Magnesium Hydride Confers Osmotic Tolerance in Mung Bean Seedlings by Promoting Ascorbate–Glutathione Cycle
Abstract
1. Introduction
2. Results
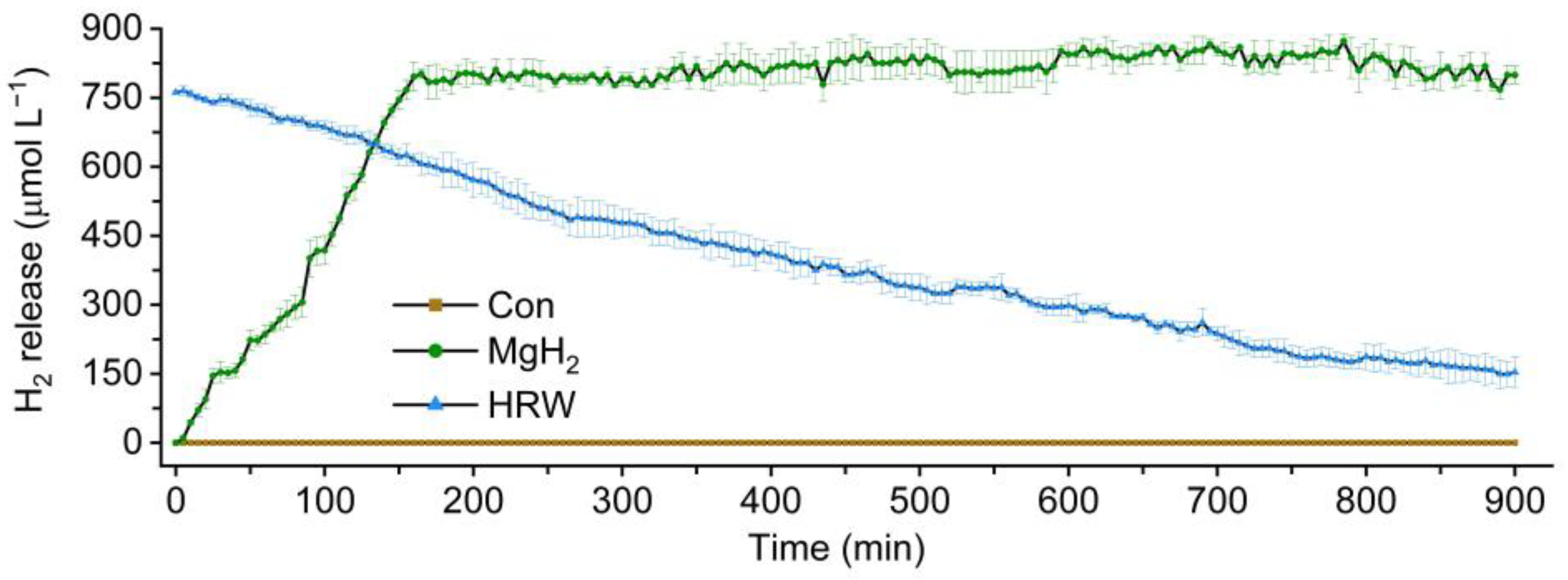
2.1. Characterization of H2 Release in HRW and MgH2 Solution
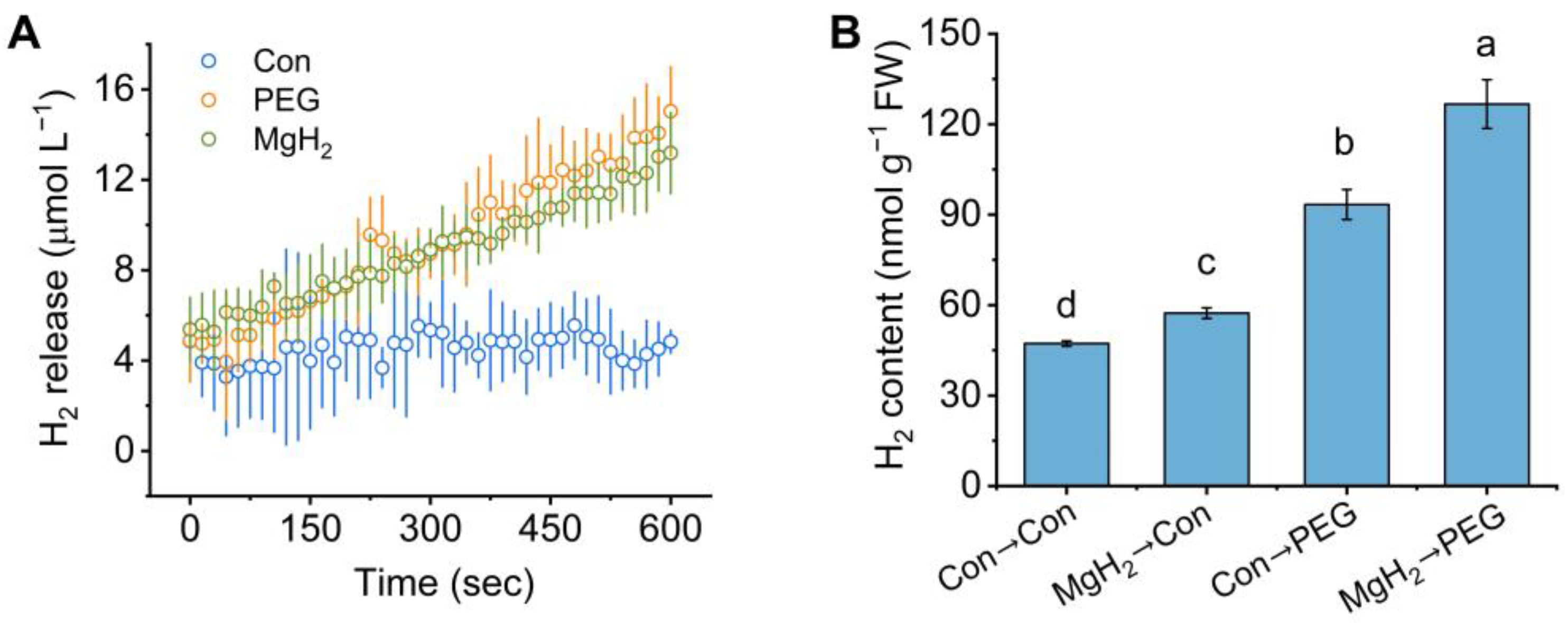
2.2. Osmotic Stress-Induced H2 Production Was Promoted by MgH2
2.3. Osmotic Tolerance of Mung Bean Seedlings Achieved by MgH2
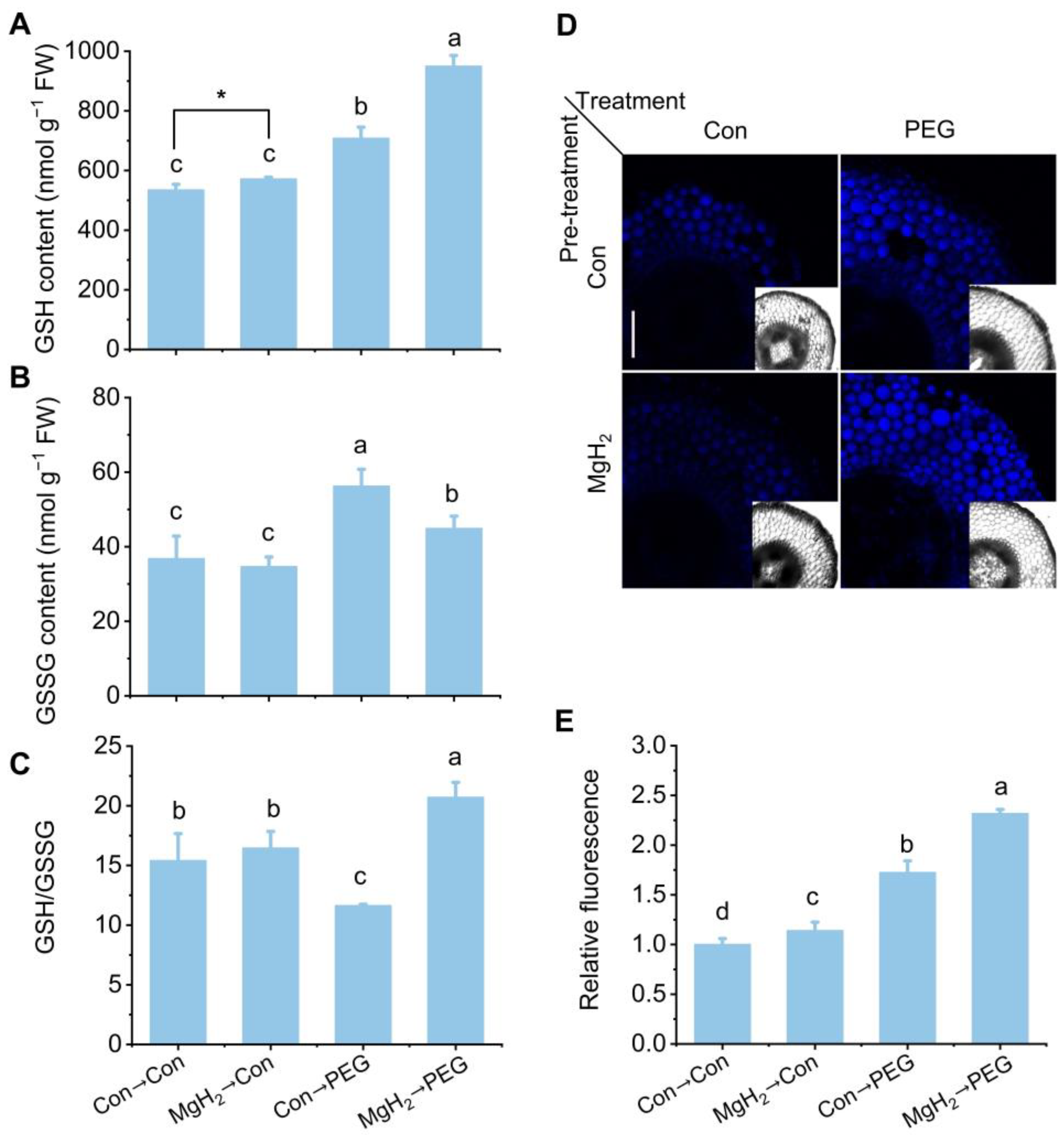
2.4. MgH2 Conferred Redox Homeostasis in Response to Osmotic Stress
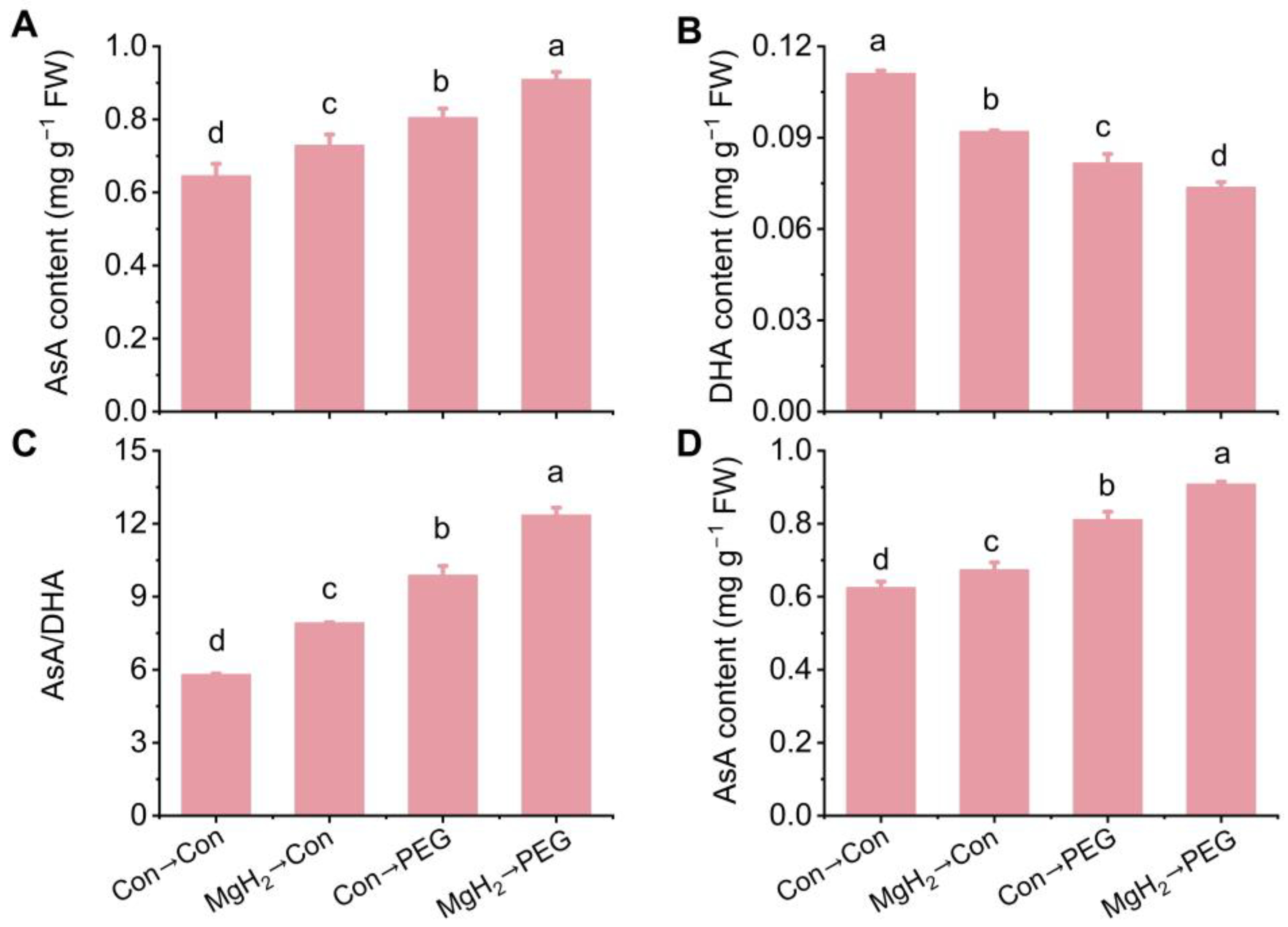
2.5. MgH2-Alleviated Oxidative Damage Was Dependent on AsA-GSH Cycle
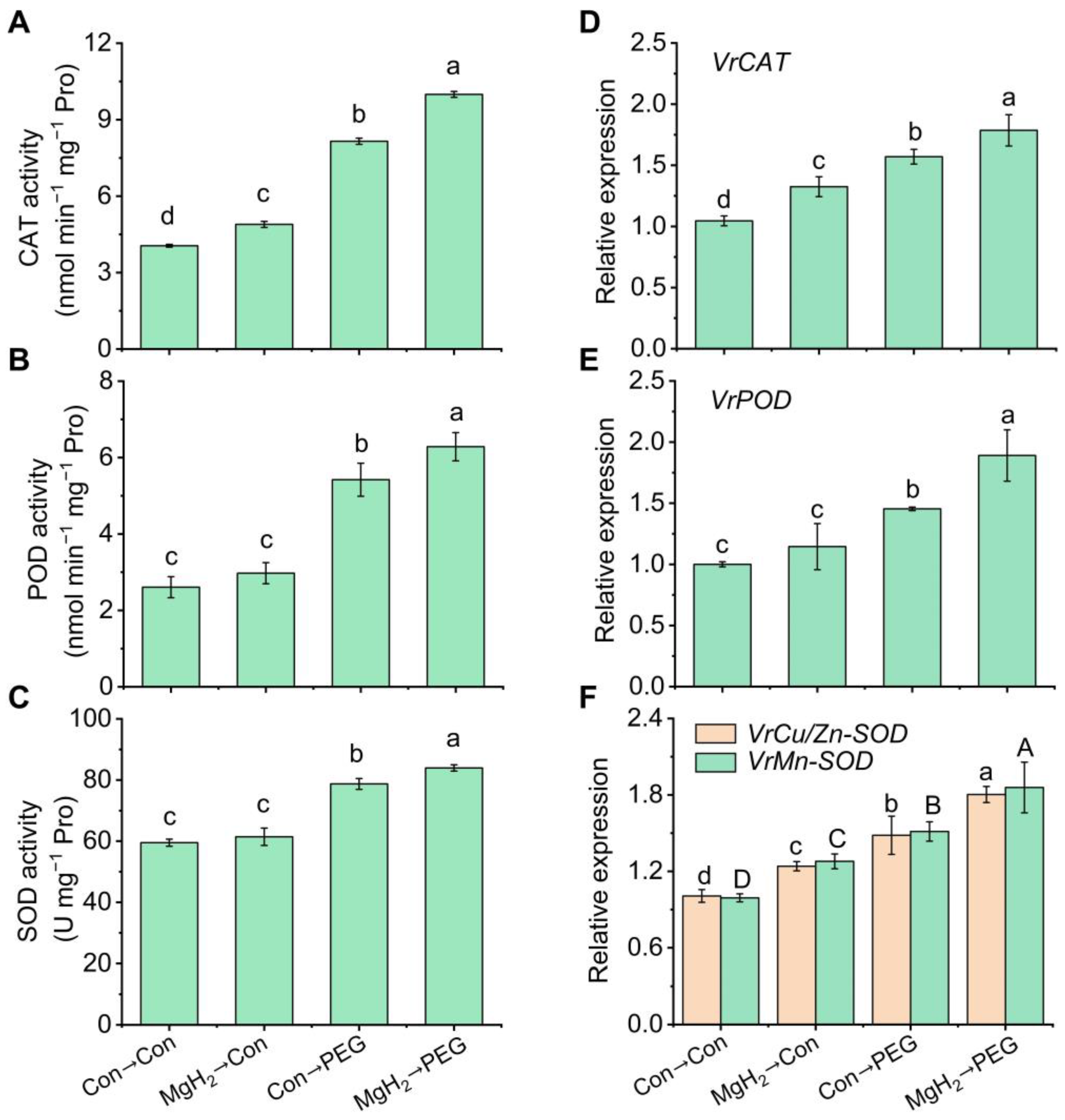
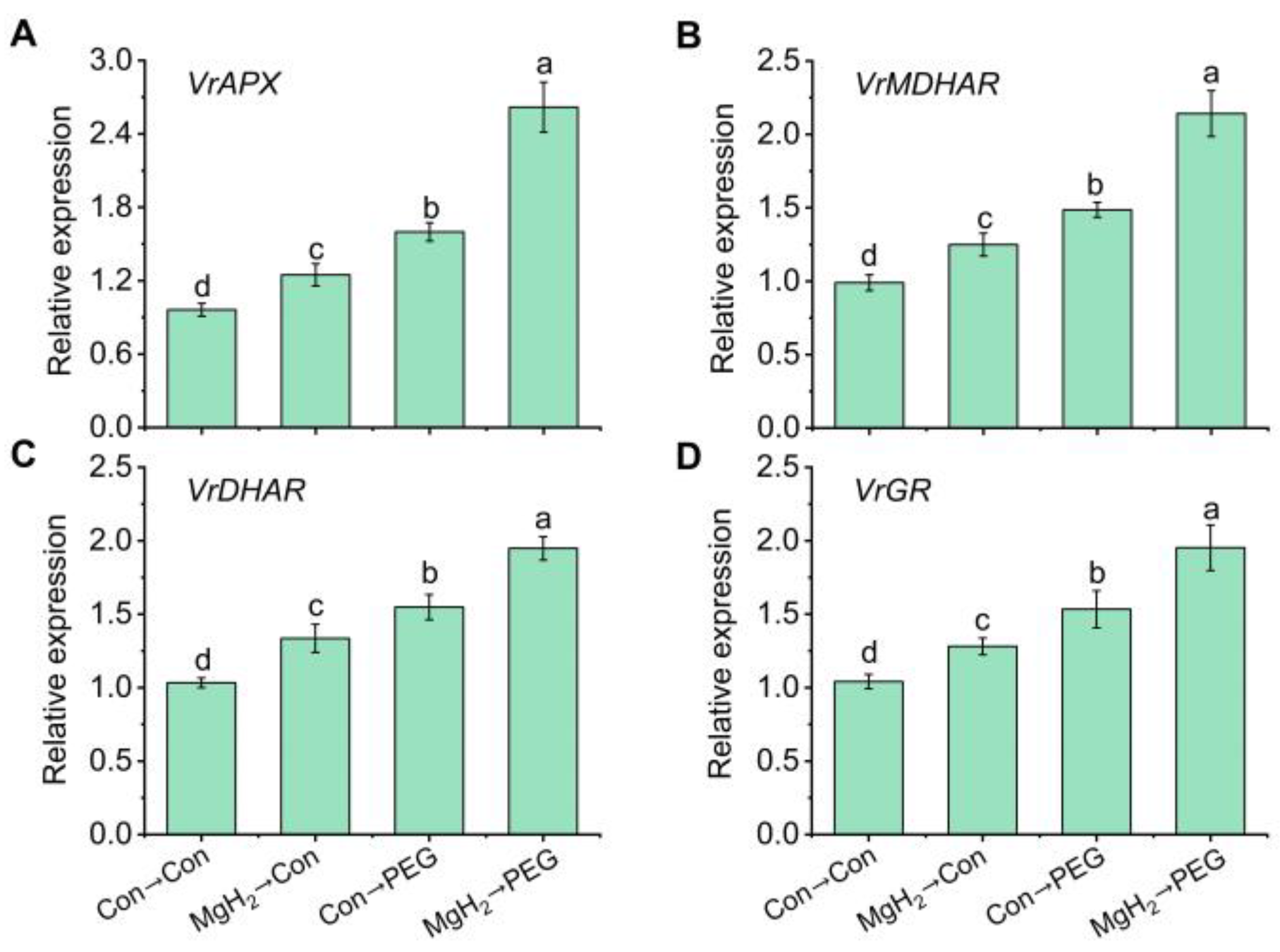
2.6. Regulation of AsA-GSH Cycle-Involved Key Enzymes and Genes by MgH2
3. Discussion
4. Materials and Methods
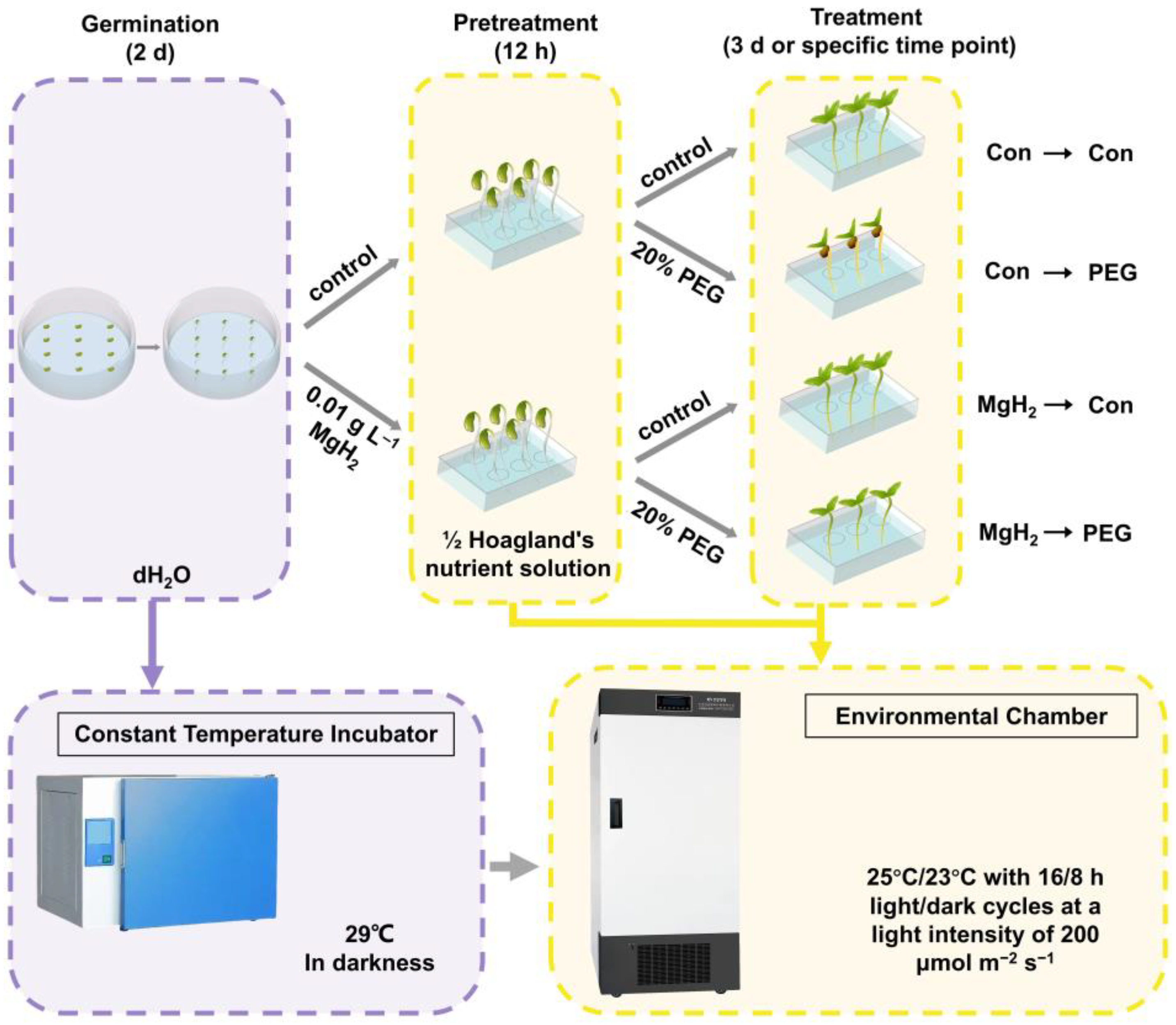
4.1. Plant Materials and Growth Conditions
4.2. Detection of H2 Release and Content
4.3. Oxidative Damage Assay
4.4. Analysis of ROS
4.5. Determination of Antioxidant Enzyme Activities
4.6. Detection of AsA and DHA Contents
4.7. Measurement of GSH and GSSG Levels
4.8. Detection of Enzyme Activities in AsA-GSH Cycle
4.9. qRT-PCR Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Swann, A.L.S. Plants and drought in a changing climate. Curr. Clim. Chang. Rep. 2018, 4, 192–201. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.R. On the essentials of drought in a changing climate. Science 2020, 368, 256–260. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef] [PubMed]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Li, B.; Fan, R.; Sun, G.; Sun, T.; Fan, Y.; Bai, S.; Guo, S.; Huang, S.; Liu, J.; Zhang, H.; et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant Soil 2021, 461, 389–405. [Google Scholar] [CrossRef]
- Labudda, M.; Azam, F.M.S. Glutathione-dependent responses of plants to drought: A review. Acta Soc. Bot. Pol. 2014, 83, 3–12. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, T.F.; Ahamed, K.U.; Nahar, K.; Al Mahmud, J.; Bhuyan, M.B.; Anee, T.I.; Fujita, M.; Hasanuzzaman, M. Mitigation of PEG-induced drought stress in rapeseed (Brassica rapa L.) by exogenous application of osmolytes. Biocatal. Agric. Biotechnol. 2019, 20, 101197. [Google Scholar] [CrossRef]
- Veziroğlu, T.N.; Şahi, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, M.; Sun, X. Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. PLoS ONE 2013, 8, e71038. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, H.; Yu, C.; Wang, F.; Sun, X. A review of experimental studies of hydrogen as a new therapeutic agent in emergency and critical care medicine. Med. Gas Res. 2014, 4, 17. [Google Scholar] [CrossRef]
- Li, L.; Lou, W.; Kong, L.; Shen, W. Hydrogen commonly applicable from medicine to agriculture: From molecular mechanisms to the field. Curr. Pharm. Des. 2021, 27, 747–759. [Google Scholar] [CrossRef]
- McCurry, M.D.; D’Agostino, G.D.; Walsh, J.T.; Bisanz, J.E.; Zalosnik, I.; Dong, X.; Morris, D.J.; Korzenik, J.R.; Edlow, A.G.; Balskus, E.P.; et al. Gut bacteria convert glucocorticoids into progestins in the presence of hydrogen gas. Cell 2024, 187, 2952–2968.e13. [Google Scholar] [CrossRef]
- Hancock, J.T.; Russell, G. Downstream signalling from molecular hydrogen. Plants 2021, 10, 367. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, K.; Cui, W.; Li, L.; Shen, W. Hydrogen-modulated stomatal sensitivity to abscisic acid and drought tolerance via the regulation of apoplastic pH in Medicago sativa. J. Plant Growth Regul. 2016, 35, 565–573. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Y.; Lai, D.; Zhang, W.; Shen, W. H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS ONE 2012, 7, e49800. [Google Scholar] [CrossRef]
- Cui, W.; Gao, C.; Fang, P.; Lin, G.; Shen, W. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J. Hazard. Mater. 2013, 260, 715–724. [Google Scholar] [CrossRef]
- Xie, Y.; Mao, Y.; Zhang, W.; Lai, D.; Wang, Q.; Shen, W. Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol. 2014, 165, 759–773. [Google Scholar] [CrossRef]
- Su, J.; Zhang, Y.; Nie, Y.; Cheng, D.; Wang, R.; Hu, H.; Chen, J.; Zhang, J.; Du, Y.; Shen, W. Hydrogen-induced osmotic tolerance is associated with nitric oxide-mediated proline accumulation and reestablishment of redox balance in alfalfa seedlings. Environ. Exp. Bot. 2018, 147, 249–260. [Google Scholar] [CrossRef]
- Felix, K.; Su, J.; Lu, R.; Zhao, G.; Cui, W.; Wang, R.; Mu, H.; Cui, J.; Shen, W. Hydrogen-induced tolerance against osmotic stress in alfalfa seedlings involves ABA signaling. Plant Soil 2019, 445, 409–423. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, P.; Wang, Y.; Li, Y.; Su, J.; Chen, Z.; Yu, X.; Shen, W. Genetic elucidation of hydrogen signaling in plant osmotic tolerance and stomatal closure via hydrogen sulfide. Free Radic. Biol. Med. 2020, 161, 1–14. [Google Scholar] [CrossRef]
- Li, L.; Huang, H.; Jin, Z.; Jiang, K.; Zeng, Y.; Pathier, D.; Cheng, X.; Shen, W. Strawberry yield improvement by hydrogen-based irrigation is functionally linked to altered rhizosphere microbial communities. Plants 2024, 13, 1723. [Google Scholar] [CrossRef]
- Shao, Y.; Lin, F.; Wang, Y.; Cheng, P.; Lou, W.; Wang, Z.; Liu, Z.; Chen, D.; Guo, W.; Lan, Y.; et al. Molecular hydrogen confers resistance to rice stripe virus. Microbiol. Spectr. 2023, 11, e04417-22. [Google Scholar] [CrossRef]
- He, J.; Cheng, P.; Wang, J.; Xu, S.; Zou, J.; Shen, W. Magnesium hydride confers copper tolerance in alfalfa via regulating nitric oxide signaling. Ecotoxicol. Environ. Saf. 2022, 231, 113197. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, C.; Bai, F.; Wang, W.; An, S.; Zhao, K.; Li, Z.; Li, J.; Sun, H. A comprehensive review of the promising clean energy carrier: Hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel 2024, 355, 129455. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C., Jr.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage–past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Baricco, M.; Bang, M.; Fichtner, M.; Hauback, B.; Linder, M.; Luetto, C.; Moretto, P.; Sgroi, M. SSH2S: Hydrogen storage in complex hydrides for an auxiliary power unit based on high temperature proton exchange membrane fuel cells. J. Power Sources 2017, 342, 853–860. [Google Scholar] [CrossRef]
- Lototskyy, M.; Nyallang, N.S.; Pasupathi, S.; Wærnhus, I.; Vik, A.; Ilea, C.; Yartys, V. A concept of combined cooling, heating and power system utilising solar power and based on reversible solid oxide fuel cell and metal hydrides. Int. J. Hydrogen Energy 2018, 43, 18650–18663. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Wang, S.; Zou, J.; Ding, W.; Shen, W. Magnesium hydride-mediated sustainable hydrogen supply prolongs the vase life of cut carnation flowers via hydrogen sulfide. Front. Plant Sci. 2020, 11, 595376. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Wang, S.; Liu, Y.; Zou, J.; Ding, W.; Du, H.; Shen, W. Magnesium hydride acts as a convenient hydrogen supply to prolong the vase life of cut roses by modulating nitric oxide synthesis. Postharvest Biol. Technol. 2021, 177, 111526. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Chen, X.; Zhang, X.; Chi, Y.; Zhang, D.; Chu, S.; Zhou, P. A critical review for hydrogen application in agriculture: Recent advances and perspectives. Crit. Rev. Environ. Sci. Technol. 2024, 54, 222–238. [Google Scholar] [CrossRef]
- Crowl, D.A.; Jo, Y.D. The hazards and risks of hydrogen. J. Loss Prev. Process Ind. 2007, 20, 158–164. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, S.; Liu, Z.; Chen, G.; Cheng, P.; Li, L.; Xu, S.; Shen, W. H2 supplied via ammonia borane stimulates lateral root branching via phytomelatonin signaling. Plant Physiol. 2024, 194, 884–901. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Yu, Y.; Liu, W.; Lu, L.; Jin, C.; Lin, X. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J. Integr. Plant Biol. 2015, 57, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Kunert, K. The ascorbate–glutathione cycle coming of age. J. Exp. Bot. 2024, 75, 2682–2699. [Google Scholar] [CrossRef]
- Noctor, G.; Cohen, M.; Trémulot, L.; Châtel-Innocenti, G.; Van Breusegem, F.; Mhamdi, A. Glutathione: A key modulator of plant defence and metabolism through multiple mechanisms. J. Exp. Bot. 2024, 75, 4549–4572. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Huang, X.; Ling, X.; Yu, M.; Cui, J.; Shabala, S. Hydrogen-rich water promotes elongation of hypocotyls and roots in plants through mediating the level of endogenous gibberellin and auxin. Funct. Plant Biol. 2020, 47, 771–778. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, G.; Cheng, P.; Yan, X.; Li, Y.; Cheng, D.; Wang, R.; Chen, J.; Shen, W. Nitrite accumulation during storage of tomato fruit as prevented by hydrogen gas. Int. J. Food Prop. 2019, 22, 1425–1438. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, K.; Cui, W.; Xie, Y.; Han, B.; Shen, W. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ. 2013, 36, 956–969. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, Y.; Cui, W.; Jin, Q.; Zhang, Y.; Bu, D.; Fu, J.; Wang, R.; Zhou, F.; Shen, W. Hydrogen enhances adaptation of rice seedlings to cold stress via the reestablishment of redox homeostasis mediated by miRNA expression. Plant Soil 2017, 414, 53–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.; Cheng, D.; Wang, R.; Mei, Y.; Hu, H.; Shen, W.; Zhang, Y. Nitric oxide contributes to methane-induced osmotic stress tolerance in mung bean. BMC Plant Biol. 2018, 18, 207. [Google Scholar] [CrossRef]
- Cheng, P.; Feng, L.; Zhang, S.; Li, L.; Guan, R.; Long, W.; Xian, Z.; Zhang, J.; Shen, W. Ammonia borane positively regulates cold tolerance in Brassica napus via hydrogen sulfide signaling. BMC Plant Biol. 2022, 22, 585. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kampfenkel, K.; van Montagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Smith, I.K. Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol. 1985, 79, 1044–1047. [Google Scholar] [CrossRef]
- Pasternak, M.; Lim, B.; Wirtz, M.; Hell, R.; Cobbett, C.S.; Meyer, A.J. Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. Plant J. 2008, 53, 999–1012. [Google Scholar] [CrossRef]
- Chumyam, A.; Shank, L.; Faiyue, B.; Uthaibutra, J.; Saengnil, K. Effects of chlorine dioxide fumigation on redox balancing potential of antioxidative ascorbate-glutathione cycle in ‘Daw’ longan fruit during storage. Sci. Hortic. 2017, 222, 76–83. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lu, X.; Yao, W.; Cheng, X.; Wang, Q.; Feng, Y.; Shen, W. Magnesium Hydride Confers Osmotic Tolerance in Mung Bean Seedlings by Promoting Ascorbate–Glutathione Cycle. Plants 2024, 13, 2819. https://doi.org/10.3390/plants13192819
Zhang Y, Lu X, Yao W, Cheng X, Wang Q, Feng Y, Shen W. Magnesium Hydride Confers Osmotic Tolerance in Mung Bean Seedlings by Promoting Ascorbate–Glutathione Cycle. Plants. 2024; 13(19):2819. https://doi.org/10.3390/plants13192819
Chicago/Turabian StyleZhang, Yihua, Xing Lu, Wenrong Yao, Xiaoqing Cheng, Qiao Wang, Yu Feng, and Wenbiao Shen. 2024. "Magnesium Hydride Confers Osmotic Tolerance in Mung Bean Seedlings by Promoting Ascorbate–Glutathione Cycle" Plants 13, no. 19: 2819. https://doi.org/10.3390/plants13192819
APA StyleZhang, Y., Lu, X., Yao, W., Cheng, X., Wang, Q., Feng, Y., & Shen, W. (2024). Magnesium Hydride Confers Osmotic Tolerance in Mung Bean Seedlings by Promoting Ascorbate–Glutathione Cycle. Plants, 13(19), 2819. https://doi.org/10.3390/plants13192819






