Unveiling the Larvicidal Potential of Golpar (Heracleum persicum Desf. ex Fisch.) Essential Oil and Its Main Constituents on Aedes and Anopheles Mosquito Vectors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil (EO) Chemical Analysis
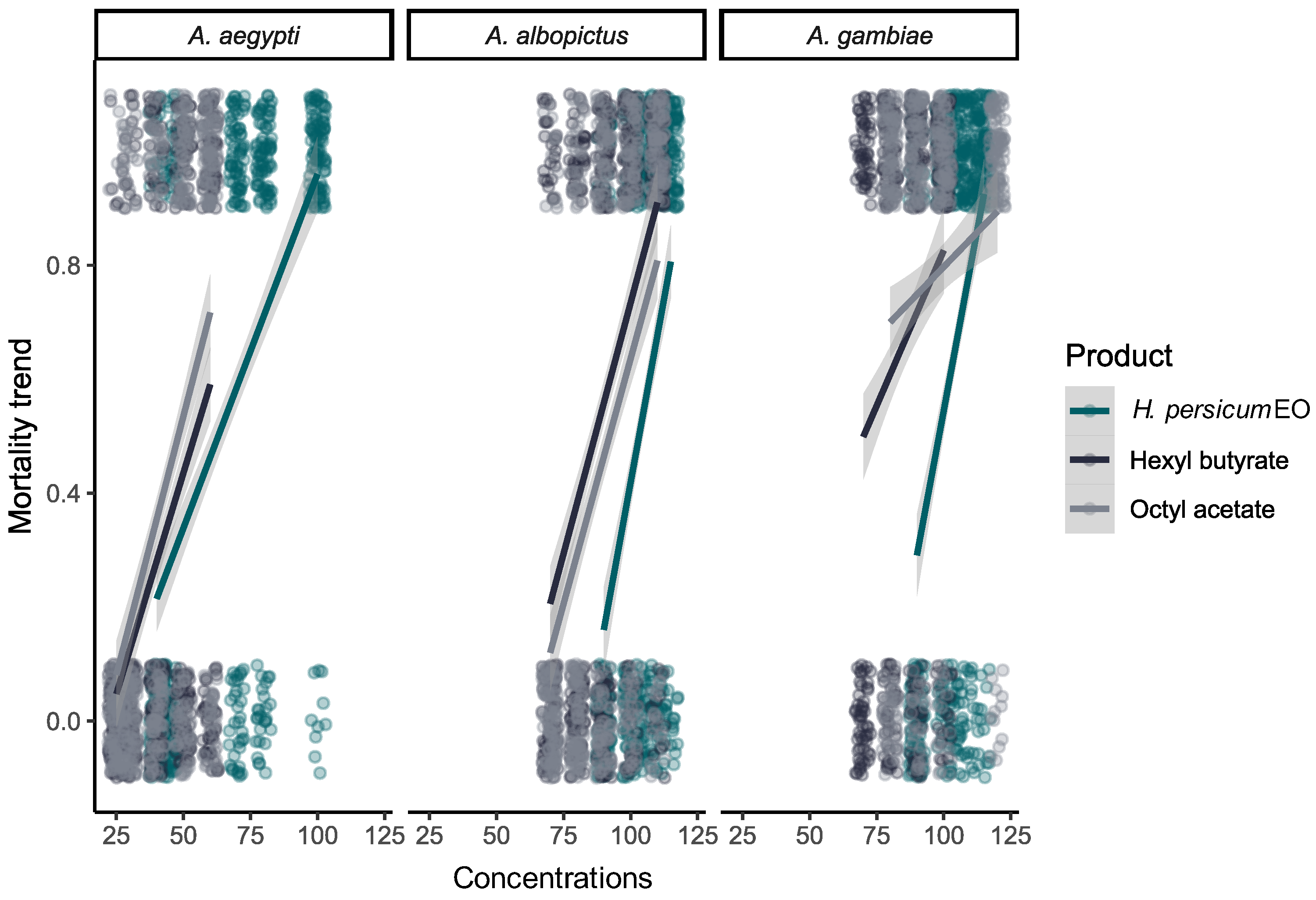
2.2. Mosquito Larvicidal Assays
2.3. Cytotoxicity Assay
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Hydrodistillation
3.4. Chemical Characterization of the EO
3.5. Mosquitoes
3.6. Larvicidal Assays
3.7. MTT Cytotoxicity Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gubler, D.J. The global threat of emergent/re-emergent vector-borne diseases. In Vector Biology, Ecology and Control; Atkinson, P.W., Ed.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany, 2010; pp. 39–62. [Google Scholar]
- Chala, B.; Hamde, F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: A review. Front. Public Health 2021, 9, 715759. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Di Giovanni, F.; Wilke, A.B.; Beier, J.C.; Pombi, M.; Mendoza-Roldan, J.A.; Desneux, N.; Canale, A.; Lucchi, A.; Dantas-Torres, F.; Otranto, D.; et al. Parasitic strategies of arthropods of medical and veterinary importance. Entomol. Gen. 2021, 41, 511–521. [Google Scholar] [CrossRef]
- Benelli, G.; Wilke, A.B.; Beier, J.C. Aedes albopictus (Asian tiger mosquito). Trends Parasitol. 2020, 36, 942–943. [Google Scholar] [CrossRef]
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Deseneux, N.; et al. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control 2022, 176, 10507. [Google Scholar] [CrossRef]
- Wilke, A.B.; Beier, J.C.; Benelli, G. Transgenic mosquitoes–fact or fiction? Trends Parasitol. 2018, 34, 456–465. [Google Scholar] [CrossRef]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef]
- Enayati, A.; Hanafi-Bojd, A.A.; Sedaghat, M.M.; Zaim, M.; Hemingway, J. Evolution of insecticide resistance and its mechanisms in Anopheles stephensi in the WHO Eastern Mediterranean Region. Malar. J. 2020, 19, 258. [Google Scholar] [CrossRef]
- Moyes, C.L.; Athinya, D.K.; Seethaler, T.; Battle, K.E.; Sinka, M.; Hadi, M.P.; Hemingway, J.; Coleman, M.; Hancock, P.A. Evaluating insecticide resistance across African districts to aid malaria control decisions. Proc. Natl. Acad. Sci. USA 2020, 117, 22042–22050. [Google Scholar] [CrossRef]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Weetman, D. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Haddi, K.; Nauen, R.; Benelli, G.; Guedes, R.N.C. Global perspectives on insecticide resistance in agriculture and public health. Entomol. Gen. 2023, 43, 495–500. [Google Scholar] [CrossRef]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef]
- Douchet, L.; Haramboure, M.; Baldet, T.; L’ambert, G.; Damiens, D.; Gouagna, L.C.; Bouyer, J.; Labbé, P.; Tran, A. Comparing sterile male releases and other methods for integrated control of the tiger mosquito in temperate and tropical climates. Sci. Rep. 2021, 11, 7354. [Google Scholar] [CrossRef]
- Şengül Demirak, M.Ş.; Canpolat, E. Plant-based bioinsecticides for mosquito control: Impact on insecticide resistance and disease transmission. Insects 2022, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Jonsell, B.; Karlsson, T. Heracleum L. In Flora Nordica (Thymelaeaceae to Apiaceae); Jonsell, B., Karlsson, T., Eds.; Bergius Foundation, Royal Swedish Academy of Sciences: Stockholm, Sweden, 2010; pp. 224–234. [Google Scholar]
- Majidi, Z.; Lamardi, S.S. Phytochemistry and biological activities of Heracleum persicum: A review. J. Integr. Med. 2018, 16, 223–235. [Google Scholar] [CrossRef]
- Zargari, A. Medicinal Plants, 3rd ed.; Tehran University Press: Tehran, Iran, 1997; pp. 59–64. [Google Scholar]
- Sedaghat, M.M.; Dehkordi, A.S.; Abai, M.R.; Khanavi, M.; Mohtarami, F.; Abadi, Y.S.; Vatandoost, H. Larvicidal activity of essential oils of Apiaceae plants against malaria vector, Anopheles stephensi. Iran. J. Arthropod Borne Dis. 2011, 5, 51. [Google Scholar]
- Manzoomi, N.; Ganbalani, G.N.; Dastjerdi, H.R.; Fathi, S.A. Fumigant toxicity of essential oils of Lavandula officinalis, Artemisia dracunculus and Heracleum persicum on the adults of Callosobruchus maculatus (Coleoptera: Bruchidae). Munn. Entomol. Zool. 2010, 5, 118–122. [Google Scholar]
- Ebadollahi, A.; Zavieh, E.A.; Nazifi, A.; Sendi, J.J.; Farjaminezhad, M.; Samadzadeh, A.; Tajmiri, P. Chemical composition and bio-pesticidal values of essential oil isolated from the seed of Heracleum persicum Desf. ex Fischer (Apiaceae). Span. J. Agric. Res. 2014, 12, 1166–1174. [Google Scholar] [CrossRef]
- Baranová, B.; Sedlák, V.; Kalemba, D.; Maciejczyk, E.; Wawrzyńczak, K.; Gruľová, D.; Kudláčková, B.; Oboňa, J. Composition of the essential oil and aqueous leachate from Heracleum mantegazzianum seeds and their larvicidal activity against an invasive mosquito Aedes japonicus. Allelopath. J. 2024, 61, 1–16. [Google Scholar] [CrossRef]
- Shaw, W.R.; Catteruccia, F. Vector biology meets disease control: Using basic research to fight vector-borne diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- NIST/EPA/NIH. Mass Spectral Library with Search Program 2017; Data ver. 17, Software ver. 2.3; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2017. [Google Scholar]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; ISBN 1118145836. [Google Scholar]
- Hazrati, S.; Mollaei, S.; Rabbi Angourani, H.; Hosseini, S.J.; Sedaghat, M.; Nicola, S. How do essential oil composition and phenolic acid profile of Heracleum persicum fluctuate at different phenological stages? Food Sci. Nutr. 2020, 8, 6192–6206. [Google Scholar] [CrossRef]
- Sefidkon, F.; Dabiri, M.; Mohammad, N. Analysis of the oil of Heracleum persicum L. (leaves and flowers). J. Essent. Oil Res. 2002, 14, 295–297. [Google Scholar] [CrossRef]
- Firuzi, O.; Asadollahi, M.; Gholami, M.; Javidnia, K. Composition and biological activities of essential oils from four Heracleum species. Food Chem. 2010, 122, 117–122. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Sajjadi, S.E.; Heshmati, M. Anti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydroalcoholic extract in animal models. J. Ethnopharmacol. 2009, 124, 475–480. [Google Scholar] [CrossRef]
- Davari, M.; Ezazi, R. Chemical composition and antifungal activity of the essential oil of Zhumeria majdae, Heracleum persicum and Eucalyptus sp. against some important phytopathogenic fungi. J. Mycol. Med. 2017, 27, 463–468. [Google Scholar] [CrossRef]
- Ghavam, M. Heracleum persicum Desf. ex Fisch., CA Mey. & Avé-Lall. fruit essential oil: Content, antimicrobial activity and cytotoxicity against ovarian cancer cell line. BMC Complement. Med. Ther. 2023, 23, 87. [Google Scholar] [CrossRef]
- Radjabian, T.; Salimi, A.; Rahmani, N.; Shockravi, A.; Mozaffarian, V. Essential Oil Composition of Some Wild Populations of Heracleum persicum Desf. Ex Fischer Growing in Iran. J. Essent. Oil Bear. Plants 2013, 6, 841–849. [Google Scholar] [CrossRef]
- Hasani, R.; Mehrengan, I.; Latijani, K.; Nejadsattari, T.; Scalone, R. Survey of the impacts of soil and climatic variations on the production of essential oils in Heracleum persicum. Biodiversitas 2017, 18, 365–377. [Google Scholar] [CrossRef]
- Bicchi, C.; D’Amato, A.; Frattini, C.; Cappelletti, E.M.; Caniato, R.; Filippini, R. Chemical diversity of the contents from the secretory structures of Heracleum sphondylium subsp. sphondylium. Phytochemistry 1990, 29, 1883–1887. [Google Scholar] [CrossRef]
- Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Papa, F.; Vittori, S. Composition and biological activities of hogweed [Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt] essential oil and its main components octyl acetate and octyl butyrate. Nat. Prod. Res. 2014, 28, 1354–1363. [Google Scholar] [CrossRef]
- Radjabian, T.; Salimi, A.; Rahmani, N. Essential-oil composition of the fruits of six Heracleum L. species from Iran: Chemotaxonomic significance. Chem. Biodivers. 2014, 11, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Dinparast, L.; Zengin, G. The genus Heracleum: A comprehensive review on its phytochemistry, pharmacology, and ethnobotanical values as a useful herb. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1018–1039. [Google Scholar] [CrossRef]
- Carroll, M.J.; Zangerl, A.R.; Berenbaum, M.R. Brief communication. Heritability estimates for octyl acetate and octyl butyrate in the mature fruit of the wild parsnip. J. Hered. 2000, 91, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.; Kubeczka, K.H. Über ätherische Öle der Apiaceae (Umbelliferae). Planta Med. 1979, 37, 49–56. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Cianfaglione, K.; Bruno, M.; Benelli, G. Larvicidal activity of essential oils of five Apiaceae taxa and some of their main constituents against Culex quinquefasciatus. Chem. Biodivers 2018, 15, e1700382. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Iannarelli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019, 193, 236–271. [Google Scholar] [CrossRef]
- Izakmehri, K.; Saber, M.; Mehrvar, A.; Hassanpouraghdam, B.; Vojoudi, S. Lethal and Sublethal Effects of Essential Oils from Eucalyptus camaldulensis and Heracleum persicum Against the Adults of Callosobruchus maculatus. J. Insect Sci. 2013, 13, 152. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. Eco-friendly larvicides from Indian plants: Effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotoxicol. Environ. Saf. 2016, 133, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Zangerl, A.R.; Liao, L.H.; Jogesh, T.; Berenbaum, M.R. Aliphatic esters as targets of esterase activity in the parsnip webworm (Depressaria pastinacella). J. Chem. Ecol. 2012, 38, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Caldas, E.D. Toxicological Aspects of Pesticides. In Sustainable Agrochemistry: A Compendium of Technologies; Vaz, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 275–305. [Google Scholar]
- Moshefi, M.; Sharififar, F.; Dehghan, G.R.; Ameri, A. Bioassay screening of the essential oil and various extracts of fruits of Heracleum persicum Desf. and rhizomes of Zingiber officinale Rosc. using brine shrimp cytotoxicity assay. Iran. J. Pharm. Res. 2009, 8, 59–63. [Google Scholar] [CrossRef]
- Gugliuzzo, A.; Francardi, V.; Simoni, S.; Roversi, P.F.; Ferrati, M.; Spinozzi, E.; Perinelli, D.R.; Bonacucina, G.; Maggi, F.; Tortorici, S.; et al. Role of plant essential oil nanoemulsions on host colonization by the invasive ambrosia beetle Xylosandrus compactus. Ind. Crops Prod. 2023, 195, 116437. [Google Scholar] [CrossRef]
- Damiens, D.; Benedict, M.; Wille, M.; Gilles, J. An inexpensive and effective larval diet for Anopheles arabiensis (Diptera: Culicidae): Eat Like a Horse, a Bird, or a Fish? J. Med. Entomol. 2012, 49, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report of the WHO Informal Consultation on the Evaluation on the Testing of Insecticides; CTD/WHO PES/IC/96.1; World Health Organization: Geneva, Switzerland, 1996; pp. 1026–1032. [Google Scholar]
- Benelli, G.; Pavela, R.; Petrelli, R.; Nzekoue, F.K.; Cappellacci, L.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Sut, S.; Dall’Acqua, S.; et al. Carlina oxide from Carlina acaulis root essential oil acts as a potent mosquito larvicide. Ind. Crops Prod. 2019, 137, 356–366. [Google Scholar] [CrossRef]
- Ferrati, M.; Spinozzi, E.; Baldassarri, C.; Rossi, P.; Favia, G.; Fiorini, D.; De Zordi, N.; Drenaggi, E.; De Fazi, L.; Benelli, G.; et al. Green purification of Acmella oleracea extract by wiped-film short path molecular distillation boosts the insecticidal activity on mosquito larvae. Ind. Crops Prod. 2024, 218, 118818. [Google Scholar] [CrossRef]
- Quassinti, L.; Lupidi, G.; Maggi, F.; Papa, F.; Vittori, S.; Bianco, A.; Bramucci, M. Antioxidant and antiproliferative activity of Hypericum hircinum L. subsp. majus (Aiton) N. Robson essential oil. Nat. Prod. Res. 2013, 27, 862–868. [Google Scholar] [CrossRef]
- Hlina, B.L.; Birceanu, O.; Robinson, C.S.; Dhiyebi, H.; Wilkie, M.P. The relationship between thermal physiology and lampricide sensitivity in larval sea lamprey (Petromyzon marinus). J. Great Lakes Res. 2021, 47, S272–S284. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; Benthem, K.J.; van Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.1.0. 2019. Available online: https://cran.r-project.org/web/packages/DHARMa/index.html (accessed on 23 December 2023).
- Russel, V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.7.2. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 19 December 2023).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]

| No | Compound a | LRI b | RI Lit. c | % ± SD d | ID e |
|---|---|---|---|---|---|
| 1 | n-hexanol | 865 | 863 | 1.2 ± 0.0 | Std |
| 2 | isopropyl-2-methyl butyrate | 888 | 880 | 3.2 ± 0.0 | RI, MS |
| 3 | isopropyl isovalerate | 902 | 900 | 2.9 ± 0.0 | RI, MS |
| 4 | isobutyl isobutyrate | 912 | 908 | 0.2 ± 0.0 | RI, MS |
| 5 | butyl isobutyrate | 949 | 955 | 0.5 ± 0.0 | Std |
| 6 | isobutyl butyrate | 952 | 953 | 0.4 ± 0.0 | RI, MS |
| 7 | isopropyl 3-methyl-2-butenoate | 961 | 969 | 0.8 ± 0.0 | RI, MS |
| 8 | butyl butanoate | 994 | 993 | 1.6 ± 0.0 | RI, MS |
| 9 | n-octanal | 1001 | 998 | 1.3 ± 0.0 | Std |
| 10 | hexyl acetate | 1012 | 1007 | 1.1 ± 0.1 | RI, MS |
| 11 | ρ-cymene | 1021 | 1020 | 0.7 ± 0.0 | Std |
| 12 | butyl 2-methyl butyrate | 1040 | 1044 | 0.5 ± 0.0 | RI, MS |
| 13 | butyl isovalerate | 1045 | 1047 | 0.3 ± 0.0 | RI, MS |
| 14 | γ-terpinene | 1056 | 1054 | 0.2 ± 0.1 | Std |
| 15 | 2-methylbutyl butyrate | 1057 | 1058 | 0.4 ± 0.0 | RI, MS |
| 16 | n-octanol | 1069 | 1063 | 3.4 ± 0.0 | Std |
| 17 | linalool | 1097 | 1095 | 1.2 ± 0.0 | Std |
| 18 | hexyl isobutyrate | 1148 | 1147 | 1.3 ± 0.0 | RI, MS |
| 19 | hexyl butyrate | 1191 | 1191 | 36.1 ± 0.2 | Std |
| 20 | (3Z)-3-octenol acetate | 1197 | 1190 | 3.9 ± 0.1 | RI, MS |
| 21 | decyl aldehyde | 1203 | 1204 | 0.6 ± 0.1 | RI, MS |
| 22 | octyl acetate | 1210 | 1214 | 23.7 ± 0.1 | Std |
| 23 | hexyl 2-methyl butyrate | 1236 | 1233 | 1.6 ± 0.0 | RI, MS |
| 24 | hexyl isovalerate | 1241 | 1241 | 0.2 ± 0.0 | RI, MS |
| 25 | (E)-anethole | 1282 | 1282 | 1.0 ± 0.0 | Std |
| 26 | octyl isobutyrate | 1344 | 1344 | 1.1 ± 0.0 | RI, MS |
| 27 | hexyl hexanoate | 1385 | 1382 | 1.2 ± 0.0 | RI, MS |
| 28 | octyl butyrate | 1388 | 1394 | 4.7 ± 0.1 | RI, MS |
| 29 | octyl 2-methyl butyrate | 1432 | 1434 | 1.9 ± 0.0 | RI, MS |
| 30 | octyl hexanoate | 1581 | 1575 | 0.4 ± 0.0 | RI, MS |
| Total identified (%) | 97.4 ± 0.2 | ||||
| Grouped compounds (%) | |||||
| Aliphatic compounds | |||||
| Esters | 87.9 ± 0.4 | ||||
| Aldehydes | 0.6 ± 0.1 | ||||
| Alcohols | 5.9 ± 0.0 | ||||
| Terpenes | |||||
| Monoterpene hydrocarbons | 0.9 ± 0.0 | ||||
| Oxygenated Monoterpenes | 2.1 ± 0.1 |
| Product | Species a | LC50 (95% CI c) (ppm) b | LC90 (95% CI c) (ppm) b | Intercept ± SE d | Slope ± SE d | χ2, p-Value |
|---|---|---|---|---|---|---|
| EO | Ae. albopictus | 102.97 (101.26–104.62) | 122.4 (118.66–128.02) | −34.37 ± 3.55 | 17.07 ± 1.76 | 11.410 p = 0.876 |
| Ae. aegypti | 59.09 (55.92–62.27) | 101.62 (93.43–113.42) | −9.64 ± 0.79 | 5.44 ± 0.44 | 6.639 p = 0.992 | |
| An. gambiae | 97.91 (95.88–99.60) | 116.02 (113.14–120.17) | −34.62 ± 3.53 | 17.39 ± 1.75 | 8.640 p = 0.967 | |
| Hexyl butyrate | Ae. albopictus | 85.40 (82.96–87.73) | 113.65 (108.51–121.07) | −19.95 ± 1.84 | 10.33 ± 0.94 | 7.075 p = 0.989 |
| Ae. aegypti | 53.59 (50.03–58.55) | 99.49 (85.20–125.40) | −8.24 ± 0.83 | 4.76 ± 0.50 | 8.570 p = 0.968 | |
| An. gambiae | 70.97 (63.21–75.51) | 116.48 (105.10–144.83) | −11.02 ± 2.22 | 5.95 ± 1.15 | 2.923 p = 0.999 | |
| Octyl acetate | Ae. albopictus | 91.38 (88.92–94.00) | 122.22 (115.83–131.75) | −19.89 ± 1.86 | 10.14 ± 0.95 | 10.610 p = 0.910 |
| Ae. aegypti | 47.05 (44.44–50.26) | 84.30 (74.56–100.35) | −8.46 ± 0.78 | 5.06 ± 0.48 | 10.759 p = 0.904 | |
| An. gambiae | 60.71 (34.71–71.89) | 125.45 (111.10–182.90) | −7.25 ± 2.27 | 4.06 ± 1.15 | 7.782 p = 0.900 |
| HEK293 a (IC50 ppm) b | |
|---|---|
| EO | 100.2 |
| 95% CI c | 76.30–110.4 |
| Octyl acetate | >200 |
| 95% CI | |
| Hexyl butyrate | 67.99 |
| 95% CI | 53.64–86.18 |
| Positive control | |
| Cisplatin | 3.92 |
| 95% CI | 3.69 to 4.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrati, M.; Baldassarri, C.; Rossi, P.; Favia, G.; Benelli, G.; De Fazi, L.; Morshedloo, M.R.; Quassinti, L.; Petrelli, R.; Spinozzi, E.; et al. Unveiling the Larvicidal Potential of Golpar (Heracleum persicum Desf. ex Fisch.) Essential Oil and Its Main Constituents on Aedes and Anopheles Mosquito Vectors. Plants 2024, 13, 2974. https://doi.org/10.3390/plants13212974
Ferrati M, Baldassarri C, Rossi P, Favia G, Benelli G, De Fazi L, Morshedloo MR, Quassinti L, Petrelli R, Spinozzi E, et al. Unveiling the Larvicidal Potential of Golpar (Heracleum persicum Desf. ex Fisch.) Essential Oil and Its Main Constituents on Aedes and Anopheles Mosquito Vectors. Plants. 2024; 13(21):2974. https://doi.org/10.3390/plants13212974
Chicago/Turabian StyleFerrati, Marta, Cecilia Baldassarri, Paolo Rossi, Guido Favia, Giovanni Benelli, Livia De Fazi, Mohammad Reza Morshedloo, Luana Quassinti, Riccardo Petrelli, Eleonora Spinozzi, and et al. 2024. "Unveiling the Larvicidal Potential of Golpar (Heracleum persicum Desf. ex Fisch.) Essential Oil and Its Main Constituents on Aedes and Anopheles Mosquito Vectors" Plants 13, no. 21: 2974. https://doi.org/10.3390/plants13212974
APA StyleFerrati, M., Baldassarri, C., Rossi, P., Favia, G., Benelli, G., De Fazi, L., Morshedloo, M. R., Quassinti, L., Petrelli, R., Spinozzi, E., & Maggi, F. (2024). Unveiling the Larvicidal Potential of Golpar (Heracleum persicum Desf. ex Fisch.) Essential Oil and Its Main Constituents on Aedes and Anopheles Mosquito Vectors. Plants, 13(21), 2974. https://doi.org/10.3390/plants13212974












