Lewinski, M.; Hallmann, A.; Staiger, D. Genome-wide identification and phylogenetic analysis of plant RNA binding proteins comprising both RNA recognition motifs and contiguous glycine residues. Mol. Genet. Genom. 2016, 291, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Sachetto-Martins, G.; Franco, L.O.; de Oliveira, D.E. Plant glycine-rich proteins: A family or just proteins with a common motif? Biochim. Biophys. Acta 2000, 1492, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Staiger, D.; Apel, K. Circadian clock-regulated expression of an RNA-binding protein in Arabidopsis: Characterisation of a minimal promoter element. Mol. Gen. Genet. 1999, 261, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Streitner, C.; Köster, T.; Simpson, C.G.; Shaw, P.; Danisman, S.; Brown, J.W.S.; Staiger, D. An hnRNP-like RNA-binding protein affects alternative splicing by in vivo interaction with target transcripts in Arabidopsis thaliana. Nucleic Acids Res. 2012, 40, 11240–11255. [Google Scholar] [CrossRef] [PubMed]
- Heintzen, C.; Nater, M.; Apel, K.; Staiger, D. AtGRP7, a nuclear RNA-binding protein as a component of a circadian-regulated negative feedback loop in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1997, 94, 8515–8520. [Google Scholar] [CrossRef]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Görlach, J. Growth Stage Based Phenotypic Analysis of Arabidopsis: A Model for High Throughput Functional Genomics in Plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef]
- Hackmann, C.; Korneli, C.; Kutyniok, M.; Köster, T.; Wiedenlübbert, M.; Müller, C.; Staiger, D. Salicylic acid-dependent and -independent impact of an RNA-binding protein on plant immunity. Plant Cell Environ. 2014, 37, 696–706. [Google Scholar] [CrossRef]
- Lummer, M.; Humpert, F.; Steuwe, C.; Schüttpelz, M.; Sauer, M.; Staiger, D. Reversible photoswitchable DRONPA-s monitors nucleocytoplasmic transport of an RNA-binding protein in transgenic plants. Traffic 2011, 12, 693–702. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steffen, A.; Dombert, K.; Iglesias, M.J.; Nolte, C.; de Leone, M.J.; Yanovsky, M.J.; Mateos, J.L.; Staiger, D. Assessing the Role of AtGRP7 Arginine 141, a Target of Dimethylation by PRMT5, in Flowering Time Control and Stress Response. Plants 2024, 13, 2771. https://doi.org/10.3390/plants13192771
Steffen A, Dombert K, Iglesias MJ, Nolte C, de Leone MJ, Yanovsky MJ, Mateos JL, Staiger D. Assessing the Role of AtGRP7 Arginine 141, a Target of Dimethylation by PRMT5, in Flowering Time Control and Stress Response. Plants. 2024; 13(19):2771. https://doi.org/10.3390/plants13192771
Chicago/Turabian StyleSteffen, Alexander, Katarzyna Dombert, María José Iglesias, Christine Nolte, María José de Leone, Marcelo J. Yanovsky, Julieta L. Mateos, and Dorothee Staiger. 2024. "Assessing the Role of AtGRP7 Arginine 141, a Target of Dimethylation by PRMT5, in Flowering Time Control and Stress Response" Plants 13, no. 19: 2771. https://doi.org/10.3390/plants13192771
APA StyleSteffen, A., Dombert, K., Iglesias, M. J., Nolte, C., de Leone, M. J., Yanovsky, M. J., Mateos, J. L., & Staiger, D. (2024). Assessing the Role of AtGRP7 Arginine 141, a Target of Dimethylation by PRMT5, in Flowering Time Control and Stress Response. Plants, 13(19), 2771. https://doi.org/10.3390/plants13192771





