Growth, Evapotranspiration, Gas Exchange and Chl a Fluorescence of Ipê-Rosa Seedlings at Different Levels of Water Replacement
Abstract
1. Introduction
2. Material and Methods
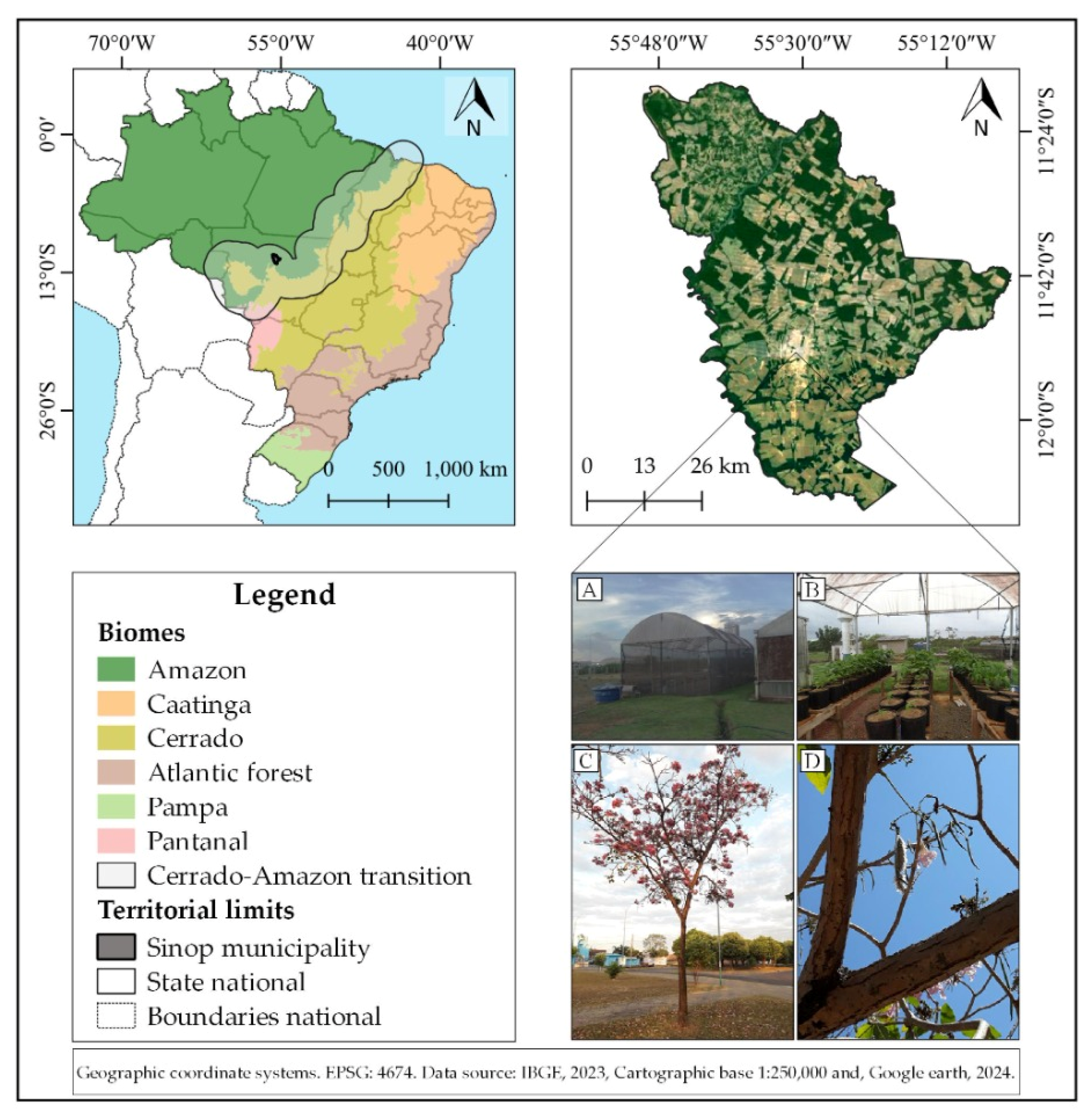
2.1. Experimental Site
2.2. Obtaining and Germinating Seeds of Handroanthus Impetiginosus
2.3. Transplanting and Substrates for Seedling Production
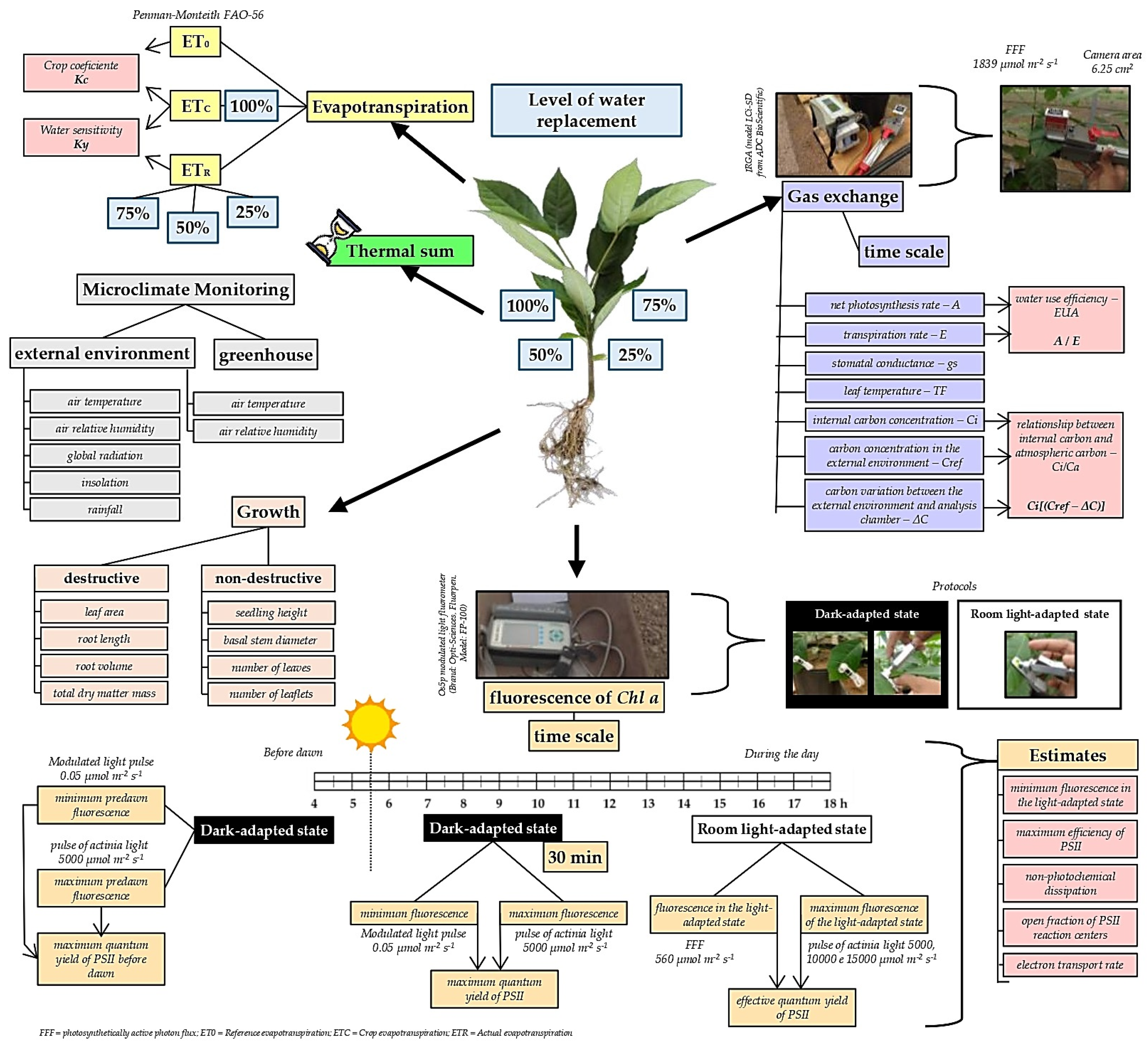
2.4. Substrate Moisture and Application of Treatments
2.5. Micrometeorological Monitoring
2.6. Reference Evapotranspiration, Crop Coefficients and Water Sensitivity
2.7. Seedling Growth Analysis
2.8. Gas Exchange and Fluorescence
2.9. Basal Temperatures and Thermal Sum
2.10. Data Analysis
3. Results
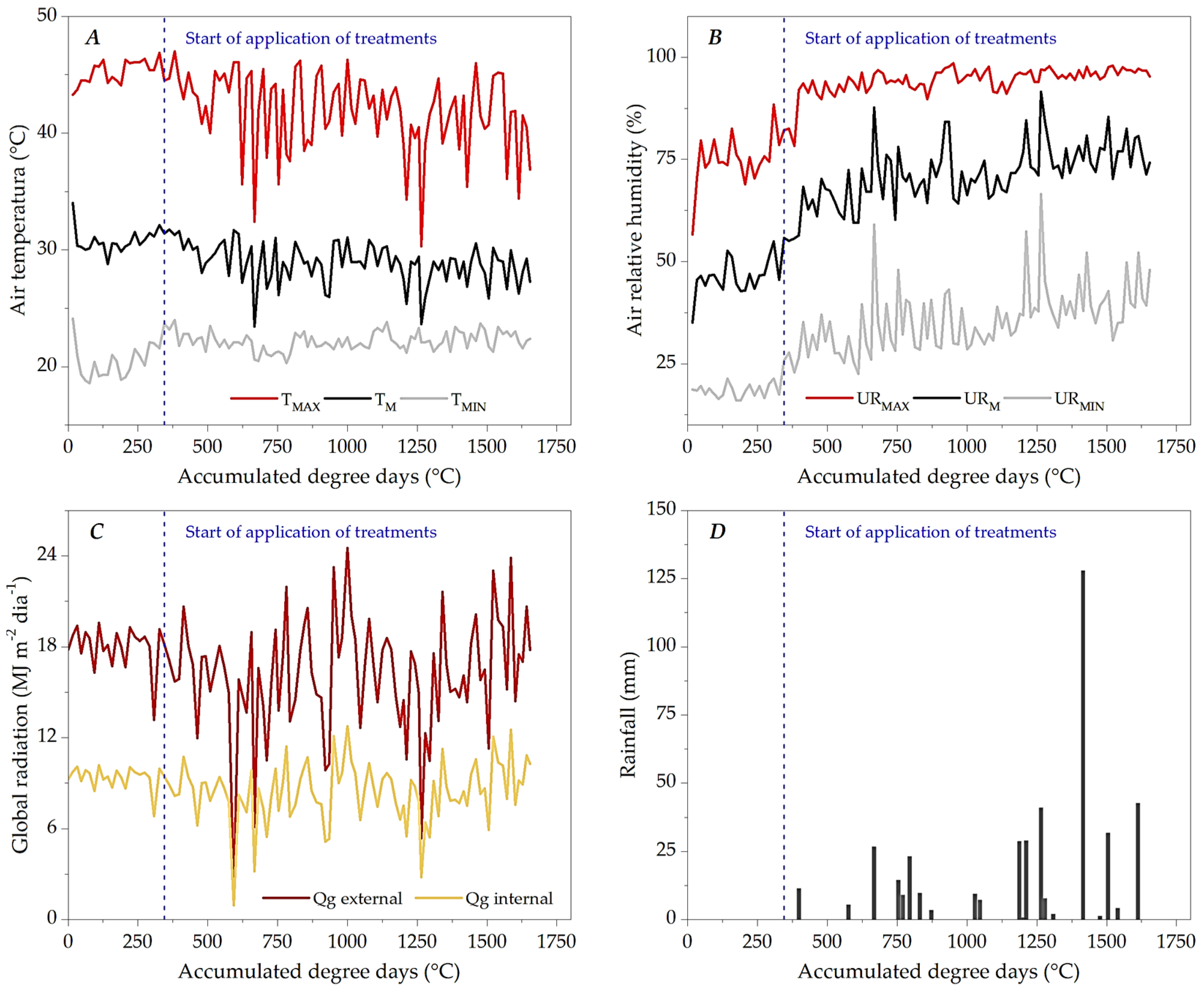
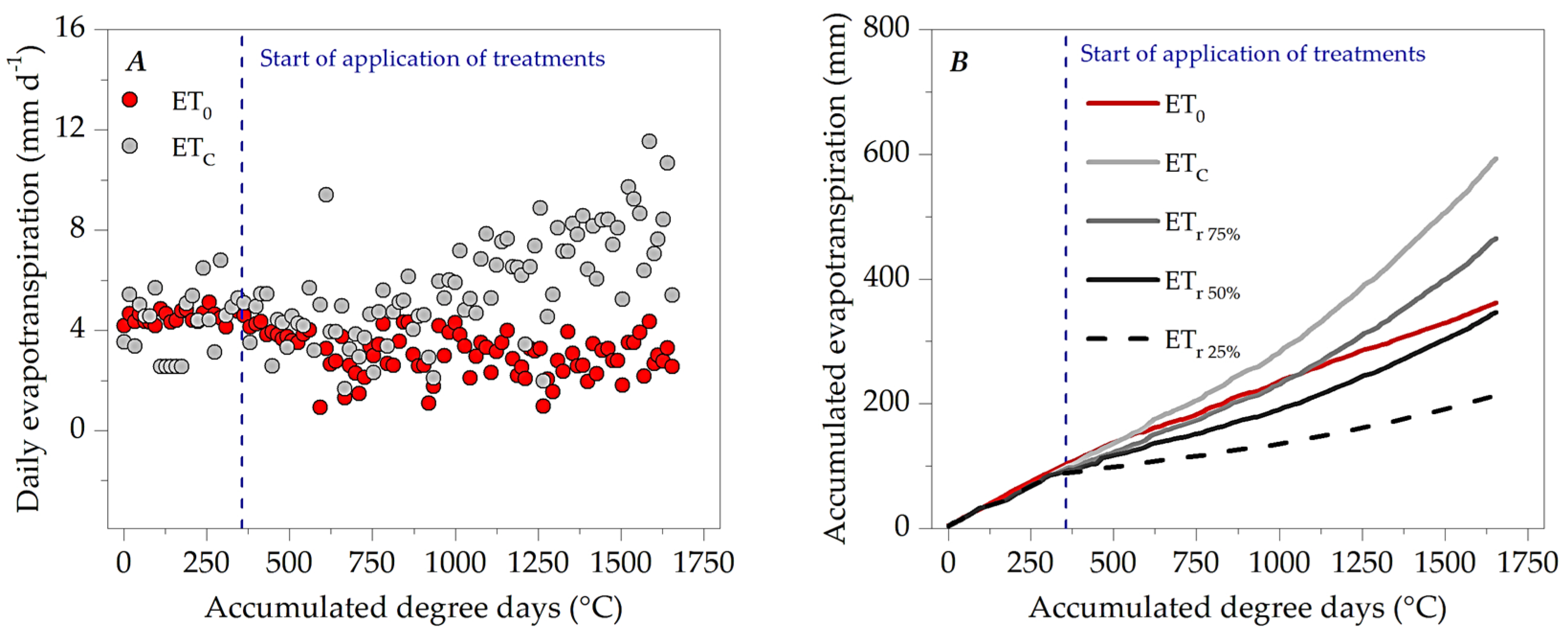
3.1. Microclimate Dynamics Throughout the Experimental Period
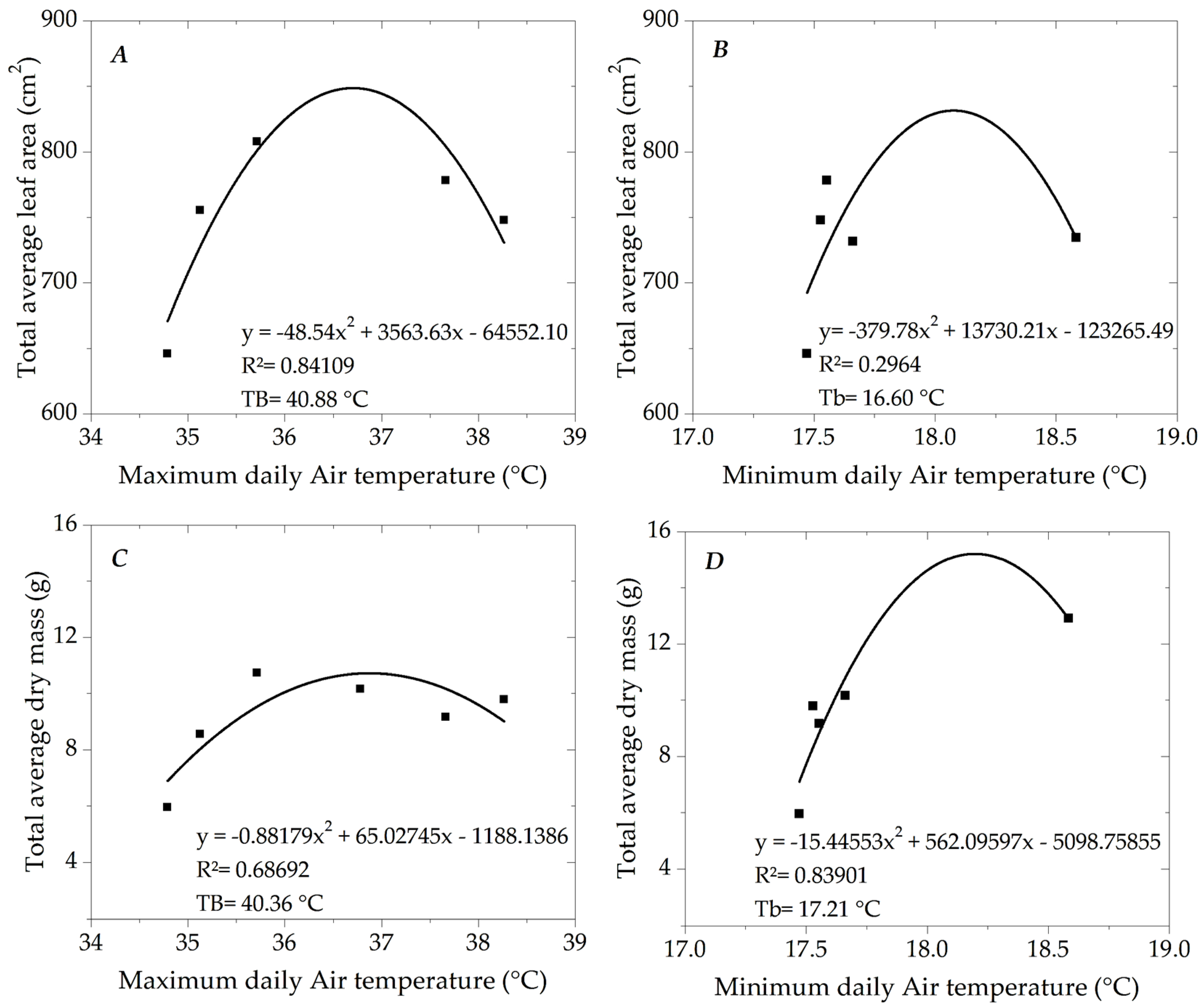
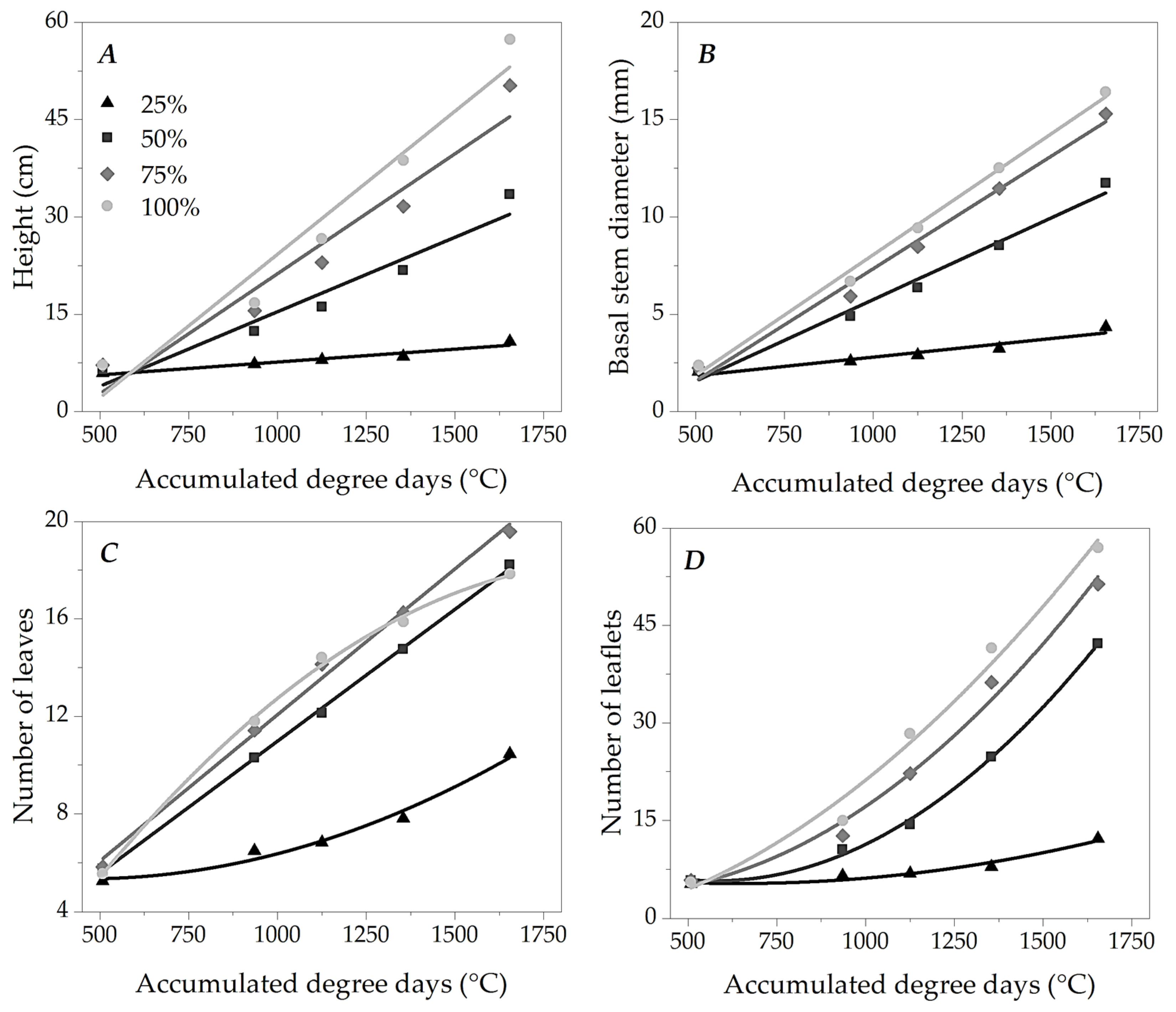
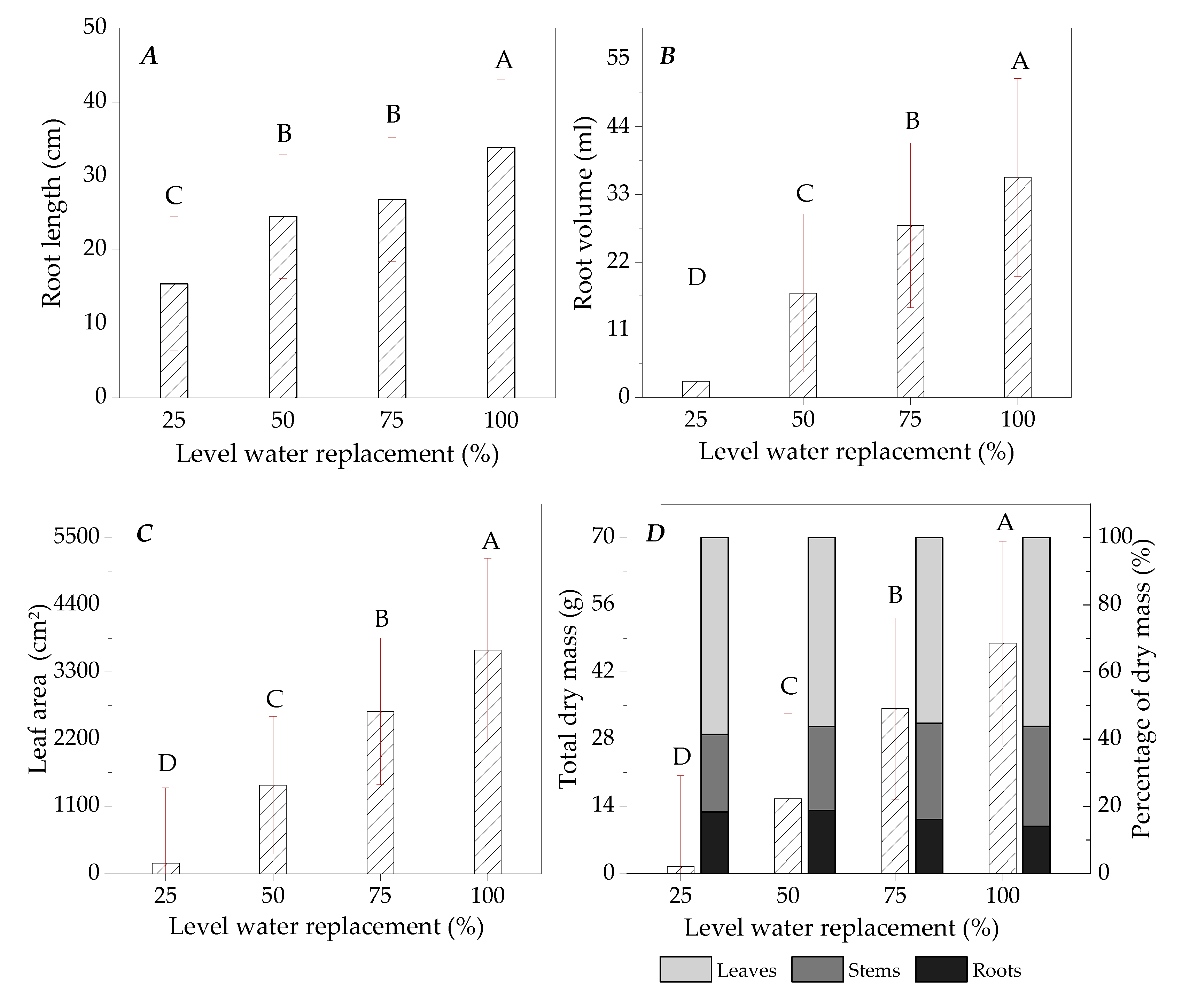
3.2. Growth of Ipê-Rosa Seedlings
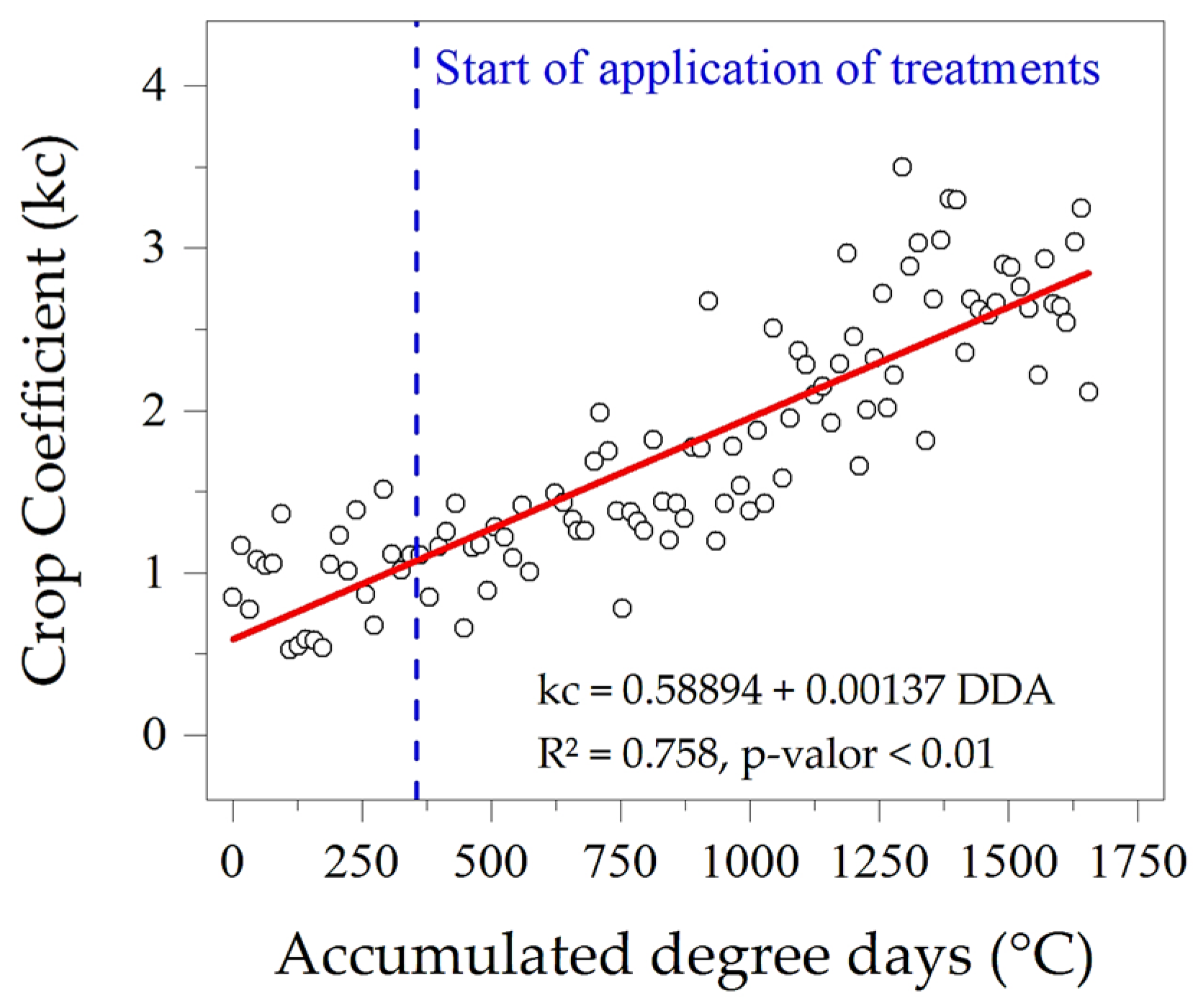
3.3. Evapotranspiration, Crop Coefficients and Water Sensitivity (Ky)
3.4. Diurnal Variations in Gas Exchange in Ipê-Rosa Seedlings
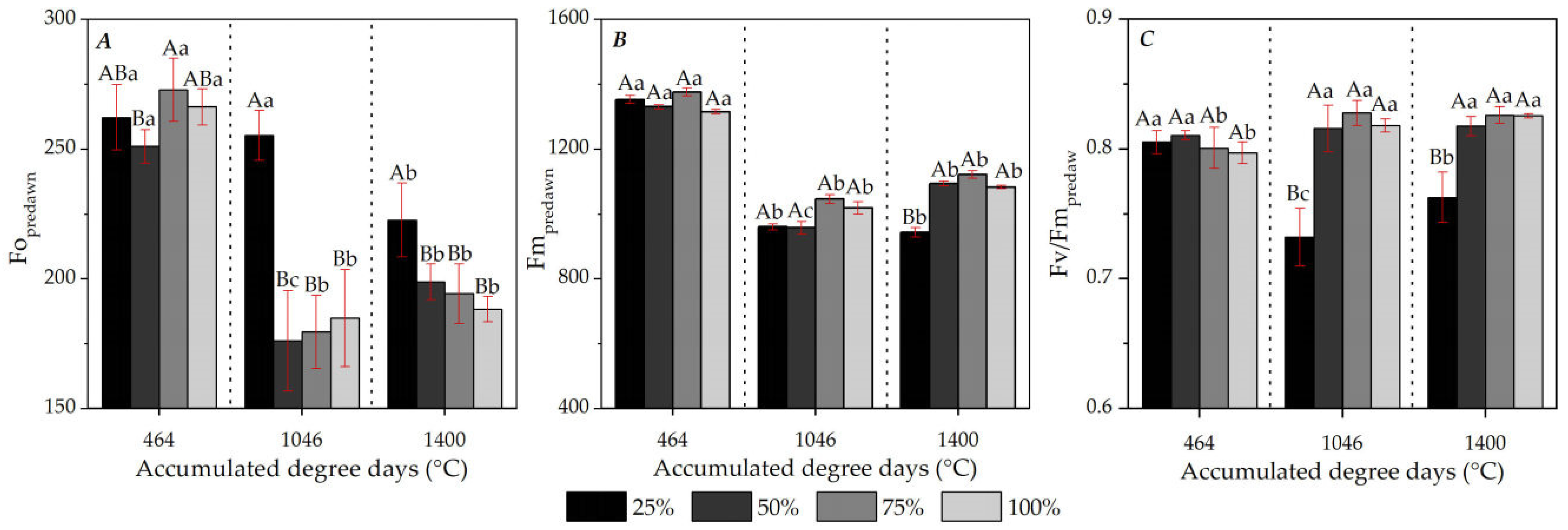
3.5. Chl a Fluorescence: Seasonality and Diurnal Variations
4. Discussion
4.1. Morphometric Growth of Ipê-Rosa Seedlings
4.2. Water Requirements and Sensitivity of the Ipê-Rosa Seedlings
4.3. Gas Exchange of Ipê-Rosa Seedlings
4.4. Chl a Fluorescence of Ipê-Rosa Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lorenzi, H. Brazilian Trees: Manual for Identification and Cultivation of Tree Plants in Brazil, 4th ed.; Instituto Plantarum: Nova Odessa, Brazil, 2002; Volume 2, 368p. [Google Scholar]
- Pereira, M.S. Technical Manual Knowing and Producing Seeds and Seedlings from the Caatinga; Association Caatinga: Fortaleza, Brazil, 2011; 60p. [Google Scholar]
- Mendonça, A.M.C.; Lira, J.M.S.; Vilela, A.L.O.; Vieira, D.A.; Melo, N.C.; Barbosa, J.P.R.A.D. High aluminum concentration and initial establishment of Handroanthus impetiginosus: Clues about an Al non-resistant species in Brazilian Cerrado. J. For. Res. 2020, 31, 2075–2082. [Google Scholar] [CrossRef]
- Benevides, D.S.; Carvalho, F.G. Survey of bee flora present in Caatinga areas in the municipality of Caraúbas-RN. Soc. Territ. 2009, 21, 44–54. [Google Scholar]
- Vieira, C.R.; Araujo, M.M.V.; Ferreira, A.F. Base saturation in the initial growth of Tabebuia impetiginosa seedlings. Rev. Estud. Ambient. 2020, 22, 6–14. [Google Scholar] [CrossRef]
- Pimenta, J.M.A.; Souza, W.M.A.T.; Ferrari, C.S.; Vieira, F.A.; Fajardo, C.G.; Pacheco, M.V. Selection of Handroanthus impetiginosus mother trees to support seed collection areas. Rev. Árvore 2023, 47, e4706. [Google Scholar] [CrossRef]
- Lisboa, M.A.N.; Silva, L.V.A.; Nascimento, A.S.; Silva, A.O.; Teixeira, M.R.A.; Ferreira, M.F.R.; Ferreira, S.C.; Silva, A.C.V.; Colares, A.V.; Calixto Júnior, J.T. Diversity, structure, and carbon sequestration potential of the woody flora of urban squares in the Brazilian semiarid region. Trees For. People 2024, 16, 100561. [Google Scholar] [CrossRef]
- Morichetti, M.; Vangi, E.; Collalti, A. Predicted Future Changes in the Mean Seasonal Carbon Cycle Due to Climate Change. Forests 2024, 15, 1124. [Google Scholar] [CrossRef]
- Santos, M.E.C.D.; Melo, R.R.D.; Correia, D.; Sousa, J.A.d.; Santos, A.M.; Silva, A.K.V.D.; Paula, E.A.D.O.; Alves, A.R.; Scatolino, M.V.; Rusch, F.; et al. Variation in the Basic Density of Woods Produced in the Brazilian Semiarid Region Subjected to Different Irrigation Regimes. Forests 2023, 14, 2168. [Google Scholar] [CrossRef]
- Castellanos, J.R.G.; Prito, J.M.; Heinrich, M. Red Lapacho (Tabebuia impetiginosa)—A global ethnopharmacological commodity? J. Ethnopharmacol. 2009, 121, 1–13. [Google Scholar] [CrossRef]
- Lourenço, J.A.; Pitangui, C.P.; Jordão, A.A.; Vannucchi, H.; Cecchi, A.O. Absence of mutagenicity and antimutagenicity of the extract obtained from the flowers of ipê-roxo Tabebuia Impetiginosa (Mart. ex DC.) Standl. Rev. Bras. Plantas Med. 2010, 12, 414–420. [Google Scholar] [CrossRef]
- Zhang, J.; Hunto, S.T.; Yang, Y.; Lee, J.; Cho, J.Y. Tabebuia impetiginosa: A Comprehensive Review on Traditional Uses, Phytochemistry, and Immunopharmacological Properties. Molecules 2020, 25, 4294. [Google Scholar] [CrossRef]
- Nahar, J.; Morshed, M.N.; Rupa, E.J.; Lee, J.H.; Kariyarath Valappil, A.; Awais, M.; Hun, K.J.; Sook, L.J.; Al-Amin, M.; Ahn, J.C.; et al. Roasting Extract of Handroanthus impetiginosus Enhances Its Anticancer Activity in A549 Lung Cancer Cells and Improves Its Antioxidant and Anti-Inflammatory Effects in Normal Cells. Appl. Sci. 2023, 13, 13171. [Google Scholar] [CrossRef]
- Santos, L.C.D.; Azevedo, L.S.; Siqueira, E.P.; Castro, A.H.F.; Lima, L.A.R.S. Chemical characterization, antioxidant activity, and cytotoxicity of fatty acids methyl esters from Handroanthus impetiginosus (Mart. Ex DC.) Mattos (Bignoniaceae) seeds. Nat. Prod. Res. 2024, 38, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.P.; Nascimento, J.W.S.; Madalena, N.S., Jr.; Serafim, E.O.; Leandro, B.S.; Pereira, L.S.; Santos, M.C.C.; Nascimento, H.H.C. Ecophysiology of Handroanthus impetiginosus seedlings subjected to different irrigation cycles. Braz. J. Dev. 2020, 6, 36563–36574. [Google Scholar] [CrossRef]
- Juárez, R.I.N.; Hodnett, M.G.; Fu, R.; Goulden, M.L.; Randow, C.V. Control of Dry Season Evapotranspiration over the Amazonian Forest as Inferred from Observations at a Southern Amazon Forest Site. J. Clim. 2007, 20, 2827–2839. [Google Scholar] [CrossRef]
- Costa, A.C.; Rezende-Silva, S.L.; Megguer, C.A.; Moura, L.M.F.; Rosa, M.; Silva, A.A. The effect of irradiance and water restriction on photosynthesis in young jatobá-do-cerrado (Hymenea stigonocarpa) plants. Phoyosynthetica 2015, 53, 118–127. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Yin, C.Y.; Pang, X.Y.; Peuke, A.D.; Wang, X.; Chen, K.; Gong, R.G. Growth and photosynthetic responses in Jatropha curcas L. seedlings of different provenances to watering regimes. Phoyosynthetica 2016, 54, 367–373. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Tang, F.; Li, P.; Liu, J.; Fu, R.; Zheng, L.; Zhang, J.; Chao, N. MaMYBR30, a Novel 1R-MYB, Plays Important Roles in Plant Development and Abiotic Stress Resistance. Plants 2024, 13, 1794. [Google Scholar] [CrossRef]
- Shirke, P.A.; Pathre, U.V. Diurnal and Seasonal Changes in Photosynthesis and Photosystem 2 Photochemical Efficiency in Prosopis juliflora Leaves Subjected to Natural Environmental Stress. Phoyosynthetica 2003, 41, 83–89. [Google Scholar] [CrossRef]
- Bandurska, H. Drought Stress Responses: Coping Strategy and Resistance. Plants 2022, 11, 922. [Google Scholar] [CrossRef]
- Oliveira, B.; Marimon Junior, B.H.; Mews, H.A.; Valadão, M.B.X.; Marimon, B.S. Unraveling the ecosystem functions in the Amazonia–Cerrado transition: Evidence of hyperdynamic nutrient cycling. Plant Ecol. 2017, 218, 225–239. [Google Scholar] [CrossRef]
- Marques, E.Q.; Marimon-Junior, B.H.; Marimon, B.S.; Matricardi, E.A.; Mews, H.A.; Colli, G.R. Redefining the Cerrado–Amazonia transition: Implications for conservation. Biodivers. Conserv. 2019, 29, 1501–1517. [Google Scholar] [CrossRef]
- Santos, W.R.; Souza, L.S.B.; Jardim, A.M.R.F.; Morais, J.E.F.; Santos, M.M.P.; Souza, C.A.A.; Silva, T.G.F. How is the water footprint of the species Vachellia farnesiana, Amburana cearensis, and Handroanthus impetiginosus influenced by abiotic stresses as water deficit and salinity? Int. J. Phytoremediat. 2024, 26, 784–792. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, I.P.; Schaaf, C.; de Setta, N. Drought Responses in Poaceae: Exploring the Core Components of the ABA Signaling Pathway in Setaria italica and Setaria viridis. Plants 2024, 13, 1451. [Google Scholar] [CrossRef]
- Scarpa, A.L.M.; Cruz, Y.C.; Duarte, V.P.; Castro, E.M.; Pasqual, M.; Oliveira, J.P.V.; Pereira, F.J. Growth Response, Gas Exchange, and Leaf Anatomy of Handroanthus spp. Seedlings in Mine Tailings Enriched with Nutrient Solution. J. Soil Sci. Plant Nutr. 2022, 22, 3774–3787. [Google Scholar] [CrossRef]
- Monteiro, E.B.; Silva, A.C.; Souza, A.P.; Tanaka, A.A.; Ferneda, B.G.; Martim, C.C. Water requirements and crop coefficients of tropical forest seedlings in different shading conditions. Rev. Bras. Eng. Agrícola Ambient. 2016, 20, 709–715. [Google Scholar] [CrossRef]
- Keffer, J.F.; Silva, C.C.; Souza, A.P.; Silva, A.C.; Bouvié, L.; Dias, T.K.R. Evapotranspiration and water sensitivity of Amazonian yellow ipe seedlings under different shading conditions. Rev. Bras. Eng. Agrícola Ambient. 2019, 23, 733–740. [Google Scholar] [CrossRef]
- Borella, D.R.; Souza, A.P.; Silva, A.C.; Pizzatto, M.; Keffer, J.F.; Lima, D.C. Water requirements of Dipteryx alata Vogel Seedlings at different solar radiation levels in Cerrado-Amazon transition. Trop. Subtrop. Agroecosyst. 2020, 23, 1–13. [Google Scholar] [CrossRef]
- Sabino, M.; Ferneda, B.G.; Martim, C.C.; Bouvié, L.; Da Silva, C.C.; Souza, A.P.; Silva, A.C.; Felipe, R.T.A. Initial growth of amazonian and brazilian Cerrado yellow ipe cultivated under different shading intensities and spectral wavelength. Interciência 2020, 45, 183–191. [Google Scholar]
- Su, X.; Yang, Z.; Zhou, C.; Geng, S.; Chen, S.; Cai, N.; Tang, J.; Chen, L.; Xu, Y. The Response and Evaluation of Morphology, Physiology, and Biochemistry Traits in Triploid Passiflora edulis Sims ‘Mantianxing’ to Drought Stress. Plants 2024, 13, 1685. [Google Scholar] [CrossRef]
- Souza, A.P.; Mota, L.L.; Zamadei, T.; Martim, C.C.; Almeida, F.T.; Paulino, J. Climate classification and climatic water balance in Mato Grosso state, Brazil. Nativa 2013, 1, 34–43. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements. In FAO Irrigation and Drainage Paper, 56; FAO: Rome, Italy, 1998; 300p. [Google Scholar]
- Martim, C.C.; Zamadei, T.; Souza, A.P.; Almeida, F.T.; Zolin, C.A. Angström-Prescott coefficients and reference evapotranspiration in the Cerrado-Amazon transition region of Mato Grosso. Rev. Bras. Climatol. 2020, 26, 579–594. [Google Scholar] [CrossRef]
- Elsheery, N.; Cao, K.F. Gas exchange, Chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol. Plant. 2008, 30, 769–777. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving Chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—Calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different Chlorophyll fluorescence parameters and the Chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Phoyosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Monteiro, E.B. Growth and Water Requirements of Forest Seedlings under Different Shading Conditions. Master’s Thesis, Federal University of Mato Grosso, Sinop, Turkey, 2015; 218p. [Google Scholar]
- Ometto, J.C. Plant Bioclimatology; Agronômica Ceres: São Paulo, Brazil, 1981; 425p. [Google Scholar]
- Souza, A.P.; Leonel, S.; Silva, A.C. Basal temperature and thermal sum in phenological phases of nectarine and peach cultivars. Pesqui. Agropecuária Bras. 2011, 46, 1588–1596. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Marenco, R.A.; Neves, T.S.; Camargo, M.A.B.; Dias, D.P.; Costa, G.F.; Rodrigues, J.C. Dynamic photoinhibition of photosynthesis in canopy trees from the Central Amazon. Rev. Bras. Biociências 2007, 5, 150–152. [Google Scholar]
- Souza, A.P.; Zamadei, T.; Monteiro, E.B.; Casavecchia, B.H. Atmospheric Transmissivity of the Global Radiation in the Amazonic Region of Mato Grosso. Rev. Bras. Meteorol. 2016, 31, 639–648. [Google Scholar] [CrossRef]
- Borella, D.R.; Souza, A.P.; Silva, K.N.C.; Santos, L.M.M.; Ximenes, E.S.O.C.; Anjos, A.M. Dynamics and estimates of air temperature and relative humidity in nurseries protected with different shading. Nativa 2021, 9, 62–75. [Google Scholar] [CrossRef]
- Monteiro, E.B.; Silva, C.C.; Silva, A.C.; Souza, A.P. Estimating Emission of Leaves Seedlings Forest in Different Shading Levels, at Conditions of Transition Amazon-Cerrado, Brazil. Am. J. Plant Sci. 2014, 5, 2330–2341. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ribeiro, A.; Silva, C.R.A.; Xavier, A.; Freitas, A.F. Modeling the growth of eucalyptus seedlings based on thermal sum. Rev. Árvore 2017, 41, e410212. [Google Scholar] [CrossRef]
- Lima, P.R.; Horbach, M.A.; Dranski, J.A.L.; Ecco, M.; Malavasi, M.M.; Malavasi, U.C. Morphophysiological evaluation of Handroanthus impetiginosus (Mart. ex DC.) Mattos seedlings during hardening. Floresta Ambiente 2014, 21, 316–326. [Google Scholar] [CrossRef]
- Augostinho, L.M.D.; Prado, R.M.; Rozane, D.E.; Freitas, N. Accumulation of dry mass and nutrient absorption rate in ‘Pedro Sato’ guava seedlings. Bragantia 2008, 67, 577–585. [Google Scholar] [CrossRef]
- Nogueira, R.C.; Paiva, R.; Lima, E.C.; Soares, G.A.; Oliveira, L.M.; Santos, B.R.; Emrich, E.B.; Castro, A.H.F. Curva de crescimento e análises bioquímicas de calos de murici-pequeno (Byrsonima intermedia A. Juss.). Rev. Bras. Pl. Med. 2008, 10, 44–48. [Google Scholar]
- César, F.R.C.F.; Matsumoto, S.N.; Viana, A.E.S.; Bonfim, J.A. Initial growth and quality of Pterogyne nitens Tull. seedling under artificial shading gradient. Ciência Florest. 2014, 24, 357–366. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Marenco, R.A.; Nascimento, H.C.S.; Magalhães, N.S. Stomatal conductance in Amazonian tree saplings in response to variations in the physical environment. Phoyosynthetica 2014, 52, 493–500. [Google Scholar] [CrossRef]
- Kutschera, U. Review Cell Expansion in Plant Development. Braz. J. Plant Physiol. 2000, 12, 65–98. [Google Scholar]
- Bashline, L.; Lei, L.; Li, S.; Gu, Y. Cell wall, cytoskeleton, and cell expansion in higher plants. Mol. Plant 2014, 7, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.N. Physiology of PSI cyclic electron transport in higher plants. Biochim. Biophys. Acta—Bioenerg. 2011, 1807, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.M.; Rocha, G.T.F.B.L.; Matos, F.S.; Rodrigues, F. Responses of paricá seedlings to water stress. Rev. Floresta 2016, 46, 405–412. [Google Scholar] [CrossRef]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Physiol. 2016, 67, 81–106. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, G.; He, Q.; Zhou, L.; Ji, Y.; Zhou, M. Environmental explanation of maize specific leaf area under varying water stress regimes. Environ. Exp. 2020, 171, 103932. [Google Scholar] [CrossRef]
- Santos, V.A.H.F.; Ferreira, M.J.; Rodrigues, J.V.F.C.; Garica, M.N.; Ceron, J.V.B.; Nelson, B.W.; Saleska, S.R. Causes of reduced leaf-level photosynthesis during strong El Niño drought in a Central Amazon forest. Glob. Chang. Biol. 2018, 24, 4266–4279. [Google Scholar] [CrossRef]
- Maeda, E.E.; Ma, X.; Wagner, F.H.; Kim, H.; Oki, T.; Eamus, D.; Huete, A. Evapotranspiration seasonality across the Amazon Basin. Earth Syst. Dynam. 2017, 8, 439–454. [Google Scholar] [CrossRef]
- Sabonaro, D.Z.; Galbiatti, J.A. Effect of irrigation levels in substrates for “ipê-roxo” seedlings production. Sci. For. 2007, 1, 95–102. [Google Scholar]
- Cunha, A.O.; Andrade, L.A.; Bruno, R.L.A.; Silva, J.A.L.; Souza, V.C. Efeitos de substratos e das dimensões dos recipientes na qualidade das mudas de Tabebuia impetiginosa (Mart. Ex D.C.) Standl. Rev. Árvore 2005, 29, 507–516. [Google Scholar] [CrossRef]
- Carvalho, D.F.; Lima, M.E.; Oliveira, A.D.; Rocha, H.S.; Guerra, J.G.M. Crop coefficiente and water consumption of eggplant in no-tillage system and conventional soil preparation. Eng. Agrícola 2012, 32, 784–793. [Google Scholar] [CrossRef]
- Pessoa, J.L.; Freire, A.L.O.; Costa, A.S. Gas exchange of Handroanthus impetiginosus (Mart. ex DC) Mattos plants under water stress and rehydration. Rev. Ciências Agroveterinárias 2017, 16, 269–276. [Google Scholar] [CrossRef][Green Version]
- Bueno, M.M.; Leles, P.S.S.; Abreu, J.F.G.; Santos, J.J.S.; Carvalho, D.F. Water requirement and growth indicators of forest tree species seedlings produced with automated irrigation management. PLoS ONE 2020, 15, e0238677. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.A.; Abreu, A.H.M.; Leles, P.S.S.; Oliveira, R.R.; Silva, D.T. Quality and initial growth of seedlings Mimosa caesalpiniifolia Benth. produced in different volumes of containers. Ciência Florest. 2018, 28, 47–55. [Google Scholar] [CrossRef]
- Silva, B.L.B.; Costa, E.; Binotti, F.F.S.; Benett, C.G.S.; Silva, A.G. Quality and growth of achachairu seedlings depending on substrate and shading. Pesqui. Agropecuária Trop. 2018, 48, 407–413. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Brienen, R.J.; Feldpausch, T.R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Álvarez-Dávila, E.; et al. Compositional response of Amazon forests to climate change. Glob. Chang. Biol. 2018, 25, 39–56. [Google Scholar] [CrossRef]
- Mira-García, A.B.; Romero-Triguero, C.; Gambín, J.M.B.; Sáchez-Iglesias, M.P.; Tortosa, P.A.N.; Nicolás, E.N. Estimation of stomatal conductance by infra-red thermometry in citrus trees cultivated under regulated deficit irrigation and reclaimed water. Agr. Water Manag. 2020, 276, e108057. [Google Scholar] [CrossRef]
- Marenco, R.A.; Antezana-Vera, S.A.; Gouvêa, R.S.; Camargo, M.A.B.; Oliveira, M.F.; Santos, J.K.S. Physiology of Amazon tree species: Photosynthesis, respiration and water relations. Ceres 2014, 61, 786–799. [Google Scholar] [CrossRef]
- Silva, A.M.L.; Costa, M.F.B.; Leite, V.G.; Rezende, A.A.; Teixeira, S.P. Leaf anatomy with taxonomic implications in ipe species. Hoehnea 2009, 36, 329–338. [Google Scholar] [CrossRef]
- Marenco, R.A.; Lopes, N.F. Fisiologia Vegetal: Fotossíntese, Respiração, Relações Hídricas e Nutrição Mineral, 3rd ed.; Editora UFV: Viçosa, Brazil, 2009; 486p. [Google Scholar]
- Dombroski, J.L.D.; Freitas, R.M.O.; Tomczak, V.E.; Pinto, J.R.S.; Farias, R.M. Ecophysiology of water stressed Handroanthus impetiginosus (Mart. Ex. DC) Mattos) Seedlings. Sci. For. 2014, 42, 155–163. [Google Scholar]
- Souza, A.F.; Rocha Junior, E.O.; Laura, V.A. Early development and efficiency in water and nitrogen use by seedlings of Calophyllum brasiliense, Eucalyptus urograndis, Tabebuia impetiginosa and Toona ciliata. Ciência Florest. 2018, 28, 1465–1477. [Google Scholar] [CrossRef]
- Shimpl, F.C.; Ferreira, M.J.; Jaquetti, R.K.; Martins, S.C.V.; Gonçalves, J.F.C. Physiological responses of young Brazil nut (Bertholletia excelsa) plants to drought stress and subsequent rewatering. Flora 2019, 252, 10–17. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and Chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Melo, H.F.; Souza, E.R.; Cunha, J.C. Fluorescence of Chlorophyll a and photosynthetic pigments in Atriplex nummularia under abiotic stresses. Rev. Bras. De Eng. Agrícola E Ambient. 2017, 21, 232–237. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.J. Effects of drought stress on growth and Chlorophyll fluorescence of Lycium ruthenicum Murr. Seedlings. Phoyosynthetica 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Dias, D.P.; Marenco, R.A. Photoinhibition of photosynthesis in Minquartia guianensis and Swietenia macrophylla inferred by monitoring the initial fluorescence. Phoyosynthetica 2006, 44, 235–240. [Google Scholar] [CrossRef]
- Dongsansuk, A.; Lütz, C.; Neuner, G. Effects of temperature and irradiance on quantum yield of PSII photochemistry and xanthophyll cycle in a tropical and a temperate species. Phoyosynthetica 2013, 51, 118–127. [Google Scholar] [CrossRef]
- Nelson, N.; Yocum, C.F. Structure and function of photosystems I and II. Annu. Rev. Plant Physiol. 2006, 57, 521–565. [Google Scholar] [CrossRef]
- Liberato, M.A.R.; Gonçalves, J.F.C.; Chevreuil, L.R.; Nina, A.R., Jr.; Fernandes, A.V.; Santos, U.M., Jr. Leaf water potential, gas exchange and Chlorophyll a fluorescence in acariquara seedlings (Minquartia guianensis Aubl.) under water stress and recovery. Braz. J. Plant Physiol. 2006, 18, 315–323. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017; 888p. [Google Scholar]
- Thapper, A.; Mamedov, F.; Mokvist, F.; Hammarström, L.; Styring, S. Defining the Far-Red Limit of Photosystem II in Spinach. Plant Cell 2009, 21, 2391–2401. [Google Scholar] [CrossRef]
- Ouzounis, T.; Parjikolaei, B.R.; Fretté, X.; Rosenqvist, E.; Ottosen, C.O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 19. [Google Scholar] [CrossRef]
- Franco, A.; Lüttge, U. Midday depression in savanna trees: Coordinated adjustments in photochemical efficiency, photorespiration, CO2 assimilation and water use efficiency. Oecologia 2002, 31, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Bacarin, M.A.; Martinazzo, E.G.; Cassol, D.; Falqueto, A.R.; Silva, D.M. Daytime variations of Chlorophyll a fluorescence in Pau d’alho seedlings. Rev. Árvore 2016, 40, 1023–1030. [Google Scholar] [CrossRef]
- Weng, J.H.; Chen, Y.N.; Liao, T.S. Relationships between Chlorophyll fluorescence parameters and photochemical reflectance index of tree species adapted to different temperature regimes. Plant Funct. Evol. Biol. 2006, 33, 241–246. [Google Scholar] [CrossRef]
- Silva, L.K.S.; Alves, M.C.J.L.; Costa, R.N.; Silva, D.M.R.; Santos, J.C.C.S.; Moura, F.B.P.; Silva, J.M., Jr.; Silva, J.V. Gas exchange and photochemical efficiency of Caatinga plants submitted to different water management strategies. J. Agric. Sci. 2019, 11, 53–67. [Google Scholar] [CrossRef]
- Morais, R.R.; Gonçalvez, J.F.C.; Santos, U.M., Jr.; Santos, A.L.W. Chloroplastid pigment contents and Chlorophyll a fluorescence in Amazonian tropical three species. Rev. Árvore 2007, 31, 959–966. [Google Scholar] [CrossRef]
- Ortiz, D.; Moreno, F.; Díez, M.C. Photosynthesis, growth, and survival in seedlings of four tropical fruit-tree species under intense radiation. Acta Amaz. 2021, 51, 1–9. [Google Scholar] [CrossRef]
- Reis, L.C.; Scalon, S.P.Q.; Dresch, D.M.; Foresti, A.C.; Santos, C.C.; Pereira, Z.V. Chlorophyll a fluorescence as an indicator of water stress in Calophyllum brasiliense. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 210–2020. [Google Scholar] [CrossRef]
- Reis, L.A.C.; Oliveira, J.A.; Farnese, F.S.; Rosado, A.M.; Reis, L.A.C. Chlorophyll fluorescence and water content parameters are good biomarkers for selecting drought tolerant eucalyptus clones. For. Ecol. Manag. 2021, 481, 118682. [Google Scholar] [CrossRef]
| LWR | Etr 1 | Etm 2 | (1 − ETr/ETp) | Yr 3 | Ym 4 | (1 − Yr/Yp) | Ky 5 |
|---|---|---|---|---|---|---|---|
| (mm) | (g m−2) | ||||||
| 75% | 465.41 | 593.2 | 0.22 | 802.47 | 1121.32 | 0.28 | 1.32 |
| 50% | 346.15 | 593.2 | 0.42 | 364.27 | 1121.32 | 0.68 | 1.62 |
| 25% | 212.99 | 593.2 | 0.64 | 34.4 | 1121.32 | 0.97 | 1.51 |
| Hour | Level of Water Replacement | |||
|---|---|---|---|---|
| 25% | 50% | 75% | 100% | |
| Net photosynthesis rate—µmol m−2 s−1 | ||||
| 09:30 | 6.11 Ab | 12.54 Aa | 11.12 Aa | 10.1 Aa |
| 12:30 | 5.36 Ab | 6.92 Bb | 10.30 Aa | 10.41 Aa |
| 13:30 | 4.92 Aa | 7.15 Ba | 6.75 Ba | 8.02 Aa |
| 16:00 | 3.50 Ab | 6.28 Bab | 6.56 Bab | 8.05 Aa |
| Transpiration rate—mol m−2 s−1 | ||||
| 09:30 | 2.95 Ab | 5.47 Aa | 4.88 Aa | 5.03 Aa |
| 12:30 | 2.65 ABc | 3.93 BAcb | 4.92 Aab | 5.81 Aa |
| 13:30 | 2.43 ABb | 3.48 BCab | 3.28 Bab | 4.51 ABa |
| 16:00 | 1.26 Bb | 2.22 Cab | 2.23 Bab | 3.09 Ba |
| Water use efficiency—µmol CO2 mol−1 H2O | ||||
| 09:30 | 2.21 Ba | 2.29 Ba | 2.29 Ba | 1.94 Ba |
| 12:30 | 2.26 ABa | 1.82 Ba | 2.16 Ba | 1.81 Ba |
| 13:30 | 1.97 Ba | 2.12 Ba | 2.06 Ba | 1.78 Ba |
| 16:00 | 2.75 Aa | 2.83 Aa | 3.15 Aa | 2.77 Aa |
| Stomatal conductance—mol m−2 s−1 | ||||
| 09:30 | 0.10 Ab | 0.23 Aa | 0.19 Aa | 0.21 Aa |
| 12:30 | 0.07 Ab | 0.10 Bb | 0.14 ABab | 0.19 Aa |
| 13:30 | 0.06 Ab | 0.09 Bab | 0.09 Bab | 0.16 Aa |
| 16:00 | 0.06 Ab | 0.13 Bb | 0.13 ABb | 0.24 Aa |
| Internal carbon to atmospheric carbon ratio | ||||
| 09:30 | 0.64 Bb | 0.71 Aab | 0.68 ABab | 0.72 ABa |
| 12:30 | 0.50 Cc | 0.64 Bab | 0.57 Cb | 0.66 Ba |
| 13:30 | 0.62 Bb | 0.59 Bb | 0.63 BCb | 0.71 ABa |
| 16:00 | 0.69 Aa | 0.67 Aa | 0.76 Aa | 0.78 Aa |
| Leaf temperature—°C | ||||
| 09:30 | 40.37 Ba | 40.03 Ba | 40.40 Ba | 40.81 Aa |
| 12:30 | 41.94 Aa | 42.02 Aa | 41.97 Aa | 41.90 Aa |
| 13:30 | 39.53 Ba | 40.41 Ba | 39.47 Ba | 39.43 Ba |
| 16:00 | 33.63 Ca | 33.47 Ca | 33.83 Ca | 34.19 Ca |
| 100% Level of Water Replacement | ||||||||
| TM | dPv | A | E | EUA | gs | Ci/Ca | Tf | |
| TM | 1.00 | 1.00 ** | 0.19 NS | 0.37 ** | −0.52 ** | −0.08 NS | −0.51 ** | 0.78 ** |
| dPv | 1.00 | 0.19 NS | 0.37 ** | −0.52 ** | −0.06 NS | −0.50 ** | 0.77 ** | |
| A | 1.00 | 0.93 ** | 0.07 NS | 0.86 ** | 0.13 NS | 0.28 * | ||
| E | 1.00 | −0.28 * | 0.75 ** | 0.02 NS | 0.56 ** | |||
| EUA | 1.00 | 0.15 NS | 0.24 NS | −0.78 ** | ||||
| gs | 1.00 | 0.55 ** | −0.03 NS | |||||
| Ci/Ca | 1.00 | −0.61 ** | ||||||
| Tf | 1.00 | |||||||
| 75% Level of Water Replacement | ||||||||
| TM | dPv | A | E | EUA | gs | Ci/Ca | Tf | |
| TM | 1.00 | 1.00 ** | 0.51 ** | 0.61 ** | −0.39 ** | 0.24 NS | −0.45 ** | 0.76 ** |
| dPv | 1.00 | 0.52 ** | 0.61 ** | −0.37 ** | 0.25 NS | −0.45 ** | 0.75 ** | |
| A | 1.00 | 0.85 ** | 0.00 NS | 0.80 ** | −0.22 NS | 0.41 ** | ||
| E | 1.00 | −0.48 ** | 0.73 ** | −0.19 NS | 0.70 ** | |||
| EUA | 1.00 | −0.09 NS | 0.05 NS | −0.68 ** | ||||
| gs | 1.00 | 0.28 * | 0.09 NS | |||||
| Ci/Ca | 1.00 | −0.58 ** | ||||||
| Tf | 1.00 | |||||||
| 50% Level of Water Replacement | ||||||||
| TM | dPv | A | E | EUA | gs | Ci/Ca | Tf | |
| TM | 1.00 | 1.00 ** | 0.57 ** | 0.67 ** | −0.65 ** | 0.43 ** | −0.15 NS | 0.75 ** |
| dPv | 1.00 | 0.55 ** | 0.66 ** | −0.63 ** | 0.41 ** | −0.16 NS | 0.74 ** | |
| A | 1.00 | 0.83 ** | −0.24 NS | 0.86 ** | 0.13 NS | 0.41 ** | ||
| E | 1.00 | −0.68 ** | 0.77 ** | 0.11 NS | 0.71 ** | |||
| EUA | 1.00 | −0.22 NS | 0.13 NS | −0.87 ** | ||||
| gs | 1.00 | 0.57 ** | 0.17 NS | |||||
| Ci/Ca | 1.00 | −0.49 ** | ||||||
| Tf | 1.00 | |||||||
| 25% Level of Water Replacement | ||||||||
| TM | dPv | A | E | EUA | gs | Ci/Ca | Tf | |
| TM | 1.00 | 1.00 ** | 0.13 NS | 0.28 * | −0.37 ** | 0.14 NS | −0.29 * | 0.74 ** |
| dPv | 1.00 | 0.13 NS | 0.26 * | −0.36 ** | 0.13 NS | −0.30 * | 0.73 ** | |
| A | 1.00 | 0.94 ** | 0.11 NS | 0.95 ** | −0.01 NS | 0.26 * | ||
| E | 1.00 | −0.21 NS | 0.94 ** | 0.10 NS | 0.45 ** | |||
| EUA | 1.00 | −0.05 NS | −0.42 ** | −0.57 ** | ||||
| gs | 1.00 | 0.23 NS | 0.19 NS | |||||
| Ci/Ca | 1.00 | −0.38 ** | ||||||
| Tf | 1.00 | |||||||
| Solar Time | 28 DATs (464 DDAs) | 66 DATs (1046 DDAs) | 90 DATs (1400 DDAs) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25% | 50% | 75% | 100% | 25% | 50% | 75% | 100% | 25% | 50% | 75% | 100% | |
| Maximum quantum yield of PSII (Fv/Fm) | ||||||||||||
| 07:00 | 0.799 | 0.797 | 0.800 | 0.800 | 0.722 | 0.799 | 0.809 | 0.810 | 0.761 | 0.819 | 0.825 | 0.828 |
| 09:00 | 0.759 | 0.763 | 0.753 | 0.755 | 0.663 | 0.765 | 0.767 | 0.770 | 0.731 | 0.800 | 0.804 | 0.813 |
| 11:00 | 0.735 | 0.742 | 0.736 | 0.736 | 0.634 | 0.684 | 0.729 | 0.748 | 0.734 | 0.805 | 0.810 | 0.819 |
| 13:00 | 0.723 | 0.740 | 0.740 | 0.734 | 0.658 | 0.749 | 0.765 | 0.781 | 0.730 | 0.805 | 0.806 | 0.815 |
| 15:00 | 0.757 | 0.763 | 0.762 | 0.749 | - | - | - | - | - | - | - | - |
| 17:00 | 0.799 | 0.799 | 0.792 | 0.789 | - | - | - | - | - | - | - | - |
| Effective quantum yield of PSII (ΦPSII) | ||||||||||||
| 07:00 | 0.378 | 0.423 | 0.423 | 0.417 | 0.188 | 0.444 | 0.503 | 0.405 | 0.298 | 0.357 | 0.366 | 0.356 |
| 09:00 | 0.281 | 0.352 | 0.328 | 0.284 | 0.159 | 0.273 | 0.351 | 0.385 | 0.281 | 0.426 | 0.453 | 0.497 |
| 11:00 | 0.327 | 0.335 | 0.335 | 0.364 | 0.106 | 0.294 | 0.366 | 0.385 | 0.288 | 0.438 | 0.410 | 0.396 |
| 13:00 | 0.338 | 0.358 | 0.366 | 0.375 | 0.155 | 0.410 | 0.430 | 0.513 | 0.278 | 0.468 | 0.480 | 0.506 |
| 15:00 | 0.383 | 0.352 | 0.382 | 0.349 | - | - | - | - | - | - | - | - |
| 17:00 | 0.235 | 0.228 | 0.211 | 0.185 | - | - | - | - | - | - | - | - |
| Electron transport rate (µmol m−2 s−1) | ||||||||||||
| 07:00 | 89.27 | 100.01 | 99.84 | 98.40 | 44.32 | 104.86 | 118.90 | 95.57 | 70.48 | 84.28 | 86.45 | 84.14 |
| 09:00 | 66.50 | 83.10 | 77.47 | 67.09 | 37.67 | 64.45 | 83.034 | 90.91 | 66.43 | 100.69 | 107.10 | 117.42 |
| 11:00 | 77.36 | 79.13 | 79.24 | 85.90 | 25.12 | 69.46 | 86.499 | 91.00 | 68.13 | 103.48 | 96.76 | 93.59 |
| 13:00 | 79.90 | 84.51 | 86.42 | 88.59 | 36.56 | 96.97 | 101.67 | 121.21 | 65.69 | 110.58 | 113.49 | 119.46 |
| 15:00 | 90.47 | 83.05 | 90.19 | 82.37 | - | - | - | - | - | - | - | - |
| 17:00 | 55.48 | 53.85 | 49.92 | 43.64 | - | - | - | - | - | - | - | - |
| Maximum efficiency of PSII (Fv′/Fm′) | ||||||||||||
| 07:00 | 0.511 | 0.496 | 0.501 | 0.545 | 0.422 | 0.644 | 0.719 | 0.623 | 0.575 | 0.637 | 0.733 | 0.674 |
| 09:00 | 0.484 | 0.509 | 0.479 | 0.469 | 0.341 | 0.465 | 0.508 | 0.529 | 0.475 | 0.599 | 0.685 | 0.678 |
| 11:00 | 0.479 | 0.492 | 0.495 | 0.524 | 0.296 | 0.428 | 0.479 | 0.497 | 0.497 | 0.697 | 0.672 | 0.635 |
| 13:00 | 0.463 | 0.475 | 0.490 | 0.528 | 0.339 | 0.526 | 0.561 | 0.611 | 0.466 | 0.656 | 0.660 | 0.702 |
| 15:00 | 0.525 | 0.497 | 0.527 | 0.505 | - | - | - | - | - | - | - | - |
| 17:00 | 0.584 | 0.606 | 0.581 | 0.605 | - | - | - | - | - | - | - | - |
| Non-photochemical dissipation (NPQ) | ||||||||||||
| 07:00 | 3.242 | 3.281 | 3.172 | 2.383 | 2.637 | 1.200 | 0.67431 | 1.638 | 1.432 | 1.611 | 0.742 | 1.356 |
| 09:00 | 2.379 | 2.155 | 2.366 | 2.523 | 2.939 | 2.774 | 2.268 | 2.070 | 2.109 | 1.698 | 0.967 | 1.086 |
| 11:00 | 2.069 | 2.015 | 1.897 | 1.646 | 3.390 | 2.050 | 2.005 | 2.046 | 1.873 | 0.859 | 1.123 | 1.681 |
| 13:00 | 2.116 | 2.208 | 2.017 | 1.519 | 2.892 | 1.932 | 1.601 | 1.346 | 2.274 | 1.188 | 1.206 | 0.874 |
| 15:00 | 1.903 | 2.285 | 1.965 | 1.998 | - | - | - | - | - | - | - | - |
| 17:00 | 1.876 | 1.642 | 1.807 | 1.494 | - | - | - | - | - | - | - | - |
| Fraction of open reaction centers of the PSII (qL) | ||||||||||||
| 07:00 | 0.600 | 0.764 | 0.757 | 0.608 | 0.317 | 0.444 | 0.401 | 0.416 | 0.326 | 0.324 | 0.22 | 0.280 |
| 09:00 | 0.418 | 0.525 | 0.533 | 0.449 | 0.367 | 0.439 | 0.530 | 0.558 | 0.425 | 0.496 | 0.378 | 0.474 |
| 11:00 | 0.535 | 0.52 | 0.518 | 0.515 | 0.296 | 0.551 | 0.630 | 0.636 | 0.410 | 0.346 | 0.342 | 0.377 |
| 13:00 | 0.604 | 0.618 | 0.597 | 0.542 | 0.356 | 0.629 | 0.597 | 0.670 | 0.440 | 0.460 | 0.471 | 0.438 |
| 15:00 | 0.566 | 0.552 | 0.554 | 0.544 | - | - | - | - | - | - | - | - |
| 17:00 | 0.239 | 0.232 | 0.233 | 0.167 | - | - | - | - | - | - | - | - |
| 100% Level of Water Replacement | ||||||||
| TM | DPV | Fv/Fm | ΦPSII | NPQ | ETR | Fv′/Fm′ | qL | |
| TM | 1.00 | 0.99 ** | −0.87 ** | −0.04 NS | 0.46 ** | −0.04 NS | −0.72 ** | 0.82 ** |
| DPV | 1.00 | −0.91 ** | −0.10 NS | 0.45 ** | −0.10 NS | −0.76 ** | 0.83 ** | |
| Fv/Fm | 1.00 | 0.17 NS | −0.50 ** | 0.17 NS | 0.80 ** | −0.84 ** | ||
| ΦPSII | 1.00 | −0.68 ** | 1.00 ** | 0.55 ** | 0.18 NS | |||
| NPQ | 1.00 | −0.68 ** | −0.91 ** | 0.48 ** | ||||
| ETR | 1.00 | 0.55 ** | 0.18 NS | |||||
| Fv′/Fm′ | 1.00 | −0.71 ** | ||||||
| qL | 1.00 | |||||||
| 75% Level of Water Replacement | ||||||||
| TM | DPV | Fv/Fm | ΦPSII | NPQ | ETR | Fv′/Fm′ | qL | |
| TM | 1.00 | 0.99 ** | −0.82 ** | −0.12 NS | 0.57 ** | −0.12 NS | −0.74 ** | 0.84 ** |
| DPV | 1.00 | −0.85 ** | −0.17 NS | 0.59 ** | −0.17 NS | −0.77 ** | 0.84 ** | |
| Fv/Fm | 1.00 | 0.27 NS | −0.56 ** | 0.27 NS | 0.82 ** | −0.81 ** | ||
| ΦPSII | 1.00 | −0.70 ** | 1.00 ** | 0.60 ** | 0.08 NS | |||
| NPQ | 1.00 | −0.70 ** | −0.93 ** | 0.54 ** | ||||
| ETR | 1.00 | 0.60 ** | 0.08 NS | |||||
| Fv′/Fm′ | 1.00 | −0.73 ** | ||||||
| qL | 1.00 | |||||||
| 50% Level of Water Replacement | ||||||||
| TM | DPV | Fv/Fm | ΦPSII | NPQ | ETR | Fv′/Fm′ | qL | |
| TM | 1.00 | 0.98 ** | −0.79 ** | −0.36 * | 0.42 ** | −0.36 * | −0.70 ** | 0.62 ** |
| DPV | 1.00 | −0.83 ** | −0.39 * | 0.42 ** | −0.39 * | −0.72 ** | 0.62 ** | |
| Fv/Fm | 1.00 | 0.50 ** | −0.38 * | 0.50 ** | 0.79 ** | −0.57 ** | ||
| ΦPSII | 1.00 | −0.76 ** | 1.00 ** | 0.78 ** | 0.07 NS | |||
| NPQ | 1.00 | −0.76 ** | −0.86 ** | 0.37 * | ||||
| ETR | 1.00 | 0.78 ** | 0.07 NS | |||||
| Fv′/Fm′ | 1.00 | −0.55 ** | ||||||
| qL | 1.00 | |||||||
| 25% Level of Water Replacement | ||||||||
| TM | DPV | Fv/Fm | ΦPSII | NPQ | ETR | Fv′/Fm′ | qL | |
| TM | 1.00 | 0.99 ** | −0.72 ** | −0.77 ** | 0.58 ** | −0.77 ** | −0.80 ** | −0.20 NS |
| DPV | 1.00 | −0.73 ** | −0.78 ** | 0.57 ** | −0.78 ** | −0.79 ** | −0.24 NS | |
| Fv/Fm | 1.00 | 0.79 ** | −0.35 * | 0.79 ** | 0.79 ** | 0.34 ** | ||
| ΦPSII | 1.00 | −0.65 ** | 1.00 ** | 0.88 ** | 0.51 ** | |||
| NPQ | 1.0 | −0.65 ** | −0.84 ** | 0.20 NS | ||||
| ETR | 1.00 | 0.88 ** | 0.51 ** | |||||
| Fv′/Fm′ | 1.00 | 0.06 NS | ||||||
| qL | 1.00 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, K.N.C.; Silva, A.C.d.; Borella, D.R.; Carneiro, S.S.; Santos, L.M.M.d.; Jorge, M.C.B.; Magosso, B.F.; Pizzatto, M.; Souza, A.P.d. Growth, Evapotranspiration, Gas Exchange and Chl a Fluorescence of Ipê-Rosa Seedlings at Different Levels of Water Replacement. Plants 2024, 13, 2850. https://doi.org/10.3390/plants13202850
Silva KNC, Silva ACd, Borella DR, Carneiro SS, Santos LMMd, Jorge MCB, Magosso BF, Pizzatto M, Souza APd. Growth, Evapotranspiration, Gas Exchange and Chl a Fluorescence of Ipê-Rosa Seedlings at Different Levels of Water Replacement. Plants. 2024; 13(20):2850. https://doi.org/10.3390/plants13202850
Chicago/Turabian StyleSilva, Kalisto Natam Carneiro, Andréa Carvalho da Silva, Daniela Roberta Borella, Samuel Silva Carneiro, Leonardo Martins Moura dos Santos, Matheus Caneles Batista Jorge, Beatriz Feltrin Magosso, Mariana Pizzatto, and Adilson Pacheco de Souza. 2024. "Growth, Evapotranspiration, Gas Exchange and Chl a Fluorescence of Ipê-Rosa Seedlings at Different Levels of Water Replacement" Plants 13, no. 20: 2850. https://doi.org/10.3390/plants13202850
APA StyleSilva, K. N. C., Silva, A. C. d., Borella, D. R., Carneiro, S. S., Santos, L. M. M. d., Jorge, M. C. B., Magosso, B. F., Pizzatto, M., & Souza, A. P. d. (2024). Growth, Evapotranspiration, Gas Exchange and Chl a Fluorescence of Ipê-Rosa Seedlings at Different Levels of Water Replacement. Plants, 13(20), 2850. https://doi.org/10.3390/plants13202850








