Assessment of Vegetable Species for Microgreen Production in Unheated Greenhouses: Yield, Nutritional Composition, and Sensory Perception
Abstract
:1. Introduction
2. Results
2.1. Earliness and Yield
2.2. Biometric and Productive Analysis
2.3. Nutritional and Mineralogical Analysis
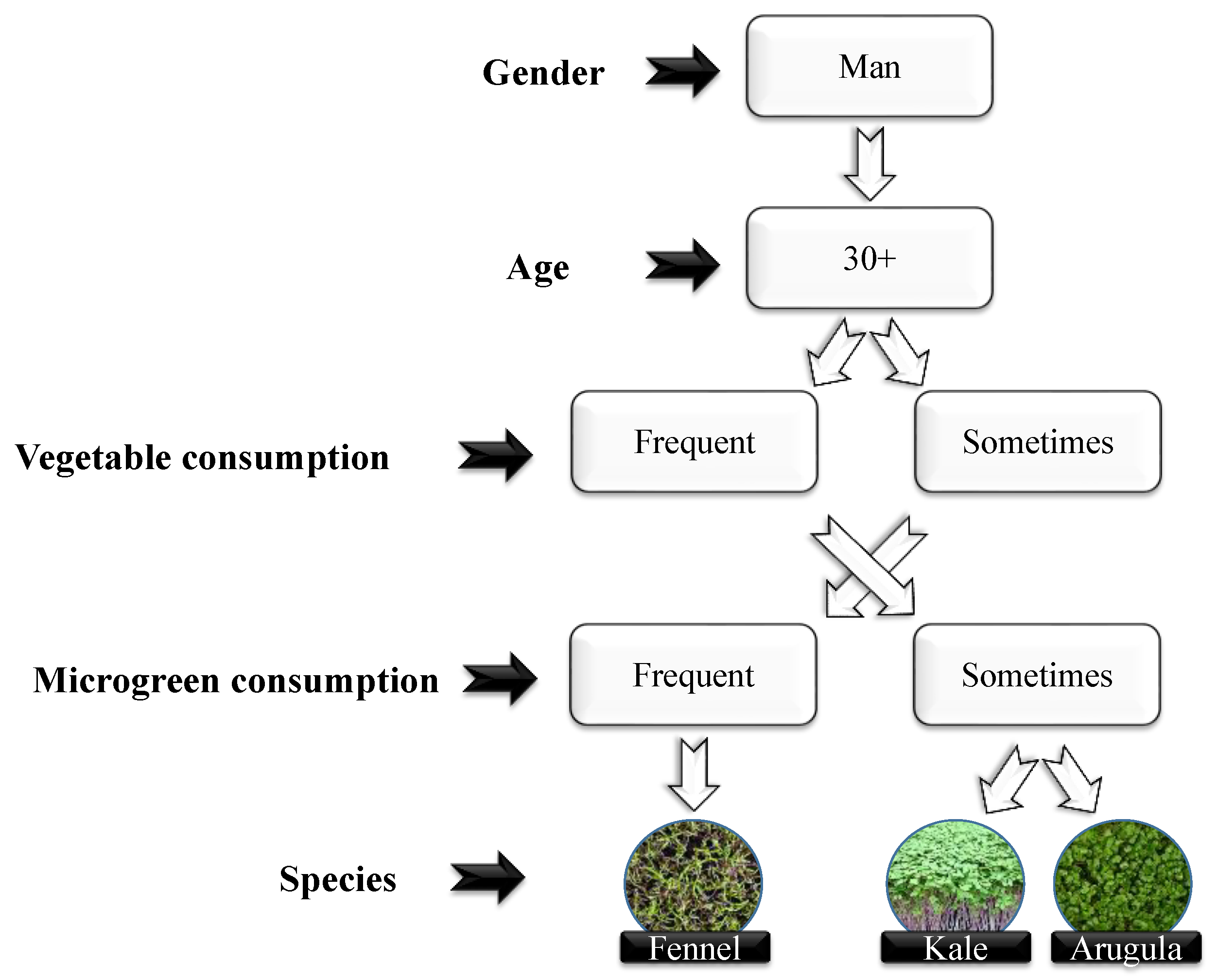
2.4. Organoleptic Properties and Preference for Purchasing Vegetables
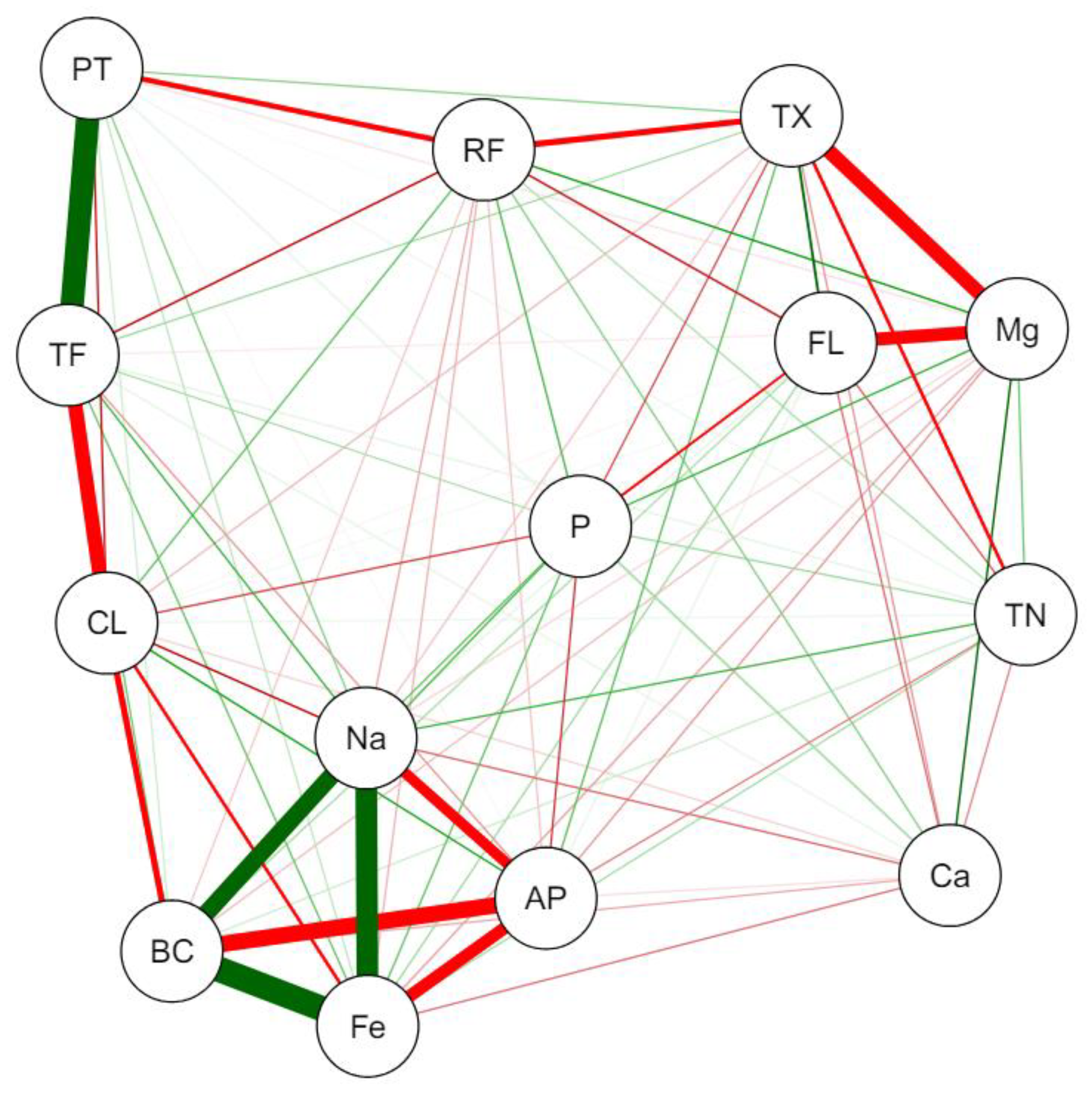
2.5. Correlation Analysis
3. Discussion
3.1. Biometric and Productive Analysis
3.2. Nutritional and Mineralogical Analysis
3.3. Organoleptic Properties and Preference for Purchasing Vegetables
3.4. Correlation Analysis
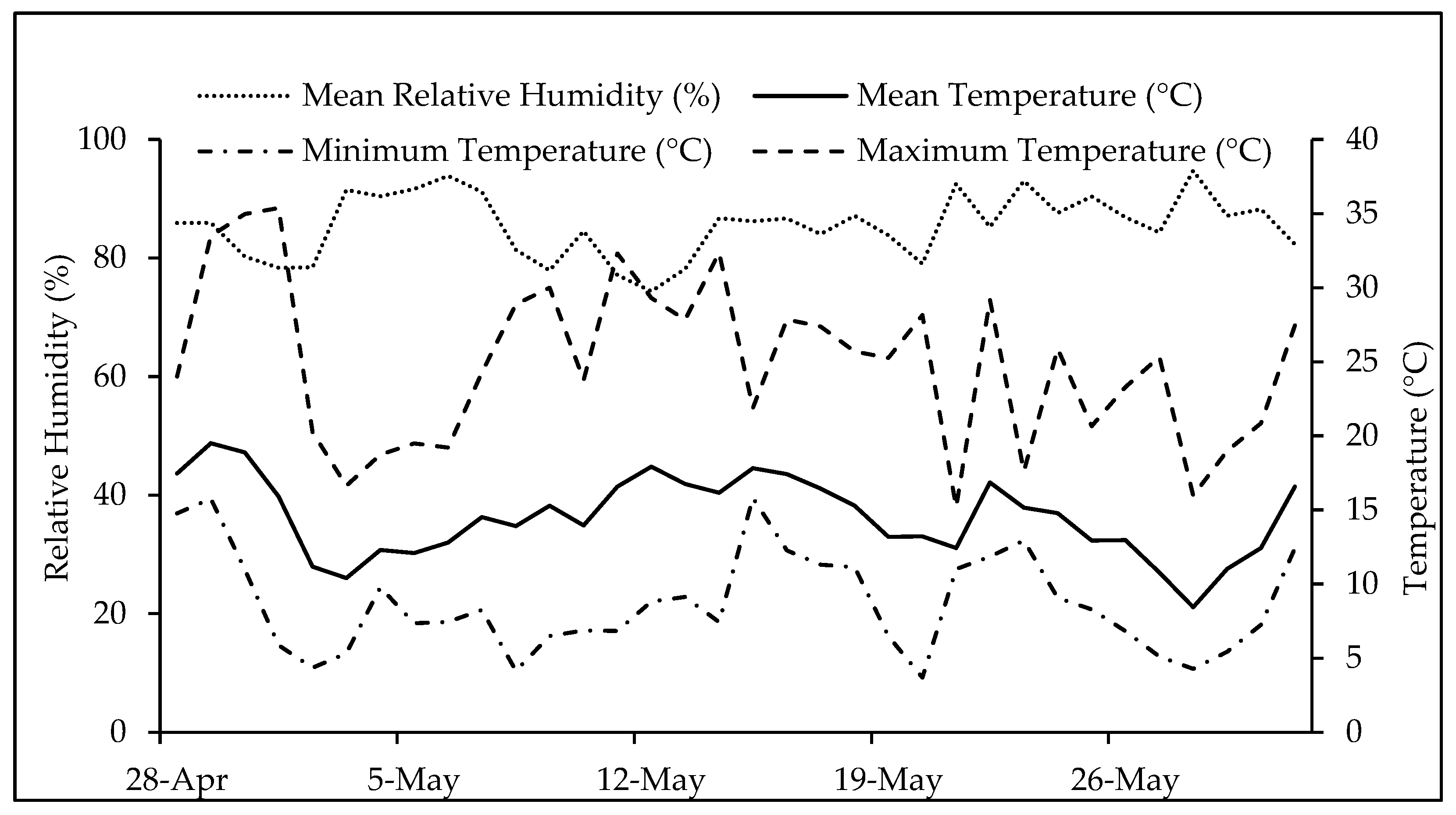
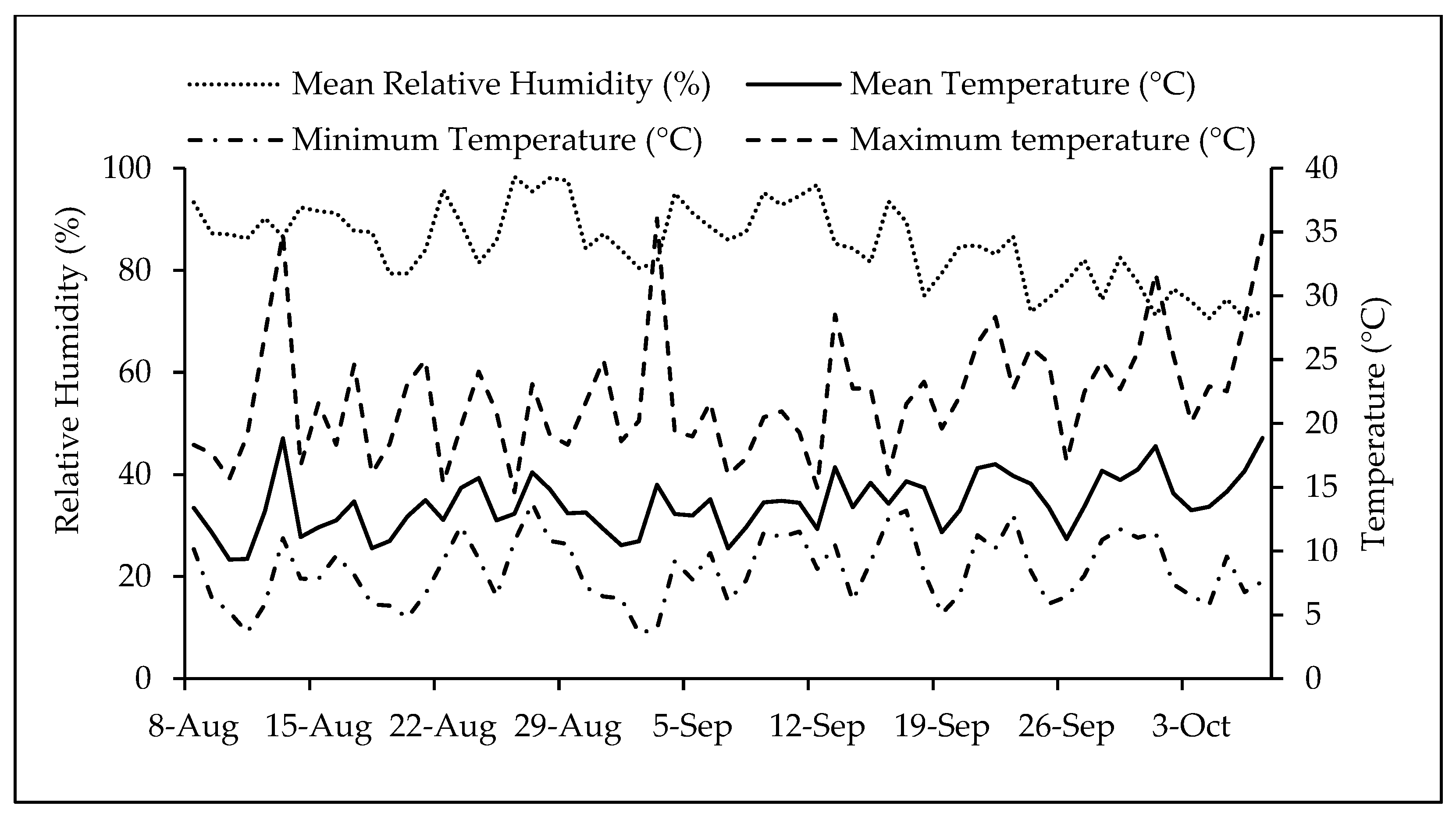
4. Materials and Methods
| Code | Sensory Scale | Purchase Preference Scale |
|---|---|---|
| 1 | Extremely pleasant | I would definitely buy it |
| 2 | Very pleasant | I would probably buy it |
| 3 | Moderately pleasant | May be maybe not |
| 4 | Sightly pleasant | I probably would not buy it |
| 5 | It does not matter to me | I definitely would not buy it |
| 6 | Slightly unpleasant | - |
| 7 | Moderately unpleasant | - |
| 8 | Very unpleasant | - |
| 9 | Extremely unpleasant | - |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral Composition and Content of 30 Varieties. J. Food Compos. Anal. 2016, 49, 87–93. [Google Scholar] [CrossRef]
- Partap, M.; Sharma, D.; Hn, D.; Thakur, M.; Verma, V.; Ujala, B.; Bhargava, B. Microgreen: A Tiny Plant with Superfood Potential. J. Funct. Foods 2023, 107, 105697. [Google Scholar] [CrossRef]
- Carrasco, G.; Manríquez, P.; Galleguillos, F.; Fuentes-Peñailillo, F.; Urrestarazu, M. Evolution of Soilless Culture in Chile. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science (ISHS). Leuven, Belgium, 9 September 2021; pp. 267–274. [Google Scholar]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Katoch, V.; Kumar, S.; Chatterjee, S. Functional Relationship of Vegetable Colors and Bioactive Compounds: Implications in Human Health. J. Nutr. Biochem. 2021, 92, 108615. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, F.; El-Nakhel, C.; Raimondo, M.; Kyriacou, M.C.; Cembalo, L.; de Pascale, S.; Rouphael, Y. Sensory Attributes and Consumer Acceptability of 12 Microgreens Species. Agronomy 2020, 10, 1043. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A Review on the Effects of Light-Emitting Diode (LED) Light on the Nutrients of Sprouts and Microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional Quality in Novel Food Sources: Genotypic Variation in the Nutritive and Phytochemical Composition of Thirteen Microgreens Species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The Science behind Microgreens as an Exciting New Food for the 21st Century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Caruso, G.; Stoleru, V.; De Pascale, S.; Cozzolino, E.; Pannico, A.; Giordano, M.; Teliban, G.; Cuciniello, A.; Rouphael, Y. Production, Leaf Quality and Antioxidants of Perennial Wall Rocket as Affected by Crop Cycle and Mulching Type. Agronomy 2019, 9, 194. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef]
- Ferrón-Carrillo, F.; Guil-Guerrero, J.L.; González-Fernández, M.J.; Lyashenko, S.; Battafarano, F.; da Cunha-Chiamolera, T.P.L.; Urrestarazu, M. LED Enhances Plant Performance and Both Carotenoids and Nitrates Profiles in Lettuce. Plant Foods Hum. Nutr. 2021, 76, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Peñailillo, F.; Gutter, K.; Vega, R.; Silva, G.C. New Generation Sustainable Technologies for Soilless Vegetable Production. Horticulturae 2024, 10, 49. [Google Scholar] [CrossRef]
- Parkes, M.G.; Azevedo, D.L.; Cavallo, A.C.; Domingos, T.; Teixeira, R.F.M. Life Cycle Assessment of Microgreen Production: Effects of Indoor Vertical Farm Management on Yield and Environmental Performance. Sci. Rep. 2023, 13, 11324. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, G.; Fuentes-Peñailillo, F.; Pérez, R.; Rebolledo, P.; Manríquez, P. An Approach to a Vertical Farming Low-Cost to Reach Sustainable Vegetable Crops. In Proceedings of the 2022 IEEE International Conference on Automation/XXV Congress of the Chilean Association of Automatic Control (ICA-ACCA), Curico, Chile, 24–28 October 2022; pp. 1–6. [Google Scholar]
- Maluin, F.N.; Hussein, M.Z.; Nik Ibrahim, N.N.L.; Wayayok, A.; Hashim, N. Some Emerging Opportunities of Nanotechnology Development for Soilless and Microgreen Farming. Agronomy 2021, 11, 1213. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Zheng, Y. Growth and Appearance Quality of Four Microgreen Species under Light-Emitting Diode Lights with Different Spectral Combinations. HortScience 2020, 55, 1399–1405. [Google Scholar] [CrossRef]
- Frąszczak, B.; Kleiber, T. Microgreens Biometric and Fluorescence Response to Iron (Fe) Biofortification. Int. J. Mol. Sci. 2022, 23, 14553. [Google Scholar] [CrossRef]
- Hoang, G.M.; Vu, T.T. Selection of Suitable Growing Substrates and Quality Assessment of Brassica Microgreens Cultivated in Greenhouse. Acad. J. Biol. 2022, 44, 133–142. [Google Scholar] [CrossRef]
- Sharma, A.; Hazarika, M.; Heisnam, P.; Pandey, H.; Devadas, V.S.; Wangsu, M.; Kartha, B.D. Factors Affecting Production, Nutrient Translocation Mechanisms, and LED Emitted Light in Growth of Microgreen Plants in Soilless Culture. ACS Agric. Sci. Technol. 2023, 3, 701–719. [Google Scholar] [CrossRef]
- Kołota, E.; Adamczewska-Sowińska, K.; Czerniak, K. Yield and Nutritional Value of Swiss Chard Grown for Summer and Autumn Harvest. J. Agric. Sci. 2010, 2, 120–124. [Google Scholar]
- Abaajeh, A.R.; Kingston, C.E.; Harty, M. Environmental Factors Influencing the Growth and Pathogenicity of Microgreens Bound for the Market: A Review. Renew. Agric. Food Syst. 2023, 38, e12. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient Composition, Oxalate Content and Nutritional Ranking of Ten Culinary Microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Komeroski, M.R.; Portal, K.A.; Comiotto, J.; Klug, T.V.; Flores, S.H.; Rios, A.D.O. Nutritional Quality and Bioactive Compounds of Arugula (Eruca Sativa L.) Sprouts and Microgreens. Int. J. Food Sci. Technol. 2023, 58, 5089–5096. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Soteriou, G.A.; Colla, G.; Rouphael, Y. The Occurrence of Nitrate and Nitrite in Mediterranean Fresh Salad Vegetables and Its Modulation by Preharvest Practices and Postharvest Conditions. Food Chem. 2019, 285, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen Nutrition, Food Safety, and Shelf Life: A Review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef]
- Fabek Uher, S.; Radman, S.; Opačić, N.; Dujmović, M.; Benko, B.; Lagundžija, D.; Mijić, V.; Prša, L.; Babac, S.; Šic Žlabur, J. Alfalfa, Cabbage, Beet and Fennel Microgreens in Floating Hydroponics—Perspective Nutritious Food? Plants 2023, 12, 2098. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Mele, M.A.; Lee, Y.-T.; Islam, M.Z. Consumer Preference, Quality, and Safety of Organic and Conventional Fresh Fruits, Vegetables, and Cereals. Foods 2021, 10, 105. [Google Scholar] [CrossRef]
- Vogel, J.T.; Tieman, D.M.; Sims, C.A.; Odabasi, A.Z.; Clark, D.G.; Klee, H.J. Carotenoid Content Impacts Flavor Acceptability in Tomato (Solanum Lycopersicum). J. Sci. Food Agric. 2010, 90, 2233–2240. [Google Scholar] [CrossRef]
- Leksrisompong, P.P.; Whitson, M.E.; Truong, V.D.; Drake, M.A. Sensory Attributes and Consumer Acceptance of Sweet Potato Cultivars with Varying Flesh Colors. J. Sens. Stud. 2012, 27, 59–69. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Zanon, A.J.; Tironi, L.F.; Streck, N.A.; Richards, N.S.P.D.S. Nutritional Quality and Sensory Acceptance of Biofortified Cassava. Braz. J. Food Technol. 2021, 24, e2020247. [Google Scholar] [CrossRef]
- Olusanya, R.N.; Kolanisi, U.; Ngobese, N.Z. Mineral Composition and Consumer Acceptability of Amaranthus Leaf Powder Supplemented Ujeqe for Improved Nutrition Security. Foods 2023, 12, 2182. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Bozdar, B.; Chachar, S.; Rai, M.; Li, J.; Li, Y.; Hayat, F.; Chachar, Z.; Tu, P. The Power of Magnesium: Unlocking the Potential for Increased Yield, Quality, and Stress Tolerance of Horticultural Crops. Front. Plant Sci. 2023, 14, 1285512. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Hossain, M.M.; Oba, S. Nutritional and Antioxidant Components and Antioxidant Capacity in Green Morph Amaranthus Leafy Vegetable. Sci. Rep. 2020, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Fayezizadeh, M.R.; Ansari, N.A.; Sourestani, M.M.; Hasanuzzaman, M. Biochemical Compounds, Antioxidant Capacity, Leaf Color Profile and Yield of Basil (Ocimum sp.) Microgreens in Floating System. Plants 2023, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Oxford University Press: Arlington, VA, USA, 1990; ISBN 0-935584-42-0. [Google Scholar]
- Food and Agriculture Organization. FAO Food and Nutrition Papers: 14/7 Food Analysis: General Techniques, Additives, Contaminants and Composition; FAO: Rome, Italy, 1986. [Google Scholar]
- Martínez Guijarro, M.R. Análisis Instrumental. Espectrometría de Absorción Atómica (EAA); Universitat Politècnica de València: Valencia, Spain, 2020. [Google Scholar]
- Meilgaard, M.C.; Carr, B.T.; Civille, G.V. Sensory Evaluation Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1999; ISBN 9781003040729. [Google Scholar]
- Lopes Bhering, L. Rbio: A Tool for Biometric and Statistical Analysis Using the R Platform. Crop Breed. Appl. Biotechnol. 2017, 17, 187–190. [Google Scholar] [CrossRef]

| Commercial Name | Sowing Density (g m−2) | Germination Index (%) | Germination Time (days) | |
|---|---|---|---|---|
| Experiment 1 | Chard | 640 | 20 | 9 |
| Carrot | 130 | 85 | 9 | |
| Radish | 320 | 85 | 3 | |
| Lettuce | 90 | 85 | 8 | |
| Kale | 190 | 85 | 3 | |
| Arugula | 128 | 95 | 3 | |
| Experiment 2 | Mizuna | 255 | 100 | 3 |
| Fennel | 155 | 80 | 8 | |
| Spinach | 255 | 100 | 3 | |
| Broccoli | 320 | 95 | 3 | |
| Anise | 180 | 75 | 8 |
| Commercial Name | Height (cm) * | Fresh Mass (g tray−1) * | Yield (g m−2) * |
|---|---|---|---|
| Autumn experiment | |||
| Chard | 3.6 d | 116 bc | 756 bc |
| Carrot | 2.9 e | 96 c | 624 c |
| Radish | 6.6 a | 341 a | 2216 a |
| Lettuce | 2.9 e | 295 a | 1994 a |
| Kale | 4.4 c | 88 c | 673 c |
| Arugula | 6.2 b | 136 b | 1171 b |
| p-value | 0.000 | 0.000 | 0.000 |
| Spring experiment | |||
| Mizuna | 7.9 a | 1367 a | 8877 a |
| Fennel | 8.4 a | 808 b | 5247 b |
| Spinach | 6.7 b | 995 b | 6461 b |
| Broccoli | 8.5 a | 1346 a | 8805 a |
| p-value | 0.004 | 0.003 | 0.000 |
| Commercial Name | Total Proteins (%) | Total Phenols (mg kg−1 of GAE) | Nitrates (g L−1) | Raw Fiber (%) | β-Carotene (mg equiv 100 g−1) |
|---|---|---|---|---|---|
| Autumn experiment 1 | |||||
| Chard | 4.4 a | 3335.9 a | 3281.9 a | 0.5 a | 39,966 a |
| Carrot | 4.6 a | 3083.6 a | 2508.5 a | 0.5 a | 15,379 d |
| Radish | 3.7 a | 2001.2 b | 1099.5 b | 0.6 a | 17,438 c |
| Lettuce | 3.8 a | 2060.5 b | 4169.3 a | 0.7 a | 12,550 e |
| Kale | 4.6 a | 3269.6 a | 2489.1 a | 0.6 a | 19,369 b |
| Arugula | 4.8 a | 3304.6 a | 2442.2 a | 0.4 a | 12,612 e |
| p-value | n.s. | 0.007 | 0.000 | n.s. | 0.000 |
| Spring experiment 2 | |||||
| Mizuna | 2.94 | 5663.8 | 4707.8 | 0.5 | 1451 |
| Fennel | 3.50 | 6164.5 | 5600.0 | 0.6 | 3474 |
| Spinach | 2.42 | 11,025.0 | 3866.7 | 0.5 | 3629 |
| Broccoli | 2.67 | 4538.9 | 4266.0 | 0.5 | 2498 |
| Commercial Name | Fe (mg kg−1) | Mg (mg kg−1) | Ca (mg kg−1) | Na (mg kg−1) | P (mg kg−1) |
|---|---|---|---|---|---|
| Autumn experiment 1 | |||||
| Chard | 70.6 bc | 207.4 bc | 308.9 c | 32.9 a | 1.1 a |
| Carrot | 75.8 bc | 203.3 bc | 313.6 c | 26.3 ab | 1.1 a |
| Radish | 79.9 b | 202.2 c | 410.9 b | 23.5 b | 0.9 a |
| Lettuce | 156.8 a | 268.5 ab | 353.1 c | 26.2 ab | 1.0 a |
| Kale | 80.6 b | 300.0 a | 552.8 a | 26.2 ab | 1.2 a |
| Arugula | 69.1 c | 202.6 bc | 380.7 bc | 25.7 ab | 0.8 a |
| p-value | 0.000 | 0.001 | 0.000 | 0.015 | n.s. |
| Spring experiment 2 | |||||
| Mizuna | 79.2 | 292.1 | 846.3 | 25.7 | 0.9 |
| Fennel | 54.5 | 279.4 | 587.6 | 25.9 | 0.4 |
| Spinach | 33.8 | 207.8 | 281.6 | 31.1 | 0.4 |
| Broccoli | 68.4 | 253.7 | 252.1 | 5.6 | 0.4 |
| Commercial Name | Appearance * | Color * | Texture * | Flavor * |
|---|---|---|---|---|
| Autumn experiment | ||||
| Chard | 2.3 a | 2.1 a | 3.0 a | 4.3 ab |
| Kale | 2.7 ab | 2.3 ab | 2.8 a | 3.2 a |
| Lettuce | 2.9 ab | 3.1 c | 2.6 a | 3.5 ab |
| Radish | 3.0 ab | 2.9 bc | 3.2 a | 4.5 b |
| Arugula | 3.1 b | 2.6 abc | 3.2 a | 4.5 b |
| Carrot | 3.2 b | 2.6 abc | 3.2 a | 3.7 ab |
| p-value | 0.000 | 0.000 | n.s. | 0.000 |
| Spring experiment | ||||
| Mizuna | 2.2 a | 2.0 b | 2.6 ab | 3.4 ab |
| Fennel | 2.7 ab | 2.7 ab | 3.1 a | 4.4 a |
| Spinach | 3.2 b | 3.3 a | 2.2 b | 2.7 b |
| p-value | 0.05 | 0.02 | 0.05 | 0.01 |
| Commercial Name | Purchase Intention * | Result of Purchase Intention |
|---|---|---|
| Autumn experiment | ||
| Chard | 2.2 a | I would probably buy it |
| Kale | 2.4 a | I would probably buy it |
| Lettuce | 2.5 a | I would probably buy it |
| Radish | 2.6 a | I would probably buy it |
| Arugula | 2.8 a | I would probably buy it |
| Carrot | 2.8 a | I would probably buy it |
| p-value | n.s. | |
| Spring experiment | ||
| Mizuna | 2.0 a | I would definitely buy it |
| Fennel | 2.6 a | I would probably buy it |
| Spinach | 2.5 a | I would probably buy it |
| p-value | n.s. | |
| Commercial Name | Scientific Name | Commercial Variety | |
|---|---|---|---|
| Autumn experiment | Chard | Beta vulgaris L. var. cicla | Penca roja |
| Carrot | Daucus carota L. | Chantenay Royal 2 | |
| Radish | Raphanus sativus L. | Pablo | |
| Lettuce | Lactuca Sativa L. | Tango roja | |
| Kale | Brassica oleracea L. var. sabellica | Verde rizado | |
| Arugula | Eruca sativa Mill. | Astro | |
| Spring experiment | Mizuna | Brassica rapa L. subsp. nipposinica | Verde |
| Fennel | Foeniculum vulgare L. | Etrusco | |
| Spinach | Spinacia oleracea L. | Woodduck | |
| Anise | Pimpinella anisum L. | - | |
| Broccoli | Brassica oleracea L. var. italica | Italian Green Sprouting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebolledo, P.; Carrasco, G.; Moggia, C.; Gajardo, P.; Sant’Ana, G.R.; Fuentes-Peñailillo, F.; Urrestarazu, M.; Vendruscolo, E.P. Assessment of Vegetable Species for Microgreen Production in Unheated Greenhouses: Yield, Nutritional Composition, and Sensory Perception. Plants 2024, 13, 2787. https://doi.org/10.3390/plants13192787
Rebolledo P, Carrasco G, Moggia C, Gajardo P, Sant’Ana GR, Fuentes-Peñailillo F, Urrestarazu M, Vendruscolo EP. Assessment of Vegetable Species for Microgreen Production in Unheated Greenhouses: Yield, Nutritional Composition, and Sensory Perception. Plants. 2024; 13(19):2787. https://doi.org/10.3390/plants13192787
Chicago/Turabian StyleRebolledo, Pabla, Gilda Carrasco, Claudia Moggia, Pedro Gajardo, Gabriela Rodrigues Sant’Ana, Fernando Fuentes-Peñailillo, Miguel Urrestarazu, and Eduardo Pradi Vendruscolo. 2024. "Assessment of Vegetable Species for Microgreen Production in Unheated Greenhouses: Yield, Nutritional Composition, and Sensory Perception" Plants 13, no. 19: 2787. https://doi.org/10.3390/plants13192787









