Chemical Composition of Wild Collected and Cultivated Edible Plants (Sonchus oleraceus L. and Sonchus tenerrimus L.)
Abstract
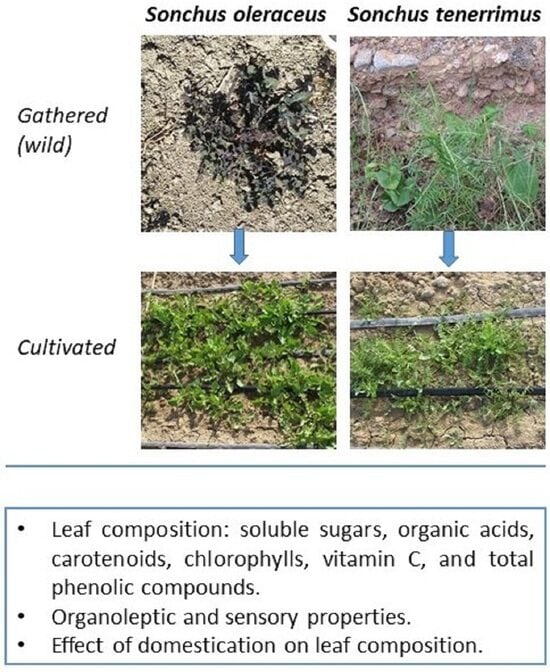
:1. Introduction
2. Results and Discussion
2.1. Germination
2.2. Nutritional Value
2.3. Sensory Attributes
2.4. Wild-Gathered vs. Cultivated Plants
3. Materials and Methods
3.1. Wild Plant Gathering
3.2. Germination Assay in Petri Dishes
3.3. Field Germination Assays
3.4. Cultivation Trial
3.5. Metabolite Analysis
3.6. Sensory Attributes
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Traditional Medicine Strategy 2014–2023; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- FAO. Biodiversity and the Ecosystem Approach in Agriculture, Forestry and Fisheries. In Proceedings of the Ninth Regular Session of the Commission on Genetic Resources for Food and Agriculture, Rome, Italy, 12–13 October 2002; Inter-Departmental Working Group on Biological Diversity for Food and Agriculture: Rome, Italy, 2002. [Google Scholar]
- Sánchez-Mata, M.C.; Tardio, J. (Eds.) Mediterranean wild edible plants. In Ethnobotany and Food Composition Tables; Springer Nature Switzerland AG: New York, NY, USA, 2016. [Google Scholar]
- Romojaro, A.; Botella, M.A.; Obon, C.; Pretel, M.T. Nutritional and antioxidant properties of wild edible plants and their use as potential ingredients in the modern diet. Int. J. Food Sci. Nutr. 2013, 64, 944–952. [Google Scholar] [CrossRef]
- de Medeiros, P.M.; Figueiredo, K.F.; Santos Goncalves, P.H.; Caetano, R.d.A.; da Costa Santos, E.M.; Cota dos Santos, G.M.; Barbosa, D.M.; de Paula, M.; Mapeli, A.M. Wild plants and the food-medicine continuum-an ethnobotanical survey in Chapada Diamantina (Northeastern Brazil). J. Ethnobiol. Ethnomed. 2021, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Villalba, J.; Burlo, F.; Hernandez, F.; Carbonell-Barrachina, A.A. Valorization of wild edible plants as food ingredients and their economic value. Foods 2023, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.F.; Akhigbe, R.E.; Adewumi, O.M.; Okeleji, L.O.; Mujaidu, K.B.; Olaleye, S.B. Effect of ethanolic extract of Cryptolepis sanguinolenta stem on in vivo and in vitro glucose absorption and transport: Mechanism of its antidiabetic activity. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S1), S91–S96. [Google Scholar] [CrossRef]
- Li, W.; Sun, H.; Zhou, J.; Zhang, Y.; Liu, L.; Gao, Y. Antibacterial activities, antioxidant contents and antioxidant properties of three traditional Chinese medicinal extracts. Bangladesh J. Pharmacol. 2015, 10, 131–137. [Google Scholar] [CrossRef]
- Huyan, T.; Li, Q.; Wang, Y.L.; Li, J.; Zhang, J.Y.; Liu, Y.X.; Shahid, M.R.; Yang, H.; Li, H.Q. Anti-tumor effect of hot aqueous extracts from Sonchus oleraceus (L.) L. and Juniperus sabina L.—Two traditional medicinal plants in China. J. Ethnopharmacol. 2016, 185, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, Ł.; Pieroni, A. Nutritional Ethnobotany in Europe: From Emergency Foods to Healthy Folk Cuisines and Contemporary Foraging Trends. In Mediterranean Wild Edible Plants; Sánchez-Mata, M.C., Tardío, J., Eds.; Springer Science + Business Media: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Zhu, G.; Mosyakin, S.L.; Clemants, S.E. Flora of China; Science Press: Beijing, China, 1999; Volume 126, p. 60. [Google Scholar]
- Fashir, G.A.; Abdalla, N.I.; and Fangama, I.M. Assessment the consumption of Sonchus cornutus (Hochst) in Khartoum State, Sudan. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 833–839. [Google Scholar]
- Seal, T.; Chaudhuri, K.; Pillai, B. Nutritional and toxicological aspects of selected wild edible plants and significance for this society. S. Afr. J. Bot. 2023, 159, 219–230. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Giménez-Giménez, A.; Rodríguez-García, I.; Torija-Isasa, M.E. Nutritional composition of Sonchus species (S asper L, S oleraceus L and S tenerrimus L). J. Sci. Food Agric. 1998, 76, 628–632. [Google Scholar] [CrossRef]
- Li, X.M.; Yang, P.L. Research progress of Sonchus species. Int. J. Food Prop. 2018, 21, 162–172. [Google Scholar] [CrossRef]
- Khan, R.A. Protective effects of Sonchus asper (L.) Hill, (Asteraceae) against CCl4-induced oxidative stress in the thyroid tissue of rats. BMC Complement. Altern. Med. 2012, 12, 181. [Google Scholar] [CrossRef]
- de Paula Filho, G.X.; Barreira, T.F.; Pinheiro-Sant’Ana, H.M. Chemical composition and nutritional value of three Sonchus species. Int. J. Food Sci. 2022, 2022, 4181656. [Google Scholar] [CrossRef]
- Li, Q.; Dong, D.D.; Huang, Q.P.; Li, J.; Du, Y.Y.; Li, B.; Li, H.Q.; Ting, H. The anti-inflammatory effect of Sonchus oleraceus aqueous extract on lipopolysaccharide stimulated RAW 264.7 cells and mice. Pharm. Biol. 2017, 55, 799–809. [Google Scholar] [CrossRef]
- Elhady, S.S.; Abdelhameed, R.F.A.; Mehanna, E.T.; Wahba, A.S.; Elfaky, M.A.; Koshak, A.E.; Noor, A.O.; Bogari, H.A.; Malatani, R.T.; Goda, M.S. Metabolic profiling, chemical composition, antioxidant capacity, and in vivo hepato- and nephroprotective effects of Sonchus cornutus in mice exposed to cisplatin. Antioxidants 2022, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Vecchia, C.A.D.; Locateli, G.; Serpa, P.Z.; Gomes, D.B.; Ernetti, J.; Miorando, D.; Zanatta, M.E.D.C.; Silva Nunes, R.K.; Wildner, S.M.; Gutierrez, M.V. Sonchus oleraceus L. promotes gastroprotection in rodents via antioxidant, anti-inflammatory, and antisecretory activities. Evid.-Based Complement. Altern. Med. 2022, 2022, 7413231. [Google Scholar] [CrossRef]
- Jimoh, F.O.; Adedapo, A.A.; Afolayan, A.J. Comparison of the nutritive value, antioxidant and antibacterial activities of Sonchus asper and Sonchus oleraceus. Rec. Nat. Prod. 2011, 5, 29–42. [Google Scholar]
- Klos, M.; van de Venter, M.; Milne, P.J.; Traore, H.N.; Meyer, D.; Oosthuizen, V. In vitro anti-HIV activity of five selected South African medicinal plant extracts. J. Ethnopharmacol. 2009, 124, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Singh, G.K. Preliminary phytochemical screening and in vitro antioxidant activity of extracts of whole plant of Sonchus oleraceus Asteraceae. Res. J. Pharm. Sci. 2014, 3, 1–12. [Google Scholar]
- Karar, E.G.M. Phytochemical Characterization and Antimicrobial Activity of Sudanese Medicinal Plants. Doctoral Dissertation, Jacobs University Bremen, Bremen, Germany, 2015. [Google Scholar]
- Al Juhaimi, F.; Ghafoor, K.; Ahmed, I.A.M.; Babiker, E.E.; Ozcan, M.M. Comparative study of mineral and oxidative status of Sonchus oleraceus, Moringa oleifera and Moringa peregrina. J. Food Meas. Charact. 2017, 11, 1745–1751. [Google Scholar] [CrossRef]
- Cardoso Vilela, F.; Soncini, R.; Giusti-Paiva, A. Anxiolytic-like effect of Sonchus oleraceus L. in mice. J. Ethnopharmacol. 2009, 124, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.Q.; Rades, T.; McDowell, A. Anti-ageing effects of Sonchus oleraceus L. (puha) leaf extracts on H2O2-induced cell senescence. Molecules 2015, 20, 4548–4564. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, Y.H.; Wang, B.; Chen, C.Y.; Zhang, H.M.; Kang, J.X. Identification of a sustainable two-plant diet that effectively prevents age-related metabolic syndrome and extends lifespan in aged mice. J. Nutr. Biochem. 2018, 51, 16–26. [Google Scholar] [CrossRef]
- Tardio, J.; Pardo-De-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Lentini, F.; Venza, F. Wild food plants of popular use in Sicily. J. Ethnobiol. Ethnomed. 2007, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean wild edible plants: Weeds or “New functional crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.; López, A.; Fenoll, J.; Hellín, P.; Kelly, S. Classification of organic and conventional sweet peppers and lettuce using a combination of isotopic and bio-markers with multivariate analysis. J. Food Compos. Anal. 2013, 31, 217–225. [Google Scholar] [CrossRef]
- López, A.; Javier, G.A.; Fenoll, J.; Hellín, P.; Flores, P. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. J. Food Compos. Anal. 2014, 33, 39–48. [Google Scholar] [CrossRef]
- Schmitzer, V.; Senica, M.; Slatnar, A.; Stampar, F.; Jakopic, J. Changes in metabolite patterns during refrigerated storage of lamb’s lettuce Valerianella locusta L. Betcke). Front. Nutr. 2021, 8, 731869. [Google Scholar] [CrossRef]
- Spinardi, A.; Ferrante, A. Effect of storage temperature on quality changes of minimally processed baby lettuce. J. Food Agric. Environ. 2012, 10, 38–42. [Google Scholar]
- Fabian, F.W.; Blum, H.B. Relative taste potency of some basic food constituents and their competitive and compensatory action. J. Food Sci. 2006, 8, 179–193. [Google Scholar] [CrossRef]
- Schifferstein, H.N.J.; Frijters, J.E.R. Sensory integration in citric-acid sucrose mixtures. Chem. Senses 1990, 15, 87–109. [Google Scholar] [CrossRef]
- Bonnans, S.; Noble, A.C. Effect of sweetener type and of sweetener and acid levels on temporal perception of sweetness, sourness and fruitiness. Chem. Senses 1993, 18, 273–283. [Google Scholar] [CrossRef]
- Pangborn, R.M. Relative taste intensities of selected sugars and organic acids. J. Food Sci. 1963, 28, 726–733. [Google Scholar] [CrossRef]
- Derrien, M.; Aghabararnejad, M.; Gosselin, A.; Desjardins, Y.; Angers, P.; Boumghar, Y. Optimization of supercritical carbon dioxide extraction of lutein and chlorophyll from spinach by-products using response surface methodology. LWT-Food Sci. Technol. 2018, 93, 79–87. [Google Scholar] [CrossRef]
- Znidarcic, D.; Ban, D.; Sircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011, 129, 1164–1168. [Google Scholar] [CrossRef]
- Leite, A.C.; Ferreira, A.M.; Morais, E.S.; Khan, I.; Freire, M.G.; Coutinho, J.A.P. Cloud point extraction of chlorophylls from spinach leaves using aqueous solutions of non-ionic surfactants. ACS Sustain. Chem. Eng. 2018, 6, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Böhm, V.; Courtney, P.D.; Schwartz, S.J. Antioxidant and antimutagenic activity of dietary chlorophyll derivatives determined by radical scavenging and bacterial reverse mutagenesis assays. J. Food Sci. 2002, 67, 2589–2595. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella Thermophila: Optimization of process parameters and modelling by artificial neural network. Process Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Panfili, G.; Niro, S.; Bufano, A.; D’Agostino, A.; Fratianni, A.; Paura, B.; Falasca, L.; Cinquanta, L. Bioactive compounds in wild Asteraceae edible plants consumed in the Mediterranean Diet. Plant Foods Hum. Nutr. 2020, 75, 540–546. [Google Scholar] [CrossRef]
- Kandlakunta, B.; Rajendran, A.; Thingnganing, L. Carotene content of some common (cereals, pulses, vegetables, spices and condiments) and unconventional sources of plant origin. Food Chem. 2008, 106, 85–89. [Google Scholar] [CrossRef]
- Gayathri, G.N.; Platel, K.; Prakash, J.; Srinivasan, K. Influence of antioxidant spices on the retention of β-carotene in vegetables during domestic cooking processes. Food Chem. 2004, 84, 35–43. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A and Provitamin A Carotenoids. In Present Knowledge in Nutrition, 11th ed.; Marriott, B.P., Birt, D.F., Stallings, V.A., Yates, A.A., Eds.; Wiley-Blackwell: Cambridge, MA, USA, 2020; pp. 73–91. [Google Scholar]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic review of β-carotene, lutein, and zeaxanthin in eye health and disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Mercadante, A.Z.; Rodriguez-Amaya, D.B. Carotenoid composition and vitamin A value of some native Brazilian green leafy vegetables. Int. J. Food Sci. Technol. 1990, 25, 213–219. [Google Scholar] [CrossRef]
- Paradiso, R.; Di Mola, I.; Cozzolino, E.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Nutrient and nutraceutical quality of rocket as a function of greenhouse cover film, nitrogen dose and biostimulant application. Agronomy 2023, 13, 638. [Google Scholar] [CrossRef]
- Kimura, M.; Rodriguez-Amaya, D.B. A scheme for obtaining standards and HPLC quantification of leafy vegetable carotenoids. Food Chem. 2002, 78, 389–398. [Google Scholar] [CrossRef]
- Calvo, M.M. Lutein: A valuable ingredient of fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2005, 45, 671–696. [Google Scholar] [CrossRef]
- Lakshminarayana, R.; Raju, M.; Krishnakantha, T.P.; Baskaran, V. Determination of major carotenoids in a few Indian leafy vegetables by high-performance liquid chromatography. J. Agric. Food Chem. 2005, 53, 2838–2842. [Google Scholar] [CrossRef]
- Hernández, V.; Botella, M.A.; Hellín, P.; Cava, J.; Fenoll, J.; Mestre, T.; Martínez, V.; Flores, P. Phenolic and carotenoid profile of lamb’s lettuce and improvement of the bioactive content by preharvest conditions. Foods 2021, 10, 188. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J.; Wenzel, A.J. Genetic variability for lutein concentrations in leafy vegetable crops can influence serum carotenoid levels and macular pigment optical density in human subjects. In Proceedings of the II International Symposium on Human Health Effects of Fruits and Vegetables: Favhealth 2007, Houston, TX, USA, 31 August 2009; Volume 841, pp. 113–117. [Google Scholar]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Bartlett, H.E. Xanthophylls and the eye. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Lopez-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and Lutein: Photoprotectors, anti-Inflammatories, and brain food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef] [PubMed]
- de Sá, M.C.; Rodriguez-Amaya, D.B. Carotenoid composition of cooked green vegetables from restaurants. Food Chem. 2003, 83, 595–600. [Google Scholar] [CrossRef]
- Rumengan, A.P.; Mandiangan, E.S.; Tanod, W.A. Identification of pigment profiles and antioxidant activity of Rhizophora mucronata mangrove leaves origin Lembeh, North Sulawesi, Indonesia. Biodiversitas 2021, 22, 2805–2816. [Google Scholar] [CrossRef]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Molnar, P.; Deli, J.; Tanaka, T.; Kann, Y.; Tani, S.; Gyemant, N.; Molnar, J.; Kawase, M. Carotenoids with anti-Helicobacter pylori activity from Golden delicious apple. Phytother. Res. 2010, 24, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 201–209. [Google Scholar] [CrossRef]
- Upreti, S.; Prusty, J.S.; Pandey, S.C.; Kumar, A.; Samant, M. Identification of novel inhibitors of angiotensin-converting enzyme 2 (ACE-2) receptor from Urtica dioica to combat coronavirus disease 2019 (COVID-19). Mol. Divers. 2021, 25, 1795–1809. [Google Scholar] [CrossRef]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Antioxidant phytochemicals in lettuce grown in high tunnels and open field. Hortic. Environ. Biotechnol. 2011, 52, 133–139. [Google Scholar] [CrossRef]
- Gutiérrez-Velázquez, M.; Almaraz-Abarca, N.; Herrera-Arrieta, Y.; Ávila-Reyes, J.A.; González-Valdez, L.S.; Torres-Ricario, R.; Uribe-Soto, J.N.; Monreal-García, H.M. Comparison of the phenolic contents and epigenetic and genetic variability of wild and cultivated watercress (Rorippa nasturtium var. aquaticum L.). Electron. J. Biotechnol. 2018, 34, 9–16. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Incrocci, L.; Pardossi, A.; Venturi, F.; Taglieri, I.; Ferroni, G.; Guidi, L. Comparison of three domestications and wild-harvested plants for nutraceutical properties and sensory profiles in five wild edible herbs: Is domestication possible? Foods 2020, 9, 1065. [Google Scholar] [CrossRef]
- Riquelme, J.; Antonio Olaeta, J.; Galvez, L.; Undurraga, P.; Fuentealba, C.; Osses, A.; Orellana, J.; Gallardo, J.; Pedreschi, R. Nutritional and functional characterization of wild and cultivated Sarcocornia neei grown in Chile. Cienc. Investig. Agrar. 2016, 43, 283–293. [Google Scholar] [CrossRef]
- Paschoalinotto, B.H.; Polyzos, N.; Compocholi, M.; Rouphael, Y.; Alexopoulos, A.; Dias, M.I.; Barros, L.; Petropoulos, S.A. Domestication of wild edible species: The response of Scolymus hispanicus plants to different fertigation regimes. Horticulturae 2023, 9, 103. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Sailendra Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Fenoll, J.; Martínez, A.; Hellín, P.; Flores, P. Simultaneous determination of ascorbic and dehydroascorbic acids in vegetables and fruits by liquid chromatography with tandem-mass spectrometry. Food Chem. 2011, 127, 340–344. [Google Scholar] [CrossRef]
- Flores, P.; Hellín, P.; Fenoll, J. Determination of organic acids in fruits and vegetables by liquid chromatography with tandem-mass spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Sancho, J.; Bota, E.; Castro, J. Introducción al Análisis Sensorial de los Alimentos; Editorial Alfaomega: Barcelona, Spain, 1999. [Google Scholar]





| Metabolite | S. oleraceus L. | S. tenerrimus L. | Sig. |
|---|---|---|---|
| Glucose | 2414.8 ± 309.5 | 2406.4 ± 129.5 | n.s. |
| Sucrose | 748.7 ± 558.8 | 2743.2 ± 61.1 | * |
| Fructose | 524.1 ± 62.7 | 707.8 ± 79.2 | * |
| Citric | 875.6 ± 26.0 | 893.1 ± 214.7 | n.s. |
| Malic | 774.5 ± 30.3 | 1343.8 ± 280.4 | * |
| Tartaric | 356.0 ± 14.3 | 205.4 ± 70.1 | ** |
| Fumaric | 71.6 ± 9.5 | 42.7 ± 8.5 | * |
| Succinic | 65.5 ± 2.8 | 67.7 ± 21.4 | n.s. |
| Quinic | 29.5 ± 2.4 | 38.0 ± 9.5 | n.s. |
| Malonic | 24.0 ± 2.3 | 10.7 ± 3.3 | ** |
| Isocitric | 17.5 ± 0.2 | 20.0 ± 7.5 | n.s. |
| Ketoglutaric | 6.6 ± 0.6 | 21.8 ± 6.6 | ** |
| Glutamic | 3.9 ± 0.2 | 5.5 ± 1.9 | n.s. |
| Shikimic | 3.0 ± 0.2 | 6.3 ± 0.9 | ** |
| Chlorophyll a | 130.5 ± 41.7 | 147.4 ± 3.7 | n.s. |
| Chlorophyll b | 51.6 ± 16.4 | 56.2 ± 1.9 | n.s. |
| All-trans-β-carotene | 42.9 ± 6.5 | 49.8 ± 1.2 | n.s. |
| Lutein | 20.3 ± 3.2 | 18.9 ± 0.1 | n.s. |
| All-trans-violaxanthin | 9.6 ± 0.8 | 11.9 ± 1.3 | n.s. |
| 9 cis-Neoxanthin | 4.9 ± 1.0 | 5.1 ± 0.5 | n.s. |
| 9 cis-β-carotene | 4.5 ± 0.7 | 5.5 ± 0.3 | n.s. |
| Luteoxanthin | 3.8 ± 0.3 | 5.6 ± 2.1 | n.s. |
| 13 cis-β-carotene | 1.8 ± 0.3 | 2.4 ± 0.7 | n.s. |
| Vitamin C | 527.7 ± 16.9 | 436.1 ± 4.4 | ** |
| Total phenolics | 188.7 ± 9.2 | 328.6 ± 17.7 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botella, M.Á.; Hellín, P.; Hernández, V.; Dabauza, M.; Robledo, A.; Sánchez, A.; Fenoll, J.; Flores, P. Chemical Composition of Wild Collected and Cultivated Edible Plants (Sonchus oleraceus L. and Sonchus tenerrimus L.). Plants 2024, 13, 269. https://doi.org/10.3390/plants13020269
Botella MÁ, Hellín P, Hernández V, Dabauza M, Robledo A, Sánchez A, Fenoll J, Flores P. Chemical Composition of Wild Collected and Cultivated Edible Plants (Sonchus oleraceus L. and Sonchus tenerrimus L.). Plants. 2024; 13(2):269. https://doi.org/10.3390/plants13020269
Chicago/Turabian StyleBotella, M. Ángeles, Pilar Hellín, Virginia Hernández, Mercedes Dabauza, Antonio Robledo, Alicia Sánchez, José Fenoll, and Pilar Flores. 2024. "Chemical Composition of Wild Collected and Cultivated Edible Plants (Sonchus oleraceus L. and Sonchus tenerrimus L.)" Plants 13, no. 2: 269. https://doi.org/10.3390/plants13020269
APA StyleBotella, M. Á., Hellín, P., Hernández, V., Dabauza, M., Robledo, A., Sánchez, A., Fenoll, J., & Flores, P. (2024). Chemical Composition of Wild Collected and Cultivated Edible Plants (Sonchus oleraceus L. and Sonchus tenerrimus L.). Plants, 13(2), 269. https://doi.org/10.3390/plants13020269