Differences in Grain Yield and Nitrogen Uptake between Tetraploid and Diploid Rice: The Physiological Mechanisms under Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Crop Management
2.3. Sampling and Measurement
2.3.1. Sampling during Rice Growth and Development
2.3.2. Dynamic Changes in Tillers
2.3.3. SPAD Value Measurement
2.3.4. Gas Exchange Measurement
2.3.5. Measurement of Grain Yield and Yield-Related Traits
2.3.6. Nitrogen Uptake
2.4. Statistical Analysis
3. Results
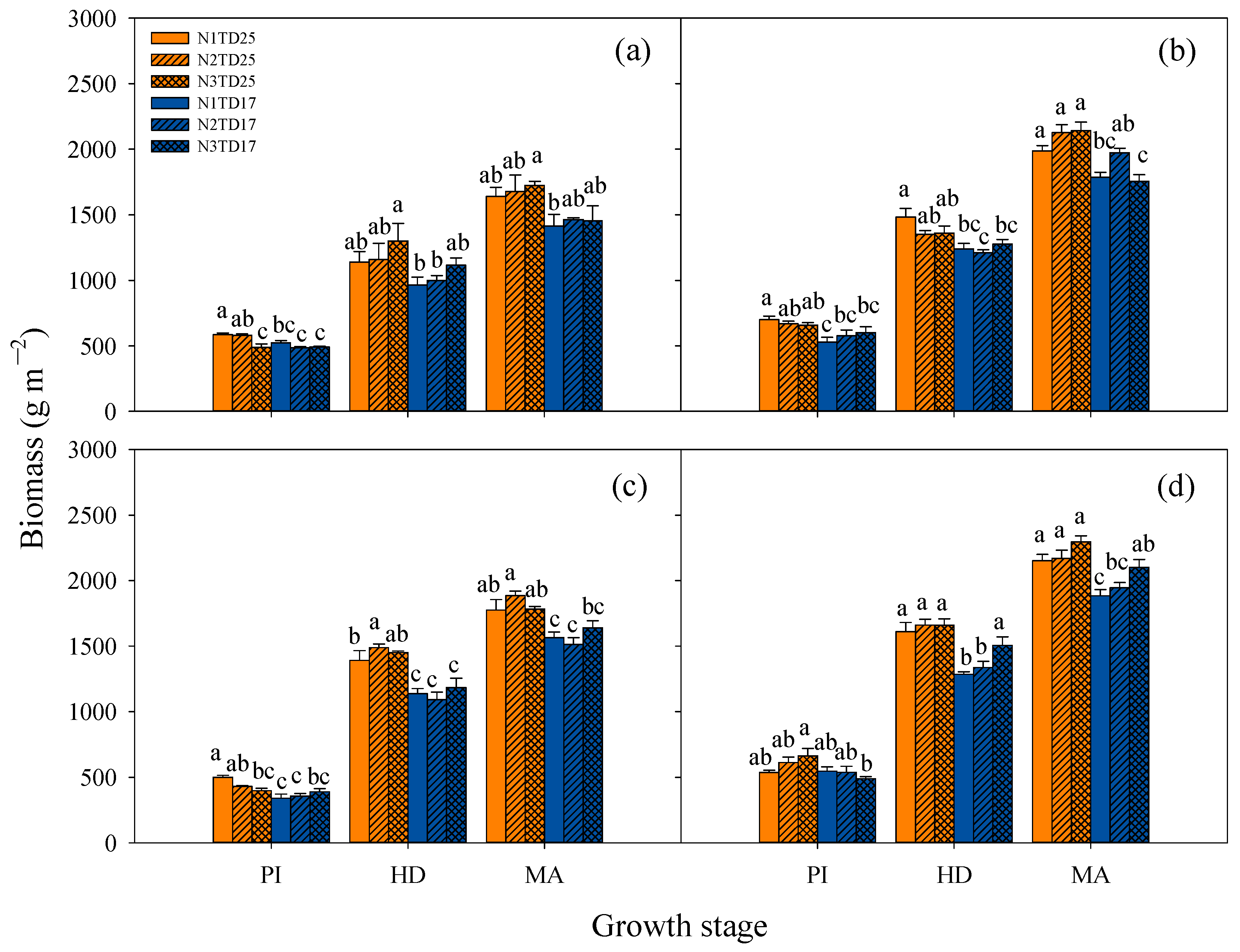
3.1. Grain Yield and Yield Components
3.2. Nitrogen Uptake and NUEg
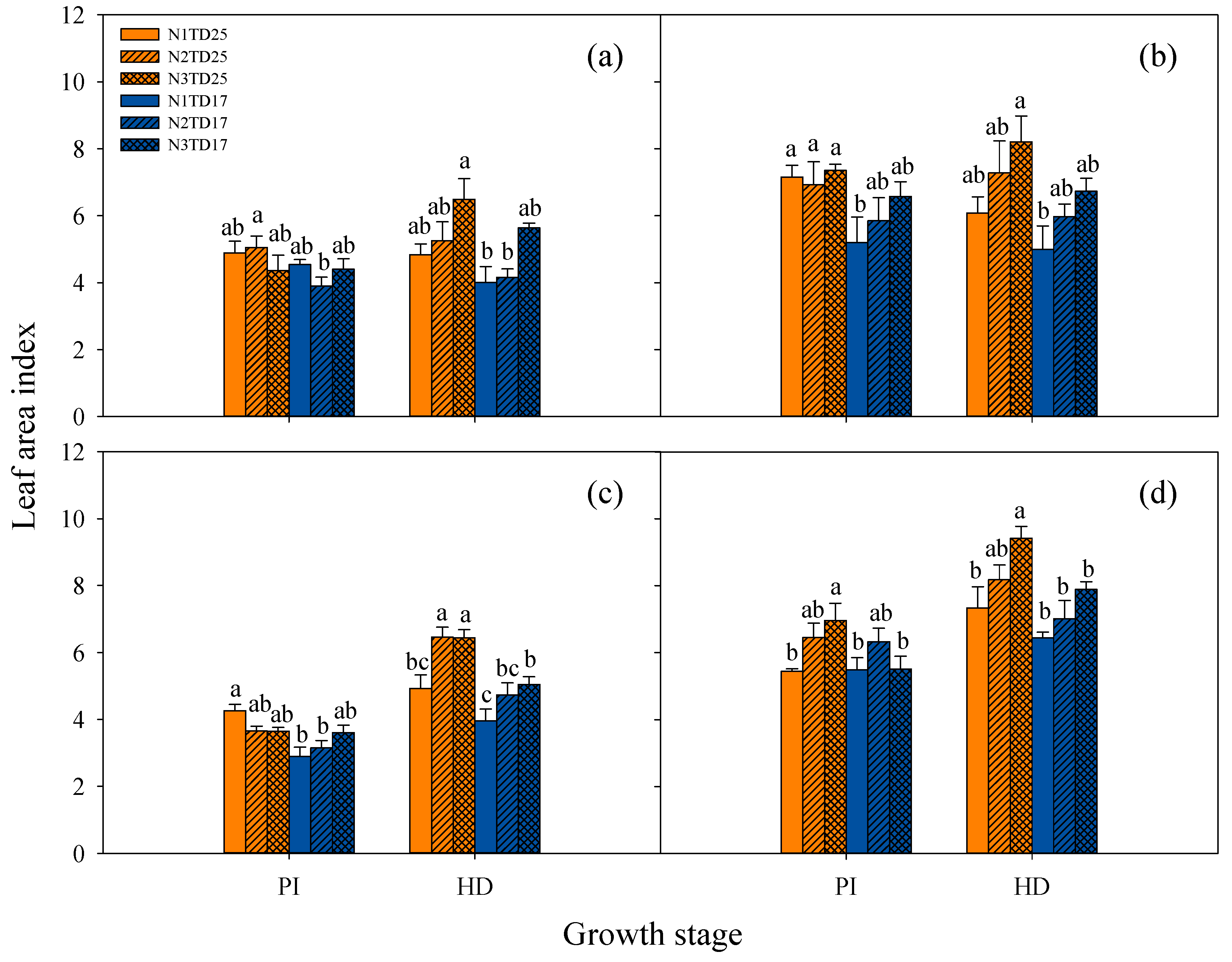
3.3. Dynamics of Tillering, Growth, and Development of Tetraploid Rice
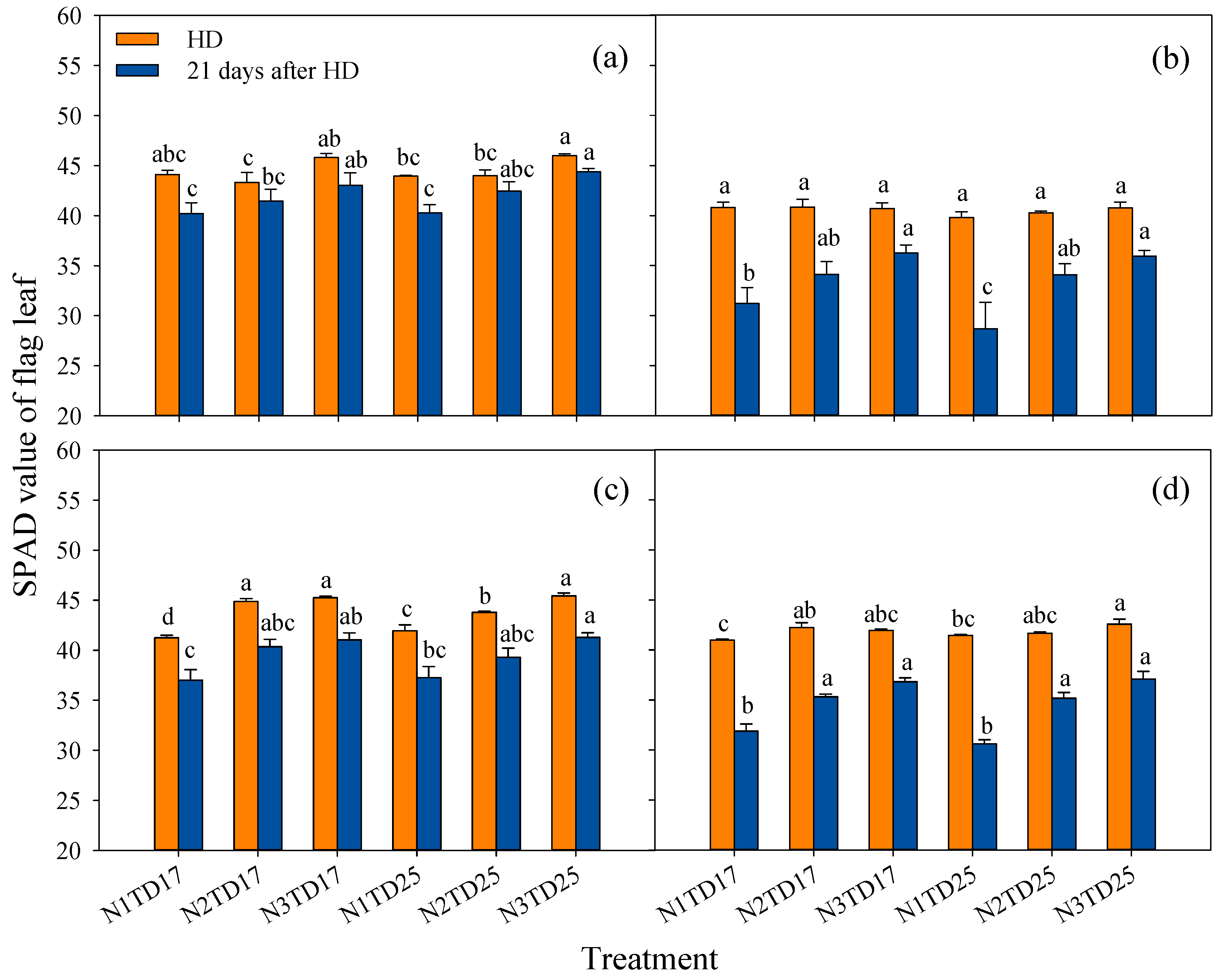
3.4. Net Photosynthetic Rate and SPAD Value
4. Discussion
4.1. Comparison of Yield and Yield Components between Diploid and Tetraploid Rice
4.2. Effect of Nitrogen Application and Planting Density on the Yield of Diploid and Tetraploid Rice
4.3. Response of Nitrogen Uptake and Application to Nitrogen and Planting Density Treatments in Tetraploid and Diploid Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, G.H.; Zhang, J.; Yang, C.D.; Liu, Z.H.; Wang, S.H.; Ding, Y.F. Population characteristics of high-yielding rice under different densities. Agron. J. 2016, 108, 1415–1423. [Google Scholar] [CrossRef]
- Oka, H. Studies on tetraploid rice IV. F1 hybrids between tetraploid varieties of rice. J. Genet. 1954, 29, 101–108. (In Japanese) [Google Scholar]
- Hou, W.; Khan, M.R.; Zhang, J.; Lu, J.; Ren, T.; Cong, R.; Li, X. Nitrogen rate and plant density interaction enhances radiation interception, yield and nitrogen use efficiency of mechanically transplanted rice. Agric. Ecosyst. Environ. 2019, 269, 183–192. [Google Scholar] [CrossRef]
- Peng, X.L.; Yang, Y.M.; Yu, C.L.; Chen, L.N.; Zhang, M.C.; Liu, Z.L.; Sun, Y.K.; Luo, S.G.; Liu, Y.Y. Crop management for increasing rice yield and nitrogen use efficiency in Northeast China. Agron. J. 2015, 107, 82–1690. [Google Scholar] [CrossRef]
- Wang, L.F.; Cao, S.; Wang, P.T.; Lu, K.N.; Song, Q.X.; Zhao, F.J.; Chen, Z.J. DNA hypomethylation in tetraploid rice potentiates stress-responsive gene expression for salt tolerance. Proc. Natl. Acad. Sci. USA 2021, 118, e2023981118. [Google Scholar] [CrossRef] [PubMed]
- Normile, D. Reinventing Rice to feed the world. Science 2008, 321, 330–333. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.Q.; Liu, G.; Li, J.; Naeem, M.; Liu, X.D. Heterosis and gene action study of agronomic traits in diploid and autotetraploid rice. Acta Agric. Scand. B—Soil Plant Sci. 2011, 61, 23–32. [Google Scholar] [CrossRef]
- Guo, X.P.; Qin, R.Z.; Chen, X. Factors affecting production of autotetraploid rice. J. Plant Genet. Resour. 2002, 3, 30–33. [Google Scholar]
- Xie, X.; Shan, S.; Wang, Y.; Cao, F.; Chen, J.; Huang, M.; Zou, Y. Dense planting with reducing nitrogen rate increased grain yield and nitrogen use efficiency in two hybrid rice varieties across two light conditions. Field Crops Res. 2019, 236, 24–32. [Google Scholar] [CrossRef]
- Ding, C.; You, J.; Chen, L.; Wang, S.; Ding, Y. Nitrogen fertilizer increases spikelet number per panicle by enhancing cytokinin synthesis in rice. Plant Cell Rep. 2014, 33, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Forrester, N.J.; Rebolleda-Gomez, M.; Sachs, J.L.; Ashman, T.L. Polyploid plants obtain greater fitness benefits from a nutrient acquisition mutualism. New Phytol. 2020, 227, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.; von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Chen, J.; Chen, D.; Dai, B.; Zhang, W.; Song, Z.; Yang, Z.; Du, C.; Tang, Z.; He, Y.; et al. The breeding of two polyploid rice lines with the characteristic of polyploid meiosis stability. Sci. China Ser. C 2007, 50, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Otto, S.P. The evolutionary consequences of polyploidy. Cell 2007, 131, 52–62. [Google Scholar] [CrossRef]
- Bao, W.K.; Qin, R.Z.; Wu, D.Y.; Chen, Z.Y.; Song, W.C.; Zhang, Y.H. High yielding tetraploid rice clones. Sci. Agric. Sin. 1985, 18, 64–66. (In Chinese) [Google Scholar]
- Shahid, M.Q.; Xu, H.M.; Lin, S.Q.; Chen, Z.X.; Naeem, M.; Li, Y.J.; Liu, X.D. Genetic analysis and hybrid vigor study of grain yield and other quantitative traits in autotetraploid rice. Pak. J. Bot. 2012, 44, 237–246. [Google Scholar]
- Song, W.C.; Zhang, Y.H. Rice tetraploidy and its effect on agronomic traits and nutritional constituents. Acta Agron. Sin. 1992, 18, 137–144. (In Chinese) [Google Scholar]
- Song, Z.; Du, C.; Dai, B.; Chen, D.; Chen, J.; Cai, D. Studies on the Growth Habits and Characteristics of Two Polyploid Indica-Japonica Hybrid Rice with Powerful Heterosis. Agric. Sci. China 2007, 6, 265–274. [Google Scholar] [CrossRef]
- Cassman, K.G.; DeDatta, S.K.; Amarante, S.T.; Liboon, S.P.; Samson, M.I.; Dizon, M.A. Long-term comparison of the agronomic efficiency and residual benefits of organic and inorganic nitrogen sources for tropical lowland rice. Exp. Agric. 1996, 32, 427–444. [Google Scholar] [CrossRef]
- Huang, M.; Yang, C.; Ji, Q.; Jiang, L.; Tan, J.; Li, Y. Tillering responses of rice to plant density and nitrogen rate in a subtropical environment of southern China. Field Crops Res. 2013, 149, 187–192. [Google Scholar] [CrossRef]
- Qin, R.Z.; Cheng, Z.J.; Guo, X.P. The establishment of mutant pool using anther culture of autotetrapolyploid rice. Acta Agron. Sin. 2005, 31, 392–394. (In Chinese) [Google Scholar]
- Ahmed, S.; Humphreys, E.; Salim, M.; Chauhan, B.S. Growth, yield and nitrogen use efficiency of dry-seeded rice as influenced by nitrogen and seed rates in Bangladesh. Field Crops Res. 2016, 186, 18–31. [Google Scholar] [CrossRef]
- Huang, M.; Zou, Y.B. Integrating mechanization with agronomy and breeding to ensure food security in China. Field Crops Res. 2018, 224, 22–27. [Google Scholar] [CrossRef]
- Sun, W.; Xu, X.H.; Li, Y.P.; Xie, L.X.; He, Y.N.; Li, W.; Lu, X.B.; Sun, H.W.; Xie, X.Z. OsmiR530 acts downstream of OsPIL15 to regulate grain yield in rice. New Phytol. 2020, 226, 823–837. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Wu, D.Y.; Song, W.C.; Zhang, Y.H.; Qin, R.Z.; Bao, W.K. Recent advanced in the autotetraploid rice breeding. Sci. Agric. Sin. 1987, 20, 20–24. (In Chinese) [Google Scholar]
- Fang, Z.; Morrell, P.L. Domestication: Polyploidy boosts domestication. Nat. Plants 2016, 2, 16116. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Morita, S.; Kitagawa, H.; Wada, H.; Takahashi, M. Grain yield response to planting density in forage rice with a large number of spikelets. Crops Sci. 2012, 52, 345–350. [Google Scholar] [CrossRef]
- Fageria, N.K.; Santos, A.B. Yield and yield components of lowland rice genotypes as influenced by nitrogen fertilization. Commun. Soil. Sci. Plant Anal. 2015, 46, 1723–1735. [Google Scholar] [CrossRef]
- Khush, G.S. Strategies for increasing the yield potential of cereals: Case of rice as an example. Plant Breed. 2013, 132, 433–436. [Google Scholar] [CrossRef]
- Salmon, A.; Flagel, L.; Ying, B.; Udall, J.A.; Wendel, J.F. Homoeologous nonreciprocal recombination in polyploid cotton. New Phytol. 2010, 186, 123–134. [Google Scholar] [CrossRef]
- Liu, J.X.; Chen, J.G.; Chen, D.L.; Song, Z.J.; Dai, B.C.; Cai, D.T. Studies on growth and flowering characteristics of polyploid hybrid rice parents with strong heterosis. Sci. Agric. Sin. 2008, 41, 3456–3464. (In Chinese) [Google Scholar]
- Nelson, G.C.; Rosegrant, M.W.; Koo, J.; Robertson, R.; Sulser, T.; Zhu, T.; Ringler, C.; Msangi, S.; Palazzo, A.; Batka, M. Climate Change: Impact on Agriculture and Costs of Adaptation; International Food Policy Research Institute: Washington, DC, USA, 2009; Volume 21. [Google Scholar]
- Peng, S.; Tang, Q.; Zou, Y. Current status and challenges of rice production in China. Plant Prod. Sci. 2009, 12, 3–8. [Google Scholar] [CrossRef]
- Ju, C.; Buresh, R.J.; Wang, Z.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crops Res. 2015, 175, 47–55. [Google Scholar] [CrossRef]
- Rinehardt, J.M.; Edmisten, K.L.; Wells, R.; Faircloth, J.C. Response of ultra-narrow and conventional spaced cotton to variable nitrogen rates. J. Plant Nutr. 2004, 27, 743–755. [Google Scholar] [CrossRef]
- Bao, W.K.; Yan, Y.R. A preliminary report on investigations of auto-polyploids and amphidiploids in some cereal crops. J. Integr. Plant Biol. 1956, 5, 297–316. (In Chinese) [Google Scholar]
- Chen, X.; Cui, Z.; Fan, M.; Vitousek, P.; Zhao, M.; Ma, W.; Wang, Z.; Zhang, W.; Yan, X.; Yang, J. Producing more grain with lower environmental costs. Nature 2014, 514, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J. Polyploidy and ecological adaptation in wild yarrow. Proc. Natl. Acad. Sci. USA 2011, 108, 7096–7101. [Google Scholar] [CrossRef]
- He, Y.C.; Ge, J.; Wei, Q.; Jiang, A.M.; Gan, L.; Song, Z.J.; Cai, D.T. Using a polyploid meiosis stability (PMeS) line as a parent improves embryo development and the seed set rate of a tetraploid rice hybrid. Can. J. Plant Sci. 2011, 91, 325–335. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Kontturi, M.; Young, A.T. Photosynthesis by Flag Leaves of Wheat in Relation to Protein, Ribulose Bis phosphate Carboxylase Activity and Nitrogen Supply. J. Exp. Bot. 1989, 40, 43–52. [Google Scholar] [CrossRef]
- Sun, Y.J.; Ma, J.; Sun, Y.Y.; Xu, H.; Yang, Z.Y.; Liu, S.J.; Jia, X.W.; Zheng, H.Z. The effects of different water and nitrogen managements on yield and nitrogen use efficiency in hybrid rice of China. Field Crops Res. 2012, 127, 85–98. [Google Scholar] [CrossRef]
- Chen, R.; Feng, Z.; Zhang, X.; Song, Z.; Cai, D. A new way of rice breeding: Polyploid rice breeding. Plants 2021, 10, 422. [Google Scholar] [CrossRef]
- Kamiji, Y.; Yoshida, H.; Palta, J.A.; Sakuratani, T.; Shiraiwa, T. N applications that increase plant N during panicle development are highly effective in increasing spikelet number in rice. Field Crops Res. 2011, 122, 242–247. [Google Scholar] [CrossRef]
- Zhu, D.W.; Zhang, H.C.; Guo, B.W.; Xu, K.; Dai, Q.G.; Wei, H.Y.; Gao, H.; Hu, Y.J.; Cui, P.Y.; Huo, Z.Y. Effects of nitrogen level on yield and quality of japonica soft super rice. J. Integr. Agric. 2017, 16, 1018–1027. [Google Scholar] [CrossRef]
- Zheng, K.W.; Zou, J.S.; Lu, C.G. Effects of Transplanting Density and Nitrogen Fertilizer on Yield For mation and N Absorption in a Two-line Intersubspecific Hybrid Rice Liangyoupeijiu. Acta Agron. Sin. 2006, 32, 885–893. (In Chinese) [Google Scholar]
- Jiang, W.K.; Liu, Y.L.; Xia, E.H.; Gao, L.Z. Prevalent role of gene features in determining evolutionary fates of whole-genome duplication duplicated genes in flowering plants. Plant Physiol. 2013, 161, 1844–1861. [Google Scholar] [CrossRef]
- Xu, X.P.; Zhou, W.; Liang, G.Q.; Sun, J.W.; Wang, X.B.; He, P.; Xu, F.S.; Yu, X.C. Effects of nitrogen and density interactions on grain yield and nitrogen use efficiency of double-rice systems. J. Plant Nutr. Fertil. 2015, 21, 763–772. (In Chinese) [Google Scholar]
- Zhang, Y.H.; Zhang, Y.L.; Huang, Q.W.; Xu, Y.C.; Shen, Q.R. Effects of different nitrogen application rates on grain yields and nitrogen uptake and utilization by different rice cultivars. Plant Nutr. Fertil. Sci. 2006, 12, 616–621. (In Chinese) [Google Scholar]


| Year | Genotypes | Densities | N Rates | Panicle Number (m−2) | Spikelets per Panicle | Grain-Filling Rate (%) | Grain Weight (mg) | Harvest Index (%) |
|---|---|---|---|---|---|---|---|---|
| 2018 | T7 | TD17 | N1 | 204.6 b | 116.1 a | 73.7 a | 39.1 a | 39.3 a |
| N2 | 216.6 ab | 107.6 a | 71.1 a | 38.9 a | 36.1 a | |||
| N3 | 227.8 ab | 107.1 a | 72.8 a | 38.1 a | 35.8 a | |||
| TD25 | N1 | 233.8 ab | 112.9 a | 70.6 a | 39.9 a | 36.4 a | ||
| N2 | 231.3 ab | 109.9 a | 72.9 a | 38.5 a | 35.1 a | |||
| N3 | 254.2 a | 100.4 a | 69.8 a | 39.1 a | 30.8 b | |||
| Mean | 228.0 B | 109.0 B | 71.8 B | 38.9 A | 35.6 B | |||
| Analysis of variance | N/TD/N × TD | ns/*/ns | ns/ns/ns | ns/ns/ns | ns/ns/ns | */*/ns | ||
| FLY4 | TD17 | N1 | 226.9 bc | 186.4 a | 85.9 ab | 25.2 a | 46.6 ab | |
| N2 | 225.9 c | 190.0 a | 85.9 ab | 26.2 a | 45.9 ab | |||
| N3 | 230.6 bc | 181.2 a | 83.4 b | 25.5 a | 46.4 ab | |||
| TD25 | N1 | 240.0 abc | 190.7 a | 88.3 a | 24.9 a | 47.9 a | ||
| N2 | 258.8 ab | 184.1 a | 85.8 ab | 25.5 a | 44.8 b | |||
| N3 | 267.5 a | 185.3 a | 85.3 ab | 24.8 a | 45.2 b | |||
| Mean | 241.6 A | 186.3 A | 85.8 A | 25.4 B | 46.1 A | |||
| Analysis of variance | N/TD/N × TD | ns/**/ns | ns/ns/ns | ns/ns/ns | ns/ns/ns | ns/ns/ns | ||
| 2019 | T7 | TD17 | N1 | 162.5 c | 163.0 a | 75.2 ab | 37.1 b | 41.1 a |
| N2 | 168.1 c | 153.4 ab | 75.5 ab | 36.7 b | 40.8 a | |||
| N3 | 181.3 bc | 132.7 bc | 75.9 ab | 37.2 b | 35.4 c | |||
| TD25 | N1 | 201.1 ab | 133.2 bc | 73.9 b | 38.1 a | 37.9 b | ||
| N2 | 222.9 a | 124.1 c | 76.9 ab | 37.7 ab | 36.4 bc | |||
| N3 | 220.9 a | 119.9 c | 77.8 a | 37.4 ab | 37.7 bc | |||
| Mean | 192.8 B | 137.7 B | 75.9 B | 37.4 A | 38.2 B | |||
| Analysis of variance | N/TD/N × TD | ns/**/ns | */*/ns | ns/ns/ns | ns/*/ns | **/*/** | ||
| FLY4 | TD17 | N1 | 223.6 c | 198.9 abc | 91.9 a | 25.3 a | 52.8 a | |
| N2 | 221.5 c | 208.7 a | 91.8 a | 25.4 a | 53.2 a | |||
| N3 | 239.6 bc | 205.4 ab | 89.9 a | 25.5 a | 51.2 ab | |||
| TD25 | N1 | 261.5 ab | 176.9 c | 94.0 a | 25.4 a | 49.9 b | ||
| N2 | 265.6 a | 184.4 bc | 91.3 a | 25.7 a | 51.1 ab | |||
| N3 | 264.6 a | 200.6 abc | 90.5 a | 25.6 a | 51.1 ab | |||
| Mean | 246.1 A | 195.8 A | 91.6 A | 25.5 B | 51.6 A | |||
| Analysis of variance | N/TD/N × TD | ns/**/ns | ns/*/ns | ns/ns/ns | ns/ns/ns | ns/*/ns | ||
| Year | Genotypes | Densities | N Rates | Straw N Content (%) | Grain N Content (%) | Straw N Uptake (kg ha−1) | Grain N Uptake (kg ha−1) | Total N Uptake (kg ha−1) | NUEg (kg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| 2018 | T7 | TD17 | N1 | 1.06 b | 1.28 b | 74.7 d | 70.5 a | 162.9 d | 34.2 a |
| N2 | 1.06 b | 1.34 ab | 82.0 cd | 70.8 a | 170.0 cd | 31.2 ab | |||
| N3 | 1.35 a | 1.47 a | 102.0 ab | 76.4 a | 200.3 ab | 26.0 bc | |||
| TD25 | N1 | 1.00 b | 1.33 b | 85.4 bcd | 79.3 a | 184.2 bcd | 32.4 a | ||
| N2 | 1.05 b | 1.36 ab | 95.4 bc | 80.7 a | 193.3 abc | 30.6 abc | |||
| N3 | 1.18 ab | 1.35 ab | 116.4 a | 71.4 a | 211.6 a | 25.2 c | |||
| Mean | 1.12 A | 1.36 A | 92.7 A | 74.9 B | 187.0 A | 29.9 B | |||
| Analysis of variance | N/TD/N × TD | */ns/ns | */ns/ns | */*/ns | ns/ns/ns | */*/ns | **/ns/ns | ||
| FLY4 | TD17 | N1 | 0.55 bc | 1.24 b | 46.1 d | 103.4 c | 160.4 c | 52.1 a | |
| N2 | 0.75 a | 1.36 a | 72.7 abc | 123.6 ab | 206.4 ab | 43.9 b | |||
| N3 | 0.81 a | 1.34 ab | 66.4 bcd | 109.0 bc | 189.1 bc | 43.1 b | |||
| TD25 | N1 | 0.53 c | 1.23 b | 49.5 cd | 117.5 abc | 175.5 c | 54.4 a | ||
| N2 | 0.70 ab | 1.31 ab | 73.3 ab | 125.2 a | 211.6 ab | 45.0 b | |||
| N3 | 0.80 a | 1.33 ab | 83.5 a | 129.3 a | 228.0 a | 42.6 b | |||
| Mean | 0.69 B | 1.30 B | 65.3 B | 118.0 A | 195.2 A | 46.9 A | |||
| Analysis of variance | N/TD/N × TD | */ns/ns | */ns/ns | */*/ns | ns/*/ns | **/*/ns | **/ns/ns | ||
| 2019 | T7 | TD17 | N1 | 0.63 d | 1.12 ab | 48.5 c | 72.0 b | 134.3 c | 47.9 a |
| N2 | 0.87 ab | 1.19 ab | 64.9 b | 73.4 b | 153.5 b | 40.3 bc | |||
| N3 | 0.95 a | 1.22 a | 86.1 a | 70.6 b | 173.2 a | 33.6 d | |||
| TD25 | N1 | 0.68 cd | 1.10 b | 64.1 b | 74.0 b | 152.2 b | 44.3 ab | ||
| N2 | 0.77 bc | 1.21 ab | 79.1 a | 82.7 a | 179.1 a | 38.3 cd | |||
| N3 | 0.97 a | 1.20 ab | 92.3 a | 80.7 a | 189.7 a | 35.6 cd | |||
| Mean | 0.81 A | 1.17 A | 72.5 A | 75.6 B | 163.7 B | 40.0 B | |||
| Analysis of variance | N/TD/N × TD | **/ns/ns | */ns/ns | **/**/ns | ns/**/* | */**/ns | **/ns/ns | ||
| FLY4 | TD17 | N1 | 0.48 a | 1.13 b | 38.9 b | 112.7 d | 157.8 c | 63.9 a | |
| N2 | 0.48 a | 1.20 ab | 39.6 b | 123.9 bcd | 170.1 bc | 61.2 ab | |||
| N3 | 0.53 a | 1.27 a | 48.9 ab | 135.7 b | 194.1 a | 55.6 b | |||
| TD25 | N1 | 0.43 a | 1.14 b | 43.0 ab | 122.6 cd | 171.3 bc | 62.7 ab | ||
| N2 | 0.52 a | 1.21 ab | 50.1 a | 133.8 bc | 191.0 ab | 58.2 ab | |||
| N3 | 0.48 a | 1.28 a | 48.7 ab | 150.3 a | 208.6 a | 56.3 ab | |||
| Mean | 0.49 B | 1.21 A | 44.9 B | 129.8 A | 182.2 A | 59.6 A | |||
| Analysis of variance | N/TD/N × TD | ns/ns/ns | */ns/ns | */ns/ns | **/*/ns | **/*/ns | */ns/ns | ||
| Year | Genotypes | DAP (g m−2) | DTP (g m−2) | DTEP (%) | DTCP (%) |
|---|---|---|---|---|---|
| 2018 | T7 | 438.3 b | 122.1 b | 10.1 b | 22.7 b |
| FLY4 | 642.0 a | 387.2 a | 29.0 a | 37.9 a | |
| 2019 | T7 | 402.5 b | 243.0 b | 18.3 b | 37.5 a |
| FLY4 | 581.3 a | 495.2 a | 32.6 a | 45.9 a |
| Year | Genotypes | Densities | N Rates | PI-NPR (µmol m⁻2s⁻1) | BT-NPR (µmol m⁻2s⁻1) | HD-NPR (µmol m⁻2s⁻1) |
|---|---|---|---|---|---|---|
| 2018 | T7 | TD17 | N1 | 26.2 a | 27.8 a | 24.1 a |
| N2 | 26.1 a | 29.1 a | 22.3 a | |||
| N3 | 27.7 a | 28.2 a | 24.4 a | |||
| TD25 | N1 | 24.3 a | 27.4 a | 23.6 a | ||
| N2 | 25.7 a | 27.9 a | 22.4 a | |||
| N3 | 26.1 a | 29.4 a | 23.7 a | |||
| Mean | 26.0 B | 28.3 B | 23.4 A | |||
| Analysis of variance | N/TD/N × TD | ns/ns/ns | ns/ns/ns | ns/ns/ns | ||
| FLY4 | TD17 | N1 | 31.9 a | 31.1 ab | 24.8 a | |
| N2 | 28.3 a | 33.8 a | 24.1 a | |||
| N3 | 28.7 a | 29.6 b | 24.0 a | |||
| TD25 | N1 | 31.7 a | 32.7 ab | 21.6 a | ||
| N2 | 29.0 a | 30.3 ab | 25.4 a | |||
| N3 | 28.7 a | 29.6 b | 22.9 a | |||
| Mean | 29.7 A | 31.2 A | 23.8 A | |||
| Analysis of variance | N/TD/N × TD | ns/ns/ns | ns/ns/ns | ns/ns/ns | ||
| 2019 | T7 | TD17 | N1 | 23.6 a | 21.3 a | |
| N2 | 25.0 a | 20.9 a | ||||
| N3 | 25.1 a | 22.1 a | ||||
| TD25 | N1 | 23.8 a | 21.1 a | |||
| N2 | 24.9 a | 22.0 a | ||||
| N3 | 24.3 a | 22.3 a | ||||
| Mean | 24.5 B | 21.6 A | ||||
| Analysis of variance | N/TD/N × TD | ns/ns/ns | ns/ns/ns | |||
| FLY4 | TD17 | N1 | 24.9 bc | 20.1 b | ||
| N2 | 26.8 ab | 23.4 a | ||||
| N3 | 27.3 ab | 21.5 ab | ||||
| TD25 | N1 | 22.8 c | 19.6 b | |||
| N2 | 26.2 ab | 22.4 ab | ||||
| N3 | 28.5 a | 22.1 ab | ||||
| Mean | 26.1 A | 21.5 A | ||||
| Analysis of variance | N/TD/N × TD | */ns/ns | */ns/ns | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Xiong, Z.; Huang, J.; Zhang, Z.; Cai, D.; Xiong, D.; Cui, K.; Peng, S.; Huang, J. Differences in Grain Yield and Nitrogen Uptake between Tetraploid and Diploid Rice: The Physiological Mechanisms under Field Conditions. Plants 2024, 13, 2884. https://doi.org/10.3390/plants13202884
Xiao J, Xiong Z, Huang J, Zhang Z, Cai D, Xiong D, Cui K, Peng S, Huang J. Differences in Grain Yield and Nitrogen Uptake between Tetraploid and Diploid Rice: The Physiological Mechanisms under Field Conditions. Plants. 2024; 13(20):2884. https://doi.org/10.3390/plants13202884
Chicago/Turabian StyleXiao, Jian, Zhuang Xiong, Jiada Huang, Zuolin Zhang, Detian Cai, Dongliang Xiong, Kehui Cui, Shaobing Peng, and Jianliang Huang. 2024. "Differences in Grain Yield and Nitrogen Uptake between Tetraploid and Diploid Rice: The Physiological Mechanisms under Field Conditions" Plants 13, no. 20: 2884. https://doi.org/10.3390/plants13202884
APA StyleXiao, J., Xiong, Z., Huang, J., Zhang, Z., Cai, D., Xiong, D., Cui, K., Peng, S., & Huang, J. (2024). Differences in Grain Yield and Nitrogen Uptake between Tetraploid and Diploid Rice: The Physiological Mechanisms under Field Conditions. Plants, 13(20), 2884. https://doi.org/10.3390/plants13202884









