Juvenile Plant–Microbe Interactions Modulate the Adaptation and Response of Forest Seedlings to Rapid Climate Change
Abstract
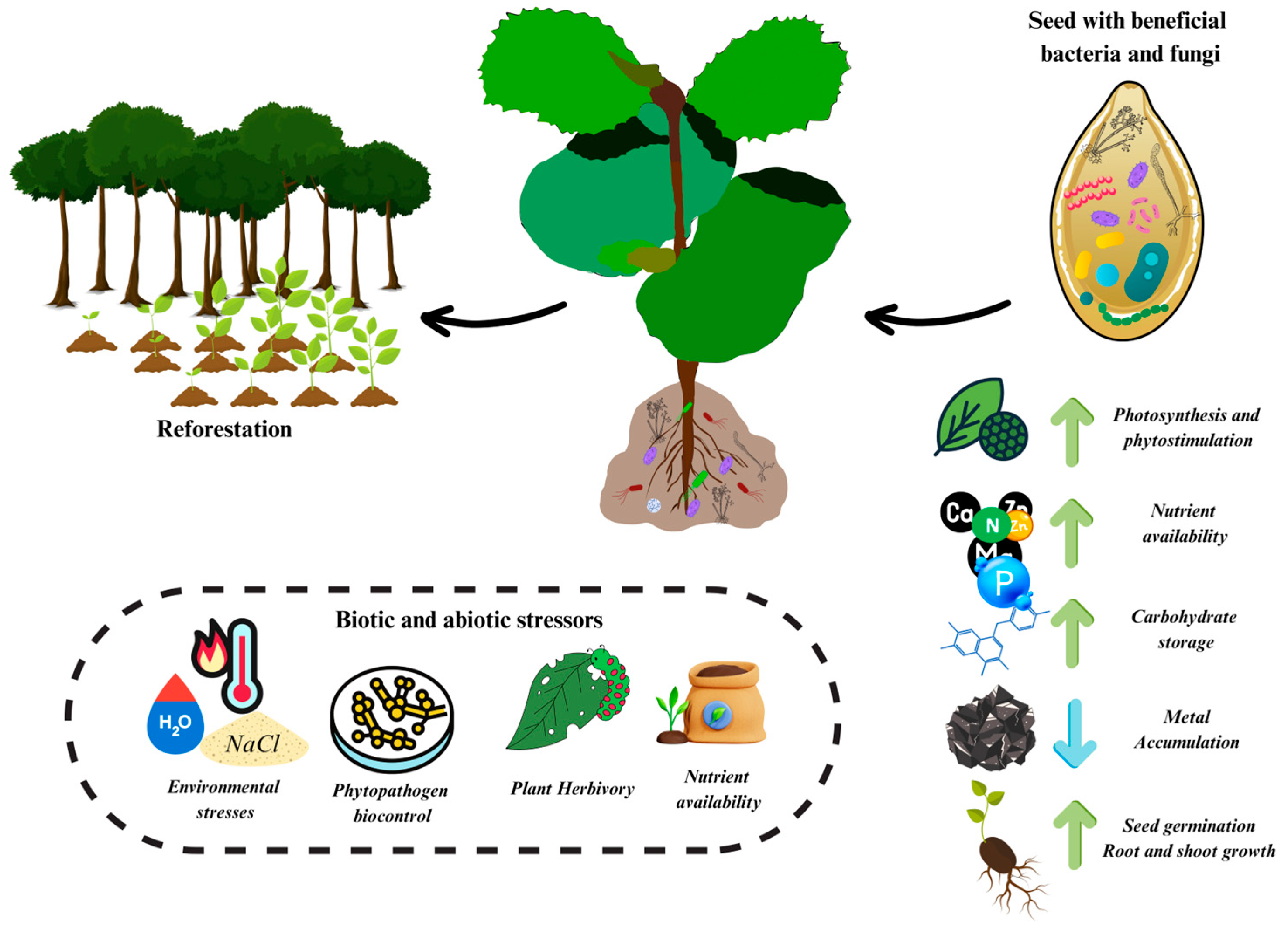
:1. Introduction
2. Juvenile Plant–Microbe Interactions
2.1. Seed-Associated Microorganisms
2.2. Beneficial Microorganisms at the Plantlet Stage
3. Metabolic Responses of Plants and Their Associated Microorganisms to Abiotic Stress
3.1. Water Deficit
3.2. Salinity
3.3. Heat Stress
3.4. Cold Stress
3.5. Mineral Deficiency
3.6. Metal(loid)s
4. Role of Juvenile Plant–Microbe Symbiosis in Native Forest Regeneration
4.1. Promoting the Adaptation of New Plantlets
4.2. Protecting the Forest Trees against New Phytopathogens
| Microorganisms | Plant Species | Mechanisms | References |
|---|---|---|---|
| Bacillus subtilis | Populus euramericana Populus deltoides × Populus nigra | Enhanced seedling height by 62% and total biomass by 37% after 120 days. The photosynthetic rate increased by 54%. | [125] |
| Rhizophagus manihotis Rhizophagus Agregatus Rhizophagus fasciculatus Acaulospora sp. | Cupressus atlantica | Increased the relative water content and water potential under water deficit stress. Increase contents of proline and of soluble sugars. Increase Superoxide dismutase (SOD) and catalase (CAT) activities. | [133] |
| Funneliformis mosseae Diversispora tortuosa | Gleditsia sinensis | Increase seedling height, basal diameter, dry biomass. Increase chlorophyll concentrations and photosynthetic rates. Increased phosphorus (P) and potassium (K) content in leaf, stem, and root, and increased nitrogen (N) content in the leaf and stem. | [148] |
| Bacillus subtilis Claroideoglomus etunicatum Rhizophagus intraradices Funneliformis mosseae | Acacia gerrardii | Induce acquired systemic resistance against adverse impact of salt stress. Improvement in the nutritional value in terms of increase in total lipids, phenols, and fiber content. Increased content of osmoprotectants such as glycine, betaine, and proline. | [137] |
| Rhizophagus intraradices Funneliformis mosseae Pseudomonas fluorescens | Cupressus arizonica | Induce resistance under Cadmium (Cd) stress condition. Increase P, K and iron concentrations, height, shoot dry weight, proline content and reduced electrolyte leakage percentage. | [131] |
| Bacillus licheniformis | Camellia oleifera | Production lytic enzymes chitinase and β-1,3-glucanase that can inhibit foliar pathogens by 37.4% (Botrytis cinerea) to 50.5% (Pestalotiopsis karstenii). Increased the total N and P contents in the soils. Increased root dry weight and production the phytohormone auxin. | [145] |
| Bacillus velezensis | Juglans regia | Production lytic enzymes chitinase, protease, and β-l,3-glucanase activity and degraded the cell wall of Colletotrichum gloeosporioides. Production indole-3-acetic acid (IAA) and exhibited the potential for ammonium production and phosphate solubilization. | [146] |
| Pseudomonas fluorescens | Santalum album | Biopriming at 100% for 8 days recorded the highest germination percentage (88%). | [127] |
| Pseudomonas aeruginosa | Pongamia pinnata | Ammonia production, IAA production, siderophore production and was observed to promote solubilization of phosphate, silicate and zinc in the plate assay. | [128] |
| Funneliformis mosseae Rhizophagus irregularis Pseudomonas putida Pseudomonas fluorescens | Myrtus communis | Drought resistance, improved leaf physiology, reduced electrolyte leakage, malondialdehyde, and proline concentrations and mitigated oxidative pigment losses under drought through upregulation of the antioxidant defense as evidenced by non-enzymatic antioxidant accumulation. | [136] |
| Funneliformis mosseae Diversispora tortuosa | Zelkova serrata | Induce resistance salt stress. Increasing the leaf photosynthetic ability and biomass accumulation by reducing sodium content, increasing P, K+, and magnesium content, as well as by enhancing photosynthetic pigments content and the stomatal conductance of leaves. | [79] |
| Pseudomonas sp. Bacillus subtilis Bacillus amyloliquefaciens | Araucaria angustifolia | IAA, Siderophores production, inorganic phosphate solubilization. | [129] |
| Rhizophagusirregularis Funneliformis mosseae Pseudomonas fluorescens | Cupressus arizonica | Reduction oxidative damage in water stress (reduce hydrogen peroxide and MDA) and increase the enzymatic antioxidants (CAT, SOD, glutathione peroxidase, ascorbate peroxidase). | [134] |
| Funneliformismosseae | Robinia pseudoacacia | Induce resistance lead (Pb) stress. Increased the root activity and root tolerance index. Inoculated plants had greater accumulation and translocation capacities for Pb in the roots and stems. | [132] |
| Bacillus spp. Paenibacillus spp. | Abies nordmanniana | Improved seed germination and produced IAA. Increased plant root growth, especially by inducing secondary root formation, under in greenhouse conditions. | [126] |
| Methylobacterium sp. Kineococcus endophyticus | Populus deltoides x (Populus trichocarpa x Populus maximowiczii) | IAA production, phosphorus solubilization. reduced the bioaccumulation of Zn and Cd. | [130] |
| Pseudomonas frederiksbergensis | Populus euramericana | Phosphate-solubilizing activity, growth rate and organic acid secretion (high concentrations of gluconic, 2-ketogluconic, pyruvic, maleic and malic acids). | [149] |
| Trichoderma harzianum Trichoderma asperiana | Cabralea canjerana Cedrela fissilis Cordia trichotoma Erythrina cristagall Luehea divaricata | Increase the supervival rates and height and diameter of plants. | [150] |
| Caballeronia sordidicola | Picea glauca x engelmannii | Help in biological nitrogen fixation in limit soil nitrogen and enhanced seedling length and biomass by nearly | [151] |
| Claroideoglomus etunicatum Acaulospora sp. Rhizobium sp. Burkholderia sp. | Schizolobium parahyba | The application of microorganisms increased wood yield by about 20% compared to the application of fertilizer alone. | [152] |
| Microbacterium sp. Streptomyces sp. | Quercus brantii | The inoculation of the bacteria increased the rate of phosphate solubilization, improving root growth and seedling weight under water stress conditions. | [135] |
| Acinetobacter lwoffii Pantoea agglomerans | Anacardium othonianum | Auxin production, phosphate solubilization, production of phosphatases, siderophores, and biocontrol against Fusarium oxysporum. | [147] |
| Caballeronia sordidicola | Pinus contorta | Inoculation of diazotrophic bacteria increased the fixation of 49-50% of the host atmospheric nitrogen, and increased seedling length and biomass up to 1.5 and 4 times, respectively. | [153] |
| Rhizophagus clarus Gigaspora margarita | Chizolobium parahyba | In the absence of P, growth variables (height, dry matter area, root dry matter, leaf area, stem diameter) increased in relation to control plants. N, P, Ca and Mg contents were also influenced by fungal inoculation. | [154] |
4.3. Promoting Nutrient Mineralization
4.4. Moving Nutrients and Signals through Shared Hyphal Networks
4.5. Stimulating Native Soil Microbial Communities
4.6. Change in the Expression of Genes and Proteins Involved in Plant Adaptation
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronese, M.; Lamperti, F.; Keller, K.; Chiaromonte, F.; Roventini, A. Evidence for sharp increase in the economic damages of extreme natural disasters. Proc. Natl. Acad. Sci. USA 2019, 116, 21450–21455. [Google Scholar] [CrossRef]
- Albrich, K.; Rammer, W.; Seidl, R. Climate change causes critical transitions and irreversible alterations of mountain forests. Glob. Chang. Biol. 2020, 26, 4013–4027. [Google Scholar] [CrossRef]
- Li, D.; Wu, S.; Liu, L.; Zhang, Y.; Li, S. Vulnerability of the global terrestrial ecosystems to climate change. Glob. Chang. Biol. 2018, 24, 4095–4106. [Google Scholar] [CrossRef] [PubMed]
- Stabile, M.C.; Guimarães, A.L.; Silva, D.S.; Ribeiro, V.; Macedo, M.N.; Coe, M.T.; Pinto, E.; Moutinho, P.; Alencar, A. Solving Brazil’s land use puzzle: Increasing production and slowing Amazon deforestation. Land Use Policy 2020, 91, 104362. [Google Scholar] [CrossRef]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous fertilizers: Impact on environment sustainability, mitigation strategies, and challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.; Gâteblé, G. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The threat of the combined effect of biotic and abiotic stress factors in forestry under a changing climate. Front. Plant Sci. 2020, 11, 1874. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Xie, M.-M.; Hashem, A.; Abd-Allah, E.F.; Wu, Q.-S. Arbuscular mycorrhizal fungi and rhizobia synergistically promote root colonization, plant growth, and nitrogen acquisition. Plant Growth Regul. 2023, 100, 691–701. [Google Scholar] [CrossRef]
- Denison, R.F.; Kiers, E.T. Life histories of symbiotic rhizobia and mycorrhizal fungi. Curr. Biol. 2011, 21, R775–R785. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, K.; Singh, V.; Singh, J.; Devi, S.; Tewari, A. Plant–Microbe symbiosis: Perspectives and applications. Plant Microbe Symbiosis Fundam. Adv. 2013, 1, 119–145. [Google Scholar] [CrossRef]
- Leung, T.L.; Poulin, R. Parasitism, commensalism, and mutualism: Exploring the many shades of symbioses. Vie Milieu/Life Environ. 2008, 58, 107–115. [Google Scholar]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Taulé, C.; Vaz-Jauri, P.; Battistoni, F. Insights into the early stages of plant–endophytic bacteria interaction. World J. Microbiol. Biotechnol. 2021, 37, 13. [Google Scholar] [CrossRef] [PubMed]
- Mertin, A.A.; Philpott, M.; Blackall, L.L.; French, K.; Liew, E.C.; van der Merwe, M.M. Integrating seed microbiome knowledge into restoration and ex situ conservation of native Australian plants. Aust. J. Bot. 2023. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.-M.; Guerrero-Galán, C.; Scholz, S.S.; Kiba, T.; Sakakibara, H.; Ludwig-Müller, J.; Krapp, A.; Oelmüller, R.; Vicente-Carbajosa, J.; Pollmann, S. Harnessing symbiotic plant–fungus interactions to unleash hidden forces from extreme plant ecosystems. J. Exp. Bot. 2020, 71, 3865–3877. [Google Scholar] [CrossRef]
- Hernández, I.; Taulé, C.; Pérez-Pérez, R.; Battistoni, F.; Fabiano, E.; Villanueva-Guerrero, A.; Nápoles, M.C.; Herrera, H. Endophytic Seed-Associated Bacteria as Plant Growth Promoters of Cuban Rice (Oryza sativa L.). Microorganisms 2023, 11, 2317. [Google Scholar] [CrossRef]
- Ganesh, J.; Singh, V.; Hewitt, K.; Kaundal, A. Exploration of the rhizosphere microbiome of native plant Ceanothus velutinus–an excellent resource of plant growth-promoting bacteria. Front. Plant Sci. 2022, 13, 979069. [Google Scholar] [CrossRef]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- González-Benítez, N.; Martín-Rodríguez, I.; Cuesta, I.; Arrayás, M.; White, J.F.; Molina, M.C. Endophytic microbes are tools to increase tolerance in Jasione plants against arsenic stress. Front. Microbiol. 2021, 12, 664271. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, K.; Madhaiyan, M.; Kim, Y.K.; Kang, Y.; Sa, T. Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of Rice. BMC Microbiol. 2017, 17, 209. [Google Scholar] [CrossRef]
- Pérez, L.I.; Gundel, P.E.; Zabalgogeazcoa, I.; Omacini, M. An ecological framework for understanding the roles of Epichloë endophytes on plant defenses against fungal diseases. Fungal Biol. Rev. 2020, 34, 115–125. [Google Scholar] [CrossRef]
- Bright, M.; Bulgheresi, S. A complex journey: Transmission of microbial symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, B.; Vandamme, P.; Merckx, V.; Smets, E.; Dessein, S. Bacterial leaf symbiosis in angiosperms: Host specificity without co-speciation. PLoS ONE 2011, 6, e24430. [Google Scholar] [CrossRef]
- Lemaire, B.; Janssens, S.; Smets, E.; Dessein, S. Endosymbiont transmission mode in bacterial leaf nodulation as revealed by a population genetic study of Psychotria leptophylla. Appl. Environ. Microbiol. 2012, 78, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.C.; Saldierna Guzmán, J.P.; Shay, J.E. Transmission of bacterial endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- Qin, Y.; Pan, X.; Yuan, Z. Seed endophytic microbiota in a coastal plant and phytobeneficial properties of the fungus Cladosporium cladosporioides. Fungal Ecol. 2016, 24, 53–60. [Google Scholar] [CrossRef]
- Sharma, P.; Aswini, K.; Sai Prasad, J.; Kumar, N.; Pathak, D.; Gond, S.; Venkadasamy, G.; Suman, A. Characterization of actinobacteria from wheat seeds for plant growth promoting traits and protection against fungal pathogens. J. Basic Microbiol. 2023, 63, 439–453. [Google Scholar] [CrossRef]
- Wassermann, B.; Cernava, T.; Müller, H.; Berg, C.; Berg, G. Seeds of native alpine plants host unique microbial communities embedded in cross-kingdom networks. Microbiome 2019, 7, 108. [Google Scholar] [CrossRef]
- Hernández, I.; Taulé, C.; Pérez-Pérez, R.; Battistoni, F.; Fabiano, E.; Rivero, D.; Nápoles, M.C. Endophytic rhizobia promote the growth of Cuban rice cultivar. Symbiosis 2021, 85, 175–190. [Google Scholar] [CrossRef]
- Kumar, K.; Pal, G.; Verma, A.; Kumar, D.; Shukla, P.; Verma, S.K. Seed vectored bacterial endophyte Bacillus pumilus protect sorghum (Sorghum bicolor L.) seedlings from a fungal pathogen Rhizoctonia solani. Biol. Control 2023, 183, 105249. [Google Scholar] [CrossRef]
- Glassner, H.; Zchori-Fein, E.; Yaron, S.; Sessitsch, A.; Sauer, U.; Compant, S. Bacterial niches inside seeds of Cucumis melo L. Plant Soil 2018, 422, 101–113. [Google Scholar] [CrossRef]
- Ai, J.; Yu, T.; Liu, X.; Jiang, Y.; Wang, E.; Deng, Z.-S. Seed associated microbiota and vertical transmission of bacterial communities from seed to nodule in Sophora davidii. Plant Soil 2023, 1–18. [Google Scholar] [CrossRef]
- Bodhankar, S.; Grover, M.; Reddy, G. In planta screening of maize seed endophytic bacteria for potential applications under dryland conditions. Indian J. Dryland Agric. Res. Dev. 2019, 34, 53–62. [Google Scholar] [CrossRef]
- Nelson, E.B. The seed microbiome: Origins, interactions, and impacts. Plant Soil 2018, 422, 7–34. [Google Scholar] [CrossRef]
- Yang, H.; Yang, Z.; Wang, Q.-C.; Wang, Y.-L.; Hu, H.-W.; He, J.-Z.; Zheng, Y.; Yang, Y. Compartment and plant identity shape tree mycobiome in a subtropical forest. Microbiol. Spectr. 2022, 10, e01347-22. [Google Scholar] [CrossRef] [PubMed]
- Ramakuwela, T.; Hatting, J.; Bock, C.; Vega, F.E.; Wells, L.; Mbata, G.N.; Shapiro-Ilan, D. Establishment of Beauveria bassiana as a fungal endophyte in pecan (Carya illinoinensis) seedlings and its virulence against pecan insect pests. Biol. Control 2020, 140, 104102. [Google Scholar] [CrossRef]
- Vitorino, L.C.; de Souza Rocha, A.F.; Bessa, L.A.; Lourenço, L.L.; da Costa, A.C.; Silva, F.G. Symbiotic microorganisms affect the resilience of Hymenaea courbaril L., a neotropical fruit tree, to water restriction. Plant Stress 2022, 5, 100092. [Google Scholar] [CrossRef]
- Faddetta, T.; Abbate, L.; Alibrandi, P.; Arancio, W.; Siino, D.; Strati, F.; De Filippo, C.; Fatta Del Bosco, S.; Carimi, F.; Puglia, A.M. The endophytic microbiota of Citrus limon is transmitted from seed to shoot highlighting differences of bacterial and fungal community structures. Sci. Rep. 2021, 11, 7078. [Google Scholar] [CrossRef]
- Alibrandi, P.; Cardinale, M.; Rahman, M.M.; Strati, F.; Ciná, P.; de Viana, M.L.; Giamminola, E.M.; Gallo, G.; Schnell, S.; De Filippo, C. The seed endosphere of Anadenanthera colubrina is inhabited by a complex microbiota, including Methylobacterium spp. and Staphylococcus spp. with potential plant-growth promoting activities. Plant Soil 2018, 422, 81–99. [Google Scholar] [CrossRef]
- Johnson, D.; Liu, X.; Burslem, D.F. Symbiotic control of canopy dominance in subtropical and tropical forests. Trends Plant Sci. 2023, 28, 995–1003. [Google Scholar] [CrossRef]
- Herrera, H.; Sanhueza, T.; da Silva Valadares, R.B.; Matus, F.; Pereira, G.; Atala, C.; Mora, M.d.l.L.; Arriagada, C. Diversity of Root-Associated Fungi of the Terrestrial Orchids Gavilea lutea and Chloraea collicensis in a Temperate Forest Soil of South-Central Chile. J. Fungi 2022, 8, 794. [Google Scholar] [CrossRef]
- Oliveira, H.; Pereira, S.; Santos, M.G. Cenostigma microphyllum seedlings in semiarid region grow faster under arbuscular mycorrhizal symbiosis, regardless of water availability. J. Arid Environ. 2023, 212, 104962. [Google Scholar] [CrossRef]
- Costa, P.H.d.O.; Nascimento, S.V.d.; Herrera, H.; Gastauer, M.; Ramos, S.J.; Caldeira, C.F.; Oliveira, G.; Valadares, R.B.d.S. Non-specific interactions of rhizospheric microbial communities support the establishment of Mimosa acutistipula var. ferrea in an Amazon rehabilitating mineland. Processes 2021, 9, 2079. [Google Scholar] [CrossRef]
- Hanif, M.; Ashraf, Z.; Bashir, S.; Riaz, F.; Amanat, R.; Yousaf, N.; Sarwar, S. Ectomycorrhizal Fungi as Biofertilizers in Forestry. In Arbuscular Mycorrhizal Fungi in Agriculture-New Insights; IntechOpen: London, UK, 2023. [Google Scholar]
- De Jesús-Velázquez, J.; Cisneros-Villaseñor, A.; Tamayo-Bustamante, R.A.; Girón-Gutiérrez, D.; Luna-Soria, H.; Cambrón-Sandoval, V.H. Effect of pre-germinative treatments on eight priority native species for reforestation in the tropical deciduous forest. Conservation 2023, 3, 277–290. [Google Scholar] [CrossRef]
- Andivia, E.; Villar-Salvador, P.; Oliet, J.A.; Puértolas, J.; Dumroese, R.K.; Ivetić, V.; Molina-Venegas, R.; Arellano, E.C.; Li, G.; Ovalle, J.F. Climate and species stress resistance modulate the higher survival of large seedlings in forest restorations worldwide. Ecol. Appl. 2021, 31, e02394. [Google Scholar] [CrossRef] [PubMed]
- Prematuri, R.; Turjaman, M.; Tawaraya, K. Effect of arbuscular mycorrhiza fungal inoculation on growth of tropical tree species under nursery and post-opencast bauxite mining field in Bintan Island, Indonesia. Int. J. Plant Soil Sci. 2020, 32, 1–13. [Google Scholar] [CrossRef]
- Panchami, P.S.; Geetha Thanuja, K.; Karthikeyan, S. Isolation and characterization of indigenous plant growth-promoting rhizobacteria (PGPR) from cardamom rhizosphere. Curr. Microbiol. 2020, 77, 2963–2981. [Google Scholar] [CrossRef]
- Araújo, V.C.; Rossati, K.F.; Xavier, L.V.; de Oliveira, V.A.; dos Santos Carmo, G.J.; de Assis, G.A.; de Oliveira Mendes, G. Enhanced growth in nursery of coffee seedlings inoculated with the rhizosphere fungus Aspergillus niger for field transplantation. Rhizosphere 2020, 15, 100236. [Google Scholar] [CrossRef]
- Liese, R.; Lübbe, T.; Albers, N.W.; Meier, I.C. The mycorrhizal type governs root exudation and nitrogen uptake of temperate tree species. Tree Physiol. 2018, 38, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Bizos, G.; Papatheodorou, E.M.; Chatzistathis, T.; Ntalli, N.; Aschonitis, V.G.; Monokrousos, N. The role of microbial inoculants on plant protection, growth stimulation, and crop productivity of the olive tree (Olea europea L.). Plants 2020, 9, 743. [Google Scholar] [CrossRef]
- Ortiz, J.; Soto, J.; Fuentes, A.; Herrera, H.; Meneses, C.; Arriagada, C. The endophytic fungus Chaetomium cupreum regulates expression of genes involved in the tolerance to metals and plant growth promotion in Eucalyptus globulus roots. Microorganisms 2019, 7, 490. [Google Scholar] [CrossRef]
- Compant, S.; Van Der Heijden, M.G.; Sessitsch, A. Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Srivastava, A.K.; Tiwari, R.K. Plant growth promoting and antifungal activity in endophytic Bacillus strains from pearl millet (Pennisetum glaucum). Braz. J. Microbiol. 2020, 51, 229–241. [Google Scholar] [CrossRef]
- Nakkeeran, S.; Vinodkumar, S.; Renukadevi, P.; Rajamanickam, S.; Jogaiah, S. Bioactive molecules from Bacillus spp.: An effective tool for plant stress management. Bioact. Mol. Plant Def. Signal. Growth Stress 2019, 1–23. [Google Scholar] [CrossRef]
- Tao, J.; Dong, F.; Wang, Y.; Chen, H.; Tang, M. Arbuscular mycorrhizal fungi enhance photosynthesis and drought tolerance by regulating MAPK gene expressions of Populus simonii× P. nigra. Physiol. Plant. 2022, 174, e13829. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, G.; Panneerselvam, P.; Ganeshamurthy, A.N. Bacterial mediated alleviation of abiotic stress in crops. In Bacteria in Agrobiology: Stress Management; Springer: Berlin/Heidelberg, Germany, 2011; pp. 205–224. [Google Scholar]
- Lawlor, D.W. Limitation to photosynthesis in water-stressed leaves: Stomata vs. metabolism and the role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Shaffique, S.; Khan, M.A.; Imran, M.; Kang, S.-M.; Park, Y.-S.; Wani, S.H.; Lee, I.-J. Research progress in the field of microbial mitigation of drought stress in plants. Front. Plant Sci. 2022, 13, 870626. [Google Scholar] [CrossRef]
- Bittencourt, P.P.; Alves, A.F.; Ferreira, M.B.; da Silva Irineu, L.E.S.; Pinto, V.B.; Olivares, F.L. Mechanisms and Applications of Bacterial Inoculants in Plant Drought Stress Tolerance. Microorganisms 2023, 11, 502. [Google Scholar] [CrossRef]
- Morcillo, R.J.; Vílchez, J.I.; Zhang, S.; Kaushal, R.; He, D.; Zi, H.; Liu, R.; Niehaus, K.; Handa, A.K.; Zhang, H. Plant transcriptome reprograming and bacterial extracellular metabolites underlying tomato drought resistance triggered by a beneficial soil bacteria. Metabolites 2021, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Quiroz, A.; Herrera, H.; Ortiz, J.; Arriagada, C.; Jorquera-Fontena, E. Rhizosphere-inhabiting fungi isolated from native plants of the atacama desert affect leaf traits of ‘chardonnay’grapevines (Vitis vinifera L.). Rhizosphere 2023, 27, 100715. [Google Scholar] [CrossRef]
- Aslam, A.; Ahmad Zahir, Z.; Asghar, H.N.; Shahid, M. Effect of carbonic anhydrase-containing endophytic bacteria on growth and physiological attributes of wheat under water-deficit conditions. Plant Prod. Sci. 2018, 21, 244–255. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S. Drought-tolerant Bacillus megaterium isolated from semi-arid conditions induces systemic tolerance of wheat under drought conditions. Plant Cell Rep. 2021, 41, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil 2020, 452, 423–440. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Hao, Y.; Wu, X.-L.; Dai, F.-J.; Abd-Allah, E.F.; Wu, Q.-S.; Liu, S.-R. Arbuscular mycorrhizal fungi improve drought tolerance of tea plants via modulating root architecture and hormones. Plant Growth Regul. 2023, 1–10. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Zhai, Y.; Jia, J.; Zhao, Q.; Wang, W.; Hu, X. Microbial diversity and functions in saline soils: A review from a biogeochemical perspective. J. Adv. Res. 2023. [Google Scholar] [CrossRef]
- Kiani-Pouya, A.; Rasouli, F.; Rabbi, B.; Falakboland, Z.; Yong, M.; Chen, Z.-H.; Zhou, M.; Shabala, S. Stomatal traits as a determinant of superior salinity tolerance in wild barley. J. Plant Physiol. 2020, 245, 153108. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Yin, M.; Ma, X.; Yu, X.; Guo, X.; Du, N.; Eller, F.; Guo, W. Soil salinity, not plant genotype or geographical distance, shapes soil microbial community of a reed wetland at a fine scale in the Yellow River Delta. Sci. Total Environ. 2023, 856, 159136. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, S.; Yoneyama, T. Effects of pH and osmotic stress on cellular polyamine contents in the soybean rhizobia Rhizobium fredii P220 and Bradyrhizobium japonicum A1017. Appl. Environ. Microbiol. 1993, 59, 1104–1109. [Google Scholar] [CrossRef]
- Tewari, S.; Sharma, S. Rhizobial exopolysaccharides as supplement for enhancing nodulation and growth attributes of Cajanus cajan under multi-stress conditions: A study from lab to field. Soil Tillage Res. 2020, 198, 104545. [Google Scholar] [CrossRef]
- Li, H.; La, S.; Zhang, X.; Gao, L.; Tian, Y. Salt-induced recruitment of specific root-associated bacterial consortium capable of enhancing plant adaptability to salt stress. ISME J. 2021, 15, 2865–2882. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Laouane, R.B.; Anli, M.; Boutasknit, A.; Wahbi, S.; Meddich, A. Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci. Hortic. 2019, 253, 429–438. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Z.; Ren, Q.; Zhu, L.; Lin, J.; Zhang, J.; Cheng, X.; Ma, J.; Yue, J. Effects of arbuscular mycorrhizal fungi on growth, photosynthesis, and nutrient uptake of Zelkova serrata (Thunb.) Makino seedlings under salt stress. Forests 2019, 10, 186. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Querejeta, J.I.; Ren, W.; Prieto, I. Vertical decoupling of soil nutrients and water under climate warming reduces plant cumulative nutrient uptake, water-use efficiency and productivity. New Phytol. 2021, 230, 1378–1393. [Google Scholar] [CrossRef]
- Xiang, J.; Chen, X.; Hu, W.; Xiang, Y.; Yan, M.; Wang, J. Overexpressing heat-shock protein OsHSP50. 2 improves drought tolerance in rice. Plant Cell Rep. 2018, 37, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Yuan, L.; Zhu, S.; Liu, S.; Ge, J.; Wang, C. Response of osmotic adjustment and ascorbate-glutathione cycle to heat stress in a heat-sensitive and a heat-tolerant genotype of wucai (Brassica campestris L.). Sci. Hortic. 2016, 211, 87–94. [Google Scholar] [CrossRef]
- Malik, S.S.; Sudalaimuthuasari, N.; Kundu, B.; AlMaskari, R.S.; Mundra, S. Contrasting genome patterns of two Pseudomonas strains isolated from the date palm rhizosphere to assess survival in a hot arid environment. World J. Microbiol. Biotechnol. 2022, 38, 207. [Google Scholar] [CrossRef]
- Mota, T.M.; Oshiquiri, L.H.; Lopes, É.C.V.; Barbosa Filho, J.R.; Ulhoa, C.J.; Georg, R.C. Hsp genes are differentially expressed during Trichoderma asperellum self-recognition, mycoparasitism and thermal stress. Microbiol. Res. 2019, 227, 126296. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Iqbal, M.A.; Li, C.; Iqbal, A.; Abbas, R.N. Overviewing Drought and Heat Stress Amelioration—From Plant Responses to Microbe-Mediated Mitigation. Sustainability 2023, 15, 1671. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; Asaf, S.; Lee, K.-E.; Park, Y.-G.; Kim, A.-Y.; Khan, M.A.; You, Y.-H.; Lee, I.-J. Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 2019, 14, 416–423. [Google Scholar] [CrossRef]
- Andrade-Linares, D.R.; Lehmann, A.; Rillig, M.C. Microbial stress priming: A meta-analysis. Environ. Microbiol. 2016, 18, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.; Ali, F.; Rafique, M.; Ali, J.; Afridi, M.S.; Smith, D.; Mehmood, S.; Amna; Souleimanov, A.; Jellani, G. Biochemical Characterization and Potential of Bacillus safensis Strain SCAL1 to Mitigate Heat Stress in Solanum lycopersicum L. J. Plant Growth Regul. 2023, 42, 523–538. [Google Scholar] [CrossRef]
- Tripathi, R.; Keswani, C.; Tewari, R. Trichoderma koningii enhances tolerance against thermal stress by regulating ROS metabolism in tomato (Solanum lycopersicum L.) plants. J. Plant Interact. 2021, 16, 116–125. [Google Scholar] [CrossRef]
- Tian, X.; Liu, X.-Q.; Liu, X.-R.; Li, Q.-S.; Abd_Allah, E.F.; Wu, Q.-S. Mycorrhizal cucumber with Diversispora versiformis has active heat stress tolerance by up-regulating expression of both CsHsp70s and CsPIPs genes. Sci. Hortic. 2023, 319, 112194. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.-J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Ind. Crops Prod. 2020, 143, 111931. [Google Scholar] [CrossRef]
- Szczerba, A.; Płażek, A.; Pastuszak, J.; Kopeć, P.; Hornyák, M.; Dubert, F. Effect of low temperature on germination, growth, and seed yield of four soybean (Glycine max L.) cultivars. Agronomy 2021, 11, 800. [Google Scholar] [CrossRef]
- Manasa, S.L.; Panigrahy, M.; Panigrahi, K.C.; Rout, G.R. Overview of cold stress regulation in plants. Bot. Rev. 2022, 88, 359–387. [Google Scholar] [CrossRef]
- Enders, T.A.; St. Dennis, S.; Oakland, J.; Callen, S.T.; Gehan, M.A.; Miller, N.D.; Spalding, E.P.; Springer, N.M.; Hirsch, C.D. Classifying cold-stress responses of inbred maize seedlings using RGB imaging. Plant Direct 2019, 3, e00104. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, J.; Zhao, X.; Zhang, Y.; Ren, J.; Xing, L.; Jiang, C.; Wang, X.; Wang, J.; Zhao, S. Research progress in membrane lipid metabolism and molecular mechanism in peanut cold tolerance. Front. Plant Sci. 2019, 10, 838. [Google Scholar] [CrossRef]
- Golizadeh, F.; Kumleh, H.H. Physiological responses and expression changes of fatty acid metabolism–related genes in Wheat (Triticum aestivum) under cold stress. Plant Mol. Biol. Report. 2019, 37, 224–236. [Google Scholar] [CrossRef]
- Acuña-Rodríguez, I.S.; Newsham, K.K.; Gundel, P.E.; Torres-Díaz, C.; Molina-Montenegro, M.A. Functional roles of microbial symbionts in plant cold tolerance. Ecol. Lett. 2020, 23, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y.; Mohamed, H.I. Inoculation with Lysinibacillus fusiformis strain YJ4 and Lysinibacillus sphaericus strain YJ5 alleviates the effects of cold stress in maize plants. Gesunde Pflanz. 2023, 75, 77–95. [Google Scholar] [CrossRef]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Bacillus velezensis 5113 induced metabolic and molecular reprogramming during abiotic stress tolerance in wheat. Sci. Rep. 2019, 9, 16282. [Google Scholar] [CrossRef]
- Tapia-Vázquez, I.; Sánchez-Cruz, R.; Arroyo-Domínguez, M.; Lira-Ruan, V.; Sánchez-Reyes, A.; del Rayo Sánchez-Carbente, M.; Padilla-Chacón, D.; Batista-García, R.A.; Folch-Mallol, J.L. Isolation and characterization of psychrophilic and psychrotolerant plant-growth promoting microorganisms from a high-altitude volcano crater in Mexico. Microbiol. Res. 2020, 232, 126394. [Google Scholar] [CrossRef]
- Wu, H.; Gu, Q.; Xie, Y.; Lou, Z.; Xue, P.; Fang, L.; Yu, C.; Jia, D.; Huang, G.; Zhu, B. Cold-adapted Bacilli isolated from the Qinghai–Tibetan Plateau are able to promote plant growth in extreme environments. Environ. Microbiol. 2019, 21, 3505–3526. [Google Scholar] [CrossRef]
- Vega-Celedón, P.; Bravo, G.; Velásquez, A.; Cid, F.P.; Valenzuela, M.; Ramírez, I.; Vasconez, I.-N.; Alvarez, I.; Jorquera, M.A.; Seeger, M. Microbial diversity of psychrotolerant bacteria isolated from wild flora of Andes mountains and Patagonia of Chile towards the selection of plant growth-promoting bacterial consortia to alleviate cold stress in plants. Microorganisms 2021, 9, 538. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Gao, Y.-L.; Wang, Y.-P.; Guo, J.-H. A consortium of three plant growth-promoting rhizobacterium strains acclimates Lycopersicon esculentum and confers a better tolerance to chilling stress. J. Plant Growth Regul. 2016, 35, 54–64. [Google Scholar] [CrossRef]
- Zubair, M.; Hanif, A.; Farzand, A.; Sheikh, T.M.M.; Khan, A.R.; Suleman, M.; Ayaz, M.; Gao, X. Genetic screening and expression analysis of psychrophilic Bacillus spp. reveal their potential to alleviate cold stress and modulate phytohormones in wheat. Microorganisms 2019, 7, 337. [Google Scholar] [CrossRef]
- Pasbani, B.; Salimi, A.; Aliasgharzad, N.; Hajiboland, R. Colonization with arbuscular mycorrhizal fungi mitigates cold stress through improvement of antioxidant defense and accumulation of protecting molecules in eggplants. Sci. Hortic. 2020, 272, 109575. [Google Scholar] [CrossRef]
- Acuña-Rodríguez, I.S.; Ballesteros, G.I.; Atala, C.; Gundel, P.E.; Molina-Montenegro, M.A. Hardening blueberry plants to face drought and cold events by the application of fungal endophytes. Agronomy 2022, 12, 1000. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Lal, S.K.; Lakra, N.; Mehta, S.; Shabek, N.; Narayan, O.P. Plant mineral transport systems and the potential for crop improvement. Planta 2021, 253, 45. [Google Scholar] [CrossRef]
- Ercoli, L.; Schüßler, A.; Arduini, I.; Pellegrino, E. Strong increase of durum wheat iron and zinc content by field-inoculation with arbuscular mycorrhizal fungi at different soil nitrogen availabilities. Plant Soil 2017, 419, 153–167. [Google Scholar] [CrossRef]
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 2016, 58, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Makar, O.; Kuźniar, A.; Patsula, O.; Kavulych, Y.; Kozlovskyy, V.; Wolińska, A.; Skórzyńska-Polit, E.; Vatamaniuk, O.; Terek, O.; Romanyuk, N. Bacterial endophytes of spring wheat grains and the potential to acquire Fe, Cu, and Zn under their low soil bioavailability. Biology 2021, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.K.; Islam, M.M.; Hore, S.; Mandal, S. Rice seed endophytes transmit into the plant seedling, promote plant growth and inhibit fungal phytopathogens. Plant Growth Regul. 2023, 99, 373–388. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Marriel, I.E.; Sousa, S.M.d.; Lana, U.G.d.P.; Mattos, B.B.; Oliveira, C.A.d.; Gomes, E.A. Endophytic Bacillus strains enhance pearl millet growth and nutrient uptake under low-P. Braz. J. Microbiol. 2018, 49, 40–46. [Google Scholar] [CrossRef]
- Chaudhary, R.; Kumar, V.; Gupta, S.; Naik, B.; Prasad, R.; Mishra, S.; Saris, P.E.J.; Kumar, V. Finger Millet (Eleusine coracana) Plant–Endophyte Dynamics: Plant Growth, Nutrient Uptake, and Zinc Biofortification. Microorganisms 2023, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.R.; Doohan, F.M.; Hodkinson, T.R. Fungal root endophytes of a wild barley species increase yield in a nutrient-stressed barley cultivar. Symbiosis 2015, 65, 1–7. [Google Scholar] [CrossRef]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef]
- Chmielowska-Bąk, J.; Deckert, J. Plant recovery after metal stress—A review. Plants 2021, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Ortiz, J.; Herrera, H.; Fuentes, A.; Almonacid, L.; Charles, T.C.; Arriagada, C. Enhanced arsenic tolerance in Triticum aestivum inoculated with arsenic-resistant and plant growth promoter microorganisms from a heavy metal-polluted soil. Microorganisms 2019, 7, 348. [Google Scholar] [CrossRef] [PubMed]
- Husna; Hussain, A.; Shah, M.; Hamayun, M.; Qadir, M.; Iqbal, A. Heavy metal tolerant endophytic fungi Aspergillus welwitschiae improves growth, ceasing metal uptake and strengthening antioxidant system in Glycine max L. Environ. Sci. Pollut. Res. 2021, 29, 15501–15515. [Google Scholar] [CrossRef]
- Khullar, S.; Reddy, M.S. Arsenic toxicity and its mitigation in ectomycorrhizal fungus Hebeloma cylindrosporum through glutathione biosynthesis. Chemosphere 2020, 240, 124914. [Google Scholar] [CrossRef]
- Saba; Rehman, Y.; Ahmed, M.; Sabri, A.N. Potential role of bacterial extracellular polymeric substances as biosorbent material for arsenic bioremediation. Bioremediation J. 2019, 23, 72–81. [Google Scholar] [CrossRef]
- Durand, A.; Leglize, P.; Lopez, S.; Sterckeman, T.; Benizri, E. Noccaea caerulescens seed endosphere: A habitat for an endophytic bacterial community preserved through generations and protected from soil influence. Plant Soil 2022, 472, 257–278. [Google Scholar] [CrossRef]
- Adeyemi, N.O.; Atayese, M.O.; Sakariyawo, O.S.; Azeez, J.O.; Sobowale, S.P.A.; Olubode, A.; Mudathir, R.; Adebayo, R.; Adeoye, S. Alleviation of heavy metal stress by arbuscular mycorrhizal symbiosis in Glycine max (L.) grown in copper, lead and zinc contaminated soils. Rhizosphere 2021, 18, 100325. [Google Scholar] [CrossRef]
- Hachani, C.; Lamhamedi, M.S.; Zine El Abidine, A.; Abassi, M.; Khasa, D.P.; Béjaoui, Z. Water relations, gas exchange, chlorophyll fluorescence and electrolyte leakage of ectomycorrhizal Pinus halepensis seedlings in response to multi-heavy metal stresses (Pb, Zn, Cd). Microorganisms 2021, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, S.-H.; Khaine, I.; Kwak, M.; Lee, H.; Lee, T.; Lee, W.; Woo, S. Physiological changes and growth promotion induced in poplar seedlings by the plant growth-promoting rhizobacteria Bacillus subtilis JS. Photosynthetica 2018, 56, 1188–1203. [Google Scholar] [CrossRef]
- Garcia-Lemos, A.M.; Grosskinsky, D.K.; Saleem Akhtar, S.; Nicolaisen, M.H.; Roitsch, T.; Nybroe, O.; Veierskov, B. Identification of root-associated bacteria that influence plant physiology, increase seed germination, or promote growth of the christmas tree species Abies nordmanniana. Front. Microbiol. 2020, 11, 566613. [Google Scholar] [CrossRef]
- Chitra, P.; Jijeesh, C. Biopriming of seeds with plant growth promoting bacteria Pseudomonas fluorescens for better germination and seedling vigour of the East Indian sandalwood. New For. 2021, 52, 829–841. [Google Scholar] [CrossRef]
- Radhapriya, P.; Ramachandran, A.; Anandham, R.; Mahalingam, S. Pseudomonas aeruginosa RRALC3 enhances the biomass, nutrient and carbon contents of Pongamia pinnata seedlings in degraded forest soil. PLoS ONE 2015, 10, e0139881. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Cardoso, E.J.B.N. Isolation, selection and characterization of root-associated growth promoting bacteria in Brazil Pine (Araucaria angustifolia). Microbiol. Res. 2012, 167, 69–78. [Google Scholar] [CrossRef]
- Vannucchi, F.; Imperato, V.; Saran, A.; Staykov, S.; D’Haen, J.; Sebastiani, L.; Vangronsveld, J.; Thijs, S. Inoculated seed endophytes modify the poplar responses to trace elements in polluted soil. Agronomy 2021, 11, 1987. [Google Scholar] [CrossRef]
- Aalipour, H.; Nikbakht, A.; Etemadi, N.; Soleimani, M.; Rejali, F. Evaluating the effect of cadmium on the decline of Arizona cypress seedlings and the enhancement role of mycorrhizal fungus and plant growth promoting rhizobacteria. JWSS-Isfahan Univ. Technol. 2019, 23, 417–431. [Google Scholar] [CrossRef]
- Huang, L.; Chen, D.; Zhang, H.; Song, Y.; Chen, H.; Tang, M. Funneliformis mosseae enhances root development and Pb phytostabilization in Robinia pseudoacacia in Pb-contaminated soil. Front. Microbiol. 2019, 10, 2591. [Google Scholar] [CrossRef]
- Zarik, L.; Meddich, A.; Hijri, M.; Hafidi, M.; Ouhammou, A.; Ouahmane, L.; Duponnois, R.; Boumezzough, A. Use of arbuscular mycorrhizal fungi to improve the drought tolerance of Cupressus atlantica G. Comptes Rendus Biol. 2016, 339, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Aalipour, H.; Nikbakht, A.; Etemadi, N.; Rejali, F.; Soleimani, M. Biochemical response and interactions between arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria during establishment and stimulating growth of Arizona cypress (Cupressus arizonica G.) under drought stress. Sci. Hortic. 2020, 261, 108923. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Rezaei, K.; Fayyaz, P.; Naghiha, R.; Namvar, Z. The effect of indigenous phosphate-solubilizing bacteria on Quercus brantii seedlings under water stress. J. Sustain. For. 2021, 40, 733–747. [Google Scholar] [CrossRef]
- Azizi, S.; Kouchaksaraei, M.T.; Hadian, J.; Abad, A.R.F.N.; Sanavi, S.A.M.M.; Ammer, C.; Bader, M.K.-F. Dual inoculations of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria boost drought resistance and essential oil yield of common myrtle. For. Ecol. Manag. 2021, 497, 119478. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.; Alqarawi, A.; Al-Huqail, A.; Shah, M. Induction of osmoregulation and modulation of salt stress in Acacia gerrardii Benth. by arbuscular mycorrhizal fungi and Bacillus subtilis (BERA 71). BioMed Res. Int. 2016, 2016, 6294098. [Google Scholar] [CrossRef] [PubMed]
- Besoain, X.; Guajardo, J.; Camps, R. First report of Diplodia mutila causing gummy canker in Araucaria araucana in Chile. Plant Dis. 2017, 101, 1328. [Google Scholar] [CrossRef]
- Balocchi, F.; Wingfield, M.J.; Ahumada, R.; Barnes, I. Pewenomyces kutranfy gen. nov. et sp. nov. causal agent of an important canker disease on Araucaria araucana in Chile. Plant Pathol. 2021, 70, 1243–1259. [Google Scholar] [CrossRef]
- Herrera, H.; Novotná, A.; Ortiz, J.; Soto, J.; Arriagada, C. Isolation and identification of plant growth-promoting bacteria from rhizomes of Arachnitis uniflora, a fully mycoheterotrophic plant in southern Chile. Appl. Soil Ecol. 2020, 149, 103512. [Google Scholar] [CrossRef]
- Sanhueza, T.; Herrera, H.; Arriagada, C. Contribution of Leaf-Associated Microorganisms from Native Andean Ericaceae against Botrytis cinerea in Vaccinium corymbosum Cultivars. J. Soil Sci. Plant Nutr. 2023, 23, 2637–2650. [Google Scholar] [CrossRef]
- Haghighatpanah, N.; Mirzaee, H.; Khodaiyan, F.; Kennedy, J.F.; Aghakhani, A.; Hosseini, S.S.; Jahanbin, K. Optimization and characterization of pullulan produced by a newly identified strain of Aureobasidium pullulans. Int. J. Biol. Macromol. 2020, 152, 305–313. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, T.-J.; Chi, Z.; Hu, Z.; Liu, G.-L.; Sun, Y.; Zhang, S.-H.; Chi, Z.-M. Macromolecular pullulan produced by Aureobasidium melanogenum 13-2 isolated from the Taklimakan desert and its crucial roles in resistance to the stress treatments. Int. J. Biol. Macromol. 2019, 135, 429–436. [Google Scholar] [CrossRef]
- Cignola, R.; Boato, A.; Sadallah, A.; Firrao, G.; Di Francesco, A. Molecular characterization of Aureobasidium spp. strains isolated during the cold season. A preliminary efficacy evaluation as novel potential biocontrol agents against postharvest pathogens. Eur. J. Plant Pathol. 2023, 167, 221–233. [Google Scholar] [CrossRef]
- Won, S.-J.; Kwon, J.-H.; Kim, D.-H.; Ahn, Y.-S. The effect of Bacillus licheniformis MH48 on control of foliar fungal diseases and growth promotion of Camellia oleifera seedlings in the coastal reclaimed land of Korea. Pathogens 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Choub, V.; Ajuna, H.B.; Won, S.-J.; Moon, J.-H.; Choi, S.-I.; Maung, C.E.H.; Kim, C.-W.; Ahn, Y.S. Antifungal activity of Bacillus velezensis CE 100 against anthracnose disease (Colletotrichum gloeosporioides) and growth promotion of walnut (Juglans regia L.) trees. Int. J. Mol. Sci. 2021, 22, 10438. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.S.A.; de Oliveira Marques, V.; Selari, P.J.R.G.; Martins, P.F.; Silva, F.G.; de Fátima Sales, J. Multifunctional potential of endophytic bacteria from Anacardium othonianum Rizzini in promoting in vitro and ex vitro plant growth. Microbiol. Res. 2021, 242, 126600. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, H.; Zhu, L.; Yuan, Y.; Xu, L.; Wang, G.G.; Zhai, L.; Yang, L.; Zhang, J. Arbuscular mycorrhizal fungi effectively enhances the growth of Gleditsia sinensis Lam. seedlings under greenhouse conditions. Forests 2019, 10, 567. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, X.; Wen, X. Identification and characterization of the rhizosphere phosphate-solubilizing bacterium Pseudomonas frederiksbergensis JW-SD2, and its plant growth-promoting effects on poplar seedlings. Ann. Microbiol. 2016, 66, 1343–1354. [Google Scholar] [CrossRef]
- Griebeler, A.M.; Araujo, M.M.; Tabaldi, L.A.; Steffen, G.P.; Turchetto, F.; Rorato, D.G.; Barbosa, F.M.; Berghetti, Á.L.; Nhantumbo, L.S.; Lima, M.S. Type of container and Trichoderma spp. inoculation enhance the performance of tree species in enrichment planting. Ecol. Eng. 2021, 169, 106317. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Can naturally-occurring endophytic nitrogen-fixing bacteria of hybrid white spruce sustain boreal forest tree growth on extremely nutrient-poor soils? Soil Biol. Biochem. 2020, 140, 107642. [Google Scholar] [CrossRef]
- Cely, M.V.; Siviero, M.A.; Emiliano, J.; Spago, F.R.; Freitas, V.F.; Barazetti, A.R.; Goya, E.T.; Lamberti, G.d.S.; Dos Santos, I.M.; De Oliveira, A.G. Inoculation of Schizolobium parahyba with mycorrhizal fungi and plant growth-promoting rhizobacteria increases wood yield under field conditions. Front. Plant Sci. 2016, 7, 1708. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Evaluating lodgepole pine endophytes for their ability to fix nitrogen and support tree growth under nitrogen-limited conditions. Plant Soil 2020, 455, 271–287. [Google Scholar] [CrossRef]
- Brito, V.N.; Tellechea, F.R.F.; Heitor, L.C.; Freitas, M.S.M.; Martins, M.A. arbuscular mycorrhizal fungi and phosphate fertilization on the seedling production of paricá. Ciência Florestal 2017, 27, 485–497. [Google Scholar] [CrossRef]
- Zhu, H.; Bing, H.; Wu, Y.; Sun, H.; Zhou, J. Low molecular weight organic acids regulate soil phosphorus availability in the soils of subalpine forests, eastern Tibetan Plateau. Catena 2021, 203, 105328. [Google Scholar] [CrossRef]
- Simard, S.; Asay, A.; Beiler, K.; Bingham, M.; Deslippe, J.; He, X.; Philip, L.; Song, Y.; Teste, F. Resource transfer between plants through ectomycorrhizal fungal networks. Mycorrhizal Netw. 2015, 224, 133–176. [Google Scholar] [CrossRef]
- Domínguez-Núñez, J.A.; Berrocal-Lobo, M.; Albanesi, A.S. Ectomycorrhizal fungi: Role as biofertilizers in forestry. Biofertil. Sustain. Agric. Environ. 2019, 55, 67–82. [Google Scholar] [CrossRef]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef]
- Uroz, S.; Buee, M.; Deveau, A.; Mieszkin, S.; Martin, F. Ecology of the forest microbiome: Highlights of temperate and boreal ecosystems. Soil Biol. Biochem. 2016, 103, 471–488. [Google Scholar] [CrossRef]
- Sarmiento, C.; Zalamea, P.-C.; Dalling, J.W.; Davis, A.S.; Stump, S.M.; U’Ren, J.M.; Arnold, A.E. Soilborne fungi have host affinity and host-specific effects on seed germination and survival in a lowland tropical forest. Proc. Natl. Acad. Sci. USA 2017, 114, 11458–11463. [Google Scholar] [CrossRef]
- Nascimento, S.V.d.; Costa, P.H.d.O.; Herrera, H.; Caldeira, C.F.; Gastauer, M.; Ramos, S.J.; Oliveira, G.; Valadares, R.B.d.S. Proteomic Profiling and Rhizosphere-Associated Microbial Communities Reveal Adaptive Mechanisms of Dioclea apurensis Kunth in Eastern Amazon’s Rehabilitating Minelands. Plants 2022, 11, 712. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanhueza, T.; Hernández, I.; Sagredo-Sáez, C.; Villanueva-Guerrero, A.; Alvarado, R.; Mujica, M.I.; Fuentes-Quiroz, A.; Menendez, E.; Jorquera-Fontena, E.; Valadares, R.B.d.S.; et al. Juvenile Plant–Microbe Interactions Modulate the Adaptation and Response of Forest Seedlings to Rapid Climate Change. Plants 2024, 13, 175. https://doi.org/10.3390/plants13020175
Sanhueza T, Hernández I, Sagredo-Sáez C, Villanueva-Guerrero A, Alvarado R, Mujica MI, Fuentes-Quiroz A, Menendez E, Jorquera-Fontena E, Valadares RBdS, et al. Juvenile Plant–Microbe Interactions Modulate the Adaptation and Response of Forest Seedlings to Rapid Climate Change. Plants. 2024; 13(2):175. https://doi.org/10.3390/plants13020175
Chicago/Turabian StyleSanhueza, Tedy, Ionel Hernández, Cristiane Sagredo-Sáez, Angela Villanueva-Guerrero, Roxana Alvarado, Maria Isabel Mujica, Alejandra Fuentes-Quiroz, Esther Menendez, Emilio Jorquera-Fontena, Rafael Borges da Silva Valadares, and et al. 2024. "Juvenile Plant–Microbe Interactions Modulate the Adaptation and Response of Forest Seedlings to Rapid Climate Change" Plants 13, no. 2: 175. https://doi.org/10.3390/plants13020175
APA StyleSanhueza, T., Hernández, I., Sagredo-Sáez, C., Villanueva-Guerrero, A., Alvarado, R., Mujica, M. I., Fuentes-Quiroz, A., Menendez, E., Jorquera-Fontena, E., Valadares, R. B. d. S., & Herrera, H. (2024). Juvenile Plant–Microbe Interactions Modulate the Adaptation and Response of Forest Seedlings to Rapid Climate Change. Plants, 13(2), 175. https://doi.org/10.3390/plants13020175







