Antioxidant and Antifungal Activities and Characterization of Phenolic Compounds Using Ultra-High Performance Liquid Chromatography and Mass Spectrometry (UPLC-MS) of Aqueous Extracts and Fractions from Verbesina sphaerocephala Stems
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoid Content
2.2. Identification and Quantification of Phenolic Compounds by Ultra-High-Performance Liquid Chromatography Coupled to Mass Spectrometry (UPLC–MS)
2.3. Antioxidant Activity
2.3.1. DPPH Antioxidant Assay
2.3.2. ABTS Antioxidant Assay
2.3.3. Total Antioxidant Capacity
2.3.4. Ferric Reducing Power (FRP)
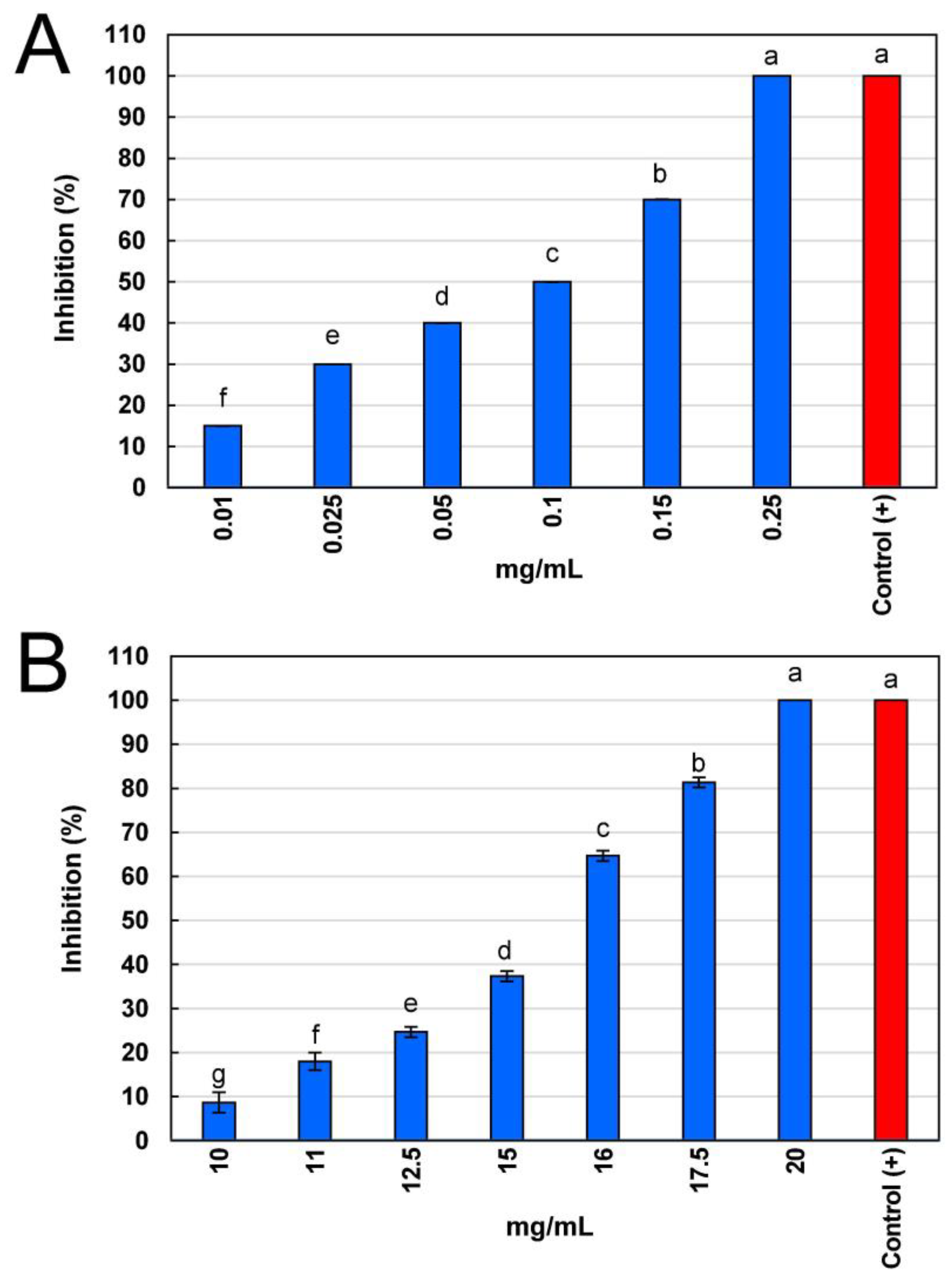
2.4. Antifungal Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Fungal Strain
3.3. Plant Material
3.4. Drying Process
3.5. Preparation of the Extracts
3.6. Liquid-Liquid Phase Separation
3.7. Determination of Total Phenolic and Flavonoid Content
3.7.1. Total Phenolic Content
3.7.2. Total Flavonoid Content
3.8. Identification and Quantification of Total Phenolic Compounds by Ultra-High-Performance Liquid Chromatography Coupled to Mass Spectrometry
3.9. Analysis of Antioxidant Activity
3.9.1. DPPH Antioxidant Assay
3.9.2. ABTS Antioxidant Assay
3.9.3. Total Antioxidant Capacity
3.9.4. Ferric Reducing Potential
3.10. Antifungal Assay
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, I. Phytopathogenic Fungi and Their Biocontrol Applications. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology: Volume 3: Fungal metabolites and Nano-Technology, 1st ed.; Kumar Sharma, V., Shah, M.P., Parmar, S., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 3, pp. 1–21. [Google Scholar]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A Review of Plant Leaf Fungal Diseases and Its Environment Speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Roca-Couso, R.; Flores-Félix, J.D.; Rivas, R. Mechanisms of Action of Microbial Biocontrol Agents against Botrytis cinerea. J. Fungi 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis Cinerea: The Cause of Grey Mould Disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Rafińska, K.; Pomastowski, P.; Wrona, O.; Górecki, R.; Buszewski, B. Medicago sativa as a Source of Secondary Metabolites for Agriculture and Pharmaceutical Industry. Phytochem. Lett. 2017, 20, 520–539. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Xue, H.; Jiang, Y.; Zhao, H.; Köllner, T.G.; Chen, S.; Chen, F.; Chen, F. Characterization of Composition and Antifungal Properties of Leaf Secondary Metabolites from Thirteen Cultivars of Chrysanthemum morifolium Ramat. Molecules 2019, 24, 4202. [Google Scholar] [CrossRef]
- Rzedowski, J.; Calderón de Rzedowski, G. Compositae: Tribu Heliantheae II. Flora Bajío Reg. Adyac. 2011, 172, 1–409. [Google Scholar]
- Vázquez-Sánchez, M.; Medina-Medrano, J.R.; Cortez-Madrigal, H.; Angoa-Pérez, M.V.; Muñoz-Ruíz, C.V.; Villar-Luna, E. Nematicidal Activity of Wild Plant Extracts against Second-Stage Juveniles of Nacobbus aberrans. Nematropica 2018, 48, 136–144. [Google Scholar]
- Arciniegas, A.; Pérez-Castorena, A.L.; Villaseñor, J.L.; Romo de Vivar, A. Cadinenes and Other Metabolites from Verbesina sphaerocephala A. Gray. Biochem. Syst. Ecol. 2020, 93, 104183. [Google Scholar] [CrossRef]
- Rodríguez-Valdovinos, K.Y.; Salgado-Garciglia, R.; Vázquez-Sánchez, M.; Álvarez-Bernal, D.; Oregel-Zamudio, E.; Ceja-Torres, L.F.; Medina-Medrano, J.R. Quantitative Analysis of Rutin by HPTLC and in Vitro Antioxidant and Antibacterial Activities of Phenolic-Rich Extracts from Verbesina sphaerocephala. Plants 2021, 10, 475. [Google Scholar] [CrossRef]
- Aguilar-Mejía, Y. Efecto Antioxidante y Antifúngico de los Compuestos Volátiles del Hidrolato de Tallos y Hojas de Verbesina sphaerocephala (Asteraceae); Universidad Michoacana de San Nicolás de Hidalgo: Morelia, Mexico, 2023. [Google Scholar]
- Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R.; Flores-Félix, J.D. Antioxidant Activity of the Main Phenolics Found in Red Fruits: An in Vitro and in Silico Study. Food Chem. 2024, 452, 139459. [Google Scholar] [CrossRef] [PubMed]
- Razola-Díaz, M.D.C.; Aznar-Ramos, M.J.; Benítez, G.; Gómez-Caravaca, A.M.; Verardo, V. Exploring the Potential of Phenolic and Antioxidant Compounds in New Rosaceae Fruits. J. Sci. Food Agric. 2024, 104, 3705–3718. [Google Scholar] [CrossRef]
- Vek, V.; Keržič, E.; Poljanšek, I.; Eklund, P.; Humar, M.; Oven, P. Wood Extractives of Silver Fir and Their Antioxidant and Antifungal Properties. Molecules 2021, 26, 6412. [Google Scholar] [CrossRef]
- Lal, M.; Sutradhar, D. Extraction of Kaempferol Derivatives from Zygophyllum paulayanum and Its Diverse Biological Activities. Nat. Prod. Res. 2024, 0, 1–5. [Google Scholar] [CrossRef]
- Mehal, K.K.; Kaur, A.; Singh, H.P.; Batish, D.R. Investigating the Phytotoxic Potential of Verbesina encelioides: Effect on Growth and Performance of Co-Occurring Weed Species. Protoplasma 2023, 260, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Cardona Galeano, C.W.; Robledo Restrepo, C.S.M.; Rojano, C.B.A.; Alzate Guarin, C.F.; Muñoz Herrera, D.L.; Saez Vega, C.J. Actividad Leishmanicida y Antioxidante de Extractos de Piper daniel-gonzalezii Trel. (Piperaceae). Rev. Cuba. Plantas Med. 2013, 18, 268–277. [Google Scholar]
- Ramseyer, J.; Thuerig, B.; De Mieri, M.; Schärer, H.J.; Oberhänsli, T.; Gupta, M.P.; Tamm, L.; Hamburger, M.; Potterat, O. Eudesmane Sesquiterpenes from Verbesina lanata with Inhibitory Activity against Grapevine Downy Mildew. J. Nat. Prod. 2017, 80, 3296–3304. [Google Scholar] [CrossRef]
- Divya Ramakrishnan, C.K.; Doss, D.; Vijayabharathi, A. Biochemical and Antimicrobial Characterization of an Underexploited Medicinal Plant-Verbesina encelioides. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3407–3416. [Google Scholar] [CrossRef]
- Nguyen, X.H.; Naing, K.W.; Lee, Y.S.; Moon, J.H.; Lee, J.H.; Kim, K.Y. Isolation and Characteristics of Protocatechuic Acid from Paenibacillus elgii HOA73 against Botrytis cinerea on Strawberry Fruits. J. Basic Microbiol. 2015, 55, 625–634. [Google Scholar] [CrossRef]
- Alnuaimi, A.D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Mccullough, M.J. Clinical Isolates and Laboratory Reference Candida Species and Strains Have Varying Abilities to Form Biofilms. FEMS Yeast Res. 2013, 13, 689–699. [Google Scholar] [CrossRef]
- Raut, J.S.; Rajput, S.B.; Shinde, R.B.; Surwase, B.S.; Karuppayil, S.M. Vanillin Inhibits Growth, Morphogenesis and Biofilm Formation by Candida albicans. J. Biol. Act. Prod. from Nat. 2013, 3, 130–138. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.Z.; Yu-Xuan, W.; Tao, L.; Zhang, Y.D.; Wang, S.R.; Zhang, G.C.; Zhang, J. Inhibitory Effects and Mechanisms of Vanillin on Gray Mold and Black Rot of Cherry Tomatoes. Pestic. Biochem. Physiol. 2021, 175, 104859. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Mendoza, L.; Cotoras, M. Alteration of Oxidative Phosphorylation as a Possible Mechanism of the Antifungal Action of P-Coumaric Acid against Botrytis cinerea. J. Appl. Microbiol. 2017, 123, 969–976. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, F.; Wang, Y.; Li, S.; Zhang, D.; Liang, W.; Yang, Q. Scopoletin Negatively Regulates the HOG Pathway and Exerts Antifungal Activity against Botrytis cinerea by Interfering with Infection Structures, Cell Wall, and Cell Membrane Formation. Phytopathol. Res. 2024, 6, 1–14. [Google Scholar] [CrossRef]
- Yan, H.; Meng, X.; Lin, X.; Duan, N.; Wang, Z.; Wu, S. Antifungal Activity and Inhibitory Mechanisms of Ferulic Acid against the Growth of Fusarium graminearum. Food Biosci. 2023, 52, 102414. [Google Scholar] [CrossRef]
- da Rocha Neto, A.C.; Maraschin, M.; Di Piero, R.M. Antifungal Activity of Salicylic Acid against Penicillium expansum and Its Possible Mechanisms of Action. Int. J. Food Microbiol. 2015, 215, 64–70. [Google Scholar] [CrossRef]
- Khanzada, B.; Akhtar, N.; Okla, M.K.; Alamri, S.A.; Al-Hashimi, A.; Baig, M.W.; Rubnawaz, S.; Abdelgawad, H.; Hirad, A.H.; Haq, I.U.; et al. Profiling of Antifungal Activities and in Silico Studies of Natural Polyphenols from Some Plants. Molecules 2021, 26, 7164. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, G.; Li, B.; Tian, S. Effect of Cinnamic Acid for Controlling Gray Mold on Table Grape and Its Possible Mechanisms of Action. Curr. Microbiol. 2015, 71, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1–31. [Google Scholar] [CrossRef]
- Abody, S.M.; Mickymaray, S. Antibiotics Anti-Fungal E Ffi Cacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 1–42. [Google Scholar]
- Robles-Yerena, L.; Rodríguez-Mendoza, J.; Santoyo, G.; Ochoa-Alvarado, X.I.; Medina-Estrada, R.I.; Jiménez-Mejía, R.; Loeza-Lara, P.D. Phylogenetic Identification of Fungi Isolated from Strawberry and Papaya Fruits and Their Susceptibility to Fatty Acids. Can. J. Plant Pathol. 2022, 44, 828–835. [Google Scholar] [CrossRef]
- Abah, S.E.; Egwari, L.O. Methods of Extraction and Antimicrobial Susceptibility Testing of Plant Extracts. Afr. Jownal Basic Appl. Sci. 2011, 3, 205–209. [Google Scholar]
- Danmusa, U.M.; Nasir, I.A.; Abdullahi, M.I.; Ahmad, A.A.; Abdulkadir, I.S. Phytochemical Analysis and Antimicrobial Activities of Methanolic Stem Extracts of Ochna schweinfurthiana F. Hoffm. J. Pharm. Pharmacogn. Res. 2015, 3, 171–182. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Lamaison, J.L.; Carnart, A. Teneurs En Principaux Flavonoides Des Fleurs et Des Feuilles de Crataegus monogyna Jacq. et de Crataegus laevigata (Poiret) Dc. En Fonction de La Periode de Vegetation. Plantes Med. Phyther. 1991, 25, 12–16. [Google Scholar]
- Juárez-Trujillo, N.; Monribot-Villanueva, J.L.; Jiménez-Fernández, V.M.; Suárez-Montaño, R.; Aguilar-Colorado, Á.S.; Guerrero-Analco, J.A.; Jiménez, M. Phytochemical Characterization of Izote (Yucca elephantipes) Flowers. J. Appl. Bot. Food Qual. 2018, 91, 202–210. [Google Scholar] [CrossRef]
- Monribot-Villanueva, J.L.; Elizalde-Contreras, J.M.; Aluja, M.; Segura-Cabrera, A.; Birke, A.; Guerrero-Analco, J.A.; Ruiz-May, E. Endorsing and Extending the Repertory of Nutraceutical and Antioxidant Sources in Mangoes during Postharvest Shelf Life. Food Chem. 2019, 285, 119–129. [Google Scholar] [CrossRef]
- Barriada-Bernal, L.G.; Almaraz-Abarca, N.; Delgado-Alvarado, E.A.; Gallardo-Velázquez, T.; Ávila-Reyes, J.A.; Torres-Morán, M.I.; González-Elizondo, M.D.S.; Herrera-Arrieta, Y. Flavonoid Composition and Antioxidant Capacity of the Edible Flowers of Agave durangensis (Agavaceae). CYTA-J. Food 2014, 12, 105–114. [Google Scholar] [CrossRef]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and Anti-Inflammatory Activity Determination of One Hundred Kinds of Pure Chemical Compounds Using Offline and Online Screening HPLC Assay. Evid.-Based Complement. Altern. Med. 2015, 1, 165457. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Mokrani, A.; Krisa, S.; Cluzet, S.; Da Costa, G.; Temsamani, H.; Renouf, E.; Mérillon, J.M.; Madani, K.; Mesnil, M.; Monvoisin, A.; et al. Phenolic Contents and Bioactive Potential of Peach Fruit Extracts. Food Chem. 2016, 202, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Magaldi, S.; Mata-Essayag, S.; Hartung De Capriles, C.; Perez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y. Well Diffusion for Antifungal Susceptibility Testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef]
- Rutiaga-Quiñones, J.G. Chemische und Biologische Untersuchungen zum Verhalten Dauerhafter Holzarten und Ihrer Extrakte Gegenüber Holzabbauenden Pilzen; Buchverl: München, Germany, 2001. [Google Scholar]
| Extract | TPC (mg GAE/g DE) | TFC (mg RE/g DE) | DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) | TAC (mg AAE/g DE) | FRP (mg GAE/g DE) |
|---|---|---|---|---|---|---|
| Aqueous stem extract | 21.40 ± 0.36 a | 11.53 ± 0.07 a | 12.36 ± 0.07 c | 5.60 ± 0.11 a | 20.62 ± 0.09 c | 74.76 ± 1.48 b |
| Ethyl acetate fraction | 21.26 ± 0.38 a | 3.71 ± 0.03 b | 7.36 ± 0.01 a | 7.76 ± 0.09 b | 40.21 ± 0.41 a | 129.57 ± 2.08 a |
| Methanolic fraction | 13.54 ± 0.43 b | 2.12 ± 0.07 c | 8.48 ± 0.02 b | 10.26 ± 0.04 c | 31.46 ± 0.6 b | 61.48 ± 1.04 c |
| Rt (min) | Compound | µg/g DE | ||
|---|---|---|---|---|
| Aqueous Extract | Ethyl Acetate Fraction | Methanolic Fraction | ||
| 1.4 | Gallic acid | ND | ND | 4.98 ± 0.04 a |
| 2.5 | Protocatechuic acid | 93.06 ± 2.56 b | 250.54 ± 1.04 a | 16.14 ± 0.45 c |
| 3.76 | Hydroxybenzoic acid | 65.69 ± 1.14 b | 135.61 ± 0.89 a | 3.82 ± 0.12 c |
| 5.12 | Vanillic acid | 66.86 ± 0.13 b | 246.94 ± 3.46 a | 5.99 ± 0.26 c |
| 5.34 | Chlorogenic acid | 22.51 ± 0.34 c | 114.66 ± 3.16 a | 64.05 ± 1.17 b |
| 5.38 | Caffeic acid | ND | 18.36 ± 1.16 a | 4.86 ± 0.11 b |
| 6.52 | Vanillin | 10.09 ± 0.06 b | 289.27 ± 2.92 a | 0.75 ± 0.09 c |
| 7.21 | p-coumaric acid | 21.13 ± 0.45 b | 139.72 ± 2.71 a | 3.51 ± 0.10 c |
| 8.4 | Scopoletin | ND | 3.03 ± 0.34 a | ND |
| 8.6 | Ferulic acid | 2.99 ± 0.15 b | 26.05 ± 0.31 a | 0.17 ± 0.04 c |
| 9.15 | Salicylic acid | 10.97 ± 0.25 b | 40.19 ± 0.35 a | 1.38 ± 0.06 c |
| 9.16 | Sinapic acid | 1.37 ± 0.21 b | 5.74 ± 0.69 a | 1.23 ± 0.07 b |
| 10.35 | Rutin | 20.56 ± 0.73 b | 250.52 ± 8.21 a | 10.28 ± 0.18 b |
| 10.57 | Isoquercitrin (quercetin-3-O-glucoside) | ND | 69.16 ± 2.19 a | ND |
| 11.91 | Astragaline (kaempferol 3-O-glucoside) | ND | 41.61 ± 1.51 a | ND |
| 13.02 | Secoisolariciresinol | ND | 47.79 ± 1.09 a | ND |
| 14.08 | trans-Cinnamic acid | 11.46 ± 0.06 b | 59.45 ± 1.43 a | ND |
| 15.18 | Quercetin | ND | 21.25 ± 0.49 a | 6.45 ± 0.10 b |
| 15.28 | Luteolin | 1.07 ± 0.12 a | ND | ND |
| 17.81 | Kaempferol | ND | 6.94 ± 0.09 a | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Valdovinos, K.Y.; Salgado-Garciglia, R.; Hernández-García, A.; Saavedra-Molina, A.; del Río-Torres, R.E.N.; López-Meza, J.E.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; Medina-Medrano, J.R. Antioxidant and Antifungal Activities and Characterization of Phenolic Compounds Using Ultra-High Performance Liquid Chromatography and Mass Spectrometry (UPLC-MS) of Aqueous Extracts and Fractions from Verbesina sphaerocephala Stems. Plants 2024, 13, 2791. https://doi.org/10.3390/plants13192791
Rodríguez-Valdovinos KY, Salgado-Garciglia R, Hernández-García A, Saavedra-Molina A, del Río-Torres REN, López-Meza JE, Monribot-Villanueva JL, Guerrero-Analco JA, Medina-Medrano JR. Antioxidant and Antifungal Activities and Characterization of Phenolic Compounds Using Ultra-High Performance Liquid Chromatography and Mass Spectrometry (UPLC-MS) of Aqueous Extracts and Fractions from Verbesina sphaerocephala Stems. Plants. 2024; 13(19):2791. https://doi.org/10.3390/plants13192791
Chicago/Turabian StyleRodríguez-Valdovinos, Kathia Yanelly, Rafael Salgado-Garciglia, Alejandra Hernández-García, Alfredo Saavedra-Molina, Rosa Elva Norma del Río-Torres, Joel Edmundo López-Meza, Juan Luis Monribot-Villanueva, José Antonio Guerrero-Analco, and José Roberto Medina-Medrano. 2024. "Antioxidant and Antifungal Activities and Characterization of Phenolic Compounds Using Ultra-High Performance Liquid Chromatography and Mass Spectrometry (UPLC-MS) of Aqueous Extracts and Fractions from Verbesina sphaerocephala Stems" Plants 13, no. 19: 2791. https://doi.org/10.3390/plants13192791
APA StyleRodríguez-Valdovinos, K. Y., Salgado-Garciglia, R., Hernández-García, A., Saavedra-Molina, A., del Río-Torres, R. E. N., López-Meza, J. E., Monribot-Villanueva, J. L., Guerrero-Analco, J. A., & Medina-Medrano, J. R. (2024). Antioxidant and Antifungal Activities and Characterization of Phenolic Compounds Using Ultra-High Performance Liquid Chromatography and Mass Spectrometry (UPLC-MS) of Aqueous Extracts and Fractions from Verbesina sphaerocephala Stems. Plants, 13(19), 2791. https://doi.org/10.3390/plants13192791








