Screening for Resistance Resources against Bacterial Wilt in Wild Potato
Abstract
:1. Introduction
2. Results
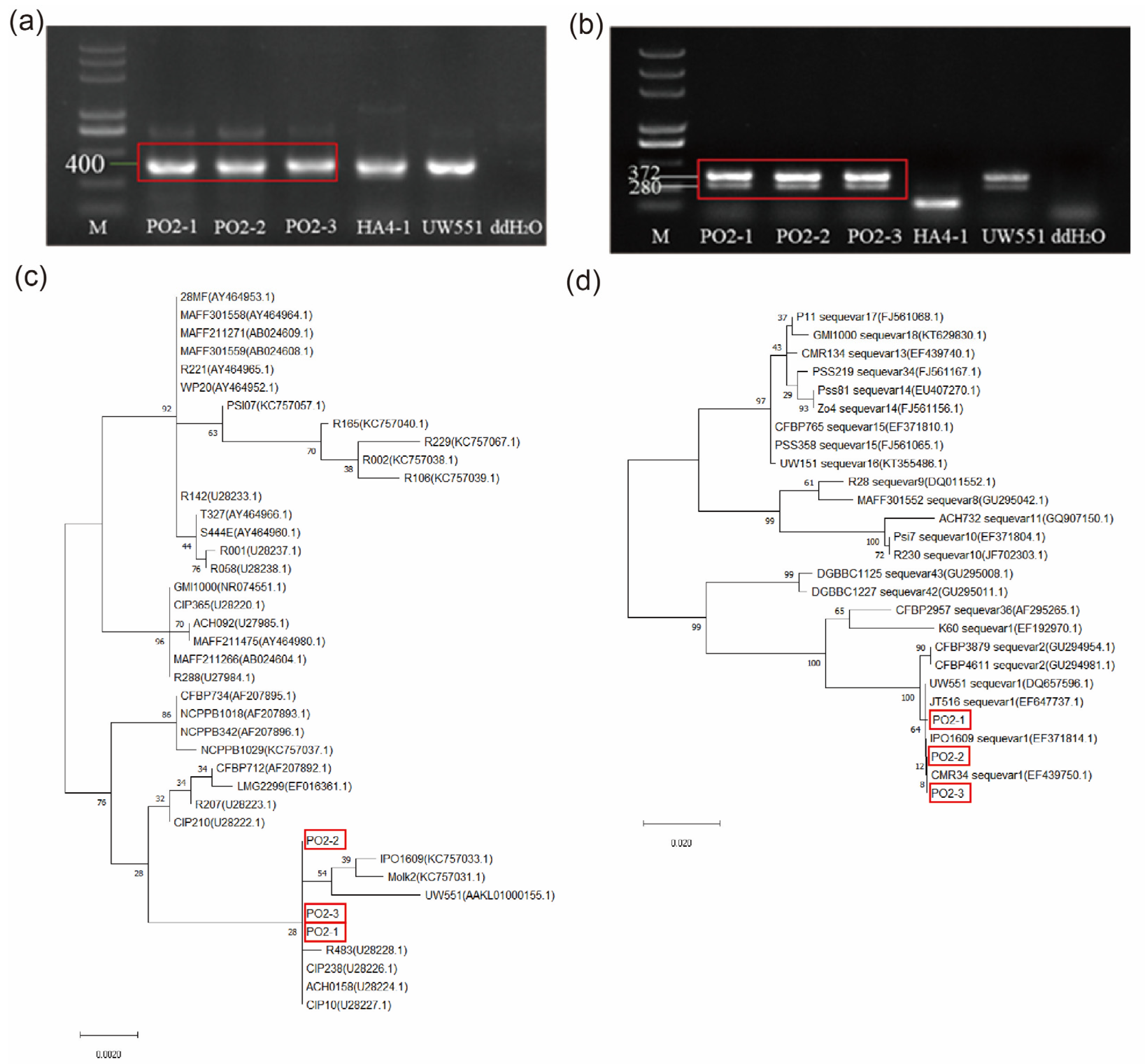
2.1. The Traditional Identification of R. solanacearum Strains
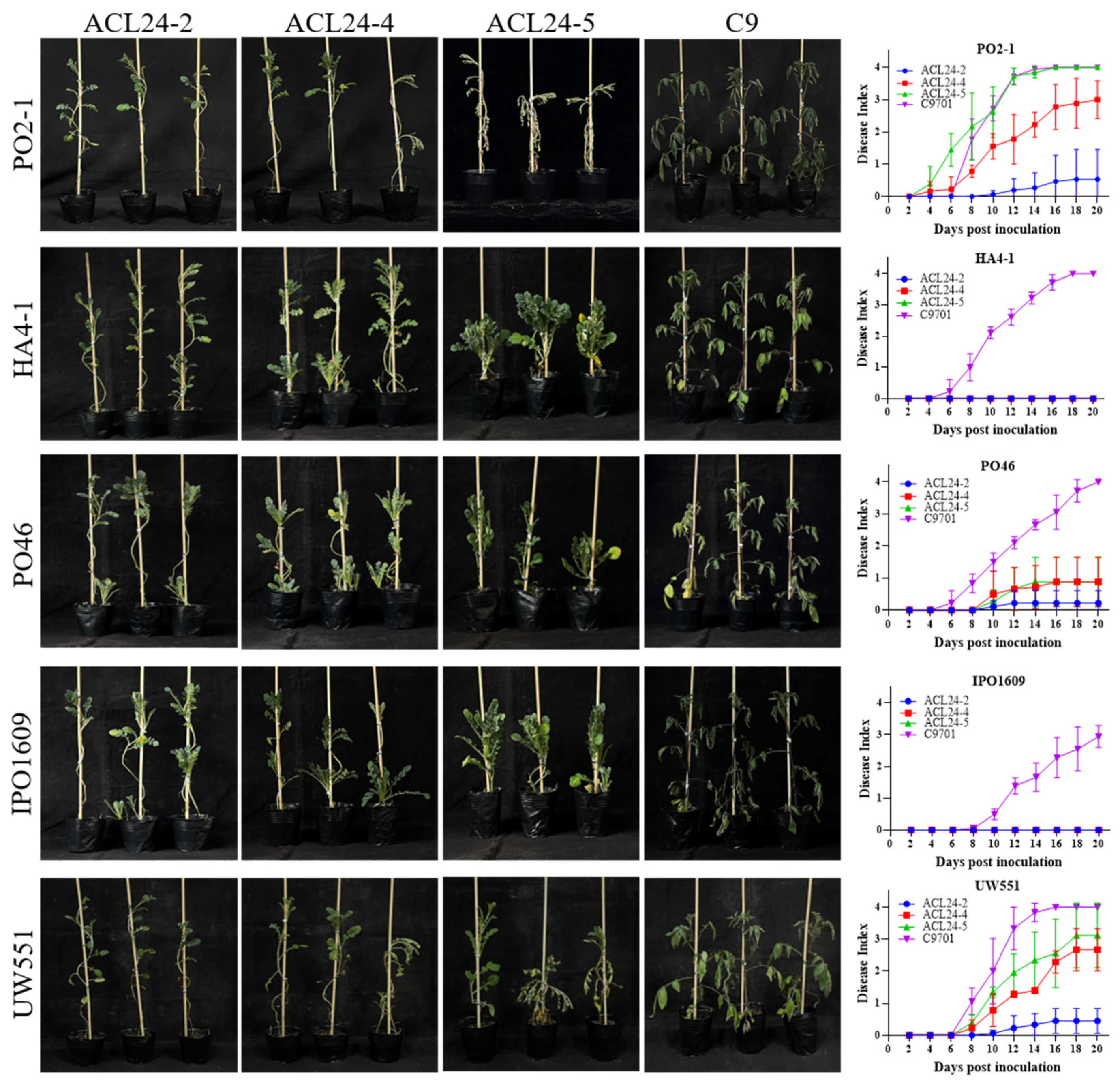
2.2. Screening of Potato Resistance Resources
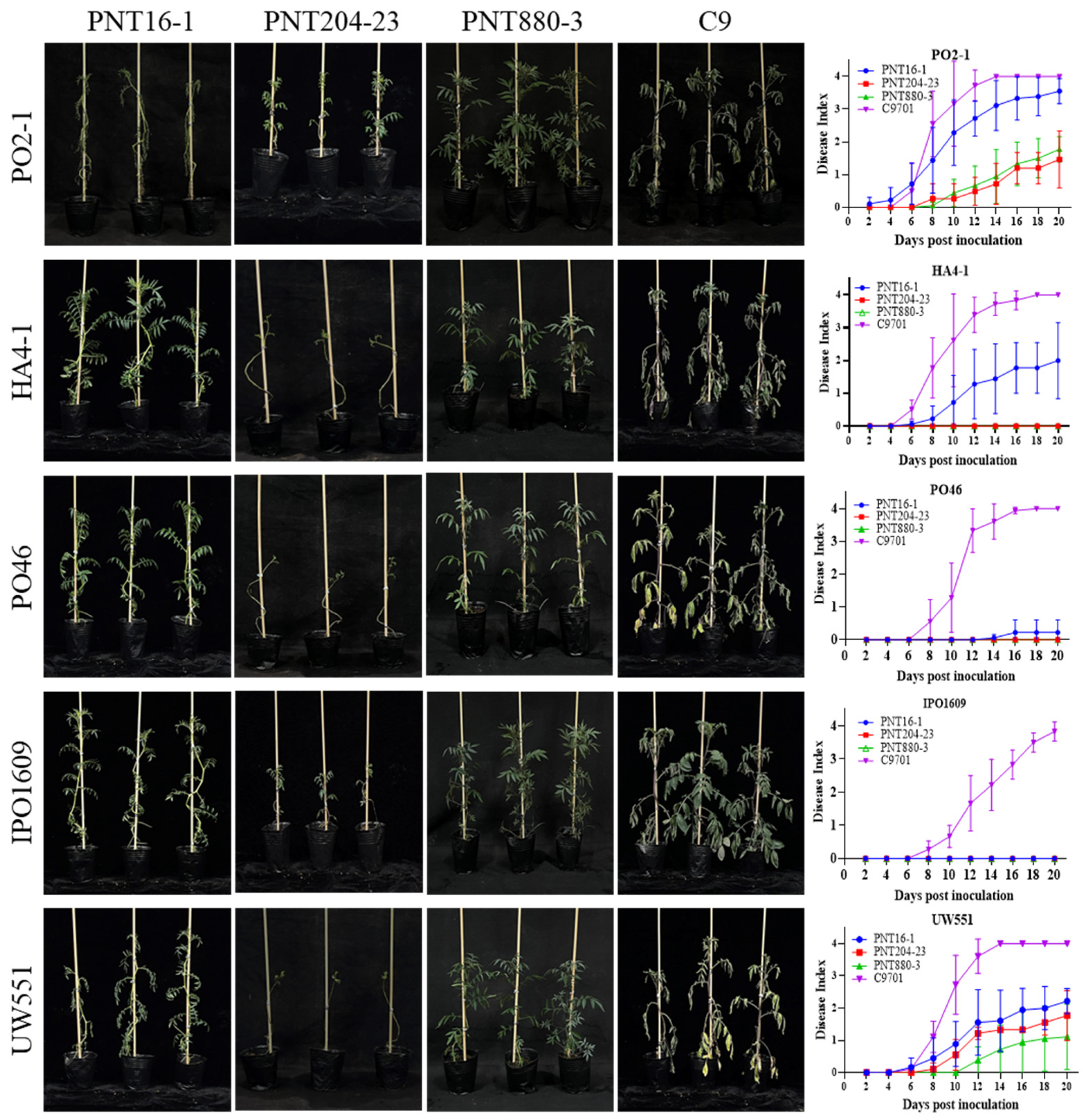
2.3. PNT880-3 Displays the Breeding Value
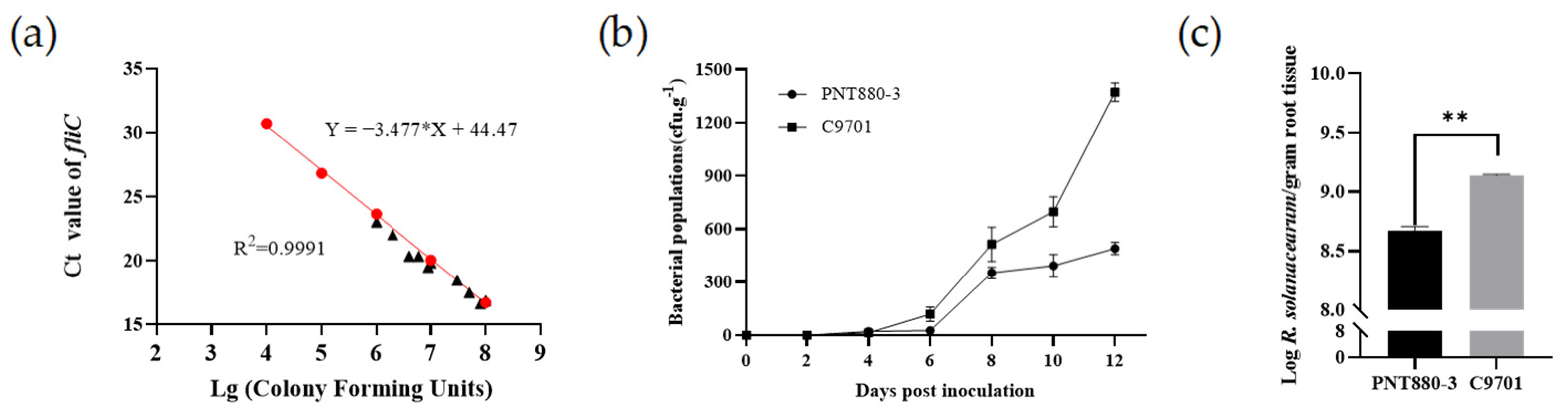
2.4. PNT880-3 Limits R. solanacearum Multiplication in Root
3. Discussion
4. Materials and Methods
4.1. Plant and Bacterial Materials
4.2. Pathogenicity Assays
4.3. Bacteria DNA Extraction, and qRT-PCR
4.4. Pollen Vigor Analysis
4.5. Determination of the Ploidy
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stokstad, E. The new potato. Science 2019, 363, 574–577. [Google Scholar] [CrossRef]
- Patil, V.U.; Gopal, J.; Singh, B.P. Improvement for Bacterial Wilt Resistance in Potato By Conventional and Biotechnological Approaches. Agric. Res. 2012, 1, 299–316. [Google Scholar] [CrossRef]
- Fegan, M.; Prior, P. How complex is the Ralstonia solanacearum species complex? In Bacterial Wilt Disease and the Ralstonia Solanacearum Species Complex; Allen, C., Prior, P., Hayward, A.C., Eds.; APS Press: StPaul, MN, USA, 2005; pp. 449–461. [Google Scholar]
- Prior, P.; Ailloud, F.; Dalsing, B.L.; Remenant, B.; Sanchez, B.; Allen, C. Genomic and proteomic evidence supporting the division of the plant pathogen Ralstonia solanacearum into three species. BMC Genom. 2016, 17, 90. [Google Scholar] [CrossRef]
- Fegan, M.; Taghavi, M.; Sly, L.; Hayward, A. Phylogeny, diversity and molecular diagnostics of Ralstonia solanacearum. In Bacterial Wilt Disease: Molecular and Ecological Aspects; Springer: Berlin/Heidelberg, Germany, 1998; pp. 19–33. [Google Scholar]
- Spooner, D.M.; Ghislain, M.; Simon, R.; Jansky, S.H.; Gavrilenko, T. Systematics, diversity, genetics, and evolution of wild and cultivated potatoes. Bot. Rev. 2014, 80, 283–383. [Google Scholar] [CrossRef]
- Machida-Hirano, R. Diversity of potato genetic resources. Breed. Sci. 2015, 65, 26–40. [Google Scholar] [CrossRef]
- Lopes, C.A.; Carvalho, A.D.; Pereira, A.S.; Azevedo, F.Q.; Castro, C.M.; Emygdio, B.M.; Silva, G.O. Performance of Solanum phureja-derived bacterial-wilt resistant potato clones in a field naturally infested with Ralstonia solanacearum in Central Brazil. Hortic. Bras. 2021, 39, 411–416. [Google Scholar] [CrossRef]
- Mori, K.; Mukojima, N.; Nakao, T.; Tamiya, S.; Sakamoto, Y.; Sohbaru, N.; Hayashi, K.; Watanuki, H.; Nara, K.; Yamazaki, K. Germplasm release: Saikai 35, a male and female fertile breeding line carrying Solanum phureja-derived cytoplasm and potato cyst nematode resistance (H1) and Potato virus Y resistance (Ry chc) genes. Am. J. Potato Res. 2012, 89, 63–72. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Mori, K.; Matsuo, Y.; Mukojima, N.; Watanabe, W.; Sobaru, N.; Tamiya, S.; Nakao, T.; Hayashi, K.; Watanuki, H. Breeding of a new potato variety ‘Nagasaki Kogane’with high eating quality, high carotenoid content, and resistance to diseases and pests. Breed. Sci. 2017, 67, 320–326. [Google Scholar] [CrossRef]
- Sequeira, L.; Rowe, P. Selection and utilization of Solanum phureja clones with high resistance to different strains of Pseudomonas solanacearum. Am. Potato J. 1969, 46, 451–462. [Google Scholar] [CrossRef]
- French, E.; Lindo, D. Resistance to Pseudomonas solanacearum in potato: Specificity and temperature sensitivity. Phytopathology 1982, 72, 1408–1412. [Google Scholar] [CrossRef]
- Lopes, C.A.; Melo, P.E.d.; Rossato, M.; Pereira, A.d.S. Breeding potatoes for resistance to bacterial blight in Brazil: A quick review in face of a more effective screening protocol. Hortic. Bras. 2018, 36, 6–12. [Google Scholar] [CrossRef]
- Fock, I.; Collonnier, C.; Purwito, A.; Luisetti, J.; Souvannavong, V.; Vedel, F.; Servaes, A.; Ambroise, A.; Kodja, H.; Ducreux, G. Resistance to bacterial wilt in somatic hybrids between Solanum tuberosum and Solanum phureja. Plant Sci. 2000, 160, 165–176. [Google Scholar] [CrossRef]
- Habe, I.; Sakamoto, Y.; Matsumoto, K. The development and efficient utilization of molecular markers for the major quantitative trait locus of bacterial wilt resistance in potato. Euphytica 2023, 219, 68. [Google Scholar] [CrossRef]
- Habe, I.; Miyatake, K. Identification and characterization of resistance quantitative trait loci against bacterial wilt caused by the Ralstonia solanacearum species complex in potato. Mol. Breed. 2022, 42, 50. [Google Scholar] [CrossRef]
- Narancio, R.; Zorrilla, P.; Robello, C.; Gonzalez, M.; Vilaro, F.; Pritsch, C.; Dalla Rizza, M. Insights on gene expression response of a characterized resistant genotype of Solanum commersonii Dun. against Ralstonia solanacearum. Eur. J. Plant Pathol. 2013, 136, 823–835. [Google Scholar] [CrossRef]
- Watanabe, K.; El-Nashaar, H.; Iwanaga, M. Transmission of bacterial wilt resistance by First Division Restitution (FDR) 2n pollen via 4x× 2x crosses in potatoes. Euphytica 1992, 60, 21–26. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Palta, J.; Bamberg, J. Freezing tolerance and tuber production in selfed and backcross progenies derived from somatic hybrids between Solanum tuberosum L. and S. commersonii Dun. Theor. Appl. Fract. Mech. 1999, 99, 100–107. [Google Scholar] [CrossRef]
- Carputo, D.; Aversano, R.; Barone, A.; Di Matteo, A.; Iorizzo, M.; Sigillo, L.; Zoina, A.; Frusciante, L. Resistance to Ralstonia solanacearum of Sexual Hybrids Between Solanum commersonii and S. tuberosum. Am. J. Potato Res. 2009, 86, 196–202. [Google Scholar] [CrossRef]
- Andino, M.; Gaiero, P.; Gonzalez-Barrios, P.; Galvan, G.; Vilaro, F.; Speranza, P. Potato introgressive hybridisation breeding for bacterial wilt resistance using Solanum commersonii Dun. as donor: Genetic and agronomic characterisation of a backcross 3 progeny. Potato Res. 2022, 65, 119–136. [Google Scholar] [CrossRef]
- Laferriere, L.T.; Helgeson, J.P.; Allen, C. Fertile Solanum tuberosum plus S-commersonii somatic hybrids as sources of resistance to bacterial wilt caused by Ralstonia solanacearum. Theor. Appl. Genet. 1999, 98, 1272–1278. [Google Scholar] [CrossRef]
- Kim-Lee, H.; Moon, J.; Hong, Y.; Kim, M.; Cho, H. Bacterial wilt resistance in the progenies of the fusion hybrids between haploid of potato and Solanum commersonii. Am. J. Potato Res. 2005, 82, 129–137. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Wang, H.; Xie, C.; Cai, X.; He, L.; Zhou, J.; Liu, J. Tetrasomic inheritance pattern of the pentaploid Solanum chacoense (+) S. tuberosum somatic hybrid (resistant to bacterial wilt) revealed by SSR detected alleles. Plant Cell Tiss. Org. Cult. 2016, 127, 315–323. [Google Scholar] [CrossRef]
- Nirula, K.; Nayar, N.; Bassi, K.; Singh, G. Reaction of tuber-bearing Solanum species to root knot nematode, Meloidogyne incognita. Am. Potato J. 1967, 44, 66–69. [Google Scholar] [CrossRef]
- Xue, W.; Haynes, K.G.; Qu, X. Characterization of early blight resistance in potato cultivars. Plant Dis. 2019, 103, 629–637. [Google Scholar] [CrossRef]
- Ritter, E.; Debener, T.; Barone, A.; Salamini, F.; Gebhardt, C. RFLP mapping on potato chromosomes of two genes controlling extreme resistance to potato virus X (PVX). Mol. Genet. Genom. 1991, 227, 81–85. [Google Scholar] [CrossRef]
- Bethke, P.C.; Halterman, D.A.; Jansky, S. Are we getting better at using wild potato species in light of new tools? Crop. Sci. 2017, 57, 1241–1258. [Google Scholar] [CrossRef]
- Yang, L.; Wang, D.; Xu, Y.; Zhao, H.; Wang, L.; Cao, X.; Chen, Y.; Chen, Q. A new resistance gene against potato late blight originating from Solanum pinnatisectum located on potato chromosome 7. Front. Plant Sci. 2017, 8, 1729. [Google Scholar] [CrossRef]
- Kuhl, J.; Hanneman, R.; Havey, M. Characterization and mapping of Rpi1, a late-blight resistance locus from diploid (1EBN) Mexican Solanum pinnatisectum. Mol. Genet. Genom. 2001, 265, 977–985. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.S.; Zhao, G.Z.; Cai, X.K.; Jabaji, S.; Seguin, P.; Chen, H.L. Genetic and Pathogenic Diversity of Ralstonia solanacearum Causing Potato Brown Rot in China. Am. J. Potato Res. 2017, 94, 403–416. [Google Scholar] [CrossRef]
- Dong, J.; Li, J.; Deng, G.; Chen, C.; Jing, S.; Song, B.; Cai, X. QTL analysis for low temperature tolerance of wild potato species Solanum commersonii in natural field trials. Sci. Hortic. 2023, 310, 111689. [Google Scholar] [CrossRef]
- Huet, G. Breeding for resistances to Ralstonia solanacearum. Front. Plant Sci. 2014, 5, 715. [Google Scholar] [CrossRef] [PubMed]
- Milling, A.; Meng, F.; Denny, T.P.; Allen, C. Interactions with hosts at cool temperatures, not cold tolerance, explain the unique epidemiology of Ralstonia solanacearum race 3 biovar 2. Phytopathology 2009, 99, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Cellier, G.; Prior, P. Deciphering phenotypic diversity of Ralstonia solanacearum strains pathogenic to potato. Phytopathology 2010, 100, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Li, C.; Li, G.; Wang, P.; Peng, Z.; Cheng, L.; Li, H.; Zhang, Z.; Li, Y.; Huang, W. Genome architecture and tetrasomic inheritance of autotetraploid potato. Mol. Plant 2022, 15, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Bryan, G.J.; McLean, K.; Waugh, R.; Spooner, D.M. Levels of intra-specific AFLP diversity in tuber-bearing potato species with different breeding systems and ploidy levels. Front. Genet. 2017, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Carputo, D.; Barone, A.; Frusciante, L. 2n gametes in the potato: Essential ingredients for breeding and germplasm transfer. Theor. Appl. Genet. 2000, 101, 805–813. [Google Scholar] [CrossRef]
- Veilleux, R. Diploid and polyploid gametes in crop plants: Mechanisms of formation and utilization in plant breeding. Plant Breed. Rev. 1985, 3, 253–288. [Google Scholar] [CrossRef]
- Jansky, S. Parental effects on the performance of cultivated × wild species hybrids in potato. Euphytica 2011, 178, 273–281. [Google Scholar] [CrossRef]
- Estrada, R. Acaphu: A tetraploid, fertile breeding line, selected from an S. acaule × S. phureja cross. Am. Potato J. 1984, 61, 1–7. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef]
- Carputo, D.; Terra, A.; Barone, A.; Esposito, F.; Fogliano, V.; Monti, L.; Frusciante, L. Glycoalkaloids and acclimation capacity of hybrids between Solanum tuberosum and the incongruent hardy species Solanum commersonii. Theor. Appl. Genet. 2003, 107, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Lebecka, R.; Zimnoch-Guzowska, E.; Kaczmarek, Z. Resistance to soft rot (Erwinia carotovora subsp. atroseptica) in tetraploid potato families obtained from 4x−2x crosses. Am. J. Potato Res. 2005, 82, 203–210. [Google Scholar] [CrossRef]
- Carlson, P.S.; Smith, H.H.; Dearing, R.D. Parasexual interspecific plant hybridization. Proc. Natl. Acad. Sci. USA 1972, 69, 2292–2294. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Bansal, M.; Kumar, V. Protoplast fusion technology and its biotechnological applications. Chem. Eng. Trans. 2008, 14, 113–120. [Google Scholar]
- Chen, L.; Guo, X.; Xie, C.; He, L.; Cai, X.; Tian, L.; Song, B.; Liu, J. Nuclear and cytoplasmic genome components of Solanum tuberosum + S. chacoense somatic hybrids and three SSR alleles related to bacterial wilt resistance. Theor. Appl. Genet. 2013, 126, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Fock, I.; Collonnier, C.; Lavergne, D.; Vaniet, S.; Ambroise, A.; Luisetti, J.; Kodja, H.; Sihachakr, D. Evaluation of somatic hybrids of potato with Solanum stenotomum after a long-term in vitro conservation. Plant Physiol. Biochem. 2007, 45, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xie, C.; Cai, X.; Song, B.; He, L.; Liu, J. Meiotic behavior of pollen mother cells in relation to ploidy level of somatic hybrids between Solanum tuberosum and S. chacoense. Plant Cell Rep. 2010, 29, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.; Peeters, N.; Vailleau, F.; Barberis, P.; Jiang, G.; Berthomé, R.; Guidot, A. Protocols. Plant pathogenicity phenotyping of Ralstonia solanacearum strains. In Host-Pathogen Interactions: Methods; Humana Press: New York, NY, USA, 2018; pp. 223–239. [Google Scholar]
- Wang, B.; He, W.; Huang, M.; Feng, J.; Li, Y.; Yu, L.; Wang, Y.; Zhou, D.; Meng, C.; Cheng, D.; et al. Ralstonia solanacearum type III effector RipAS associates with potato type one protein phosphatase StTOPP6 to promote bacterial wilt. Hortic. Res. 2023, 10, uhad087. [Google Scholar] [CrossRef]
- Dong, J.; Tu, W.; Wang, H.; Zuo, Y.; Liu, T.; Zhao, Q.; Ying, J.; Wu, J.; Liu, Y.; Cai, X. Genome sequence analysis provides insights into the mode of 2 n egg formation in Solanum malmeanum. Theor. Appl. Genet. 2023, 136, 157. [Google Scholar] [CrossRef]
- Tu, W.; Dong, J.; Zou, Y.; Zhao, Q.; Wang, H.; Ying, J.; Wu, J.; Du, J.; Cai, X.; Song, B. Interspecific potato somatic hybrids between Solanum malmeanum and S. tuberosum provide valuable resources for freezing-tolerance breeding. Plant Cell Tiss. Org. Cult. 2021, 147, 73–83. [Google Scholar] [CrossRef]

| Accessions a | Species | Disease Index b (Mean ± SD) | Resistance Level c |
|---|---|---|---|
| ACL24-2 | S. acaule | 0.53 ± 0.92 | R |
| ACL24-4 | S. acaule | 1.78 ± 0.77 | MR |
| ACL24-5 | S. acaule | 3.72 ± 0.25 | S |
| ACL25-1 | S. acaule | 3.56 ± 0.38 | S |
| ACL25-3 | S. acaule | 2.67 ± 0.67 | MS |
| ACL25-4 | S. acaule | 3.72 ± 0.48 | S |
| ACL27 | S. acaule | 3 ± 0.33 | MS |
| ACP443-2 | S. acaule punae | 2.94 ± 0.35 | MS |
| ALB26-1 | S. albicans | 2.28 ± 0.95 | MS |
| ALB26-2 | S. albicans | 2.67 ± 1.26 | MS |
| ALB26-3 | S. albicans | 2.22 ± 0.38 | MS |
| ALB27-1 | S. albicans | 1.61 ± 1.42 | MR |
| ALB27-2 | S. albicans | 1.67 ± 1.45 | MR |
| ALB27-3 | S. albicans | 1.5 ± 1.69 | MR |
| ALB28-1 | S. albicans | 3.72 ± 0.35 | S |
| ALB28-2 | S. albicans | 3.11 ± 0.92 | S |
| ALB28-3 | S. albicans | 3.17 ± 0.93 | S |
| ALB462-2 | S. albicans | 3.17 ± 0.93 | S |
| ALB464-3 | S. albicans | 1.56 ± 1.73 | MR |
| RAP17-1 | S. raphanifolium | 3.47 ± 0.92 | S |
| RAP17-2 | S. raphanifolium | 3.22 ± 1.07 | S |
| RAP18-1 | S. raphanifolium | 3.78 ± 0.38 | S |
| RAP19-3 | S. raphanifolium | 3.72 ± 0.48 | S |
| RAP20-1 | S. raphanifolium | 2.78 ± 1.84 | MS |
| RAP20-2 | S. raphanifolium | 2.8 ± 1.06 | MS |
| STO80-1 | S. stoloniferum | 3.78 ± 0.38 | S |
| STO80-3 | S. stoloniferum | 4 ± 0 | S |
| STO80-5 | S. stoloniferum | 3.78 ± 0.38 | S |
| STO80-6 | S. stoloniferum | 3.78 ± 0.38 | S |
| BLB47-1 | S. bulbocastanum | 3.33 ± 1.15 | S |
| BLB47-2 | S. bulbocastanum | 3.56 ± 0.77 | S |
| BLB47-3 | S. bulbocastanum | 3.61 ± 0.67 | S |
| PNT16-1 | S. pinnatisectum | 2.72 ± 0.54 | MS |
| PNT204-23 | S. pinnatisectum | 0.49 ± 0.43 | R |
| PNT880-3 | S. pinnatisectum | 0.67 ± 0.6 | R |
| VRN90-1 | S. vernei | 94 ± 0 | S |
| VRN90-2 | S. vernei | 4 ± 0 | S |
| VRN90-5 | S. vernei | 3.72 ± 0.35 | S |
| VRN904-6 | S. vernei | 3.06 ± 0.42 | S |
| BET4-1 | S. berthaultii | 2.11 ± 1.51 | MS |
| BET5-3 | S. berthaultii | 3.61 ± 0.35 | S |
| BET5-5 | S. berthaultii | 4 ± 0 | S |
| BET5-6 | S. berthaultii | 3.56 ± 0.38 | S |
| BLV30-1 | S. boliviense | 2.11 ± 0.75 | MS |
| BLV30-2 | S. boliviense | 2.89 ± 1.39 | MS |
| BLV32-1 | S. boliviense | 4 ± 0 | S |
| BLV32-2 | S. boliviense | 3.33 ± 1.15 | S |
| BLV32-3 | S. boliviense | 2.67 ± 1.33 | MS |
| BLV33-1 | S. boliviense | 3.28 ± 0.67 | S |
| BLV33-3 | S. boliviense | 3.89 ± 0.1 | S |
| BLV36-2 | S. boliviense | 3.67 ± 0.58 | S |
| BLV36-3 | S. boliviense | 3.83 ± 0.29 | S |
| BLV37-1 | S. boliviense | 3.2 ± 1.39 | S |
| BLV37-2 | S. boliviense | 2.93 ± 1.1 | MS |
| BLV37-3 | S. boliviense | 2.93 ± 1.85 | MS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Wang, B.; Huang, M.; Meng, C.; Wu, J.; Du, J.; Song, B.; Chen, H. Screening for Resistance Resources against Bacterial Wilt in Wild Potato. Plants 2024, 13, 220. https://doi.org/10.3390/plants13020220
He W, Wang B, Huang M, Meng C, Wu J, Du J, Song B, Chen H. Screening for Resistance Resources against Bacterial Wilt in Wild Potato. Plants. 2024; 13(2):220. https://doi.org/10.3390/plants13020220
Chicago/Turabian StyleHe, Wenfeng, Bingsen Wang, Mengshu Huang, Chengzhen Meng, Jiahui Wu, Juan Du, Botao Song, and Huilan Chen. 2024. "Screening for Resistance Resources against Bacterial Wilt in Wild Potato" Plants 13, no. 2: 220. https://doi.org/10.3390/plants13020220





