Mechanisms of Cannabis Growth Promotion by Bacillus velezensis S141
Abstract
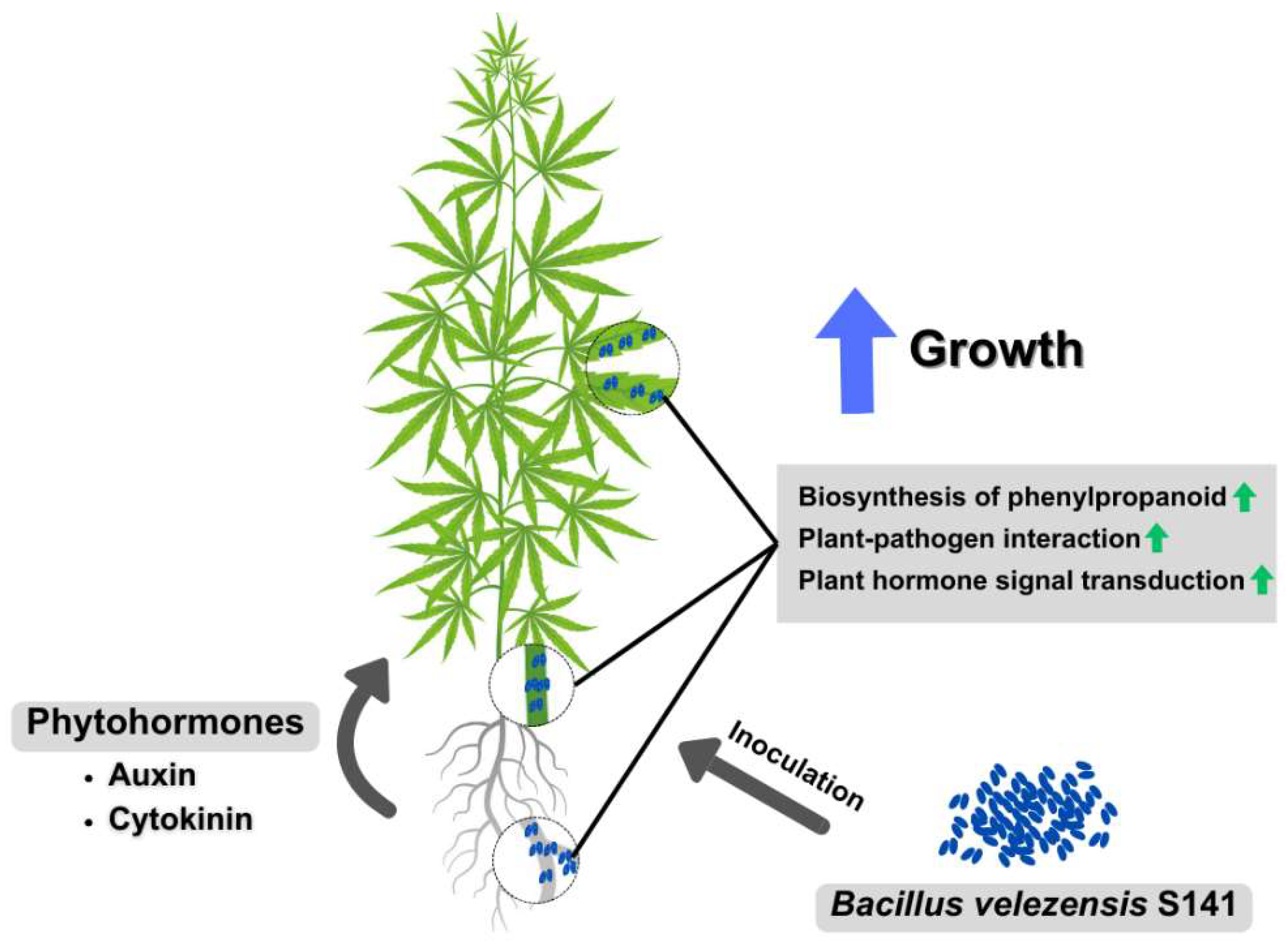
:1. Introduction
2. Results
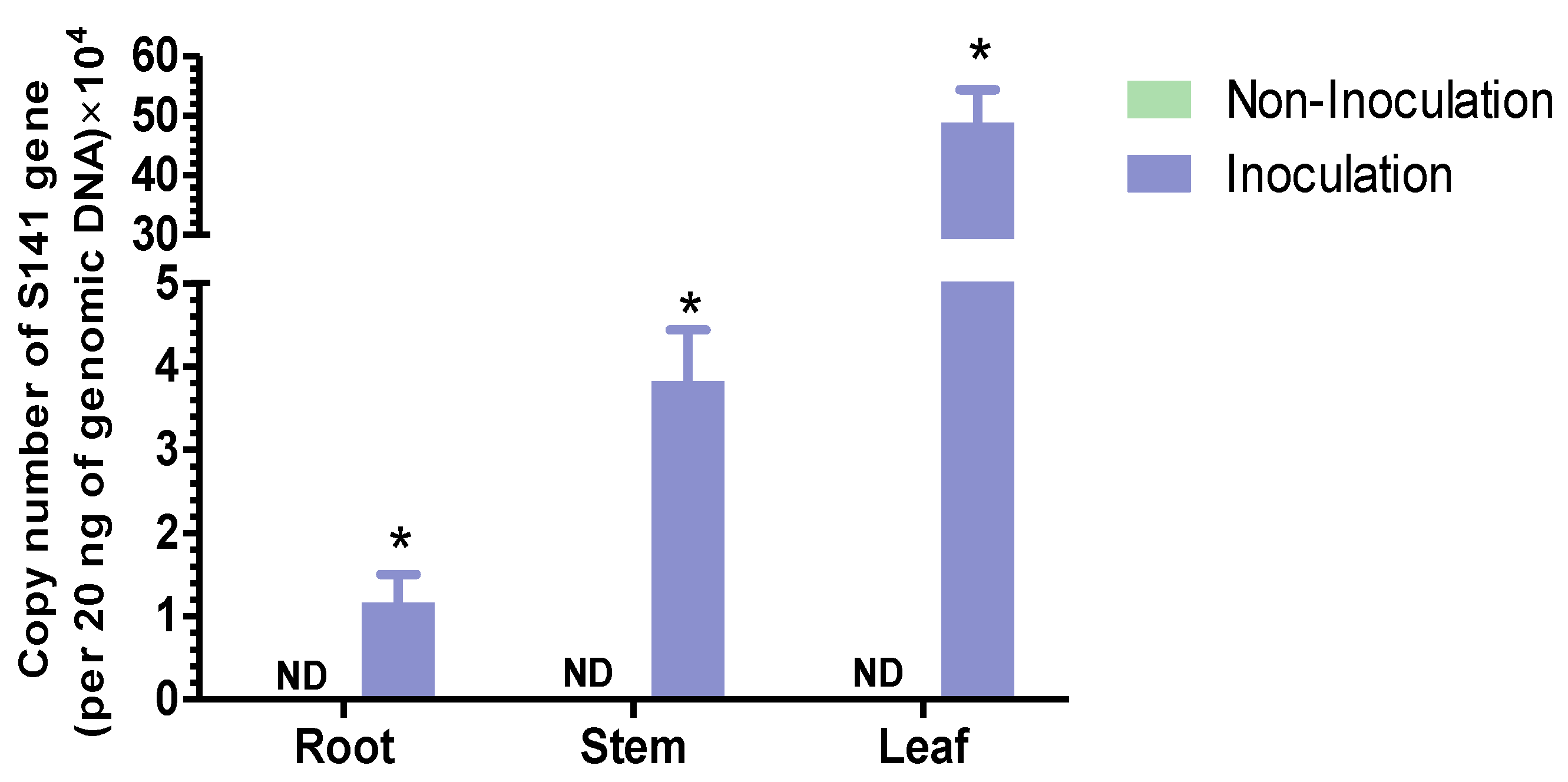
2.1. The Colonization of Bacillus velezensis S141 After Cannabis Inoculation
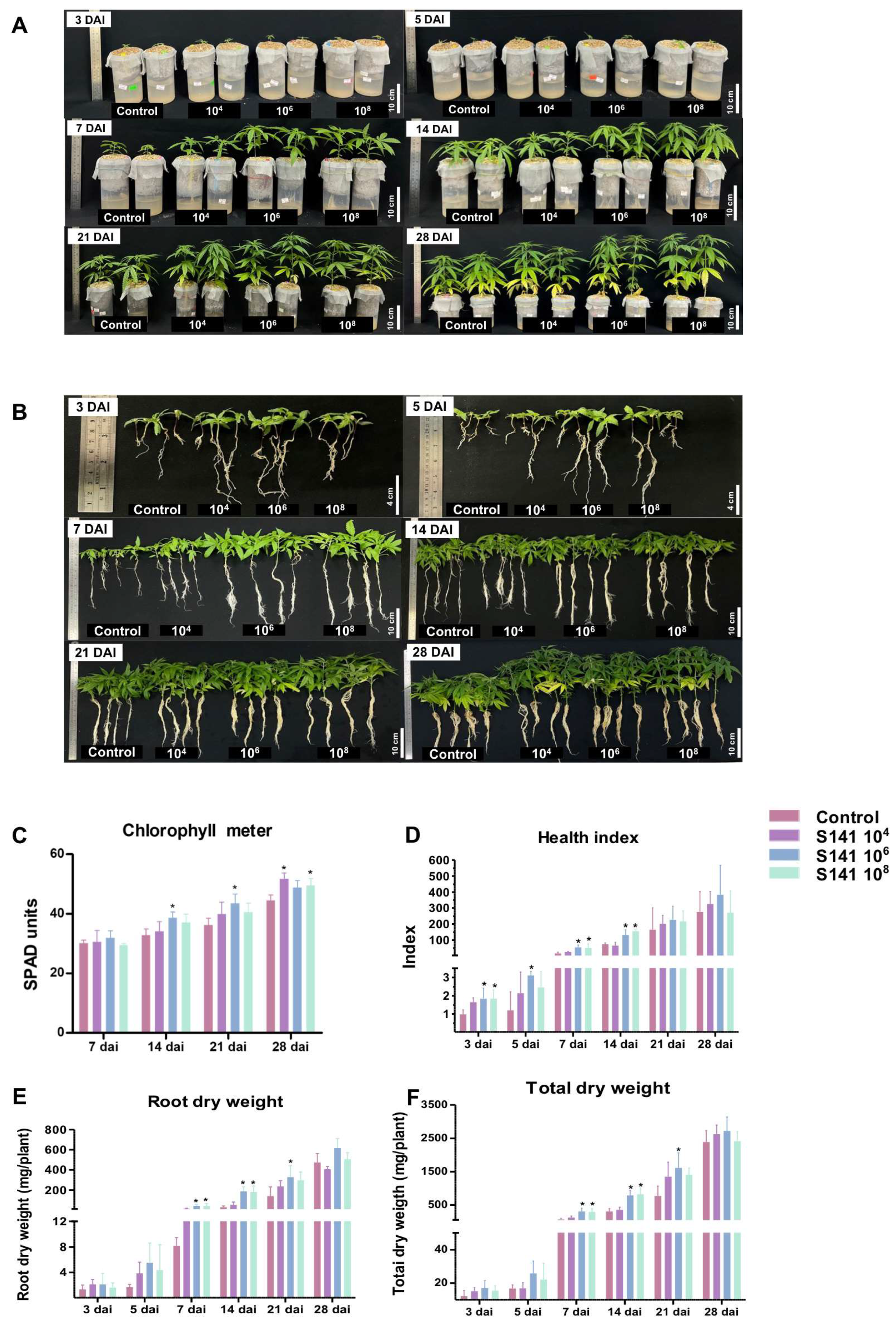
2.2. The Improved Growth Performance of the Bacillus velezensis S141-Inoculated Cannabis: Laboratorial Cultivation
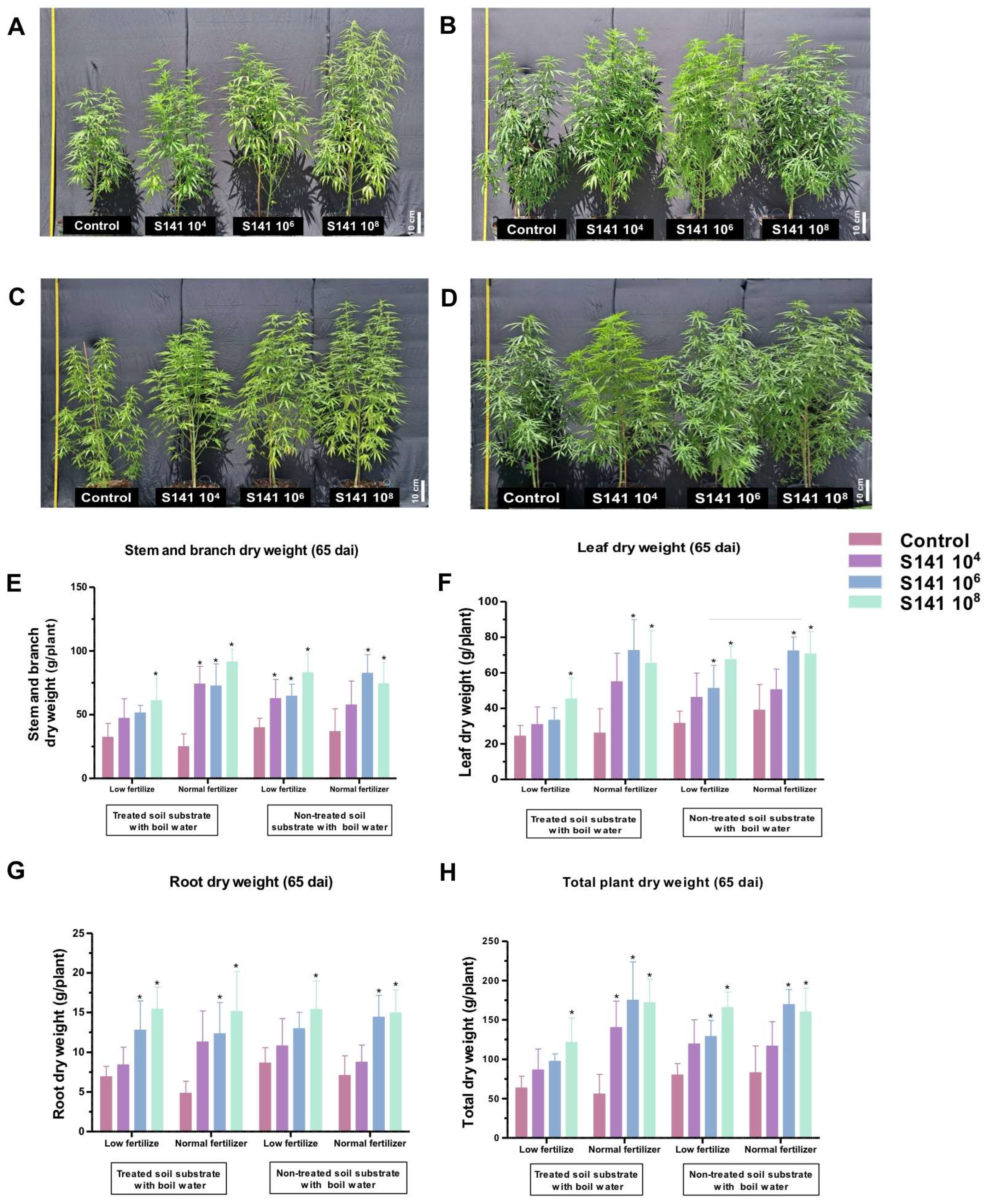
2.3. The Improved Growth Performance of the Bacillus velezensis S141-Inoculated Cannabis: Greenhouse Cultivation
2.4. Transcriptomic Analysis, GO Terms, and KEGG Pathways
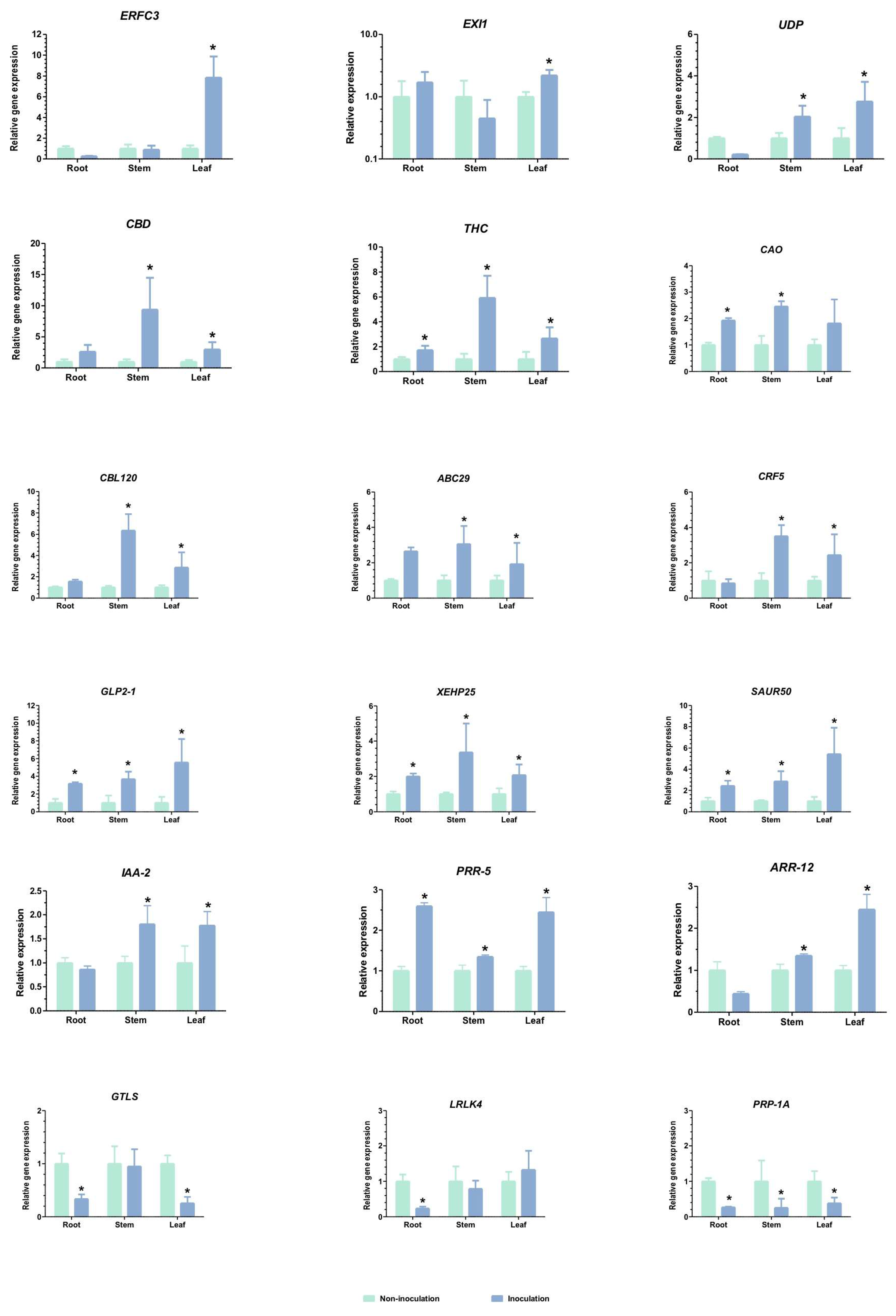
2.5. Gene Expression Validation by qRT-PCR
2.6. The Effects of Bacillus velezensis S141 Mutants on the Foi Thong Suranaree 1 Cannabis Strain Growth Under Laboratory Conditions
3. Discussion
4. Materials and Methods
4.1. The Statement of Ethics
4.2. Bacterial Strains and Growth Conditions
4.3. Cannabis Sativa Strain Used in This Study
4.4. Investigation of Plant Growth Promotion
4.5. Evaluating the Chlorophyll Content and Health Index (HI) of the Cannabis
4.6. RNA and DNA Extraction
4.7. Sequencing Analysis
4.8. Gene Expression Analysis by Quantitative Real-Time PCR (qRT-PCR)
4.9. Estimation of the Number of Endophytic S141 in Different Plant Tissues
4.10. Investigation of the Cannabis Growth Profiles Upon Inoculation With Bacillus velezensis S141 Mutants
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hartsel, J.A.; Eades, J.; Hickory, B.; Makriyannis, A. Cannabis sativa and Hemp. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 735–754. [Google Scholar]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Dariš, B.; Verboten, M.T.; Knez, Ž.; Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn. J. Basic Med. Sci. 2019, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef] [PubMed]
- Bruce, D.; Connelly, G.; Ellison, S. Different fertility approaches in organic hemp (Cannabis sativa L.) production alter floral biomass yield but not CBD: THC ratio. Sustainability 2022, 14, 6222. [Google Scholar] [CrossRef]
- Bashan, Y.; De-Bashan, L.E. How the plant growth-promoting bacterium Azospirillum promotes plant growth—A critical assessment. Adv. Agron. 2010, 108, 77–136. [Google Scholar]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Lyu, D.; Backer, R.; Smith, D. Plant growth-promoting rhizobacteria (PGPR) as plant biostimulants in agriculture. In Biostimulants for Sustainable Crop Production; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 197–226. [Google Scholar]
- Ruiz-Garcia, C.; Bejar, V.; Martinez-Checa, F.; Llamas, I.; Quesada, E. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Vélez in Málaga, southern Spain. Int. J. Syst. Evol. Microbiol. 2005, 55, 191–195. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Uhl, J.; Grosch, R.; Alquéres, S.; Pittroff, S.; Dietel, K.; Schmitt-Kopplin, P.; Borriss, R.; Hartmann, A. Cyclic lipopeptides of Bacillus amyloliquefaciens subsp. plantarum colonizing the lettuce rhizosphere enhance plant defense responses toward the bottom rot pathogen Rhizoctonia solani. Mol. Plant-Microbe Interact. 2015, 28, 984–995. [Google Scholar] [CrossRef]
- Cantoro, R.; Palazzini, J.M.; Yerkovich, N.; Miralles, D.J.; Chulze, S.N. Bacillus velezensis RC 218 as a biocontrol agent against Fusarium graminearum: Effect on penetration, growth and TRI5 expression in wheat spikes. BioControl 2021, 66, 259–270. [Google Scholar] [CrossRef]
- Fan, B.; Blom, J.; Klenk, H.-P.; Borriss, R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 2017, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wu, H.; Chen, L.; Xie, S.; Zang, H.; Borriss, R.; Gao, X. Bacilysin from Bacillus amyloliquefaciens FZB42 has specific bactericidal activity against harmful algal bloom species. Appl. Environ. Microbiol. 2014, 80, 7512–7520. [Google Scholar] [CrossRef]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-I.; Teaumroong, N. Co-inoculation of Bacillus velezensis strain S141 and Bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms 2020, 8, 678. [Google Scholar] [CrossRef]
- Songwattana, P.; Boonchuen, P.; Piromyou, P.; Wongdee, J.; Greetatorn, T.; Inthaisong, S.; Tantasawat, P.A.; Teamtisong, K.; Tittabutr, P.; Boonkerd, N. Insights into antifungal mechanisms of Bacillus velezensis S141 against Cercospora leaf spot in mungbean (V. radiata). Microbes Environ. 2023, 38, ME22079. [Google Scholar] [CrossRef]
- Kondo, T.; Sibponkrung, S.; Hironao, K.-Y.; Tittabutr, P.; Boonkerd, N.; Ishikawa, S.; Ashida, H.; Teaumroong, N.; Yoshida, K.-i. Bacillus velezensis S141, a soybean growth-promoting bacterium, hydrolyzes isoflavone glycosides into aglycones. J. Gen. Appl. Microbiol. 2023, 69, 175–183. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Balderas-Ruíz, K.A.; Bustos, P.; Santamaria, R.I.; González, V.; Cristiano-Fajardo, S.A.; Barrera-Ortíz, S.; Mezo-Villalobos, M.; Aranda-Ocampo, S.; Guevara-García, Á.A.; Galindo, E. Bacillus velezensis 83 a bacterial strain from mango phyllosphere, useful for biological control and plant growth promotion. Amb Express 2020, 10, 163. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Yue, T.; Huang, Y.; He, C.; Jiang, W.; Liu, H.; Zeng, H.; Wang, J. Plant growth-promoting rhizobacteria Bacillus velezensis JB0319 promotes lettuce growth under salt stress by modulating plant physiology and changing the rhizosphere bacterial community. J. Exp. Bot. 2023, 213, 105451. [Google Scholar] [CrossRef]
- Bagheri, N.; Ahmadzadeh, M.; Mariotte, P.; Jouzani, G.S. Behavior and interactions of the plant growth-promoting bacteria Azospirillum oryzae NBT506 and Bacillus velezensis UTB96 in a co-culture system. World J. Microbiol. Biotechnol. 2022, 38, 101. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hao, H.; Lu, X.; Zhao, X.; Wang, Y.; Zhang, Y.; Xie, Z.; Wang, R. Transcriptome profiling of genes involved in induced systemic salt tolerance conferred by Bacillus amyloliquefaciens FZB42 in Arabidopsis thaliana. Sci. Rep. 2017, 7, 10795. [Google Scholar] [CrossRef]
- Shin, J.-H.; Park, B.-S.; Kim, H.-Y.; Lee, K.-H.; Kim, K.S. Antagonistic and plant growth-promoting effects of Bacillus velezensis BS1 isolated from rhizosphere soil in a pepper field. Plant Pathol. J. 2021, 37, 307. [Google Scholar] [CrossRef]
- Kiddee, S.; Wongdee, J.; Piromyou, P.; Songwattana, P.; Greetatorn, T.; Boonkerd, N.; Teaumroong, N.; Saito, K.; Tittabutr, P. Unveiling the tripartite synergistic interaction of plant-arbuscular mycorrhizal fungus symbiosis by endophytic Bacillus velezensis S141 in Lotus japonicus. Symbiosis 2024, 92, 355–367. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Zhao, L.; Chen, A.; Li, J.; Tang, H.; Pan, G.; Chang, L.; Deng, Y.; Huang, S. Comparative transcriptome combined with physiological analyses revealed key factors for differential cadmium tolerance in two contrasting hemp (Cannabis sativa L.) cultivars. Ind. Crop. Prod. 2019, 140, 111638. [Google Scholar] [CrossRef]
- Balthazar, C.; Cantin, G.; Novinscak, A.; Joly, D.L.; Filion, M. Expression of putative defense responses in cannabis primed by Pseudomonas and/or Bacillus strains and infected by Botrytis cinerea. Front. Plant Sci. 2020, 11, 572112. [Google Scholar] [CrossRef]
- Miotti, N.; Sukhikh, N.; Laboureau, N.; Casati, P.; Pooggin, M.M. Cannabis virome reconstruction and antiviral RNAi characterization through small RNA sequencing. Plants 2023, 12, 3925. [Google Scholar] [CrossRef]
- Liao, Z.; Zhou, Z.; Li, Y.; Zhang, Y. Plant metabolism and synthetic biology. Synth. Syst. Biotechnol. 2023, 8, 563. [Google Scholar] [CrossRef]
- Geng, D.; Shen, X.; Xie, Y.; Yang, Y.; Bian, R.; Gao, Y.; Li, P.; Sun, L.; Feng, H.; Ma, F. Regulation of phenylpropanoid biosynthesis by MdMYB88 and MdMYB124 contributes to pathogen and drought resistance in apple. Hortic. Res. 2020, 7, 102. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action mechanisms of effectors in plant-pathogen interaction. Int. J. Mol. Sci. 2022, 23, 6758. [Google Scholar] [CrossRef]
- Song, S.; Chang, J.; Ma, C.; Tan, Y.-W. Single-molecule fluorescence methods to study plant hormone signal transduction pathways. Front. Plant Sci. 2017, 8, 1888. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wang, J.; Ren, W.; Zhou, Z.; Long, X.; Gao, X.; Rengel, Z. Expression of genes related to plant hormone signal transduction in jerusalem artichoke (Helianthus tuberosus L.) seedlings under salt stress. Agronomy 2022, 12, 163. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Ihsan, Z.; Shah, A.N.; Wu, C.; Yousaf, M.; Nasim, W.; Alharby, H. Exogenously applied plant growth regulators enhance the morpho-physiological growth and yield of rice under high temperature. Front. Plant Sci. 2016, 7, 1250. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, Y.; Wang, Y.; Li, H.; Liu, J.; Tan, J.; He, J.; Bai, J.; Ma, H. Evolution Analysis of the Aux/IAA Gene Family in Plants Shows Dual Origins and Variable Nuclear Localization Signals. Int. J. Mol. Sci. 2017, 18, 2107. [Google Scholar] [CrossRef]
- Ren, H.; Gray, W.M. SAUR Proteins as Effectors of Hormonal and Environmental Signals in Plant Growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef]
- Mason, M.G.; Mathews, D.E.; Argyros, D.A.; Maxwell, B.B.; Kieber, J.J.; Alonso, J.M.; Ecker, J.R.; Schaller, G.E. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 2005, 17, 3007–3018. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Diffuse Growth of Plant Cell Walls. Plant Physiol. 2018, 176, 16–27. [Google Scholar] [CrossRef]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef]
- Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The Roles of Aquaporins in Plant Stress Responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef]
- Gou, J.; Strauss, S.H.; Tsai, C.J.; Fang, K.; Chen, Y.; Jiang, X.; Busov, V.B. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell 2010, 22, 623–639. [Google Scholar] [CrossRef]
- Lee, Z.H.; Hirakawa, T.; Yamaguchi, N.; Ito, T. The Roles of Plant Hormones and Their Interactions with Regulatory Genes in Determining Meristem Activity. Int. J. Mol. Sci. 2019, 20, 4065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gong, M.; Xu, X.; Li, H.; Deng, W. Roles of Auxin in the Growth, Development, and Stress Tolerance of Horticultural Plants. Cells 2022, 11, 2761. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Burch, D.; Badenhorst, P.; Palanisamy, R.; Mason, J.; Spangenberg, G. Regulated expression of a cytokinin biosynthesis gene IPT delays leaf senescence and improves yield under rainfed and irrigated conditions in canola (Brassica napus L.). PLoS ONE 2015, 10, e0116349. [Google Scholar] [CrossRef] [PubMed]
- Glanz-Idan, N.; Lach, M.; Tarkowski, P.; Vrobel, O.; Wolf, S. Delayed Leaf Senescence by Upregulation of Cytokinin Biosynthesis Specifically in Tomato Roots. Front. Plant Sci. 2022, 13, 922106. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Limkul, S.; Phiwthong, T.; Massu, A.; Jaree, P.; Thawonsuwan, J.; Teaumroong, N.; Boonanuntanasarn, S.; Somboonwiwat, K.; Boonchuen, P. The interferon-like proteins, Vagos, in Fenneropenaeus merguiensis elicit antimicrobial responses against WSSV and VPAHPND infection. Fish Shellfish Immunol. 2022, 131, 718–728. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aunkam, P.; Sibponkrung, S.; Limkul, S.; Seabkongseng, T.; Mahanil, K.; Umnajkitikorn, K.; Boonkerd, N.; Teaumroong, N.; Sato, S.; Tittabutr, P.; et al. Mechanisms of Cannabis Growth Promotion by Bacillus velezensis S141. Plants 2024, 13, 2971. https://doi.org/10.3390/plants13212971
Aunkam P, Sibponkrung S, Limkul S, Seabkongseng T, Mahanil K, Umnajkitikorn K, Boonkerd N, Teaumroong N, Sato S, Tittabutr P, et al. Mechanisms of Cannabis Growth Promotion by Bacillus velezensis S141. Plants. 2024; 13(21):2971. https://doi.org/10.3390/plants13212971
Chicago/Turabian StyleAunkam, Phirom, Surachat Sibponkrung, Sirawich Limkul, Tuangrak Seabkongseng, Kanjana Mahanil, Kamolchanok Umnajkitikorn, Nantakorn Boonkerd, Neung Teaumroong, Shusei Sato, Panlada Tittabutr, and et al. 2024. "Mechanisms of Cannabis Growth Promotion by Bacillus velezensis S141" Plants 13, no. 21: 2971. https://doi.org/10.3390/plants13212971
APA StyleAunkam, P., Sibponkrung, S., Limkul, S., Seabkongseng, T., Mahanil, K., Umnajkitikorn, K., Boonkerd, N., Teaumroong, N., Sato, S., Tittabutr, P., & Boonchuen, P. (2024). Mechanisms of Cannabis Growth Promotion by Bacillus velezensis S141. Plants, 13(21), 2971. https://doi.org/10.3390/plants13212971




