Physiological Mechanism through Which Al Toxicity Inhibits Peanut Root Growth
Abstract
1. Introduction
2. Results
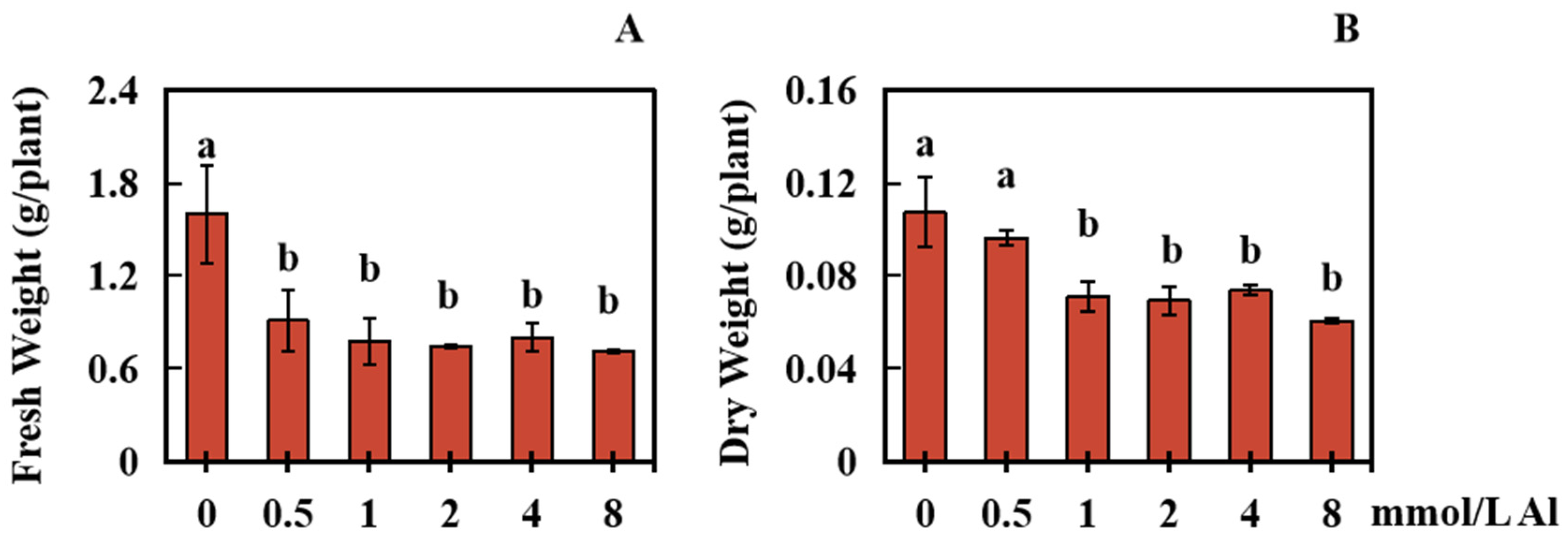
2.1. Effects of Al Poisoning on the Biomass of Roots in Peanut Plants
2.2. Influences of Al Poisoning on Development of Peanut Roots
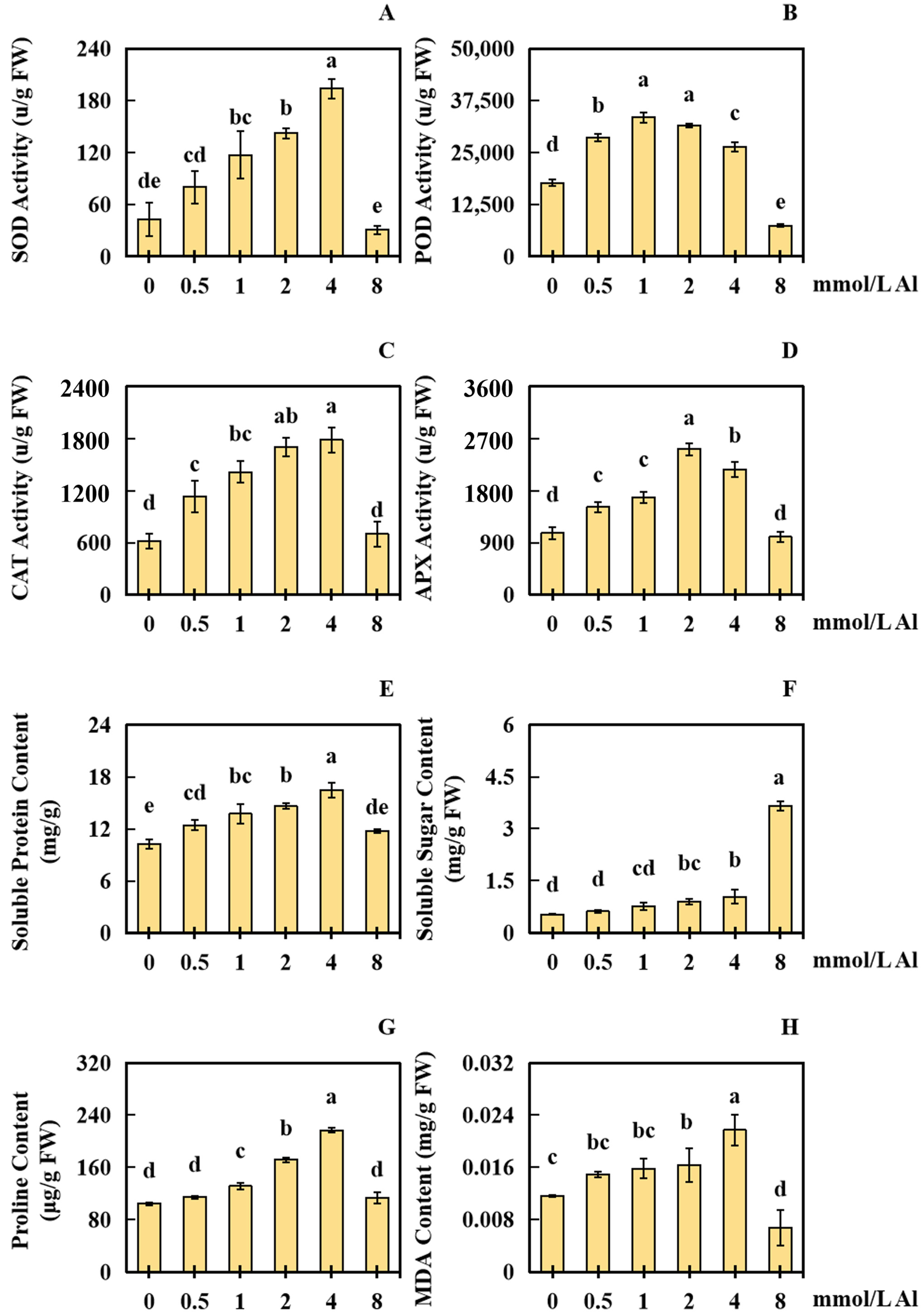
2.3. Results of Al Poisoning on the Physical Response Indices of Peanut Roots
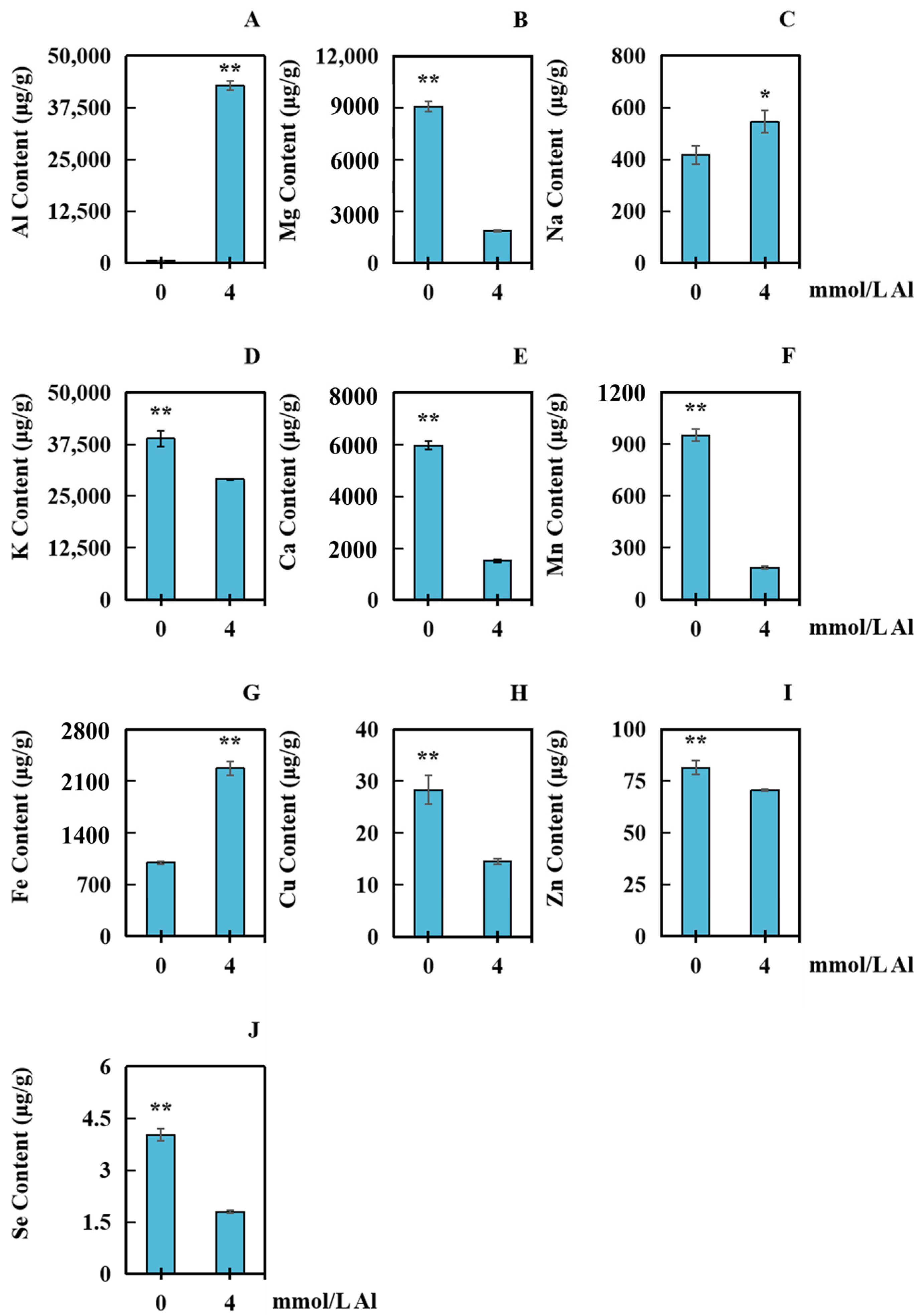
2.4. Influences of Al Poisoning on the Accumulation of Several Elements in Peanut Roots
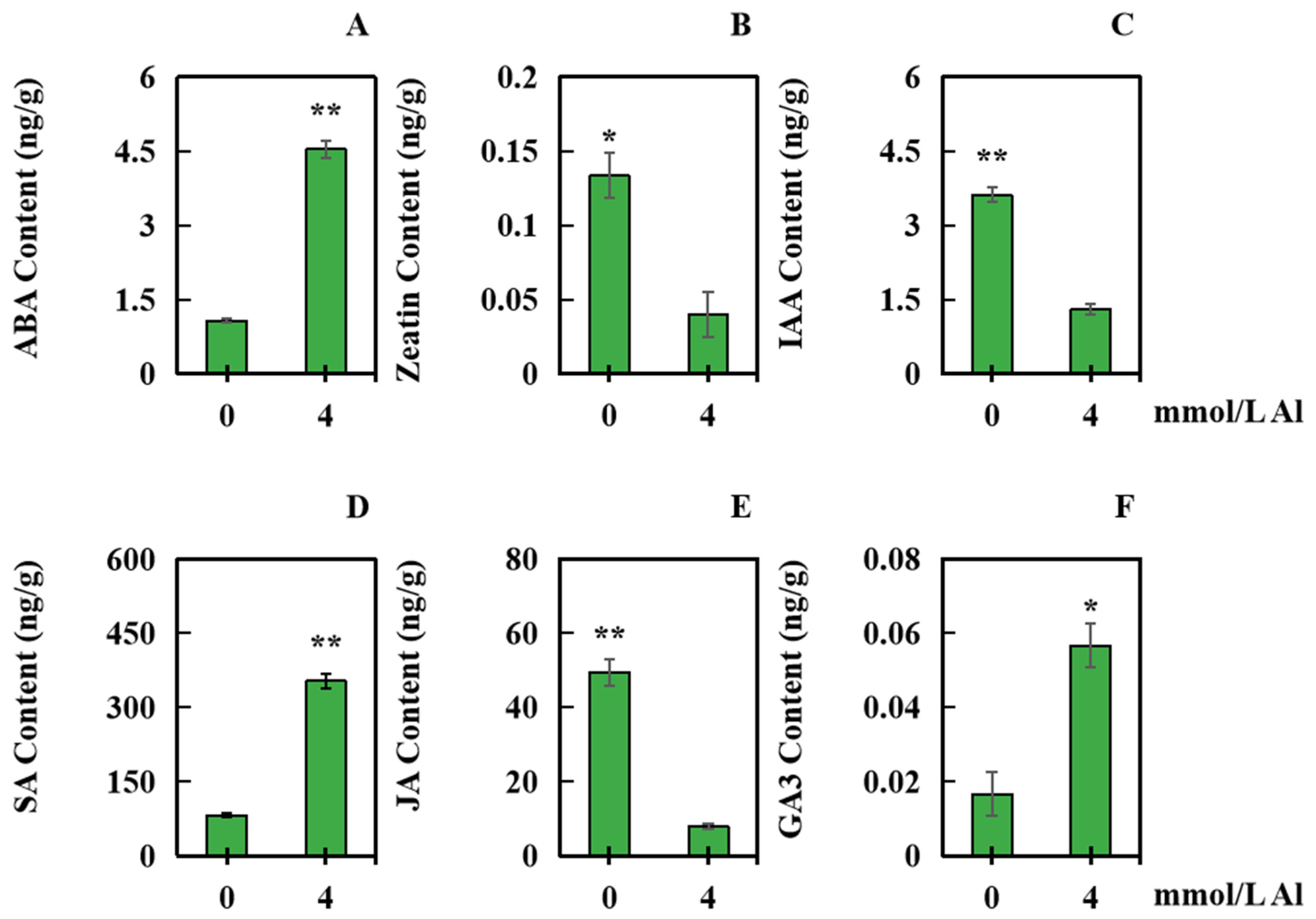
2.5. Effects of Al Toxicity Stress on the Contents of Several Hormones in Peanut Roots
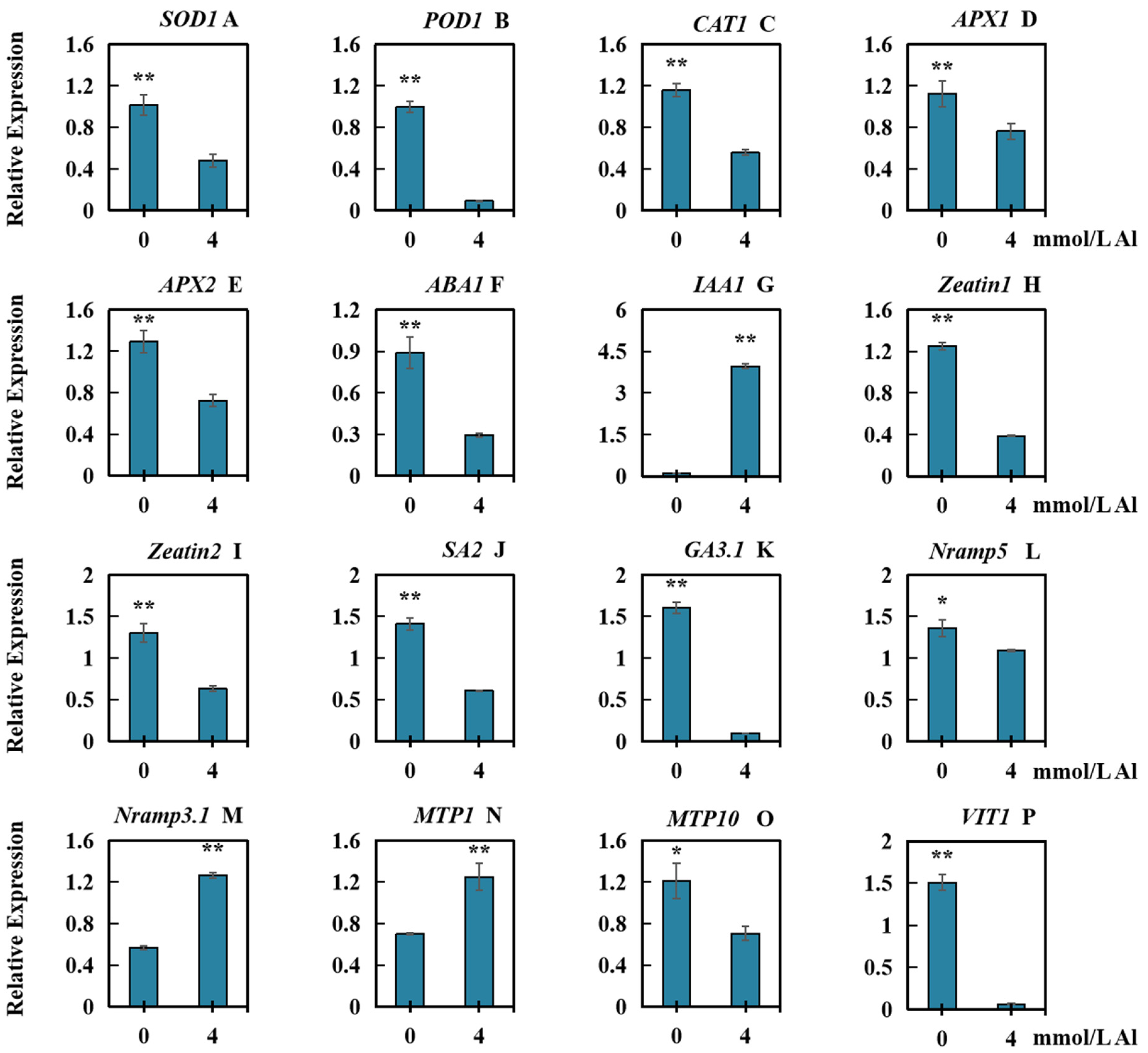
2.6. Effects of Al Toxicity Stress on the Relative Transcript Levels of Several Types of Genes in Roots of Peanut
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Hydroponic Treatment
4.2. Measurement of Dry and Fresh Weight of Roots in Peanut
4.3. Determination of Peanut Root Morphological Indices
4.4. Determination of Peanut Root Physiological Response Indices
4.5. Determination of Ion Content in Peanut Roots
4.6. Determination of the 6 Kinds of Hormones in Peanut Roots
4.7. Fluorescence Quantitative PCR (qPCR) Analysis
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, S.Q.; Li, X.X.; Luo, J.; Han, R.F.; Chen, Q.Q.; Shen, D.S.; Shentu, J.L. Soil heterogeneity influence on the distribution of heavy metals in soil during acid rain infiltration: Experimental and numerical modeling. J. Environ. Manag. 2022, 322, 116144. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.F.; Sun, X.M.; Sun, Y.Q.; Yan, J.Y.; Zhao, Y.F.; Chen, J. Soil acidification and factors controlling topsoil pH shift of cropland in central China from 2008 to 2018. Geoderma 2022, 408, 115586. [Google Scholar] [CrossRef]
- Han, T.F.; Liu, K.L.; Huang, J.; Ma, C.B.; Zheng, L.; Wang, H.Y.; Qu, X.L.; Ren, Y.; Yu, Z.K.; Zhang, H.M. Spatio-temporal evolution of soil pH and its driving factors in the main Chinese farmland during past 30 years. J. Plant Nutr. Fertil. 2020, 26, 2137–2149. [Google Scholar]
- Whitley, A.E.; Moir, J.L.; Almond, P.C. A meta-analysis of exchangeable aluminium in New Zealand soils using the National Soils Database. Soil Res. 2019, 57, 113–123. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Li, C.X.; Hou, C.C.; Chen, L.Y.; Kaskel, S.; Xu, Q. Rechargeable Al-ion batteries. EnergyChem 2021, 3, 100049. [Google Scholar] [CrossRef]
- Huang, W.J.; Yang, X.D.; Yao, S.C.; LwinOo, T.; He, H.Y.; Wang, A.Q.; Li, C.Z.; He, L.F. Reactive oxygen species burst induced by aluminum stress triggers mitochondria-dependent programmed cell death in peanut root tip cells. Plant Physiol. Biochem. 2014, 82, 76–84. [Google Scholar] [CrossRef]
- Xiong, J.; Ding, G.; Li, S.Y.; Chen, L.L.; Song, L.Q. Effects of aluminum stress on growth, development and nutrient uptake of different aluminum-tolerant rapeseed cultivars at seedling stage. Acta Agric. Boreali-Sin. 2020, 35, 165–171. (In Chinese) [Google Scholar]
- Mohanty, S.; Das, A.B.; Das, P.; Mohanty, P. Effect of a low dose of aluminum on mitotic and meiotic activity, 4C DNA content, and pollen sterility in rice, Oryza sativa L. cv. Lalat. Ecotoxicol. Environ. Saf. 2004, 59, 70–75. [Google Scholar] [CrossRef]
- Sousa, A.D.; AbdElgawad, H.; Fidalgo, F.; Teixeira, J.; Matos, M.; Tamagnini, P.; Fernandes, R.; Figueiredo, F.; Azenha, M.; Teles, L.O.; et al. Subcellular compartmentalization of aluminum reduced its hazardous impact on rye photosynthesis. Environ. Pollut. 2022, 315, 120313. [Google Scholar] [CrossRef]
- Xu, S.S.; Wu, L.H.; Li, L.Y.; Zhong, M.H.; Tang, Y.; Cao, G.Q.; Lin, K.M.; Ye, Y.Q. Aluminum-induced alterations to the cell wall and antioxidant enzymes involved in the regulation of the aluminum tolerance of Chinese Fir (Cunninghamia lanceolata). Front. Plant Sci. 2022, 13, 891117. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Ding, G.; Chen, L.L.; Li, S.Y.; Zou, X.Y.; Song, L.Q. Effects of aluminum stress on physiological characteristics and yield components of two rapeseed varieties. J. Gansu Agric. Univ. 2019, 54, 43–50. (In Chinese) [Google Scholar]
- Yan, L.; Riaz, M.; Li, S.; Cheng, J.; Jiang, C.C. Harnessing the power of exogenous factors to enhance plant resistance to aluminum toxicity; a critical review. Plant Physiol. Biochem. 2023, 203, 108064. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Li, X.; Zhou, Y.Y.; Wei, L.; Keovongkod, C.; He, H.Y.; Zhan, J.; Wang, A.Q.; He, L.F. Transcriptome analysis reveals significant difference in gene expression and pathways between two peanut cultivars under Al stress. Gene 2021, 781, 145535. [Google Scholar] [CrossRef]
- Liao, B.S. Current situation and potential analysis of peanut production in China. Chin. J. Oil Crop Sci. 2020, 42, 161–166. (In Chinese) [Google Scholar]
- Yan, M.L.; Ge, W.W.; Zhang, X.; Huang, Y.N.; Zhang, Z.D.; Zhang, Y. The situation analysis and development of oil industry in China countermeasures. China Oils Fats 2023, 48, 8–18. (In Chinese) [Google Scholar]
- Takeuchi, J.; Fukui, K.; Seto, Y.; Takaoka, Y.; Okamoto, M. Ligand–receptor interactions in plant hormone signaling. Plant J. 2021, 105, 290–306. [Google Scholar] [CrossRef]
- Zluhan-Martínez, E.; López-Ruíz, B.A.; García-Gómez, M.L.; García-Ponce, B.; Sánchez, M.D.L.P.; Álvarez-Buylla, E.R.; Garay-Arroyo, A. Integrative roles of phytohormones on cell proliferation, elongation and differentiation in the Arabidopsis thaliana primary root. Front. Plant Sci. 2021, 12, 659155. [Google Scholar] [CrossRef]
- Li, M.X.; Zhu, Y.C.; Li, S.S.; Zhang, W.; Yin, C.X.; Lin, Y.J. Regulation of phytohormones on the growth and development of plant root hair. Front. Plant Sci. 2022, 13, 865302. [Google Scholar] [CrossRef]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef]
- Takatsuka, H.; Umeda, M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014, 65, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.L.; Chen, F.; Du, H.W.; Zhang, X.K.; Wang, X.G.; Yao, G.X.; Xu, B.B. Graphene oxide and indole-3-acetic acid cotreatment regulates the root growth of Brassica napus L. via multiple phytohormone pathways. BMC Plant Biol. 2020, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Baskin, T.I.; Preston, S.; Zelinsky, E.; Yang, X.L.; Elmali, M.; Bellos, D.; Wells, D.M.; Bennett, M.J. Positioning the root elongation zone is saltatory and receives input from the shoot. iScience 2020, 23, 101309. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.; Garcia, M.N.F.; Powers, S.J.; Vaughan, S.; Bennett, M.J.; Phillips, A.L.; Thomas, S.G.; Hedden, P. Mapping sites of gibberellin biosynthesis in the Arabidopsis root tip. New Phytol. 2020, 229, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Pandey, B.K.; Li, Y.X.; Huang, G.Q.; Wang, J.; Quan, R.D.; Zhou, J.H.; Zhou, Y.; Miao, Y.C.; Zhang, D.B.; et al. Orchestration of ethylene and gibberellin signals determines primary root elongation in rice. Plant Cell 2022, 34, 1273–1288. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Shen, P.; Sun, X.W.; Wu, Z.F.; Yu, T.Y.; Feng, H.; Sun, Q.Q.; Wu, J.X.; Wang, C.B.; Wu, Y. Quantifying the role of peanut root and root nodule in nitrogen absorption and fixation under four forms of N fertilizers. J. Agric. Food Res. 2022, 9, 100334. [Google Scholar] [CrossRef]
- Muhammad, N.; Zvobgo, G.; Zhang, G.P. A review: The beneficial effects and possible mechanisms of aluminum on plant growth in acidic soil. J. Integr. Agric. 2019, 18, 1518–1528. [Google Scholar] [CrossRef]
- Kochian, V.L.; Piñeros, A.M.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, D.K.; Singh, S.; Sharma, S.; Dubey, N.K.; Chauhan, D.K.; Vaculík, M. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017, 137, 177–193. [Google Scholar] [CrossRef]
- Li, W.N.; Ullah, S.; Xu, Y.Y.; Bai, T.D.; Ye, S.M.; Jiang, W.X.; Yang, M. Effects of elevated aluminum concentration and distribution on root damage, cell wall polysaccharides, and nutrient uptake in different tolerant eucalyptus clones. Int. J. Mol. Sci. 2022, 23, 13438. [Google Scholar] [CrossRef]
- Liu, J.; Piñeros, M.A.; Kochian, L.V. The role of aluminum sensing and signaling in plant aluminum resistance. J. Integr. Plant Biol. 2014, 56, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Rehman, R.U.; Hakeem, K.R.; Alharby, H.F. Aluminium stress modulates the osmolytes and enzyme defense system in Fagopyrum species. Plant Physiol. Biochem. 2019, 144, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Killi, D.; Raschi, A.; Bussotti, F. Lipid peroxidation and chlorophyll fluorescence of photosystem II performance during drought and heat stress is associated with the antioxidant capacities of C3 sunflower and C4 maize varieties. Int. J. Mol. Sci. 2020, 21, 4846. [Google Scholar] [CrossRef]
- Ru, C.; Wang, K.F.; Hu, X.T.; Chen, D.Y.; Wang, W.; Yang, H.S. Nitrogen modulates the effects of heat, drought, and combined stresses on photosynthesis, antioxidant capacity, cell osmoregulation, and grain yield in winter wheat. J. Plant Growth Regul. 2023, 42, 1681–1703. [Google Scholar] [CrossRef]
- Dreyer, B.H.; Schippers, J.H.M. Copper-Zinc superoxide dismutases in plants: Evolution, enzymatic properties, and beyond. Annu. Plant Rev. Online 2019, 2, 933–968. [Google Scholar]
- Su, W.; Raza, A.; Gao, A.; Jia, Z.; Zhang, Y.; Hussain, M.A.; Mehmood, S.S.; Cheng, Y.; Lv, Y.; Zou, X. Genome-wide analysis and expression profile of superoxide dismutase (sod) gene family in rapeseed (Brassica napus L.) under different hormones and abiotic stress conditions. Antioxidants 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.L.; Wang, C.P.; Khan, N.; Chen, M.; Fu, W.; Guan, L.; Leng, X. Genome-wide identification of the class III POD gene family and their expression profiling in grapevine (Vitis vinifera L.). BMC Genom. 2020, 21, 444. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Ding, X.P.; Ding, Z.H.; Tie, W.W.; Yan, Y.; Wang, Y.; Yang, H.; Hu, W. The class III peroxidase (POD) gene family in cassava: Identification, phylogeny, duplication, and expression. Int. J. Mol. Sci. 2019, 20, 2730. [Google Scholar] [CrossRef]
- Yang, T.T.; Zhang, P.Y.; Pan, J.H.; Amanullah, S.; Luan, F.S.; Han, W.H.; Liu, H.Y.; Wang, X.Z. Genome-wide analysis of the peroxidase gene family and verification of lignin synthesis-related genes in watermelon. Int. J. Mol. Sci. 2022, 23, 642. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.J.; Yun, L.; Ji, L.; Li, G.H.; Ji, M.C.; Shi, Y.; Zheng, X. Catalase (CAT) gene family in wheat (Triticum aestivum L.): Evolution, expression pattern and function analysis. Int. J. Mol. Sci. 2022, 23, 542. [Google Scholar] [CrossRef]
- Raza, A.; Su, W.; Gao, A.; Mehmood, S.S.; Hussain, M.A.; Nie, W.L.; Lv, Y.; Zhang, X.L. Catalase (CAT) gene family in rapeseed (Brassica napus L.): Genome-wide analysis, identification, and expression pattern in response to multiple hormones and abiotic stress conditions. Int. J. Mol. Sci. 2021, 22, 4281. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Sharif, Y.; Chen, K.; Wang, L.H.; Fu, H.W.; Zhuang, Y.H.; Chitikineni, A.; Chen, H.; Zhang, C.; Varshney, R.K.; et al. Genome-wide characterization of ascorbate peroxidase gene family in peanut (Arachis hypogea L.) revealed their crucial role in growth and multiple stress tolerance. Front. Plant Sci. 2022, 13, 962182. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.Y.; Tan, J.H.; Chen, H.; Yang, Z.Q.; Luo, Q.F.; Jia, J. Physiology, transcriptome and root exudates analysis of response to aluminum stress in Pinus massoniana. Forests 2023, 14, 1410. [Google Scholar] [CrossRef]
- Jiang, X.F.; Chen, H.; Liao, Y.C.; Ye, Z.Q.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef]
- Qiu, G.K.; Han, Z.M.; Wang, Q.Y.; Wang, T.Y.; Sun, Z.H.; Yu, Y.; Han, X.R.; Yu, H.W. Toxicity effects of nanoplastics on soybean (Glycine max L.): Mechanisms and transcriptomic analysis. Chemosphere 2022, 313, 137571. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Tian, Y.; Wang, X.R.; Geng, J.J.; Jiang, J.L.; Yu, H.X.; Wang, C. Lead-contaminated soil induced oxidative stress, defense response and its indicative biomarkers in roots of Vicia faba seedlings. Ecotoxicology 2010, 19, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, X.J.; Wu, W. Effects of malondialdehyde-induced protein oxidation on the structural characteristics of rice protein. Int. J. Food Sci. Technol. 2020, 55, 760–768. [Google Scholar] [CrossRef]
- Sun, C.L.; Lu, L.L.; Yu, Y.; Liu, L.J.; Hu, Y.; Ye, Y.Q.; Jin, C.W.; Lin, X.Y. Decreasing methylation of pectin caused by nitric oxide leads to higher aluminium binding in cell walls and greater aluminium sensitivity of wheat roots. J. Exp. Bot. 2016, 67, 979–989. [Google Scholar] [CrossRef]
- Tian, X.Y.; He, D.D.; Bai, S.; Zeng, W.Z.; Wang, Z.; Wang, M.; Wu, L.Q.; Chen, Z.C. Physiological and molecular advances in magnesium nutrition of plants. Plant Soil 2021, 468, 1–17. [Google Scholar] [CrossRef]
- Muhammad, N.; Cai, S.G.; Shah, J.M.; Zhang, G.P. The combined treatment of Mn and Al alleviates the toxicity of Al or Mn stress alone in barley. Acta Physiol. Plant. 2016, 38, 277. [Google Scholar] [CrossRef]
- Muhammad, N.; Zvobgo, G.; Fu, L.B.; Lwalaba, J.L.W.; Zhang, G.P. Physiological mechanisms for antagonistic interaction of manganese and aluminum in barley. J. Plant Nutr. 2019, 42, 466–476. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.Q.; Xu, Q.S.; Pan, L.; Kong, Y.L.; Zhu, L.F.; Cao, X.C.; Tian, W.H.; Liu, J.; Jin, Q.Y.; Xiang, X.J.; et al. Mechanism of interaction between calcium ions and hydrogen sulfide to alleviate the inhibitory effect of aluminum on root elongation of rice. Chin. J. Rice Sci. 2023, 37, 142–152. (In Chinese) [Google Scholar]
- Liu, Y.; Chen, J.Y.; Li, X.H.; Yang, S.X.; Wu, Z.W.; Xue, Y.B.; Chen, J.P. Physiological mechanisms in which manganese toxicity inhibits root growth in soybean. J. Soil Sci. Plant Nutr. 2023, 23, 4141–4156. [Google Scholar] [CrossRef]
- Tan, Z.J.; Li, J.X.; Guan, J.H.; Wang, C.H.; Zhang, Z.; Shi, G.R. Genome-wide identification and expression analysis reveals roles of the NRAMP gene family in iron/cadmium interactions in peanut. Int. J. Mol. Sci. 2023, 24, 1713. [Google Scholar] [CrossRef]
- Qin, L.; Han, P.P.; Chen, L.Y.; Walk, T.C.; Li, Y.S.; Hu, X.J.; Xie, L.H.; Liao, H.; Liao, X. Genome-wide identification and expression analysis of NRAMP family genes in soybean (Glycine max L.). Front. Plant Sci. 2017, 8, 1436. [Google Scholar] [CrossRef]
- Wang, X.Q.; Wang, C.H.; Zhang, Z.; Shi, G.R. Genome-wide identification of metal tolerance protein genes in peanut: Differential expression in the root of two contrasting cultivars under metal stresses. Front. Plant Sci. 2022, 13, 791200. [Google Scholar] [CrossRef]
- El-Sappah, A.H.; Abbas, M.; Rather, S.A.; Wani, S.H.; Soaud, N.; Noor, Z.; Huang, Q.L.; Eldomiaty, A.S.; Mir, R.R.; Li, J. Genome-wide identification and expression analysis of metal tolerance protein (MTP) gene family in soybean (Glycine max) under heavy metal stress. Mol. Biol. Rep. 2023, 50, 2975–2990. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Liu, Y.K.; Luo, J.P.; Li, J.X.; Kováč, J.; Li, T.Q. Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii. Plant Cell Environ. 2019, 42, 1425–1440. [Google Scholar] [CrossRef]
- Sussmilch, F.C.; Atallah, N.M.; Brodribb, T.J.; Banks, J.A.; McAdam, S.A.M. Abscisic acid (ABA) and key proteins in its perception and signaling pathways are ancient, but their roles have changed through time. Plant Signal. Behav. 2017, 12, e1365210. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takasaki, H.; Takahashi, F.; Suzuki, T.; Iuchi, S.; Mitsuda, N.; Ohme-Takagi, M.; Ikeda, M.; Seo, M.; Yamaguchi-Shinozaki, K.; et al. Arabidopsis thaliana NGATHA1 transcription factor induces ABA biosynthesis by activating NCED3 gene during dehydration stress. Proc. Natl. Acad. Sci. USA 2018, 115, 11178–11187. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Léran, S.; Corratgé-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA transport and transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, W.; Wang, Q.W.; Cao, Y.Y.; Xu, F.Y.; Dodd, I.C.; Xu, W.F. ABA regulation of root growth during soil drying and recovery can involve auxin response. Plant Cell Environ. 2022, 45, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef]
- Nordström, A.; Tarkowski, P.; Tarkowska, D.; Norbaek, R.; Åstot, C.; Dolezal, K.; Sandberg, G. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: A factor of potential importance for auxin–cytokinin-regulated development. Proc. Natl. Acad. Sci. USA 2004, 101, 8039–8044. [Google Scholar] [CrossRef]
- Kotov, A.A.; Kotova, L.M.; Romanov, G.A. Signaling network regulating plant branching: Recent advances and new challenges. Plant Sci. 2021, 307, 110880. [Google Scholar] [CrossRef]
- Antoniadi, I.; Mateo-Bonmati, E.; Pernisova, M.; Brunoni, F.; Antoniadi, M.; Villalonga, M.G.A.; Ament, A.; Karády, M.; Turnbull, C.; Doležal, K.; et al. IPT9, a cis-zeatin cytokinin biosynthesis gene, promotes root growth. Front. Plant Sci. 2022, 13, 932008. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; La, S.K.; Bishi, S.K.; Singh, A.K. Phytohormone signalling and cross-talk to alleviate aluminium toxicity in plants. Plant Cell Rep. 2021, 40, 1331–1343. [Google Scholar] [CrossRef]
- Figueiredo, M.R.A.D.; Strader, L.C. Intrinsic and extrinsic regulators of Aux/IAA protein degradation dynamics. Trends Biochem. Sci. 2022, 47, 865–874. [Google Scholar] [CrossRef]
- Hu, J.; Su, H.L.; Cao, H.; Wei, H.B.; Fu, X.K.; Jiang, X.M.; Song, Q.; He, X.H.; Xu, C.Z.; Luo, K.M. AUXIN RESPONSE FACTOR7 integrates gibberellin and auxin signaling via interactions between DELLA and AUX/IAA proteins to regulate cambial activity in poplar. Plant Cell 2022, 34, 2688–2707. [Google Scholar] [CrossRef] [PubMed]
- Li, G.J.; Song, H.Y.; Li, B.H.; Kronzucker, H.J.; Shi, W.M. Auxin resistant1 and PIN-FORMED2 protect lateral root formation in Arabidopsis under iron stress. Plant Physiol. 2015, 169, 2608–2623. [Google Scholar] [PubMed]
- Zhao, J.J.; Wang, W.Y.; Zhou, H.K.; Wang, R.L.; Zhang, P.; Wang, H.C.; Pan, X.L.; Xu, J. Mn toxicity inhibited root growth by disrupting auxin biosynthesis and transport in Arabidopsis. Front. Plant Sci. 2017, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Budimir, J.; Trefon, K.; Nair, A.; Thurow, C.; Gatz, C. Redox–active cysteines in TGACG-BINDING FACTOR 1 (TGA1) do not play a role in salicylic acid or pathogen-induced expression of TGA1-regulated target genes in Arabidopsis thaliana. New Phytol. 2021, 230, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.Y.; Ma, Z.M.; Wang, D.Y.; Sun, Y.B.; Wen, C.J.; Huang, D.Q.; Chen, Z.C.; Yang, L.; Tan, S.T.; Li, R.X.; et al. Salicylic acid regulates PIN2 auxin transporter hyperclustering and root gravitropic growth via Remorin-dependent lipid nanodomain organisation in Arabidopsis thaliana. New Phytol. 2021, 229, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Ulloa-Inostroza, E.M.; Alberdi, M.; Meriño-Gergichevich, C.; Reyes-Díaz, M. Low doses of exogenous methyl jasmonate applied simultaneously with toxic aluminum improve the antioxidant performance of Vaccinium corymbosum. Plant Soil 2017, 412, 81–96. [Google Scholar] [CrossRef]
- Zhang, N.L.; Zhou, S.; Yang, D.Y.; Fan, Z.J. Revealing shared and distinct genes responding to JA and SA signaling in Arabidopsis by meta-analysis. Front. Plant Sci. 2020, 11, 908. [Google Scholar] [CrossRef]
- Maurer, F.; Müller, S.; Bauer, P. Suppression of Fe defciency gene expression by jasmonate. Plant Physiol. Biochem. 2011, 49, 530–536. [Google Scholar] [CrossRef]
- Bagale, P.; Pandey, S.; Regmi, P.; Bhusal, S. Role of plant growth regulator “Gibberellins” in vegetable production: An overview. Int. J. Hortic. Sci. Technol. 2022, 9, 291–299. [Google Scholar]
- Teshome, S.; Kebede, M. Analysis of regulatory elements in GA2ox, GA3ox and GA20ox gene families in Arabidopsis thaliana: An important trait. Biotechnol. Biotechnol. Equip. 2021, 35, 1603–1612. [Google Scholar] [CrossRef]
- Víctor, Q. Advances in the molecular mechanisms of abscisic acid and gibberellins functions in plants. Int. J. Mol. Sci. 2021, 22, 6080. [Google Scholar]
- Wang, P.; Zhou, S.J.; Li, A.; Xie, L.B. Influence of aluminum at low pH on the rhizosphere processes of Masson pine (Pinus massoniana Lamb). Plant Growth Regul. 2022, 97, 499–510. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.H.; Li, J.Y.; Chen, J.Y.; Yang, S.X.; Zhao, M.; Xue, Y.B. Transcriptome sequencing analysis of root in soybean responding to Mn poisoning. Int. J. Mol. Sci. 2023, 24, 12727. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Li, J.Y.; Pan, Y.H.; Chen, J.Y.; Liu, Y. Metabolic responses to manganese toxicity in soybean roots and leaves. Plants 2023, 12, 3615. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Y.B.; Xie, B.X.; Zhu, S.N.; Lu, X.; Liang, C.Y.; Tian, J. Complex gene regulation between young and old soybean leaves in responses to manganese toxicity. Plant Physiol. Biochem. 2020, 155, 231–242. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, M.; Chen, J.Y.; Yang, S.X.; Chen, J.P.; Xue, Y.B. Comparative transcriptome analysis reveals complex physiological response and gene regulation in peanut roots and leaves under manganese toxicity stress. Int. J. Mol. Sci. 2023, 24, 1161. [Google Scholar] [CrossRef]
- Pornaro, C.; Macolino, S.; Menegon, A.; Richardson, M. WinRHIZO technology for measuring morphological traits of bermudagrass stolons. Agron. J. 2017, 109, 3007–3010. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, R.M.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol. Plant. 2019, 41, 23. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Zheng, D.F.; Feng, N.J.; Zhou, H.; Mu, D.W.; Liu, L.; Zhao, L.M.; Shen, X.F.; Rao, G.H.; Li, T.Z. Effects of exogenous salicylic acid and abscisic acid on growth, photosynthesis and antioxidant system of rice. Chil. J. Agric. Res. 2022, 82, 21–32. [Google Scholar] [CrossRef]
- Rácz, A.; Hideg, É.; Czégény, G. Selective responses of class III plant peroxidase isoforms to environmentally relevant UV-B doses. J. Plant Physiol. 2018, 221, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M.I. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 18. [Google Scholar] [CrossRef]
- Ekinci, M.; Ors, S.; Yildirim, E.; Turan, M.; Sahin, U.; Dursun, A.; Kul, R. Determination of physiological indices and some antioxidant enzymes of chard exposed to nitric oxide under drought stress. Russ. J. Plant Physiol. 2020, 67, 740–749. [Google Scholar] [CrossRef]
- Li, C.G.; Li, J.X.; Du, X.H.; Zhang, J.X.; Zou, Y.R.; Liu, Y.D.; Li, Y.; Lin, H.Y.; Li, H.; Liu, D.; et al. Chloroplast thylakoidal ascorbate peroxidase, PtotAPX, has enhanced resistance to oxidative stress in Populus tomentosa. Int. J. Mol. Sci. 2022, 23, 3340. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Manousi, N.; Zachariadis, G.A. Determination of the toxic and nutrient element content of almonds, walnuts, hazelnuts and pistachios by ICP-AES. Separations 2021, 8, 28. [Google Scholar] [CrossRef]
- Khew, C.Y.; Mori, I.C.; Matsuura, T.; Hirayama, T.; Harikrishna, J.A.; Lau, E.T.; Mercer, Z.J.A.; Hwang, S.S. Hormonal and transcriptional analyses of fruit development and ripening in different varieties of black pepper (Piper nigrum). J. Plant Res. 2020, 133, 73–94. [Google Scholar] [CrossRef]
- Xue, Y.B.; Zhuang, Q.L.; Zhu, S.N.; Xiao, B.X.; Liang, C.Y.; Liao, H.; Tian, J. Genome wide transcriptome analysis reveals complex regulatory mechanisms underlying phosphate homeostasis in soybean nodules. Int. J. Mol. Sci. 2018, 19, 2924. [Google Scholar] [CrossRef]
- Zhuang, Q.L.; Xue, Y.B.; Yao, Z.F.; Zhu, S.N.; Liang, C.Y.; Liao, H.; Tian, J. Phosphate starvation responsive GmSPX5 mediates nodule growth through interaction with GmNF-YC4 in soybean (Glycine max). Plant J. 2021, 108, 1422–1438. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, M.; Shi, J.N.; Yang, S.X.; Xue, Y.B. Genome-wide identification of AhMDHs and analysis of gene expression under manganese toxicity stress in Arachis hypogaea. Genes 2023, 14, 2109. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Zhao, M.; Zhang, F.; Feng, D.; Yang, S.; Xue, Y.; Liu, Y. Physiological Mechanism through Which Al Toxicity Inhibits Peanut Root Growth. Plants 2024, 13, 325. https://doi.org/10.3390/plants13020325
Shi J, Zhao M, Zhang F, Feng D, Yang S, Xue Y, Liu Y. Physiological Mechanism through Which Al Toxicity Inhibits Peanut Root Growth. Plants. 2024; 13(2):325. https://doi.org/10.3390/plants13020325
Chicago/Turabian StyleShi, Jianning, Min Zhao, Feng Zhang, Didi Feng, Shaoxia Yang, Yingbin Xue, and Ying Liu. 2024. "Physiological Mechanism through Which Al Toxicity Inhibits Peanut Root Growth" Plants 13, no. 2: 325. https://doi.org/10.3390/plants13020325
APA StyleShi, J., Zhao, M., Zhang, F., Feng, D., Yang, S., Xue, Y., & Liu, Y. (2024). Physiological Mechanism through Which Al Toxicity Inhibits Peanut Root Growth. Plants, 13(2), 325. https://doi.org/10.3390/plants13020325




