Floral Elegance Meets Medicinal Marvels: Traditional Uses, Phytochemistry, and Pharmacology of the Genus Lagerstroemia L.
Abstract
1. Introduction
2. Taxonomy and Botanical Profile of Lagerstroemia Species
3. Traditional Uses of Lagerstroemia
4. Nutritional and Phytochemical Profile of Lagerstroemia
4.1. Nutritional Profile
4.2. Phytochemical Profile
5. Pharmacological Activity
5.1. Antidiabetic Effects
5.2. Anti-Obesity and Blood Lipid-Lowering Effects
5.3. Antitumor Effects
5.4. Antiviral Effects
5.5. Antioxidant Effects
5.6. Antimicrobial Effects
5.7. Anti-Inflammatory and Analgesic Effects
5.8. Hepatoprotective Effects
5.9. Anti-Hyperuricemia Effects
5.10. Other Effects
6. The Toxicity of Lagerstroemia Plants
7. Discussion
8. Method
9. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Zheng, T.; Cai, M.; Feng, L.; Chi, X.; Shen, P.; Wang, X.; Wan, Z.; Yuan, C.; Zhang, M.; et al. Genome Assembly and Resequencing Analyses Provide New Insights into the Evolution, Domestication and Ornamental Traits of Crape Myrtle. Hortic. Res. 2023, 10, uhad146. [Google Scholar] [CrossRef] [PubMed]
- Ashnagar, A.; Ghanad, A.R.; Motakefpour, M. Isolation and Identification of Major Chemical Components Found in the Leaves of Lagerstroemia indica Plant Grown in the City of Tehran, Iran. Int. J. ChemTech Res. 2013, 5, 478–481. [Google Scholar]
- Guo, S.; Ren, X.; He, K.; Chen, X.; Zhang, S.; Roller, M.; Zheng, B.; Zheng, Q.; Ho, C.-T.; Bai, N. The Anti-Diabetic Effect of Eight Lagerstroemia speciosa Leaf Extracts Based on the Contents of Ellagitannins and Ellagic Acid Derivatives. Food Funct. 2020, 11, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, N.; Chaturvedi, A. Qualitative and Quantitative Determination of Secondary Metabolites of Lagerstroemia parviflora Roxb Leaves. Res. J. Pharm. Technol. 2020, 13, 4740–4742. [Google Scholar] [CrossRef]
- Anandhu, K.S.; Jose, M.; Kuriakose, S.; Jayalakshmi, P.M. Phytochemical Analysis and In Vitro Antidiabetic Activity of Aqueous Extract of Lagerstroemia speciosa and Aegle Marmelos. Res. J. Pharm. Technol. 2021, 14, 4697–4701. [Google Scholar]
- Ashnagar, A.; Motakefpour, M.; Rahimi, A.; Mehregan, I.; Ghannadi, A. Persian Common Crape Myrtle Leaves; Phytochemical Screening and Flavonoid Patterns. J. Curr. Chem. Pharm. Sc. 2012, 2, 240–243. [Google Scholar]
- Zhou, Y.; Wang, B.; Zhang, Q.; Chen, H. Chemical Constituents of Lagerstroemia balansae Koehne. Chin. Pharm. J. 2012, 45, 169–171. [Google Scholar]
- Faramayuda, F.; Hermanto, F.; Windyaswari, A.S.; Riyanti, S.; Nurhayati, V.A. Larvacide Activity of Bungur Plants (Lagerstroemia loudonii T. & B.). J. Pharmascience 2022, 9, 18. [Google Scholar] [CrossRef]
- Suzuki, Y.; Unno, T.; Ushitani, M.; Hayashi, K.; Kakuda, T. Antiobesity Activity of Extracts from Lagerstroemia speciosa L. Leaves on Female KK-Ay Mice. J. Nutr. Sci. Vitaminol. 1999, 45, 791–795. [Google Scholar] [CrossRef]
- Chandra, M. Antimicrobial Activity of Different Maturity of Lagerstroemia indica L. on Pathogenic Bacteria. Int. J. Basic Appl. Biol. 2014, 2, 213–216. [Google Scholar]
- Song, J.H.; Park, K.S.; Kwon, D.H.; Choi, H.J. Anti–Human Rhinovirus 2 Activity and Mode of Action of Quercetin-7-Glucoside from Lagerstroemia speciosa. J. Med. Food 2013, 16, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.; Awasthi, S. Anticancer, Antimicrobial and Hemolytic Assessment of Zinc Oxide Nanoparticles Synthesized from Lagerstroemia indica. BioNanoScience 2021, 11, 1030–1048. [Google Scholar] [CrossRef]
- Esfahani, B.N.; Hozoorbakhsh, F.; Rashed, K.; Havaei, S.A.; Heidari, K.; Moghim, S. Effect of Lagerstroemia tomentosa and Diospyros virginiana Methanolic Extracts on Different Drug-Resistant Strains of Mycobacterium Tuberculosis. Res. Pharm. Sci. 2014, 9, 193–198. [Google Scholar] [PubMed]
- Afifah, R.A.; Niwat, C. Phenolic Contents and Antioxidant Activities of Various Infused Tea Liquids Made from Leaves of Green Tea (Camellia sinensis), Banaba (Lagestroemia speciosa) and Moringa (Moringa oleifera L.). J. Teknol. Pengolah. Pertan. 2020, 2, 14–19. [Google Scholar] [CrossRef]
- Kolakul, P.; Sripanidkulchai, B. Phytochemicals and Anti-Aging Potentials of the Extracts from Lagerstroemia speciosa and Lagerstroemia floribunda. Ind. Crops Prod. 2017, 109, 707–716. [Google Scholar] [CrossRef]
- Rahman, M.A.; Uddin, N.; Hasanuzzaman, M.; Rahman, A.A. Antinociceptive, Antidiarrhoeal and Cytotoxic Activities of Lagerstroemia speciosa (L.) Pers. Pharmacologyonline 2011, 1, 604–612. [Google Scholar]
- Bhusnure, O.G.; Alagawadi, K.R.; Giram, P.S.; Poul, B.N. Study of Analgesic and Anti-Inflammatory Activities of Lagerstroemia lanceolata Wall Seed Extract. Int. J. Pharm. Clin. Res. 2009, 1, 127–130. [Google Scholar]
- Pal, L.C.; Kumar, A.; Pande, V.; Ch, V.; Rao, R. Hepatoprotective Effect of Bioactive Fraction of Lagerstroemia speciosa (L.) Pers. Bark Against Monosodium Glutamate-Induced Liver Toxicity. Pharmacogn. J. 2020, 12, 1630–1640. [Google Scholar] [CrossRef]
- Hussain, F.; Ganguly, A.; Hossain, M.S.; Rahman, S.A. Analgesic and Anti-Diarrhoeal Activities of Lagerstroemia speciosa Roots in Experimental Animal Model. Dhaka Univ. J. Pharm. Sci. 2014, 13, 57–62. [Google Scholar] [CrossRef]
- Garcia, F. On the Hypoglycemic Effect of Decoction of Lagerstroemia speciosa Leaves (Banaba) Administered Orally. J. Philipp. Isl. Med. Assoc. 1940, 20, 395–402. [Google Scholar]
- Jayakumar, K.S.; Sajan, J.S.; Aswati Nair, R.; Padmesh Pillai, P.; Deepu, S.; Padmaja, R.; Agarwal, A.; Pandurangan, A.G. Corosolic Acid Content and SSR Markers in Lagerstroemia speciosa (L.) Pers.: A Comparative Analysis among Populations Across the Southern Western Ghats of India. Phytochemistry 2014, 106, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Carew, D.P.; Chin, T.F. Constituents of Lagerstroemia flos-Reginae retz. Nature 1961, 190, 1108–1109. [Google Scholar] [CrossRef] [PubMed]
- Guizhou Provincial Drug Administration. Quality Standard of Chinese Medicinal Materials and Ethnic Medicinal Materials in Guizhou, 1st ed.; Guizhou Science and Technology Publishing House: Guizhou, China, 2003.
- Sichuan Provincial Health Bureau. Processing Standards for Traditional Chinese Medicine Pieces in Sichuan, 1st ed.; Sichuan People’s Publishing House: Sichuan, China, 1977.
- Graham, S.A.; Hall, J.; Sytsma, K.; Shi, S. Phylogenetic Analysis of the Lythraceae Based on Four Gene Regions and Morphology. Int. J. Plant Sci. 2005, 166, 995–1017. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Liu, Y.; Shi, J.; Li, W.; Suo, Z. Chloroplast Phylogenomics and Divergence Times of Lagerstroemia (Lythraceae). BMC Genom. 2021, 22, 434. [Google Scholar] [CrossRef]
- Razvi, S.S.; Aziem, S.; Prakash, R.; Mir, N.A.; Shalla, S.A.; Mahato, S. Propagation of Lagerstroemia speciosa (A Medicinal Plant) Using Juvenile Branch Cuttings: A Vulnerable Species of Southeast Asia. IJCS 2018, 6, 794–797. [Google Scholar]
- Mohamed, S.A.; Bashir, F.G.E. Effects of Indole Butyric Acid (IBA), Wounding, Cutting Position and Rooting Medium on Rooting of Giant Crape Myrtle (Lagerstroemia flos-Reginae retz) Stem Cuttings. Arab J. Water Ethics 2023, 6, 63–76. [Google Scholar] [CrossRef]
- Myint, P.P.; Soe, M.T.; Hlaing, H.H. A Study of Phytoconstituents, α-Glucosidase Inhibitory Effect and Antioxidant Activity of Lagerstroemia speciosa L. Leaf and Fruit. J. Pharmacogn. Phytochem. 2017, 6, 528–533. [Google Scholar]
- Wu, F.; Liu, D.; Wang, M.M.; Li, P.; Zhao, M.; Zhao, S. Research on the Comprehensive Evaluation and Landscape Application of 62 Lagerstroemia cultivars. In Proceedings of the II International Symposium on Germplasm of Ornamentals 1185, Beijing, China, 16–20 July 2012; pp. 305–314. [Google Scholar]
- Singh, H.; Savita; Sharma, R.; Sinha, S.; Kumar, M.; Kumar, P.; Verma, A.; Sharma, S.K. Physiological Functioning of Lagerstroemia speciosa L. under Heavy Roadside Traffic: An Approach to Screen Potential Species for Abatement of Urban Air Pollution. 3 Biotech 2017, 7, 61. [Google Scholar] [CrossRef]
- Allkanjari, O.; Menniti-Ippolito, F.; Ippoliti, I.; Di Giacomo, S.; Piccioni, T.; Vitalone, A. A Descriptive Study of Commercial Herbal Dietary Supplements Used for Dyslipidemia—Sales Data and Suspected Adverse Reactions. Phytother. Res. 2022, 36, 2583–2604. [Google Scholar] [CrossRef]
- Lee, K.-H.; Wang, H.-K.; Itokawa, H.; Morris-Natschke, S.L. Current Perspectives on Chinese Medicines and Dietary Supplements in China, Japan and the United States. J. Food Drug Anal. 2000, 8, 219–228. [Google Scholar] [CrossRef]
- Kantor, E.D.; Rehm, C.D.; Du, M.; White, E.; Giovannucci, E.L. Trends in Dietary Supplement Use among US Adults from 1999–2012. JAMA 2016, 316, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Prin. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, B.; Pal, S.; Kumar, A. Ethno-Medicinal, Phytochemical and Antimicrobial Studies of Euphorbia tirucalli L. J. Phytol. 2010, 2, 65–77. [Google Scholar]
- Boonphong, S. Waxes and Triterpene Acids from Lagerstroemia loudonii Fruit. NU. Int. J. Sci. 2013, 10, 33–43. [Google Scholar]
- Faramayuda, F.; Hermanto, F.; Windyaswari, A.S.; Riyanti, S.; Nurhayati, V.A. Identification of the Secondary Metabolites and Characterization of Lagerstroemia loudonii T. & B. J. Islam. Pharm. 2021, 6, 1–6. [Google Scholar] [CrossRef]
- Dalimartha, S. Atlas of Indonesian Medicinal Plants, 1st ed.; Puspa Swara: Depok, Indonesia, 2003. [Google Scholar]
- Park, J.-W.; Kwon, O.-K.; Yuniato, P.; Marwoto, B.; Lee, J.; Oh, S.-R.; Kim, J.-H.; Ahn, K.-S. Amelioration of an LPS-Induced Inflammatory Response Using a Methanolic Extract of Lagerstroemia ovalifolia to Suppress the Activation of NF-κB in RAW264. 7 Macrophages. Int. J. Mol. Med. 2016, 38, 482–490. [Google Scholar] [CrossRef]
- Jaisinghani, H.; Magarde, P. A Rare Medicinal Herb Lagerstroemia parviflora (ROXB). J. Med. Plants 2022, 10, 27–29. [Google Scholar]
- Jain, S.K.; Tarafder, C.R. Medicinal Plant-Lore of the Santals (A Revival of P. O. Bodding’s Work). Econ. Bot. 1970, 24, 241–278. [Google Scholar] [CrossRef]
- Bhakuni, D.S.; Dhar, M.L.; Dhar, M.M.; Dhawan, B.N.; Mehrotra, B.N. Screening of Indian Plants for Biological Activity: Part II. Indian J. Exp. Biol. 1969, 7, 250–262. [Google Scholar]
- Zhang, Y.; Jin, Y.; Liu, Z.; Huang, L.; Yu, Y.; Wang, T. Extraction Process, Chemical Composition and Antimicrobial Properties of Volatile oil from Lagerstroemia indica Fruit. Fine Chem. 2022, 39, 1641–1647. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Tan, L.N.; Wong, S.K. Phytochemistry and Pharmacology of Lagerstroemia speciosa: A Natural Remedy for Diabetes. Int. J. Herb. Med. 2014, 2, 100–105. [Google Scholar]
- Deshpande, A.; Shirsat, M.; Jeyabalan, G. A Review of Lagerstroemia speciosa: Pride of India. Int. J. Contemp. Res. Rev. 2022, 9, 20181–20185. [Google Scholar]
- Al-Snafi, A. Medicinal Value of Lagerstroemia speciosa: An Updated Review. Int. J. Curr. Pharm. Res. 2019, 11, 18–26. [Google Scholar] [CrossRef]
- Vivek, M.; Sunil, S.; Pramod, N.; Prashith, K.; Mukunda, S.; Mallikarjun, N. Anticariogenic Activity of Lagerstroemia speciosa (L.). Sci. Technol. Arts Res. J. 2013, 1, 53. [Google Scholar] [CrossRef]
- Murthy, J.S.; Lalitha, B.R.; Sharma, A. Phyto Pharmacognostic Study of Lagerstroemia speciosa—An Analytical Study. J. Ayurveda Integr. Med. Sci. 2020, 5, 206–213. [Google Scholar] [CrossRef]
- Laruan, L.M.V.; Balangcod, T.; Balangcod, K.; Patacsil, M.; Apostol, O.; Manuel, J.; Cortez, S.; Vallejo, V. Phytochemical and Antibacterial Study of Lagerstroemia speciosa (L.) Pers. and Its Ethnomedicinal Importance to Indigenous Communities of Benguet Province, Philippines. Indian J. Tradit. Know. 2013, 12, 379–383. [Google Scholar]
- Morshed, A.; Hossain, M.H.; Shakil, S.; Nahar, K.; Rahman, S.; Ferdausi, D.; Hossain, T.; Ahmad, I.; Chowdhury, M.H.; Rahmatullah, M. Evaluation of Antinociceptive Activity of Two Bangladeshi Medicinal Plants, Kalanchoe pinnata (Lam.) Pers. and Lagerstroemia speciosa (L.) Pers. Adv Nat Appl Sci 2010, 4, 193–197. [Google Scholar]
- Pavithra, G.M.; Siddiqua, S.; Naik, A.S.; TR, P.K.; Vinayaka, K.S. Antioxidant and Antimicrobial Activity of Flowers of Wendlandia thyrsoidea, Olea dioica, Lagerstroemia speciosa and Bombax malabaricum. J. Appl. Pharm. Sci. 2013, 3, 114–120. [Google Scholar]
- Faruk, M.J.; Nahur, N.; Aziz, M.A.; Mosihuzzaman, N.; Rashid, M.A. Two New Ellagic Acids from Lagerstroemia speciosa Linn. Plant. J. Bangladesh Chem Soc 2002, 15, 73–78. [Google Scholar]
- Thambi, P.; Sabu, M.C.; Chungath, J. Hepatoprotective and Free Radical Scavenging Activities of Lagerstroemia speciosa Linn. Leaf Extract. Adv. Tradit. Med. 2009, 9, 225–231. [Google Scholar] [CrossRef]
- Editorial Committee of Zhonghua Bencao. Zhonghua Bencao, 2nd ed.; Shanghai Scientific and Technological Publishing House: Shanghai, China, 1999. [Google Scholar]
- Riyanti, S.; Dewi, P.S.; Windyaswari, A.S.; Azizah, S.A.N. Alpha-Glucosidase Inhibitory Activities of Bungur (Lagerstroemia loudonii Teijsm. & Binn.) Leaves and Fruits. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 012042. [Google Scholar] [CrossRef]
- Riyanti, S.; Ratnawati, J.; Shaleh, M.I.; Suganda, A.G. Potensi Kulit Batang Bungur (Lagerstroemia loudonii Teijsm and Binn.) Sebagai Herbal Antidiabetes Dengan Mekanisme Penghambat Alfa-Glukosidase. Talent. Conf. Ser. Trop. Med. 2018, 1, 117–120. [Google Scholar] [CrossRef]
- Riyanti, S.; Suganda, A.G.; Sukandar, E.Y. Dipeptidyl Peptidase-IV Inhibitory Activity of Some Indonesian Medicinal Plants. Asian J. Pharm. Clin. Res. 2016, 9, 375–377. [Google Scholar]
- Min, J.-H.; Kim, S.-M.; Park, J.-W.; Kwon, N.H.; Goo, S.H.; Ngatinem; Ningsih, S.; Paik, J.-H.; Choi, S.; Oh, S.-R.; et al. Lagerstroemia ovalifolia Exerts Anti-Inflammatory Effects in Mice of LPS Induced ALI via Downregulating of MAPK and NF-κB Activation. J. Microbiol. Biotechnol. 2021, 31, 1501–1507. [Google Scholar] [CrossRef]
- Lee, H.-S.; Paik, J.-H.; Kwon, O.-K.; Paryanto, I.; Yuniato, P.; Ryu, H.W.; Choi, S.-H.; Oh, S.-R.; Han, S.-B.; Park, J.-W. Anti-Inflammatory Effects of Lagerstroemia ovalifolia Teijsm. & Binn. in TNFα/IFNγ-Stimulated Keratinocytes. Evid. -Based Complement. Altern. Med. 2021, 2021, 2439231. [Google Scholar] [CrossRef]
- Sirikhansaeng, P.; Tanee, T.; Sudmoon, R.; Chaveerach, A. Major Phytochemical as γ -Sitosterol Disclosing and Toxicity Testing in Lagerstroemia species. Evid. -Based Complement. Altern. Med. 2017, 2017, 7209851. [Google Scholar] [CrossRef]
- Bawane, A.A.; Tiwari, O.P.; Jain, A.P. Phytochemical Analysis and in Vitro Antioxidant Studies of Lagerstroemia parviflora Roxb Bark. J. Adv. Sci. Res. 2020, 11, 174–178. [Google Scholar]
- Parkhe, G.; Bharti, D. In Vitro Antioxidant Activity, Total Phenolic and Flavonoid Contents of Hydroalcoholic Extract of Leaves of Lagerstroemia parviflora Roxb. J. Drug Deliv. Ther. 2019, 9, 708–711. [Google Scholar] [CrossRef]
- Mazumder, A.; Saha, B.P.; Basu, S.P.; Mazumder, R. Evaluation of Antipyretic Potential of Lagerstroemia parviflora. Extract in Rats. Pharm. Biol. 2005, 43, 64–66. [Google Scholar] [CrossRef]
- Mazumder, A.; Saha, B.P.; Basu, S.P.; Mazumder, R.; Devi, B.P.; Mandal, S.C. Evaluation of Antitussive Activity of Lagerstroemia parviflora Leaf Extract. Phytother. Res. 2004, 18, 780–782. [Google Scholar] [CrossRef]
- Stohs, S.J.; Miller, H.; Kaats, G.R. A Review of the Efficacy and Safety of Banaba (Lagerstroemia speciosa L.) and Corosolic Acid. Phytother. Res. 2012, 26, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, A.; Ramu, B.; Rahman, F.; Basheer, S.; Mastanaiah, J. The Bioactive Compounds of Fruit of Lagerstroemia speciosa L. Act as Potential Antimicrobial Agent. Int. J. Pharm. Res. Health Sci. 2014, 2, 476–480. [Google Scholar]
- Kouzi, S.A.; Yang, S.; Nuzum, D.S.; Dirks-Naylor, A.J. Natural Supplements for Improving Insulin Sensitivity and Glucose Uptake in Skeletal Muscle. Front. Biosci. -Elite 2015, 7, 107–121. [Google Scholar] [CrossRef]
- Ikeda, Y.; Chen, J.-T.; Matsuda, T. Effectiveness and Safety of Banabamin Tablet Containing Extract from Banaba in Patients with Mild Type 2 Diabetes. Jpn. Pharmacol. Ther. 1999, 27, 67–74. [Google Scholar]
- Cheng, L.; Ji, T.; Zhang, M.; Fang, B. Recent Advances in Squalene: Biological Activities, Sources, Extraction, and Delivery Systems. Trends Food Sci. Technol. 2024, 146, 104392. [Google Scholar] [CrossRef]
- Bansal, S.; Vyas, S.; Bhattacharya, S.; Sharma, M. Catechin Prodrugs and Analogs: A New Array of Chemical Entities with Improved Pharmacological and Pharmacokinetic Properties. Nat. Prod. Rep. 2013, 30, 1438. [Google Scholar] [CrossRef]
- Labib, R.M.; Ayoub, N.A.; Singab, A.B.; Al-Azizi, M.M.; Sleem, A. Chemical Constituents and Pharmacological Studies of Lagerstroemia indica. Phytopharmacology 2013, 4, 373–389. [Google Scholar]
- Van Laar, A.D.; Grootaert, C.; Van Camp, J. Rare Mono-and Disaccharides as Healthy Alternative for Traditional Sugars and Sweeteners? Crit. Rev. Food Sci. Nutr. 2021, 61, 713–741. [Google Scholar] [CrossRef]
- Niranjan, M.H.; Sudarshana, M.S. Preliminary Phytochemical Studies of Lagerstroemia indica Linn. J. Pharm. Res. 2010, 3, 216–218. [Google Scholar]
- Thambi, P.; Chacko, S.M.; Chungath, J.I. Studies on Diuretic Effect of Lagerstroemia speciosa Linn. Leaf Extracts in Normal Rats. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 61–69. [Google Scholar]
- Basu, S.; Kundu, P.; Sinhababu, A. Characterization of Fatty Acid and Sterol Composition of Seed Lipid of Lagerstroemia speciosa Pers. Res. Chem. Intermed. 2015, 41, 6511–6522. [Google Scholar] [CrossRef]
- Zong, W.; Xia, W. Physicochemical Properties of Banaba Seed Oil and Its Fatty Acid Composition Determined by GC/MS. China Oils Fats 2004, 29, 65–67. [Google Scholar] [CrossRef]
- Jehan, C.M.; Daulatabad, D.; Mirajkar, A.M. A Keto Fatty Acid from Lagerstroemia speciosa Seed Oil. Phytochemistry 1990, 29, 2323–2324. [Google Scholar] [CrossRef]
- Oloyede, G.K.; Oladosu, I.A.; Oloyade, O.O. Chemical Composition and Cytotoxic Effect of Largerstroemia speciosa Fruits Essential Oils. Int. J. Biol. Chem. Sci. 2010, 4, 1851–1854. [Google Scholar] [CrossRef][Green Version]
- Thambi, P.T.; Sabu, M.C.; Chungath, J.I. Essential Oils Composition and Cytotoxic Effect of Lagerstroemia speciosa Linn Flowers. J. Pharmacol. Toxicol. Stud. 2016, 4, 1–5. [Google Scholar]
- Sharopova, M.A.; Uzakov, Z.Z.; Xaitov, I.Y. Preliminary Results on the Chemical Components of Lagerstroemia indica L. in the Conditions of Southern Uzbekistan. E3S Web Conf. 2024, 510, 03032. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Zong, W.; Zhao, G. Analysis of the Protein and Amino Acid Contents in the Seed of Lagerstroemia speciosa. Mod. Food Sci. Technol. 2006, 22, 227–228. [Google Scholar] [CrossRef]
- Harahap, U.; Syahputra, R.A.; Ahmed, A.; Nasution, A.; Wisely, W.; Sirait, M.L.; Dalimunthe, A.; Zainalabidin, S.; Taslim, N.A.; Nurkolis, F.; et al. Current Insights and Future Perspectives of Flavonoids: A Promising Antihypertensive Approach. Phytother. Res. 2024, 38, 3146–3168. [Google Scholar] [CrossRef]
- Lu, L.; Wang, J.; Wang, C.; Zhu, J.; Wang, H.; Liao, L.; Zhao, Y.; Wang, X.; Yang, C.; He, Z.; et al. Plant-Derived Virulence Arresting Drugs as Novel Antimicrobial Agents: Discovery, Perspective, and Challenges in Clinical Use. Phytother. Res. 2024, 38, 727–754. [Google Scholar] [CrossRef]
- Sandeep; Misra, R.C.; Chanotiya, C.S.; Mukhopadhyay, P.; Ghosh, S. Oxidosqualene cyclase and CYP716 Enzymes Contribute to Triterpene Structural Diversity in the Medicinal Tree Banaba. New Phytol. 2019, 222, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.W.; Cha, J.M.; Choi, S.U.; Lee, K.R. A New Triterpene Glycoside from the Stems of Lagerstroemia indica. Arch. Pharmacal Res. 2016, 39, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, S.; Khuroo, M.A. A New Pentacyclic Triterpenoid from Lagerstroemia indica. Chem. Nat. Compd. 2014, 50, 681–683. [Google Scholar] [CrossRef]
- Hou, W.; Li, Y.; Zhang, Q.; Wei, X.; Peng, A.; Chen, L.; Wei, Y. Triterpene Acids Isolated from Lagerstroemia speciosa Leaves as α-Glucosidase Inhibitors. Phytother. Res. 2009, 23, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, L.; Chen, Y.; Qiao, X.; Zhao, S.; Li, P.; Liu, J.; Wen, X.; Yang, J. Corosolic Acid Inhibits Colorectal Cancer Cells Growth as a Novel HER2/HER3 Heterodimerization Inhibitor. Br. J. Pharmacol. 2021, 178, 1475–1491. [Google Scholar] [CrossRef]
- Qian, X.-P.; Zhang, X.-H.; Sun, L.-N.; Xing, W.-F.; Wang, Y.; Sun, S.-Y.; Ma, M.-Y.; Cheng, Z.-P.; Wu, Z.-D.; Xing, C.; et al. Corosolic Acid and Its Structural Analogs: A Systematic Review of Their Biological Activities and Underlying Mechanism of Action. Phytomedicine 2021, 91, 153696. [Google Scholar] [CrossRef]
- Hudlikar, R.R.; Sargsyan, D.; Wu, R.; Su, S.; Zheng, M.; Kong, A.-N. Triterpenoid Corosolic Acid Modulates Global CpG Methylation and Transcriptome of Tumor Promotor TPA Induced Mouse Epidermal JB6 P + Cells. Chem.-Biol. Interact. 2020, 321, 109025. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, H.; Wang, Y.; Zhao, A.; Zhu, Z.; Bao, X.; Sun, Y.; Li, L.; Zhang, Q. Corosolic Acid, a Natural Triterpenoid, Induces ER Stress-Dependent Apoptosis in Human Castration Resistant Prostate Cancer Cells via Activation of IRE-1/JNK, PERK/CHOP and TRIB3. J. Exp. Clin. Canc. Res. 2018, 37, 210. [Google Scholar] [CrossRef]
- Żwawiak, J.; Pawełczyk, A.; Olender, D.; Zaprutko, L. Structure and Activity of Pentacyclic Triterpenes Codrugs. A Review. Mini Rev. Med. Chem. 2021, 21, 1509–1526. [Google Scholar] [CrossRef]
- Yang, J.; Leng, J.; Li, J.-J.; Tang, J.; Li, Y.; Liu, B.-L.; Wen, X.-D. Corosolic Acid Inhibits Adipose Tissue Inflammation and Ameliorates Insulin Resistance via AMPK Activation in High-Fat Fed Mice. Phytomedicine 2016, 23, 181–190. [Google Scholar] [CrossRef]
- Li, X.Q.; Tian, W.; Liu, X.X.; Zhang, K.; Huo, J.C.; Liu, W.J.; Li, P.; Xiao, X.; Zhao, M.G.; Cao, W. Corosolic Acid Inhibits the Proliferation of Glomerular Mesangial Cells and Protects against Diabetic Renal Damage. Sci Rep 2016, 6, 26854. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, D.; Zheng, Y.; Hu, B.; Zhang, Z.; Ye, Q.; Liu, C.; Shan, Q.; Wang, Y. Ursolic Acid Attenuates D-Galactose-Induced Inflammatory Response in Mouse Prefrontal Cortex through Inhibiting AGEs/RAGE/NF-κB Pathway Activation. Cereb. Cortex 2010, 20, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, B.; Li, P.; Wen, X.; Yang, J. Maslinic Acid Inhibits Colon Tumorigenesis by the AMPK–mTOR Signaling Pathway. J. Agric. Food Chem. 2019, 67, 4259–4272. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Malavasi, G.; Palla, G.; Marseglia, A.; Tognolini, M.; Bruni, R. A Simple GC-MS Method for the Screening of Betulinic, Corosolic, Maslinic, Oleanolic and Ursolic Acid Contents in Commercial Botanicals Used as Food Supplement Ingredients. Food Chem. 2013, 136, 735–741. [Google Scholar] [CrossRef]
- Kim, M.O.; Su, U.L.; Yuk, H.J.; Jang, H.J.; Ryu, H.W. Metabolomics Approach to Identify the Active Substances Influencing the Antidiabetic Activity of Lagerstroemia species. J. Funct. Foods 2019, 64, 103684. [Google Scholar] [CrossRef]
- Mallavadhani, U.V.; Mohapatra, S.; Mahapatra, A. Quantitative Analysis of Corosolic Acid, a Type-II Anti-Diabetic Agent, in Different Parts of Lagerstroemia speciosa Linn. J. Planar Chromatogr. -Mod. Tlc 2008, 21, 461–464. [Google Scholar] [CrossRef]
- Sonar, M.P.; Rathod, V.K. Extraction of Type II Antidiabetic Compound Corosolic Acid from Lagerstroemia speciosa by Batch Extraction and Three Phase Partitioning. Biocatal. Agric. Biotechnol. 2020, 27, 101694. [Google Scholar] [CrossRef]
- Choi, J.; Ku, P.-T.; Cho, K.-S.; Huh, M.-K. Comparison of Chemicals in Lagerstroemia spezzciosa (L.) Pers. at Growing Stage Levels by GC-MS. Korean J. Crop Sci. 2010, 55, 200–206. [Google Scholar]
- Thitikornpong, W.; Phadungcharoen, T.; Sukrong, S. Pharmacognostic Evaluations of Lagerstroemia speciosa Leaves. J. Med. Plant Res 2011, 5, 1330–1337. [Google Scholar]
- Okada, Y.; Omae, A.; Okuyama, T. A New Triterpenoid Isolated from Lagerstronemia speciosa (L.) Pers. Chem. Pharm. Bull. 2003, 34, 452–454. [Google Scholar] [CrossRef]
- Koshio, K.; Murai, Y.; Sanada, A.; Taketomi, T.; Yamazaki, M.; Kim, T.S.; Boo, H.O.; Obuchi, M.; Iwashina, T. Positive Relationship between Anthocyanin and Corosolic Acid Contents in Leaves of Lagerstroemia speciosa Pars. Trop. Agric. Dev. 2012, 56, 49–52. [Google Scholar]
- Rajic, A.; Kweifio-Okai, G.; Macrides, T.; Sandeman, R.; Chandler, D.; Polya, G. Inhibition of Serine Proteases by Anti-Inflammatory Triterpenoids. Planta Med. 2000, 66, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J. Biologically Active Pentacyclic Triterpenes and Their Current Medicine Signification. J. Appl. Biomed 2003, 1, 7–12. [Google Scholar] [CrossRef]
- Otuki, M.F.; Ferreira, J.; Lima, F.V.; Meyre-Silva, C.; Malheiros, A.; Muller, L.A.; Cani, G.S.; Santos, A.R.; Yunes, R.A.; Calixto, J.B. Antinociceptive Properties of Mixture of α-Amyrin and β-Amyrin Triterpenes: Evidence for Participation of Protein Kinase C and Protein Kinase A Pathways. J. Pharmacol. Exp. Ther. 2005, 313, 310–318. [Google Scholar] [CrossRef]
- Fukushima, M.; Matsuyama, F.; Ueda, N.; Egawa, K.; Takemoto, J.; Kajimoto, Y.; Yonaha, N.; Miura, T.; Kaneko, T.; Nishi, Y.; et al. Effect of Corosolic Acid on Postchallenge Plasma Glucose Levels. Diabetes Res. Clin. Pract. 2006, 73, 174–177. [Google Scholar] [CrossRef]
- Kesavanarayanan, K.S.; Sathiya, S.; Ranju, V. In Vitro Cytotoxic, Antioxidative and Alpha-Glucosidase Inhibitory Potential of a Herbal Mixture Comprised of Allium sativum and Lagerstroemia speciosa. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 58–68. [Google Scholar]
- Erdoğan, Ü.; Karaboyacı, M. Determination of Total Antioxidant Capacity and Total Phenolic Content of Lagerstroemia indica L. Leaves. In Proceedings of the International Conferences on Science and Technology Engineering Sciences and Technology (ICONST EST 2022), Budva, Montenegro, 7–9 September 2022. [Google Scholar]
- Kranjanasurat, P.; Sripanidkulchai, B. Total Phenolic Contents, Antioxidative Activity and Inhibitory on Effect Matrix Metalloproteinase-2 and-9 of Ethanolic Extract from Thai Flowers. Isan J. Pharm. Sci. 2013, 9, 1. [Google Scholar]
- Zhang, J.; Wang, L.; Gao, J.; Shu, Q.; Li, C.; Yao, J.; Hao, Q.; Zhang, J. Determination of Anthocyanins and Exploration of Relationship between Their Composition and Petal Coloration in Crape Myrtle (Lagerstroemia Hybrid). J. Integr. Plant Biol. 2008, 50, 581–588. [Google Scholar] [CrossRef]
- Chen, H.; Liang, L.; Xie, J. Study on Extraction and Stability of Pigment from Lagerstroemia speciosa Flower. Food Sci. Technol. 2007, 32, 201–204. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Zhang, D. Studies on the Chemical Constituents and Bioactivities in Stems and Leaves of Lagerstroemia indica L. Master’s Dissertation, Huaqiao University, Quanzhou, China, 2016. [Google Scholar]
- Chang, M.; Ahmed, A.F.; Cui, L. The Hypoglycemic Effect of Lagerstroemia indica L. and Lagerstroemia indica L. f. Alba (Nichols.) Rehd. In Vitro and In Vivo. J. Future Foods 2023, 3, 273–277. [Google Scholar] [CrossRef]
- Yan, F. Study on the Bioactive Constituents of the Fruits of Lagerstroemia speciosa (L.) Pers. Master’s Dissertation, Guangdong Pharmaceutical University, Guangdong, China, 2020. [Google Scholar]
- Trinh, B.T.D.; Staerk, D.; Jäger, A.K. Screening for Potential α-Glucosidase and α-Amylase Inhibitory Constituents from Selected Vietnamese Plants Used to Treat Type 2 Diabetes. J. Ethnopharmacol. 2016, 186, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q. Study on the Hypoglycemic Constituents from Leaves of Lagerstroemia speciosa. Master’s Dissertation, Second Military Medical University, Shanghai, China, 2008. [Google Scholar]
- Ajaib, M.; Arooj, T.; Khan, K.; Farid, S.; Ishtiaq Ch, D.M.; Perveen, S.; Shah, S.; Pakistan, L. Phytochemical, Antimicrobial and Antioxidant Screening of Fruits, Bark and Leaves of Lagerstroemia indica. J. Chem. Soc. Pak. 2016, 38, 538–545. [Google Scholar]
- Diab, Y.; Atalla, K.; Elbanna, K. Antimicrobial Screening of Some Egyptian Plants and Active Flavones from Lagerstroemia Indica Leaves. Drug Discov. Ther. 2012, 6, 212–217. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Bharadwaj, N. Antioxidant and Antimicrobial Activities of Methanolic Leaves Extract of Lagerstroemia parviflora. J. Drug Deliv. Ther. 2020, 10, 206–210. [Google Scholar] [CrossRef]
- Rabi, S.; Islam, M.N.; Islam, M.D.; Sutradhar, R.K. Bioactive Terpenoid from the Fruits of Lagerstroemia speciosa and Its Molecular Docking Study. Chem. Nat. Compd. 2022, 58, 485–490. [Google Scholar] [CrossRef]
- Sharmin, T.; Rahman, M.S.; Mohammadi, H. Investigation of Biological Activities of the Flowers of Lagerstroemia speciosa, the Jarul Flower of Bangladesh. Bmc Complement. Altern. Med. 2018, 18, 231. [Google Scholar] [CrossRef]
- Ambujakshi, H.R.; Surendra, V.; Haribabu, T.; Goli, D. Antibacterial Activity of Leaves of Lagerstroemia speciosa (L) Pers. J. Pharm. Res. 2009, 2, 1028. [Google Scholar]
- Yang, E.J.; Lee, J.-S.; Song, B.B.; Yun, C.-Y.; Kim, D.-H.; Kim, I.S. Anti-Inflammatory Effects of Ethanolic Extract from Lagerstroemia indica on Airway Inflammation in Mice. J. Ethnopharmacol. 2011, 136, 422–427. [Google Scholar] [CrossRef]
- Priya, T.T.; Sabu, M.C.; Jolly, C.I. Free Radical Scavenging and Anti-Inflammatory Properties of Lagerstroemia speciosa (L). Inflammopharmacology 2008, 16, 182–187. [Google Scholar] [CrossRef]
- Thakur, R.S.; Devaraj, E. Lagerstroemia speciosa (L.) Pers. Triggers Oxidative Stress Mediated Apoptosis via Intrinsic Mitochondrial Pathway in HepG2 Cells. Environ. Toxicol. 2020, 35, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Sai Saraswathi, V.; Rajaguru, P.; Santhakumar, K. Solar Catalysed Activity against Methyl Orange Dye, Cytotoxicity Activity of MCF-7 Cell Lines and Identification of Marker Compound by HPTLC of Lagerstroemia speciosa. J. Photochem. Photobiol. B 2017, 170, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.M.; El-Sammad, N.M.; Abdel-Halim, A.H.; Anwar, N.; Khalil, W.K.B.; Nawwar, M.; Hashim, A.N.; Elsayed, E.A.; Hassan, S.K. Lagerstroemia speciosa (L.) Pers Leaf Extract Attenuates Lung Tumorigenesis via Alleviating Oxidative Stress, Inflammation and Apoptosis. Biomolecules 2019, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kwon, M.J.; Yoo, J.Y.; Choi, H.-J.; Ahn, Y.-J. Antiviral Activity and Possible Mode of Action of Ellagic Acid Identified in Lagerstroemia speciosa Leaves toward Human Rhinoviruses. BMC Complement. Altern. Med. 2014, 14, 171. [Google Scholar] [CrossRef]
- Choi, H.J.; Bae, E.Y.; Song, J.H.; Baek, S.H.; Kwon, D.H. Inhibitory Effects of Orobol 7-O-d-Glucoside from Banaba (Lagerstroemia speciosa L.) on Human Rhinoviruses Replication. Lett. Appl. Microbiol. 2010, 51, 1–5. [Google Scholar]
- Nutan; Modi, M.; Goel, T.; Das, T.; Malik, S.; Suri, S.; Rawat, A.K.S.; Srivastava, S.K.; Tuli, R.; Malhotra, S.; et al. Ellagic Acid & Gallic Acid from Lagerstroemia speciosa L. Inhibit HIV-1 Infection through Inhibition of HIV-1 Protease & Reverse Transcriptase Activity. Indian J. Med. Res. 2013, 137, 540–548. [Google Scholar]
- Chistokhodova, N.; Nguyen, C.; Calvino, T.; Kachirskaia, I.; Cunningham, G.; Howard Miles, D. Antithrombin Activity of Medicinal Plants from Central Florida. J. Ethnopharmacol. 2002, 81, 277–280. [Google Scholar] [CrossRef]
- Unno, T.; Sugimoto, A.; Kakuda, T. Xanthine Oxidase Inhibitors from the Leaves of Lagerstroemia speciosa (L.) Pers. J. Ethnopharmacol. 2004, 93, 391–395. [Google Scholar] [CrossRef]
- Unno, T.; Sakane, I.; Kakuda, T. Inhibition of Xanthine Oxidase by an Aqueous Extract of Banaba Leaves (Lagerstroemia speciosa). Nippon Shokuhin Kagaku Kogaku Kaishi 2000, 47, 740–743. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.; Wang, B.; Liang, H.; Zhao, Y.; Zhang, Q. Sesquiterpenoid and Phenolic Glucoside Gallates from Lagerstroemia balansae. Planta Med. 2011, 77, 1944–1946. [Google Scholar] [CrossRef]
- Lopez-Murillo, L.D.; Gonzalez-Ortiz, M.; Martinez-Abundis, E.; Cortez-Navarrete, M.; Perez-Rubio, K.G. Effect of Banaba (Lagerstroemia speciosa) on Metabolic Syndrome, Insulin Sensitivity, and Insulin Secretion. J. Med. Food 2021, 25, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.K.; Amresh, G.; Chandra, P. Pharmacodynamic Evaluation of Self Micro-Emulsifying Formulation of Standardized Extract of Lagerstroemia speciosa for Antidiabetic Activity. J. Ayurveda Integr. Med. 2018, 9, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Veeresham, C.; Asres, K. In Vitro and In Vivo Inhibitory Activities of Four Indian Medicinal Plant Extracts and Their Major Components on Rat Aldose Reductase and Generation of Advanced Glycation Endproducts. Phytother. Res. 2013, 27, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Bhuiyan, M.N.H.; Mazumder, K.; Haque, K.M.F. Hypoglycemic Activity of Lagerstroemia speciosa L. Extract on Streptozotocin-Induced Diabetic Rat: Underlying Mechanism of Action. Bangl. J. Pharmacol. 2009, 4, 79–83. [Google Scholar] [CrossRef]
- Park, M.Y.; Lee, K.S.; Sung, M.K. Effects of Dietary Mulberry, Korean Red Ginseng, and Banaba on Glucose Homeostasis in Relation to PPAR-Alpha, PPAR-Gamma, and LPL mRNA Expressions. Life Sci 2005, 77, 3344–3354. [Google Scholar] [CrossRef]
- Judy, W.V.; Hari, S.P.; Stogsdill, W.W.; Judy, J.S.; Naguib, Y.M.A.; Passwater, R. Antidiabetic Activity of a Standardized Extract (GlucosolTM) from Lagerstroemia speciosa Leaves in Type II Diabetics: A Dose-Dependence Study. J. Ethnopharmacol. 2003, 87, 115–117. [Google Scholar] [CrossRef]
- Tjokroprawiro, A.; Murtiwi, S.; Tjandrawinata, R.R. DLBS3233, a Combined Bioactive Fraction of Cinnamomum burmanii and Lagerstroemia speciosa, in Type-2 Diabetes Mellitus Patients Inadequately Controlled by Metformin and Other Oral Antidiabetic Agents. J. Complement. Integr. Med. 2016, 13, 413–420. [Google Scholar] [CrossRef]
- Kakuda, T.; Sakane, I.; Takihara, T.; Ozaki, Y.; Takeuchi, H.; Kuroyanagi, M. Hypoglycemic Effect of Extracts from Lagerstroemia speciosa L. Leaves in Genetically Diabetic KK-AY Mice. Biosci. Biotechnol. Biochem. 1996, 60, 204–208. [Google Scholar] [CrossRef]
- Lieberman, S.; Spahrs, R.; Stanton, A.; Martinez, L.; Grinder, M. Weight Loss, Body Measurements, and Compliance: A 12 Week Total Lifestyle Intervention Pilot Study. Altern. Complement. Ther. 2005, 11, 307–313. [Google Scholar] [CrossRef]
- Tyagi, C.K. Antiobesity Activity of Methanolic Extract of Lagerstroemia parviflora Roxb. (Leaves) on Wistar Albino Rat Model. Int. J. Green Pharm. 2021, 15, 77–86. [Google Scholar]
- Chaudhary, G.; Mahajan, U.B.; Goyal, S.N.; Ojha, S.; Patil, C.R.; Subramanya, S.B. Protective Effect of Lagerstroemia speciosa against Dextran Sulfate Sodium Induced Ulcerative Colitis in C57BL/6 Mice. Am. J. Transl. Res. 2017, 9, 1792–1800. [Google Scholar] [PubMed]
- Saumya, S.M.; Mahaboob, B.P.; Basha, P. In Vitro Evaluation of Free Radical Scavenging Activities of Panax ginseng and Lagerstroemia speciosa: A Comparative Analysis. Int. J. Pharm. Pharm. Sci. 2011, 3, 165–169. [Google Scholar]
- Amresh, G.; Agarwal, V.K.; Rao, C.V. Self Microemulsifying Formulation of Lagerstroemia speciosa against Chemically Induced Hepatotoxicity. J. Tradit. Complement. Med. 2018, 8, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Elsawi, S.A.; Aly, H.F.; Elbatanony, M.M.; Maamoun, A.A.; Mowawad, D.M. Phytochemical Evaluation of Lagerstroemia indica (L.) Pers Leaves as Anti-Alzheimer’s. J. Mater. Environ. Sci. 2018, 9, 2575–2586. [Google Scholar]
- Prabhu, V.V.; Chidambaranathan, N.; Nalini, G.; Venkataraman, S.; Jayaprakash, S.; Nagarajan, M. Evaluation of Anti-Fibrotic Effect of Lagerstroemia speciosa (L) Pers. on Carbon Tetrachloride Induced Liver Fibrosis. Curr. Pharma Res. 2010, 1, 7–12. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Khan, S.; Wani, S.A. Controlling Diabetes with the Aid of Medicinal Herbs: A Critical Compilation of a Decade of Research. Crit. Rev. Food Sci. Nutr. 2023, 63, 12552–12566. [Google Scholar] [CrossRef]
- Lester, M.; O’Kell, A.L. Exploratory Analysis of Anti-insulin Antibodies in Diabetic Dogs Receiving Recombinant Human Insulin. J. Small Anim. Pract. 2020, 61, 236–240. [Google Scholar] [CrossRef]
- Alkahtani, S.; Hasnain, M.S.; Algamdy, H.; Aljarba, N.H.; AlKahtane, A. Acute and Sub-Acute Oral Toxicity Lagerstroemia speciosa in Sprague-Dawley Rats. Saudi J. Biol. Sci. 2022, 29, 1585–1591. [Google Scholar] [CrossRef]
- Azad, A.K.; Rahman, M.K.; Sunzida, N.K. Acute Oral Toxicity Study on Malaysian Traditional Herb: Lagerstroemia speciosa L. (Banaba). J. Pharmacogn. Phytochem. 2015, 4, 228–232. [Google Scholar]
- Zong, W.; Xia, W.; Cui, B.; Wan, J. Screening of Lagerstroemia specious Leaves Constituents Hypoglycemic Activity. J. Food Sci. Biotechnol. 2006, 25, 67–71. [Google Scholar] [CrossRef]
- Hayashi, T.; Maruyama, H.; Kasai, R.; Hattori, K.; Takasuga, S.; Hazeki, O.; Yamasaki, K.; Tanaka, T. Ellagitannins from Lagerstroemia speciosa as Activators of Glucose Transport in Fat Cells. Planta Medica 2002, 68, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kim, J.; Li, J.; Liu, F.; Liu, X.; Himmeldirk, K.; Ren, Y.; Wagner, T.E.; Chen, X. Natural Anti-Diabetic Compound 1,2,3,4,6-Penta-O-Galloyl-d-Glucopyranose Binds to Insulin Receptor and Activates Insulin-Mediated Glucose Transport Signaling Pathway. Biochem. Biophys. Res. Commun. 2005, 336, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.; Kim, J.; Himmeldirk, K.; Cao, Y.; Chen, X. Antidiabetes and Anti-Obesity Activity of Lagerstroemia speciosa. Evid. -Based Complement. Altern. Med. 2007, 4, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Saravanan, R. Glucose Uptake through Translocation and Activation of GLUT4 in PI3K/Akt Signaling Pathway by Asiatic Acid in Diabetic Rats. Hum. Exp. Toxicol. 2015, 34, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, H.; Sugimoto, A.; Suzuki, Y.; Sakane, I.; Kakuda, T. Isolation and Quantitative Analysis of the Alpha-Amylase Inhibitor in Lagerstroemia speciosa (L.) Pers. (Banaba). Yakugaku Zasshi J. Pharm. Soc. Jpn. 2003, 123, 599–605. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Kim, J.; Li, Y.; Li, J.; Liu, F.; Chen, X. Tannic Acid Stimulates Glucose Transport and Inhibits Adipocyte Differentiation in 3T3-L1 Cells. J. Nutr. 2005, 135, 165–171. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Zheng, B.; Chen, X.; Shao, Z.; Peng, T.; Zheng, Q. Active Compounds from Lagerstroemia speciosa, Insulin-like Glucose Uptake-Stimulatory/Inhibitory and Adipocyte Differentiation-Inhibitory Activities in 3T3-L1 Cells. J. Agric. Food Chem. 2008, 56, 11668–11674. [Google Scholar] [CrossRef]
- Cao, H.; Hininger-Favier, I.; Kelly, M.A.; Benaraba, R.; Dawson, H.D.; Coves, S.; Roussel, A.M.; Anderson, R.A. Green Tea Polyphenol Extract Regulates the Expression of Genes Involved in Glucose Uptake and Insulin Signaling in Rats Fed a High Fructose Diet. J. Agric. Food Chem. 2007, 55, 6372–6378. [Google Scholar] [CrossRef]
- Hattori, K.; Sukenobu, N.; Sasaki, T.; Takasuga, S.; Hayashi, T.; Kasai, R.; Yamasaki, K.; Hazeki, O. Activation of Insulin Receptors by Lagerstroemin. J. Pharmacol. Sci. 2003, 93, 69–73. [Google Scholar] [CrossRef][Green Version]
- Zong, W.; Xia, W. Effects of Total Triterpenes of Lagerstroemia specious L on Glucoseand Fat Metabolism in 3T3-L1 Cell. Food Sci. 2006, 27, 77–80. [Google Scholar] [CrossRef]
- Zong, W.; Zhao, G. Corosolic Acid Isolation from the Leaves of Eriobotrta Japonica Showing the Effects on Carbohydrate Metabolism and Differentiation of 3T3-L1 Adipocytes. Asia Pac. J. Clin. Nutr. 2007, 16, 346–352. [Google Scholar] [PubMed]
- Ms, U.; Ferdosh, S.; Haque Akanda, M.J.; Ghafoor, K.; AH, R.; Ali, M.E.; Kamaruzzaman, B.Y.; Mb, F.; Shaarani, S.; Islam Sarker, M.Z. Techniques for the Extraction of Phytosterols and Their Benefits in Human Health: A Review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar]
- Zong, W.; Dong, H. Reducing Blood Lipids of Lagerstroemia specious Seed Oil. China Oils Fats 2006, 31, 35–36. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, J.S.; Lee, M.S.; Cha, E.Y.; Kim, S.; Sul, J.Y. Corosolic Acid Reduces 5-FU Chemoresistance in Human Gastric Cancer Cells by Activating AMPK. Mol. Med. Rep. 2018, 18, 2880–2888. [Google Scholar] [CrossRef]
- Woo, S.M.; Seo, S.U.; Min, K.; Im, S.-S.; Nam, J.-O.; Chang, J.-S.; Kim, S.; Park, J.-W.; Kwon, T.K. Corosolic Acid Induces Non-Apoptotic Cell Death through Generation of Lipid Reactive Oxygen Species Production in Human Renal Carcinoma Caki Cells. Int. J. Mol. Sci. 2018, 19, 1309. [Google Scholar] [CrossRef]
- Woo, K.W.; Suh, W.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Phenolic Derivatives from the Stems of Lagerstroemia indica and Their Biological Activity. Heterocycles 2015, 91, 2355–2366. [Google Scholar] [CrossRef]
- Raju, L.; Lipin, R.; Eswaran, R. Identification, ADMET Evaluation and Molecular Docking Analysis of Phytosterols from Banaba (Lagerstroemia speciosa (L.)Pers) Seed Extract against Breast Cancer. Silico Pharmacol. 2021, 9, 43. [Google Scholar] [CrossRef]
- Sai Saraswathi, V.; Santhakumar, K. Photocatalytic Activity against Azo Dye and Cytotoxicity on MCF-7 Cell Lines of Zirconium Oxide Nanoparticle Mediated Using Leaves of Lagerstroemia speciosa. J. Photochem. Photobiol. B 2017, 169, 47–55. [Google Scholar] [CrossRef]
- Sai Saraswathi, V.; Saravanan, D.; Santhakumar, K. Isolation of Quercetin from the Methanolic Extract of Lagerstroemia speciosa by HPLC Technique, Its Cytotoxicity against MCF-7 Cells and Photocatalytic Activity. J. Photochem. Photobiol. B 2017, 171, 20–26. [Google Scholar] [CrossRef]
- Sai Saraswathi, V.; Kamarudheen, N.; Bhaskara Rao, K.V.; Santhakumar, K. Biofilm Inhibition Formation of Clinical Strains of Pseudomonas Aeruginosa Mutans, Photocatalytic Activity of Azo Dye and GC–MS Analysis of Leaves of Lagerstroemia speciosa. J. Photochem. Photobiol. B 2017, 169, 148–160. [Google Scholar] [CrossRef]
- Da Silva, M.C.A.; Paiva, S.R. Antioxidant Activity and Flavonoid Content of Clusia Fluminensis Planch.&Triana. An. Acad. Bras. Ciências 2012, 84, 609–616. [Google Scholar] [CrossRef]
- Chan, E.; Eng, S.Y.; Tan, Y.P.; Wong, Z.C.; Lye, P.Y.; Tan, L.N. Antioxidant and Sensory Properties of Thai Herbal Teas with Emphasis on Thunbergia Laurifolia lindl. Chiang Mai. J. Sci. 2012, 39, 599–609. [Google Scholar]
- Chan, E.W.C.; LYE, P.; Tan, L.N.; Eng, S.Y.; Tan, Y.; Wong, Z. Effects of Drying Method and Particle Size on the Antioxidant Properties of Leaves and Teas of Morus Alba, Lagerstroemia speciosa and Thunbergia laurifolia. Chem. Ind. Chem. Eng. Q. 2012, 18, 465–472. [Google Scholar] [CrossRef]
- Chiang, E.C.W.; Yan, L.P.; Ngar, T.L. Analysis and Evaluation of Antioxidant Properties of Thai Herbal Teas. Int. J. Adv. Sci. Arts 2011, 2, 8–15. [Google Scholar]
- Budholiya, P.; Sharma, H.K. Comparative Phytochemical Screening and Estimation of Bioactive Constituents of Leaves of Lagerstroemia parviflora, Gardenia latifolia and Terminalia tomentosa. J. Drug Deliv. Ther. 2019, 9, 674–678. [Google Scholar] [CrossRef]
- Zong, W.; Zhao, G.; Zhang, W. Application of Polyphenols of Lagerstroemia speciosa on Chinese Sausage. Meat Ind. 2006, 7–8. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Fakhri, S.; Tomas, M.; Capanoglu, E.; Hussain, Y.; Abbaszadeh, F.; Lu, B.; Hu, X.; Wu, J.; Zou, L.; Smeriglio, A.; et al. Antioxidant and Anticancer Potentials of Edible Flowers: Where Do We Stand? Crit. Rev. Food Sci. Nutr. 2022, 62, 8589–8645. [Google Scholar] [CrossRef]
- Ghosh, J.; Sil, P.C. Arjunolic Acid: A New Multifunctional Therapeutic Promise of Alternative Medicine. Biochimie 2013, 95, 1098–1109. [Google Scholar] [CrossRef]
- Kalidas, S.; Kameswari, B.; Devi, P.; Madhumitha, B.; Meera, R.; Merlin, N.J. Phyto-Physico Chemical Evaluation, Antioxidant Activities and Diuretic Activity of Leaves of Lagerstroemia reginae. Asian J. Res. Chem. 2008, 1, 83–87. [Google Scholar]
- Azad, A.K.; Uddin, A.H. Phytochemical and Cytotoxicity Evaluation of Lagerstroemia speciosa (L.) Leaves Extract by MCF-7 Cell Line and Brine Shrimp Lethality Bioassay. Technology 2020, 10, 40–44. [Google Scholar]
- Zheng, J.-Q.; Zheng, C.-M.; Lu, K.-C. Corosolic Acid–Induced Acute Kidney Injury and Lactic Acidosis in a Patient with Impaired Kidney Function. Am. J. Kidney Dis. 2010, 56, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Talbott, S.M.; Hughes, K. The Health Professional’s Guide to Dietary Supplements; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Tanquilut, M.M.; Tanquilut, N.; Elauria, J.; Peralta, E. Performance Evaluation of Banaba Lagerstroemia speciosa (L.) Pers.) Leaf Extract Spray Drying. Philipp. J. Agric. Biosyst. Eng. 2010, 8, 19–33. [Google Scholar]
- Talapatra, B.; Chaudhuri, P.K.; Mallik, A.K.; Talapatra, S.K. Lagerenyl Acetate and Lagerenol Two Tetracyclic Triterpenoids with the Cycloartane Skeleton from Lagerstroemia lancasteri. Phytochemistry 1983, 22, 2559–2562. [Google Scholar] [CrossRef]
- Qi, S.; Wu, D.; Ma, Y.; Wu, S.; Mei, W.; Luo, X. Studies on Chemical Constituents of Lagerstroemia guilinensis. Chin. Tradit. Herb. Drugs 2002, 33, 17–18. [Google Scholar] [CrossRef]
- Sai Saraswathi, V.; Thirumalai, D.; Malipeddi, H.; Asharani, I.V.; Rao, K.V.B.; Kumar, S.R.S. Silver Nanoparticle Biosynthesis and Antibacterial Activity of Aqueous Leaf Extract of Lagerstroemia speciosa. Int. J. Adv. Pharm. Res. 2013, 4, 1995–1999. [Google Scholar]
- Cao, X.; Shi, K.; Xu, Y.; Zhang, P.; Zhang, H.; Pan, S. Integrated Metabolomics and Network Pharmacology to Reveal Antioxidant Mechanisms and Potential Pharmacological Ingredients of Citrus Herbs. Food Res. Int. 2023, 174, 113514. [Google Scholar] [CrossRef]
- Zong, W.; Xia, W.; Cui, B. Determination of Corosolic and Maslinic Acids in Lagerstroemia speciosa Leaves by TLC/HPLC Method. Pharm. Chem. J. 2007, 41, 222–224. [Google Scholar] [CrossRef]
- Zong, W.; Xia, W. An Ultrasonic Extraction Method of Total Triterpenes in Lagerstroemia specious Leaves. Food Mach. 2006, 22, 14–16. [Google Scholar] [CrossRef]
- Lin, A.; Zhu, P.; Li, R.; Xia, Z.; Long, X.; Guo, S.; Fan, Y. Box-Behnken Response Surface Methodology for Optimization of Extraction Process of Corosolic Acid from Lagerstroemia speciosa. Chin. J. Ethnomed. Ethnopharmacy 2023, 32, 37–42+47. [Google Scholar]
- Patel, A.J.; Shah, M.B.; Milan, P. Application of Different Extraction Techniques on Lagerstroemia speciosa. Pharma Sci. Monit. 2018, 9, 71–85. [Google Scholar]
- Kim, C.-H.; Kim, J.-A.; Song, J.-Y.; Choi, H.-J. Method for Production of Corosolic Acid in Suspension Culture of Plant Cells. U.S. Patent 8,101,411 B2, 30 June 2006. [Google Scholar]
- Chen, Y.; Li, S.-W.; Yin, F.-Z.; Yang, M.; Huan, X.-J.; Miao, Z.-H.; Wang, X.-M.; Guo, Y.-W. Lagerindicine, a New Pyrrole Alkaloid Isolated from the Flowers of Lagerstroemia Indica Linnaeus. Nat. Prod. Bioprospect. 2021, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.; Ngo, H.T.; Rideout, J.A. Terpenoids and Sterols from Lagerstroemia speciosa. J. Asian Nat. Prod. Res. 2005, 7, 7–12. [Google Scholar] [CrossRef]
- Yan, F.; Huang, Y.; Wang, Y.; Li, Q.; He, X. Bioactive Sterols and Triterpenoids from the Fruits of Giant Crepe-Myrtle. Ind. Crops Prod. 2019, 130, 363–370. [Google Scholar] [CrossRef]
- Hui, D.; Zhang, R.; Xu, L.; Jie, J.; Zhou, C.; Yu, Z. Constituents of Three Species of Lagerstroemia. Biochem. Syst. Ecol. 2005, 33, 639–642. [Google Scholar] [CrossRef]
- Chaudhuri, P.K. A Labdane Diterpenoid Sterol from Lagerstroemia lancasteri. Phytochemistry 1987, 26, 3361–3362. [Google Scholar] [CrossRef]
- Zhan, Q.; Wang, Y.; Li, X.; Chen, W.; Sun, L. Studies on the Chemical Constituents of Petroleum Ether Extract of Lagerstroemia speciosa (Linn.) Pers Leaves. Lishizhen Med. Mater. Med. Res. 2009, 20, 2125–2127. [Google Scholar] [CrossRef]
- Huang, G.-H.; Zhan, Q.; Li, J.-L.; Chen, C.; Huang, D.-D.; Chen, W.-S.; Sun, L.-N. Chemical Constituents from Leaves of Lagerstroemia speciosa L. Biochem. Syst. Ecol. 2013, 51, 109–112. [Google Scholar] [CrossRef]
- Lou, X.; Zhang, R.; Zhao, Y.; Dou, H.; Zou, C. Chemical Studies on the Leaves of Lagerstroemia Reginae Roxb. Nat. Prod. Res. Dev. 2006, 18, 962–963. [Google Scholar] [CrossRef]
- Barik, B.R.; Kundu, A.B. Lageflorin, a Pentacyclic Triterpene from Lagerstroemia parviflora. Phytochemistry 1988, 27, 3679–3680. [Google Scholar] [CrossRef]
- Zhan, Q.; Wang, Y.; Li, X.; Yang, Y.; Chen, W.; Sun, L. Studies on the Chemical Constituents of Ethyl Acetate Extract from Lagerstroemia speciosa (Linn.) Pers Leaves. Lishizhen Med. Mater. Med. Res. 2009, 20, 1841–1842. [Google Scholar] [CrossRef]
- Hussain, S.F.; Miana, G.A.; Saifur, R. 3,4,3′-Tri-O-Methylellagic Acid from Lagerstroemia indica. Phytochemistry 1972, 11, 2890–2891. [Google Scholar] [CrossRef]
- Saleh, N.A.M. Anthocyanins of Lagerstroemia Indica Flowers. Phytochemistry 1973, 12, 2304. [Google Scholar] [CrossRef]
- Osawa, K.; Ueda, J.; Takahashi, M. The Components of the Plants of Lagerstroemia Genus. II. Studies on the Components of the Leaves of Lagerstroemia speciosa (L.) Pers., L. subcostata Koehne., L. indica Linn., and L. Fauriei Koehne. Yakugaku zasshi. 1974, 94, 271–273. [Google Scholar] [CrossRef]
- Zhang, D. Chemical Constituents in Stem-Leaves of Lagerstroemia indica. Chinese Traditional and Herbal Drugs 2015, 46, 2209–2211. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Sakai, T.; TANAKA, T.; Nonaka, G.; Nishioka, I. Tannins and Related Compounds. CVI. Preparation of Aminoalditol Derivatives of Hydrolyzable Tannins Having α-and β-Glucopyranose Cores, and Its Application to the Structure Elucidation of New Tannins, Reginins A and B and Flosin A, Isolated from Lagerstroemia Flos-Reginae RETZ. Chem. Pharm. Bull. 1991, 39, 639–646. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.-M.; Tanaka, T.; Nonaka, G.; Nishioka, I. Tannins and Related Compounds. CVII. Structure Elucidation of Three New Monomeric and Dimeric Ellagitannins, Flosin B and Reginins C and D, Isolated from Lagerstroemia Flos-Reginae Retz. Chem. Pharm. Bull. 1991, 39, 647–650. [Google Scholar] [CrossRef]
- Tanaka, T.; Tong, H.H.; Xu, Y.M.; Ishimaru, K.; Nonaka, G.I.; Nishioka, I. Tannins and Related Compounds. CXVII. Isolation and Characterization of Three New Ellagitannins, Lagerstannins A, B and C, Having a Gluconic Acid Core, from Lagerstroemia speciosa (L.) PERS. Chem. Pharm. Bull. 1992, 40, 2975–2980. [Google Scholar] [CrossRef]
- Fuji, K.; Yamada, T.; Fujita, E.; Murata, H. Lythraceous Alkaloids. X. Alkaloids of Lagerstroemia subcostata and L. Favriei: A Contribution to the Chemotaxonomy. Chem. Pharm. Bull. 1978, 26, 2515–2521. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, I.S.; Youn, U.; Chen, Q.C.; Ngoc, T.M.; Ha, D.T.; Liu, H.; Min, B.S.; Lee, J.Y.; Seong, R.S. Biphenylquinolizidine Alkaloids from Lagerstroemia indica. J. Nat. Prod. 2009, 72, 749–752. [Google Scholar] [CrossRef]
- Watanabe, K.; Kubota, T.; Shinzato, T.; Ito, J.; Mikami, Y.; Kobayashi, J. Sarusubine A, a New Dimeric Lythraceae Alkaloid from Lagerstroemia subcostata. Tetrahedron Lett. 2007, 48, 7502–7504. [Google Scholar] [CrossRef]
- Muangsin, N.; Wisetsakdakorn, W.; Chaichit, N.; Sihanonth, P.; Petsom, A.; Sangvanich, P. Austrocortinin: Crystal Structure of a Natural Anthraquinone Pigment from Fungi. Dyes Pigm. 2008, 77, 653–656. [Google Scholar] [CrossRef]

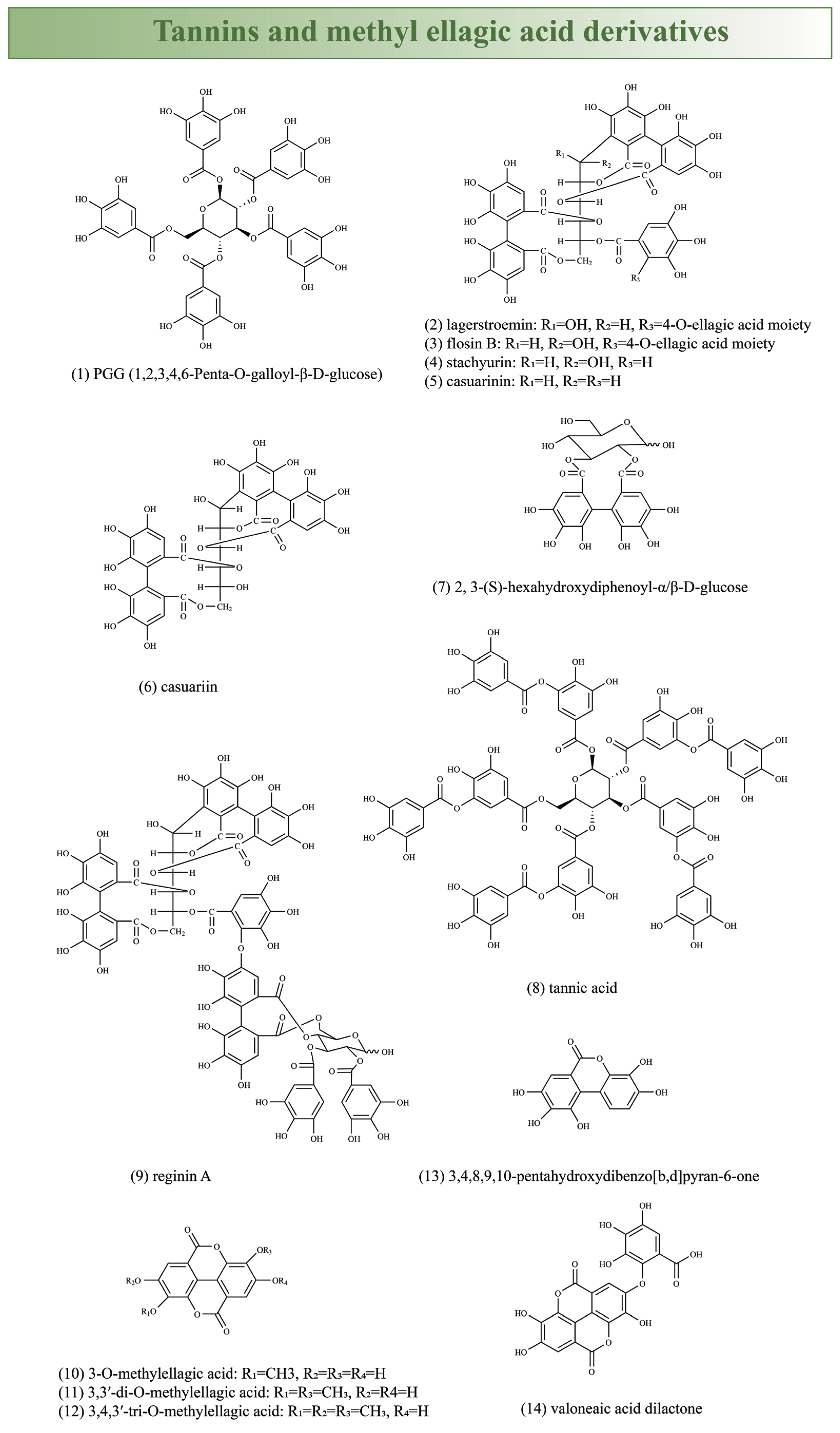
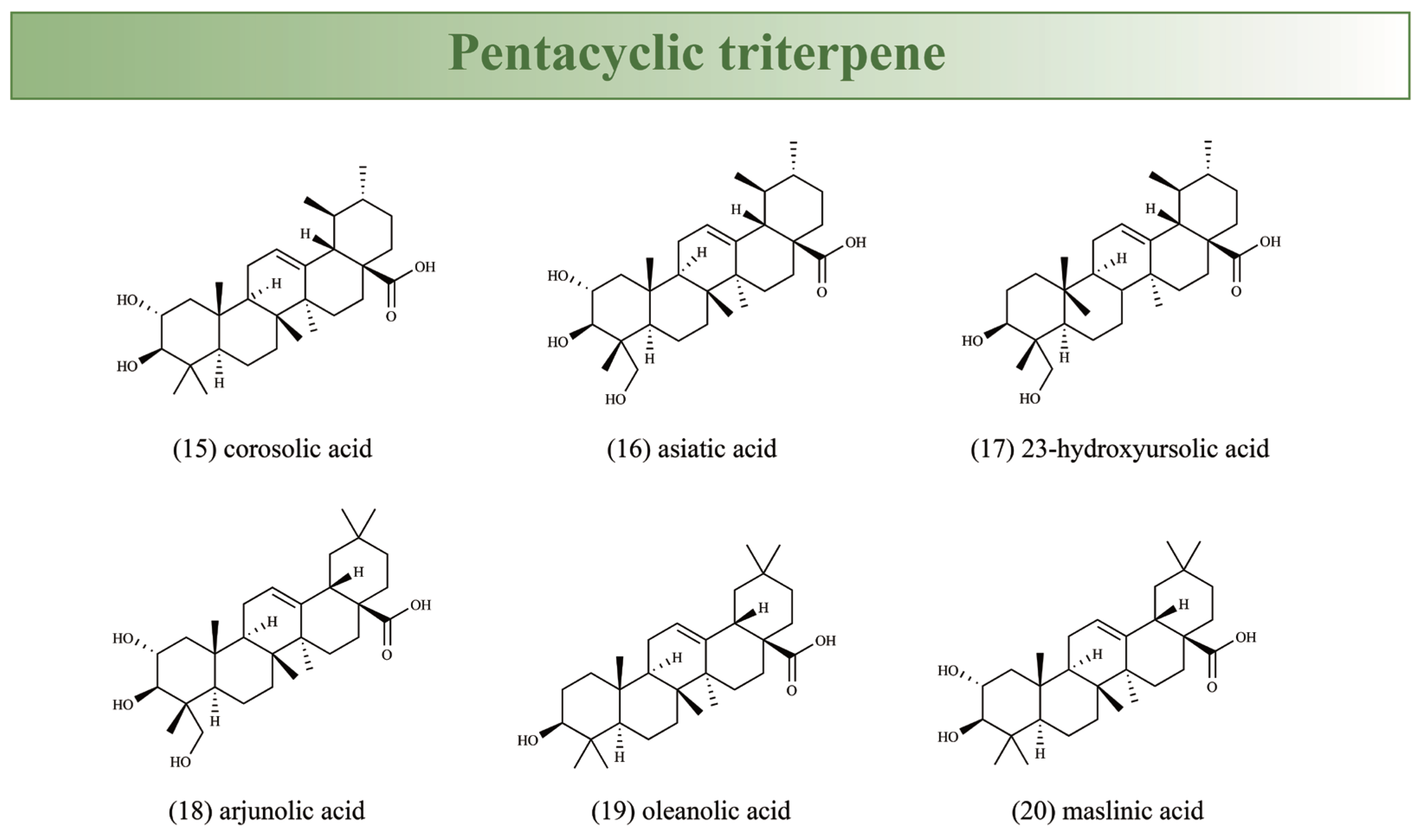
| Traditional Uses | Preparation | Plant Part Used | Country | References |
|---|---|---|---|---|
| Lagerstroemia floribunda Jack | ||||
| Treats diarrhea | - | Bark | Thailand | [37] |
| Lagerstroemia indica L. | ||||
| Febrifuge, stimulant, and styptic | - | Stem bark | - | [10] |
| Diuretic and a drastic purgative | - | Bark, flower and leaf | - | |
| Treats cuts and wounds | Applied externally | Flower | - | |
| Astringent, detoxicant, and diuretic | - | Root | - | |
| Treats cold | Decoction | Flower | - | |
| Laxative and diuretic | - | Leaf, flower and bark | - | [6] |
| Treats asthma and hemostasis and as a detoxifier | - | - | - | |
| Clears heat and detoxifies, dispels wind and relieves itching; used for sore throat, ulcers, and itchy skin rashes | - | Bark | China | [24] |
| Clears heat and detoxifies, dispels wind and dampness, promotes blood circulation, and stops bleeding; used for sore throat, abnormal vaginal discharge, erysipelas, scabies, bruises, and internal or external bleeding from injuries | Decoction for washing; powdered for topical application. | Bark | China | [23] |
| Clears heat, detoxifies, cools blood, and stops bleeding; used for abscesses, swelling and toxins, breast abscesses, dysentery, eczema, and external bleeding from injuries | Decoction for washing; crush fresh leaves and apply topically | Leaf | China | |
| Clears heat, detoxifies, cools blood, and stops bleeding; used for leukorrhea, pulmonary tuberculosis with bloody cough, childhood convulsions, pediatric fetal toxins, sores, boils, carbuncles, and scabies | Decoction for washing; crush fresh flowers and apply topically | Flower | China | |
| Lagerstroemia lanceolata Wall. | ||||
| Treats asthma, diabetes mellitus, chronic bronchitis, cold, cough, and local application for aphthae of the mouth | - | - | - | [17] |
| Narcotic | - | Seeds | - | |
| Lagerstroemia loudonii Teijsm. & Binn | ||||
| Treats diarrhea | - | Bark | Thailand | [37] |
| Treats high blood pressure and diabetes | - | Seed | Indonesia | [38] |
| Treats urination stones, diabetes, and high blood pressure | - | Leaf | Indonesia | |
| Treats diarrhea, dysentery, and urinary blood | - | Bark | Indonesia | [39] |
| Lagerstroemia ovalifolia Teijsm. & Binn | ||||
| Treats diarrhea | - | Bark | Indonesia | [40] |
| Malaria and dermatosis | - | Leaf | - | |
| Lagerstroemia parviflora Roxb. | ||||
| Heals infections and persistent sores | - | Leaf | India | [41] |
| widely employed by tribal women to address lactation challenges | - | - | India | |
| Cures gastrointestinal strangulation and syphilis | - | Whole plant | India | |
| Manages coughs, fevers, asthma, and bronchitis | - | - | India | |
| Makes a black dye | - | Bark | India | |
| Produces edible sweet chewing gum | - | - | India | |
| Treat fever | Leaf juice | Leaf | India | [42] |
| Anti-asthmatic | - | Flower | India | [43] |
| Antitussive and astringent | - | - | India | [42] |
| Lagerstroemia speciosa L. | ||||
| Treats malaria and foot fracture | Powder | Leaf | Malaysia | [44] |
| Decongestant, diuretic, and diabetes | Decoction | Leaf | Philippines, Thailand | [37,45] |
| Stimulant, antipyretic, alleviate abdominal discomfort | Decoction | Bark | Philippines | [45,46] |
| Anesthesia and pain relief of oral ulcer | Decoction | Seed, fruit | Philippines | [47,48] |
| Green leafy vegetables | - | Young leaves | Vietnam | [49] |
| ethnic medicines for lowering blood sugar | - | fruit and old leaf | ||
| Purgative and facilitate bowel movements | - | Leaf, flower and bark | Philippines | [47] |
| Kidney and bladder inflammation, urinary issues, cholesterol reduction, hypertension, and diabetes management | Decoction or infusion | Leaf | Philippines | |
| Headaches, malaria, and cracked heels | Poultice, applied directly to the lesions | Leaf | Philippines | |
| Gastrointestinal upset, hematuria, stomach ache and depression | Decoction | Bark | Philippines | |
| Astringent, stimulant, febrifuge | - | Root | Philippines | |
| Diarrhea | Decoction | Bark | Malaysia | |
| Aphthous stomatitis | gargle with decoction | Fruit and root | Philippines | |
| Stomach problems | - | Root | Philippines | |
| Mouth ulcers | - | Root | - | [45,50] |
| Decrease blood glucose levels, promote weight loss | Herbal tea | Leaf | Philippines | [45] |
| Diabetes | Decoction; 50 g to a pint of boiling water, 4 to 6 cups daily | Leaf and dried Fruit | India, Bangladesh, Philippines | [16,45,51] |
| Toppings or ingredients in salads, soups, desserts, and drinks | - | Flower | - | [52] |
| Cleansing agent | - | Leaf | - | [50] |
| urinary tract infections | - | Leaf | - | |
| Prevents HIV infection | - | - | Bangladesh | [53] |
| Used by tribal peoples to treat heart disease | - | Leaf | - | [16] |
| Treats pain, purgative | Infusion | Bark | Bangladesh | [48,51] |
| Acute jaundice | - | Leaf | India | [54] |
| Lagerstroemia subcostata Koehne | ||||
| Detoxify, dissipate blood stasis, and intercept malaria | Decoction | Flower, root | China | [55] |
| Carbuncle, snakebite, and malaria | Mash the fresh herb and apply directly to the lesions | |||
| Species | Part Used | Constituents/Preparations | Tested Pathogen/Cell | Results | Reference |
|---|---|---|---|---|---|
| Hypoglycemic | |||||
| Lagerstroemia indica L. | Stem and leaf | Ethyl acetate, chloroform, n-butyl alcohol, and water fraction of ethanol (95%) extract | α-Glucosidase and α-amylase | Inhibition rate of α-glucosidase: ethyl acetate fraction (73.60%), n-butanol alcohol fraction (59.78%), chloroform (55.26%); inhibition rate of α-amylase: chloroform fraction (61.46%) | [117] |
| L. indica | Flower | Petroleum ether, ethyl acetate, and n-butyl alcohol fraction of ethanol (70%) extract | α-Glucosidase | Showed highest inhibition rate in ethyl acetate fraction: IC50 = 4.45 μg/mL | [118] |
| Lagerstroemia indica Linn. f. alba (Nichols) | Flower | Petroleum ether, ethyl acetate, and n-butyl alcohol fraction of ethanol (70%) extract | α-Glucosidase | Showed highest inhibition rate in ethyl acetate fraction: IC50 = 4.09 μg/mL) | [118] |
| Lagerstroemia loudonii Teijsm. & Binn | Leaf and fruit | Ethanol (96%) extract | α-Glucosidase | Fruit extract: 7 times stronger inhibitor than reference drug Acarbose; leaf extract: 24 times weaker | [56] |
| L. loudonii | Stem bark | Ethanol (96%) extract | α-Glucosidase | IC50 = 79.479 ± 0.52 μg/mL | [57] |
| L. loudonii | Leaf | Ethanol (96%) extract | Dipeptidyl peptidase-IV | Inhibition rate 60.22 ± 2.01% | [58] |
| Lagerstroemia speciosa L. | Leaf | 10% acetic acid in ethanol extract | α-Amylase | IC50 = 68.19 μg/mL | [5] |
| L. speciosa | Leaf | 20% acetone/water extract | 3T3-L1 fibroblasts | Inhibited adipocyte differentiation in 3T3-L1 cells | [3] |
| L. speciosa | Fruit | Ethanol (75%) extract | α-Glucosidase | IC50 = 121.26 ± 9.71 μM for compound 21 | [119] |
| L. speciosa | Leaf and fruit | Water extract, ethanol (95%) extract | α-Glucosidase | IC50 = 4.29 μg/mL and 9.16 μg/mL for H2O extract, 2.64 μg/mL and 6.17 μg/mL for 95% EtOH extract of leaf and fruit | [29] |
| L. speciosa | Leaf | Water extract | α-Glucosidase | IC50 = 5.4 ± 0.5 μg/mL | [120] |
| L. speciosa | Leaf | Ethyl acetate extract (six pentacyclic triterpenes) | α-Glucosidase and α-amylase | Moderate inhibition of α-glucosidase (IC50 = 3.53 μg/mL) and weak inhibitory effect on α-amylase (IC50 = 100.23 μg/mL) | [89] |
| L. speciosa | Green and fallen leaf | Ethanol (80%) extract | α-Glucosidase and α-amylase | Inhibition rate of α-glucosidase: green leaf 29.06%, fallen leaf 30.49%; inhibition rate of α-amylase: green leaf 25.42%, fallen leaf 13.96% | [121] |
| Antimicrobial | |||||
| L. indica | Fruit | Low-polar solvent extract | Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Penicillium glaucum | Sensitivity to the antibacterial extract: S. aureus > E. coli > L. monocytogenes > P. glaucum | [44] |
| L. indica | Bark, leaf, and fruit | Petroleum ether, chloroform, methanol | Gram-positive bacteria (S. aureus, Bacillus subtilis), Gram-negative bacteria (E. coli, Pseudomonas aeruginosa), fungal strains (Aspergillus oryzae and A. niger) | Maximum antibacterial activity of petroleum ether extract of bark against B. subtilis (58.33 ± 0.88 mm); maximum antifungal activity of chloroform extract of bark against A. niger (40.33 ± 0.88 mm) | [122] |
| L. indica | Young, medium, and coarse leaf | Methanolic extract | B. cereus, S. aureus, Proteus mirabilis, P. aeruginosa, Salmonella typhi, E. coli, and Shigella dysenteriae | Halo range, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC): young leaf (10.2–20.6 mm, 100–145 mg/mL, 100–145 mg/mL); medium leaf (9.4–18.5 mm, 130–145 mg/mL, 130–160 mg/mL); coarse leaf (5.0–10.0 mm, 200–350 mg/mL, 145–300 mg/mL) | [10] |
| L. indica | Leaf | Methanol extract | 4 pathogenic bacteria (S. aureus, S. enteritides, E. coli, and L. monocytogenes) and Candida albicans | MLC of compound ’4-methoxy apigenin-8-C-β-D-glucopyranoside: C. albicans (32 μg/mL), S. aureus (16 μg/mL), S. enteritides (16 μg/mL), E. coli (16 μg/mL), and L. monocytogenes (16 μg/mL) | [123] |
| Lagerstroemia tomentosa C.Presl | Leaf | Methanolic (70%) extract | Mycobacterium tuberculosis | Highest inhibition rate (38%) | [13] |
| Lagerstroemia parviflora Roxb. | Leaf | Methanol extract | 2 bacteria (S. aureus and S. bongori), one fungus (A. niger) | Exhibited inhibitory activity against all tested microorganisms at different concentrations | [124] |
| L. speciosa | Fruit | CH2Cl2 extract | 3 Gram-positive bacteria (B. subtilis, B. cereus, and S. aureus), 1 Gram-negative bacterium (Klebsiella pneumonia), 4 fungal strains (Aspergilus flavus, A. niger, Rhizopus nigricans, and Fusarium equiseti) | Showed poor antibacterial and antifungal properties in compound 1 while good in compound 2 | [125] |
| L. speciosa | Flower | Methanolic extract | 5 Gram-positive bacteria, 8 Gram-negative bacteria and 2 fungi | Largest inhibition zone against S. aureus (19.0 mm) | [126] |
| L. speciosa | Fruit (without seed) | Methanol and dichloromethane extract | 4 bacterial and 3 fungi | MIC values: S. aureus (15–39 μg/mL), E. coli (16–38 μg/mL), P. aeruginosa (15–39 μg/mL), B. subtilis (14–39 μg/mL), A. niger (16–38 μg/mL), A. flavus (18–39 μg/mL), C. albicans (16–38 μg/mL) | [67] |
| L. speciosa | Flower | Methanol extract | 4 bacteria (S. aureus, B. cereus, Vibrio cholerae and E. coli), 2 fungi (C. albicans and Cryptococcus neoformans) | Zone of inhibition: S. aureus (2.2 cm), B. cereus (1.9 cm), E. coli (1.7 cm), V. cholerae (1.7 cm), C. albicans (1.5 cm), C. neoformans (1.7 cm) | [52] |
| L. speciosa | Leaf | Methanol extract | S. aureus, P. aeruginosa, S. typhimurium and E. coli | Highest activity against E. coli (15 mm), lowest activity against S. typhimurium (7 mm) | [50] |
| L. speciosa | Leaf | Ethanol and water extract | S. aureus, B. subtilis (Gram-positive) and Pseudomonas aeruginosa, E. coli, (Gram-negative bacteria) | Zone of inhibition of ethanol and water extract: S. aureus (14 mm, 15 mm), B. subtilis (12 mm, 15 mm), P. aeruginosa (14 mm, 17 mm), E. coli (16 mm, 17 mm) | [127] |
| L. speciosa | Leaf | Methanol extract | 12 oral isolates of Streptococcus mutans | Had a dose-dependent inhibition of cariogenic isolates but lower than that of standard antibiotic | [48] |
| Anti-inflammation | |||||
| Lagerstroemia ovalifolia Teijsm. & Binn | Leaf | Methanolic extract | Lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages | Inhibited the production of NO, PGE2, interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α), suppressed the mRNA and protein expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), inhibited the phosphorylation of MAPKs, reduced nuclear translocation of nuclear factor-κB (NF-κB) | [40,59] |
| L. indica | Whole plant | Ethanol (80%) extract | Jurkat cells, EoL-1 cells, and THP-1 cells | Strongly inhibited the protein release of IL-2, IL-4, IL-5, IL-13, and TNF-α in Jurkat cells | [128] |
| L. speciosa | Fruit | Ethanol (75%) extract | Macrophage BV-2 cell line | Anti-neuroinflammatory IC50 values of compounds 1 and 2: 8.29 ± 0.85 μM, 7.20 ± 1.55 μM | [119] |
| L. speciosa | Leaf | Ethyl acetate and ethanol extract | Human RBC membrane | 30.01% and 37.50% HRBC protection in hypotonic solution of 50 μg/mL ethanol and ethyl acetate extract, respectively | [129] |
| Anticancer | |||||
| L. indica | Stem | Methanolic (80%) extract | Human tumor cell lines A549, SK-OV-3, SK-MEL-2, and HCT-15, | Showed potent cytotoxicity against tumor cell lines (IC50 = 3.3–6.29 μM) | [87] |
| L. indica | Stem and leaf | Ethanol (95%) extract | A2780, NCI-H1650, BGC-832, HepG2, HCT-116 | Inhibitory effects: chloroform fraction on HepG2 (IC50 = 34.862 μg/mL), ethyl acetate fraction on HepG2 (IC50 = 43.261 μg/mL), and A2780 (IC50 = 46.673 μg/mL) | [117] |
| L. speciosa | Leaf | Extract contained 20% corosolic acid | HepG2 cells. | Caused significant cytotoxicity in HepG2 cells: membrane distortion and nuclear chromatin condensation; the loss of ΔΨm; interfere with Bax/Bcl-2 homeostasis; induce the pro-apoptotic marker genes (cytochrome c, Apaf-1, and caspases 9 and 3) | [130] |
| L. speciosa | Fruit | Ethanol (75%) extract | Tumor cell lines (HeLa, HepG2, and SGC-7901) | Showed obvious activity (IC50 = 15.90–39.13 μM) in compounds 13 and 18 when compared to the positive control cisplatin (IC50 = 3.31–16.6 μM) | [119] |
| L. speciosa | Leaf | Various solvents extract | MCF-7 cancer cell lines | Exhibited notable cytotoxicity against MCF-7 cell lines at 500 μg/mL | [131] |
| L. speciosa | Leaf | Ethanol (70%) extract | Human lung adenocarcinoma cells (A549) | Possessed cytotoxic activity against A549 cells: IC50 = 297.31 μg/mL (NRU assay), 41.23 μg/mL (MTT assay) | [132] |
| anti-HRV | |||||
| L. speciosa | Leaf | Methanol extract | HeLa cells and three rhinoviruses, HRV-2, -3, and -4 | Toxicity levels for natural ellagic acid: 1.8, 2.3, and 2.2 times higher than ribavirin against HRV-2 (38 μg/mL), HRV-3 (31 μg/mL), and HRV-4 (29 μg/mL), respectively | [133] |
| L. speciosa | Leaf | Methanol extract (quercetin 7-glucoside) | HRV2 propagated in HeLa cells | Reduced the formation of a visible cytopathic effect, inhibited virus replication during the initial stage of infection, was more effective than ribavirin | [11] |
| L. speciosa | Leaf | Methanol extract | HRV species A (HRV1B, HRV2, HRV15, and HRV40), species B (HRV3, HRV6, and HRV14), pleconaril-resistant virus (HRV5) | Possessed broad-spectrum anti-HRVs activity: IC50 = 0.58–8.80 μg/mL, CC50 (50% cytotoxicity concentration) > 100 μg/mL | [134] |
| Anti-HIV | |||||
| L. speciosa | Stem and leaf | Aqueous and 50% ethanolic extract | HIV-1-infection in TZM-bl and CEMGFP cell lines | Showed a dose-dependent inhibition (IC50 = 1–25 μg/mL), inhibited reverse transcriptase and HIV protease | [135] |
| Antithrombin activity | |||||
| L. indica | - | Methylene chloride and methanol extract | Thrombin solution | Displayed 79% and 85%anticoagulant activity of methylene chloride and methanol extract, respectively | [136] |
| Anti-hyperuricemia | |||||
| L. speciosa | Leaf | Aqueous extracts | Xanthine oxidase (XOD) | A stronger XOD-inhibitory effect of valoneic acid dilactone than a clinical drug allopurinol, with a noncompetitive inhibition pattern for the enzyme with respect to xanthine | [137] |
| L. speciosa | Leaf | Aqueous extracts | XOD | Had a stronger potential in inhibiting XOD than Aspalathus linearis (Burm.f.) R.Dahlgren, Camellia sinensis (L.) Kuntze, and Eucommia ulmoides Oliv. | [138] |
| Species | Part Used | Constituents/Preparations | Tested Subjects | Study Design | Results | Reference |
|---|---|---|---|---|---|---|
| Hypoglycemic | ||||||
| Lagerstroemia indica L. | Leaf | Distilled water extract | Adult male albino rat | Daily oral dose of 100 mg/kg b.w. extract A or B for two months | Showed a decrease in serum glucose level after 4 and 8 weeks: extract A (22.5%, 44.9%), extract B (32.2%, 58.2%), reference drug metformin (46.2%, 66.4%) | [72] |
| L. indica and Lagerstroemia indica Linn. f. alba (Nichols) | Flower | Ethanol (70%) extract | Alloxan-induced diabetic mice | Oral administration of extracts at 125, 250, and 500 mg/kg/d for 7 days | Decreased fasting blood glucose, total cholesterol level, malondialdehyde content; increased superoxide dismutase level in serum | [118] |
| Lagerstroemia balansae Koehne | Leaf | Ethanol (95%) extract | Eight-week-old male B6.Cg-m +/+Leprdb/J (db/db) mice | Oral administration of extracts at 2 g/kg/d for 6 consecutive weeks | Decreased the blood-glucose and hemoglobin Alc; improve the glucose tolerance | [139] |
| Lagerstroemia speciosa L. | Leaf | Extracts, standardized to 1.13% corosolic acid | 24 patients with metabolic syndrome | Single-center, randomized, double-blind, parallel, placebo-controlled; oral administration of extracts of 500 mg before breakfast and dinner for 12 weeks | Alleviated metabolic syndrome: significantly decreased systolic blood pressure (121.5 ± 12.9 vs. 116.3 ± 9.8 mmHg, p = 0.050), fasting plasma glucose (5.9 ± 0.4 vs. 5.7 ± 0.4 mmol/L, p = 0.034), triglycerides (2.3 ± 0.4 vs. 1.7 ± 0.5 mmol/L, p = 0.021), very low density lipoprotein (0.5 ± 0.1 vs.0.3 ± 0.1 mmol/L, p = 0.021), area under the curve of insulin (50,675 ± 14,309 vs. 37,983 ± 19,298 mmol/L, p = 0.017), and insulinogenic index (0.4 ± 0.2 vs. 0.3 ± 0.2, p = 0.047) | [140] |
| L. speciosa | Leaf | 20% acetone/water extract | Alloxan induced diabetic mice | Oral administration of extracts at 0.25, 1.0, and 4.0 g/kg/d for 21 consecutive days | Lowered levels of body weight, fasting blood glucose, tissue weight, serum biomarkers, and body fat; increased final insulin levels | [3] |
| L. speciosa | Flower | Methanolic extract | Swiss-albino mice | Oral administration of methanolic crude extract at 200 mg/kg and 400 mg/kg doses | reduce blood sugar level by 48.85 and 56.12% at 200 and 400 mg/kg, respectively | [126] |
| L. speciosa | Leaf | Self-micro-emulsifying (SME) formulation of ethanol (50%) extract | Wistar albino male diabetic rats | Oral administration of SME formulation at 50, 100 mg/kg | Elevated the pharmacodynamic performance approximately twofold in SME; exhibited dose-dependent manner; comparable to the hypoglycemic effect of glimepiride; significantly decreased serum lipid | [141] |
| L. speciosa | - | Standardized extract | Wistar albino rats | Administration with corosolic acid, boswellic acid, ellagic acid, ursolic acid, and quercetin at a dose of 10 mg/kg body weight | Inhibited lens galactitol accumulation, with ursolic acid exhibiting the most potent effect | [142] |
| L. speciosa | Leaf | Hot-water extract | Male/female Albino rats | Oral administration of extracts to streptozotocin-induced 24 h fasted rats. | Showed prominent hypoglycemic activity; inhibited gluconeogenesis and promoted glucose oxidation via the pentose phosphate pathway | [143] |
| L. speciosa | Leaf | Capsules containing 10 mg of corosolic acid | 31 subjects | Double-blind and cross-over, placebo-controlled; oral administration of corosolic acid of 10 mg | Had lower post challenge plasma glucose levels at 90 min than the placebo treatment subjects | [110] |
| L. speciosa | Leaf | Hot-water extract | 4-week-old C57BL/KsJ-db/db, male mice | Oral administration of 0.5% water extract for 12 consecutive weeks | Reduced insulin, blood glucose, triglyceride and percent HbA1c; increased expressions of liver PPAR-α mRNA, adipose tissue PPAR-γ mRNA, and liver LPL mRNA | [144] |
| L. speciosa | Leaf | Soft gel capsule formulation and dry-powder-filled hard gelatin capsule formulation, both standardized to 1% corosolic acid (GlucosolTM) | 56 type 2 diabetic volunteers | Randomized clinical trial; oral administration of a soft gelatin or hard gelatin formulation of 16, 32 and 48 mg GlucosolTM for 15 days | Exhibited a superior percent reduction in blood glucose levels of soft gel formulation (4.9–30%) compared to dry-powder formulation (3.18–20.2%) | [145] |
| L. speciosa | Leaf | Banabamin, a tablet containing extract from Banaba tea | 24 patients with mild type 2 diabetes | Crossover method; oral administration of 3 tablets or placebo t.i.d. | Significantly decreased blood glucose level | [69] |
| L. speciosa | - | Combined bioactive fraction of Cinnamomum burmanni (Nees & T.Nees) Blume and L. speciosa | patient with type 2 diabetes | Open and prospective clinical study; oral administration of fraction for 12 weeks | Improved glycemic control; enhanced insulin sensitivity, lipid profile, and adiponectin level; safe and tolerable | [146] |
| L. speciosa | Leaf | Hot-water extract | Male mice with type 2 diabetes | Oral administration of extract for 5 consecutive weeks | Regulated plasma glucose levels in noninsulin dependent diabetes mellitus | [147] |
| L. speciosa | Leaf | Water extract | Rabbits | oral administration of a decoction of leaves at 1 to 2 g/kg | Lowered the blood sugar with marked and prolonged effects in larger doses | [20] |
| Anti-obesity | ||||||
| L. speciosa | Leaf | Hot-water extract | Female KK-Ay mice | Oral administration of 5% of a hot-water extract for 12 weeks | Significantly lowered body weight gain and parametrial adipose tissue weight; suppressed hemoglobin A1C; significantly decreased total hepatic lipid contents, attributed to decreased triglyceride accumulation | [9] |
| L. speciosa | Leaf | Capsule with Banaba leaf extract | 56 participants (11 men and 45 women) | 2 tablets of the Fat Conversion Inhibitor and 1 capsule of the Carbohydrate Absorption Inhibitor (contained leaf extract of Banaba) before each meal for 3 times per day | Reduced mean total body weight, percent body fat, and waist, hip, and chest circumference | [148] |
| Lagerstroemia parviflora Roxb. | Leaf | Methanolic extract | Male Wistar albino rat | Oral administration of extract at 200 or 300 mg/kg for 12 weeks | Significantly reduced total fat, fat percentage, blood glucose, insulin resistance, and lipid profile in a dose-dependent fashion | [149] |
| Antitumor | ||||||
| L. speciosa | Leaf | Ethanol (70%) extract | Male Swiss albino mice weighing 22–25 g | Oral administration of extract at 250 mg/kg five days a week | Alleviated abnormal indicators in Benzo(a)pyrene [B(a)P]-induced lung tumor mice, such as weight, tumor-related enzymes, and genes | [132] |
| Larvicide activity | ||||||
| Lagerstroemia loudonii Teijsm. & Binn | Leaf, bark, stem, and fruit | Ethanol (96%) extract | Phase III or IV instar Aedes aegypti mosquito larvae | Administration of extract at 250, 300, 350, 400, 450, and 500 μg/mL for 24 h | Showed larvicide activity in all organ extracts with the highest effect in fruit | [8] |
| Anti-inflammation | ||||||
| Lagerstroemia ovalifolia Teijsm. & Binn | Leaf | Methanolic extract | Mice of lipopolysaccharide (LPS)-induced acute lung injury | Oral administration of extracts at 10 mg/kg, 20 mg/kg for 3 days | Suppressed inflammatory molecules and MAPK/NF-κB activation | [59] |
| L. loudonii | Seed | Purified light petroleum ether | Carrageenan-induced rat paw edema model | I.p. administration of extract at 50, 100 and 200 mg/kg | Inhibited the carrageenan induced rat paw oedema: 53.70%, 59.70%, 62.20% inhibition at the dose of 50, 100, and 200 mg/kg extracts, while 74.60% inhibition for standard drug diclofenac sodium (10 mg/kg i.p.) | [17] |
| L. indica | Stem and leaf | Ethanol (95%) extract | Rats | Administration of extract at 100 mg/kg, injected subcutaneously | Showed weak anti-inflammatory activity in ethyl acetate fraction (inhibition rate > 30%, p < 0.05) | [117] |
| L. indica | Whole plant | Ethanol (80%) extract | A mouse model of asthma | Oral injection administration of extract at 50 mg/kg, 250 mg/kg or 500 mg/kg between days 14 and 27, respectively | Significantly inhibited leukocytosis and eosinophilia in bronchoalveolar lavage (BAL) fluid and lung tissue samples; inhibited the increase in mucus secretion by goblet cells; blocked the production of reactive oxygen species in BAL fluid cells; blocked the protein expression of IL-5 in BAL fluid; weakly inhibited the concentration of ovalbumin-specific IgE in BAL fluid | [128] |
| L. speciosa | Leaf | Methanolic extract | Dextran sulfate sodium (DSS) induced ulcerative colitis in C57BL/6 mice | Oral administration of extracts at 100 and 200 mg/kg/d for 7 days | Significantly prevented DSS-induced inflammatory and ulcerative damages of the colon; reduced lipid peroxidation; restored the levels of innate antioxidants in the colon tissue | [150] |
| L. speciosa | Leaf | Ethyl acetate and ethanol extract | Female BALB/c mice | Oral administration of extract at 50 mg/kg, 2500 mg/kg | Ethyl acetate extract: had a significant dose-dependent anti-inflammatory effect against carrageen and formalin-induced paw edema in mice; ethanol extract: not in a dose-dependent manner and showed lesser activity in the formalin model | [129] |
| Antioxidant | ||||||
| L. indica | Leaf | distilled water extract | Adult male albino rats | administration of extract A or B at 100 mg/kg | Percent of blood glutathione change: extract A (3.8%), extract B (6%), reference drug vitamin E (1.4%) | [72] |
| L. speciosa | Leaf | Ethanol extract | Rats | Oral administration of extract at 50 and 250 mg/kg | Decreased the level of tissue and serum malondialdehyde in a dose-dependent manner and increased the levels of superoxide dismutase, catalase, glutathione peroxidase, and glutathione | [54] |
| L. speciosa | Leaf | Standardized aqueous leaf extract having 1% corosolic acid fraction | Adult albino mice | Oral administration of extract at 50, 100, 150, 250, 500 mg/kg/day for 2 months | Duly reduced streptozotocin generated reactive intermediates and radical species; restored normal levels of antioxidative markers like superoxide dismutase, catalase, glutathione S-transferase, and reduce glutathione | [151] |
| Hepatoprotective | ||||||
| L. indica | Leaf | Distilled water extract | Adult male albino rats | Oral administration of extract A or B at 100 mg/kg/d for 1 month | Notably decreased the liver enzymes AST, ALT, and ALP (extract A > B) | [72] |
| L. speciosa | Bark | Ethanol (70%) extract | Sprague Dawley adult male rats | Oral administration of extract at 100 and 200 mg/kg/day for 2 weeks | Enhanced liver histopathology by restoring hepatocellular architecture, reducing inflammation and mitigating vascular and cellular degeneration | [18] |
| L. speciosa | Leaf | Ethanol (50%) extract | Rats with induced liver toxicity by carbon tetrachloride | Oral administration of self-micro-emulsifying formulation of extract at 50 and 100 mg/kg every 72 h for 14 days. | Effectively protected serum enzymes; prevented the rise in levels of lipid peroxidation; increased the glutathione, superoxide dismutase and catalase contents; showed protection at the dose of 100 mg/kg comparable to normal control and standard | [152] |
| L. speciosa | Leaf | Ethanol extract | Male Wistar rats | Treated with extracts at dose of 50 and 250 mg/kg body weight for seven days | Significantly normalized serum and liver tissue parameters to near-normal levels | [54] |
| Analgesic | ||||||
| L. indica | Leaf | Distilled water extract | Adult male albino rats of Sprauge Dawely Strain | Oral administration of extract A or B | Exhibited superior analgesic activity in extract A (93.8%) compared to extract B (74.6%) | [72] |
| Lagerstroemia lanceolata Wall. | Seed | Purified light petroleum ether extract | Mice with acetic acid-induced writhing method and hot-plate method | Oral administration of extract at 10, 20 and 40 mg/kg | Acetic acid-induced writhing test: demonstrated significant analgesic effects; achieved reductions of 28.82%, 48.58%, and 75.73% at doses of 10, 20, and 40 mg/kg; outperformed a standard drug diclofenac sodium at 5 mg/kg (63.77%); hot-plate test: significantly elevated pain thresholds | [17] |
| L. speciosa | Flower, root | Methanolic extract | Acetic acid induced writhing in Swiss albino mice | Oral administration of extract at 200 and 400 mg/kg | Produced 35.38% and 53.85% (p < 0.001) of writhing inhibition at 200 and 400 mg/kg, and 70.77% inhibition in standard diclofenac sodium; exhibited dose-dependent inhibition | [19,126] |
| L. speciosa | Bark | Chloroform extract | Acetic acid-induced gastric pain model in Swiss albino mice | Oral administration of extract at 250 and 500 mg/kg | Exhibited notable inhibition of writhing (50.7%) at 500 mg/kg | [51] |
| L. speciosa | Fruit | Ethanolic extracts | Acetic acid-induced writhing model in mice | Oral administration of extract at 250 and 500 mg/kg | Produced approximately 45.95% and 70.27% writhing inhibition at 250 and 500 mg/kg, respectively | [16] |
| Anti-diarrhea | ||||||
| L. speciosa | Root | Methanolic extract | Castor oil-induced method in Swiss albino mice | Oral administration of extract at 200 and 400 mg/kg | Dose-dependently reduced diarrhea, comparable to a standard drug loperamide | [19] |
| L. speciosa | Fruit | Ethanolic extracts | Swiss albino mice | Oral administration of extract at 50 and 500 mg/kg | Delayed the onset of diarrheal episode and decreased the frequency of defecation | [16] |
| Anti-Alzheimer | ||||||
| L. indica | Leave | Ethanol (80%) extract | Aluminum chloride-induced Alzheimer’s disease rats | Oral administration of extract at 500 mg/kg | Exhibited neuro-modulating effect in Al-induced neurotoxicity | [153] |
| Antipyretic | ||||||
| L. parviflora | Leaf | Methanolic extract | Albino rats | Oral administration of extract at 100, 200, and 300 mg/kg after the yeast injection | Antipyretic effect comparable to a standard drug paracetamol | [64] |
| L. indica | Leaf | Distilled water extract | Female albino rats of Sprauge Dawely Strain | Oral administration of extract A or B at 100 mg/kg | Significantly decreased the temperature of the hyperthermal rats | [72] |
| Antitussive | ||||||
| L. parviflora | Leaf | Methanolic extract | A cough model induced by sulfur dioxide gas in mice | Oral administration of extract at 100, 200, and 300 mg/kg | Exhibited a significant dose-dependent antitussive activity, comparable to a standard drug, codeine phosphate | [65] |
| Diuretic activity | ||||||
| L. speciosa | Leaf | Ethyl acetate, ethanol, methanol, and water extract | Male Wistar rats | Oral administration of ethyl acetate, ethanol, or water extract at 250 mg/kg | Water extract: exhibited superior diuretic properties and increased urine excretion of Na+ and K+ | [75] |
| Anti-fibrotic effect | ||||||
| L. speciosa | Leaf | Ethanol extract | Male albino Wistar rats | Oral administration of extract at 100 mg/kg/d for 28 days | Reduced the hydroxyproline content of the liver, various serum enzymes level, and total bilirubin; improved the architecture of liver deranged by CCl4 | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Z.; Xu, Y.; Cai, M.; Fan, X.; Pan, H.; Zhang, D.; Zhang, Q. Floral Elegance Meets Medicinal Marvels: Traditional Uses, Phytochemistry, and Pharmacology of the Genus Lagerstroemia L. Plants 2024, 13, 3016. https://doi.org/10.3390/plants13213016
Yue Z, Xu Y, Cai M, Fan X, Pan H, Zhang D, Zhang Q. Floral Elegance Meets Medicinal Marvels: Traditional Uses, Phytochemistry, and Pharmacology of the Genus Lagerstroemia L. Plants. 2024; 13(21):3016. https://doi.org/10.3390/plants13213016
Chicago/Turabian StyleYue, Ziwei, Yan Xu, Ming Cai, Xiaohui Fan, Huitang Pan, Donglin Zhang, and Qixiang Zhang. 2024. "Floral Elegance Meets Medicinal Marvels: Traditional Uses, Phytochemistry, and Pharmacology of the Genus Lagerstroemia L." Plants 13, no. 21: 3016. https://doi.org/10.3390/plants13213016
APA StyleYue, Z., Xu, Y., Cai, M., Fan, X., Pan, H., Zhang, D., & Zhang, Q. (2024). Floral Elegance Meets Medicinal Marvels: Traditional Uses, Phytochemistry, and Pharmacology of the Genus Lagerstroemia L. Plants, 13(21), 3016. https://doi.org/10.3390/plants13213016







