Effects of Postharvest SO2 Treatment on Longan Aril Flavor and Glucosinolate Metabolites
Abstract
1. Introduction
2. Results
2.1. Effect of SO2 Treatment on Longan Fruit Storage Effect and SO2 Content in Aril
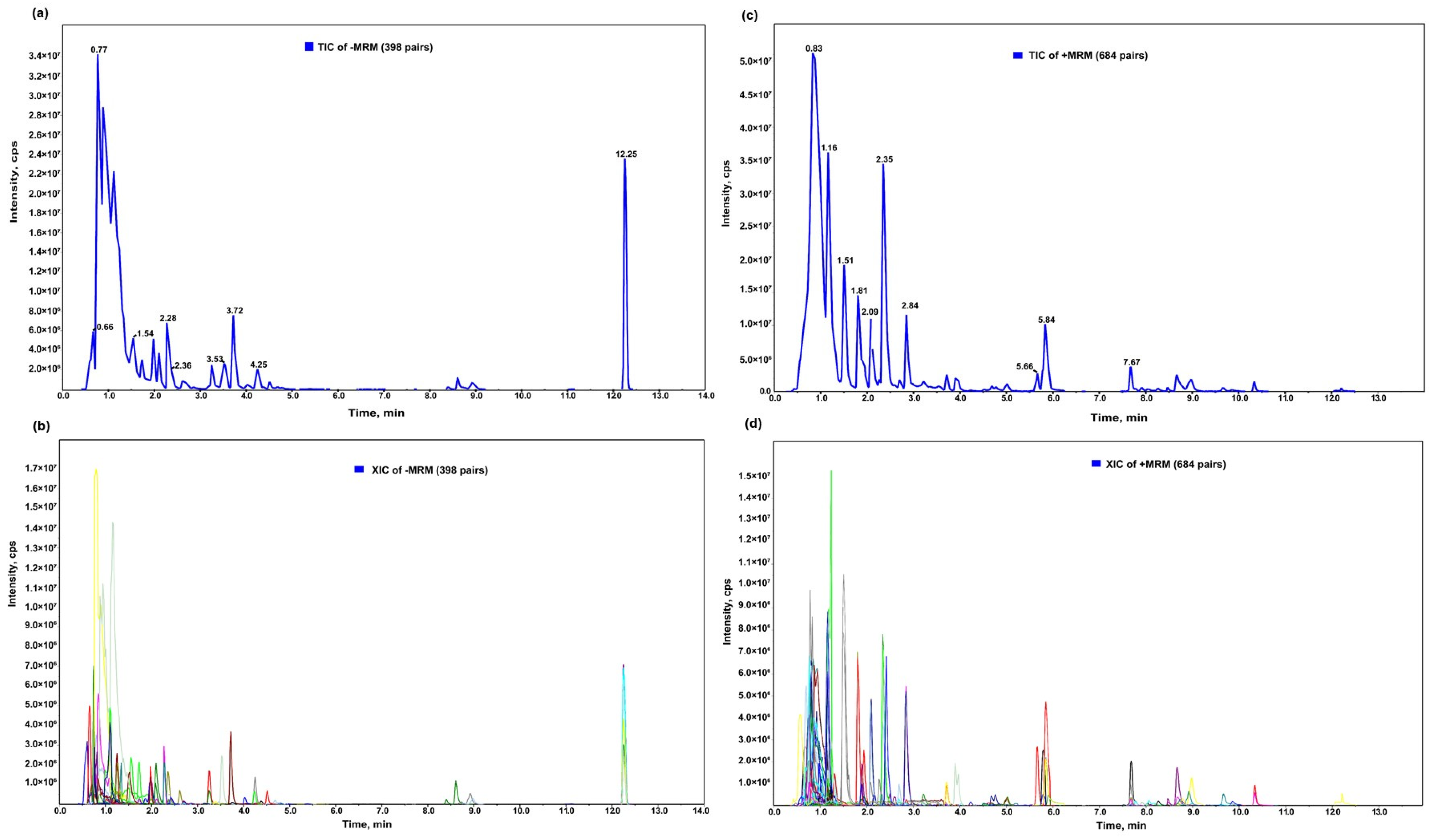
2.2. Evaluation and Analysis of the Detection Results of Sulfur-Containing Metabolites Related to Glucosinolate in the Fruits of Each Treatment Group
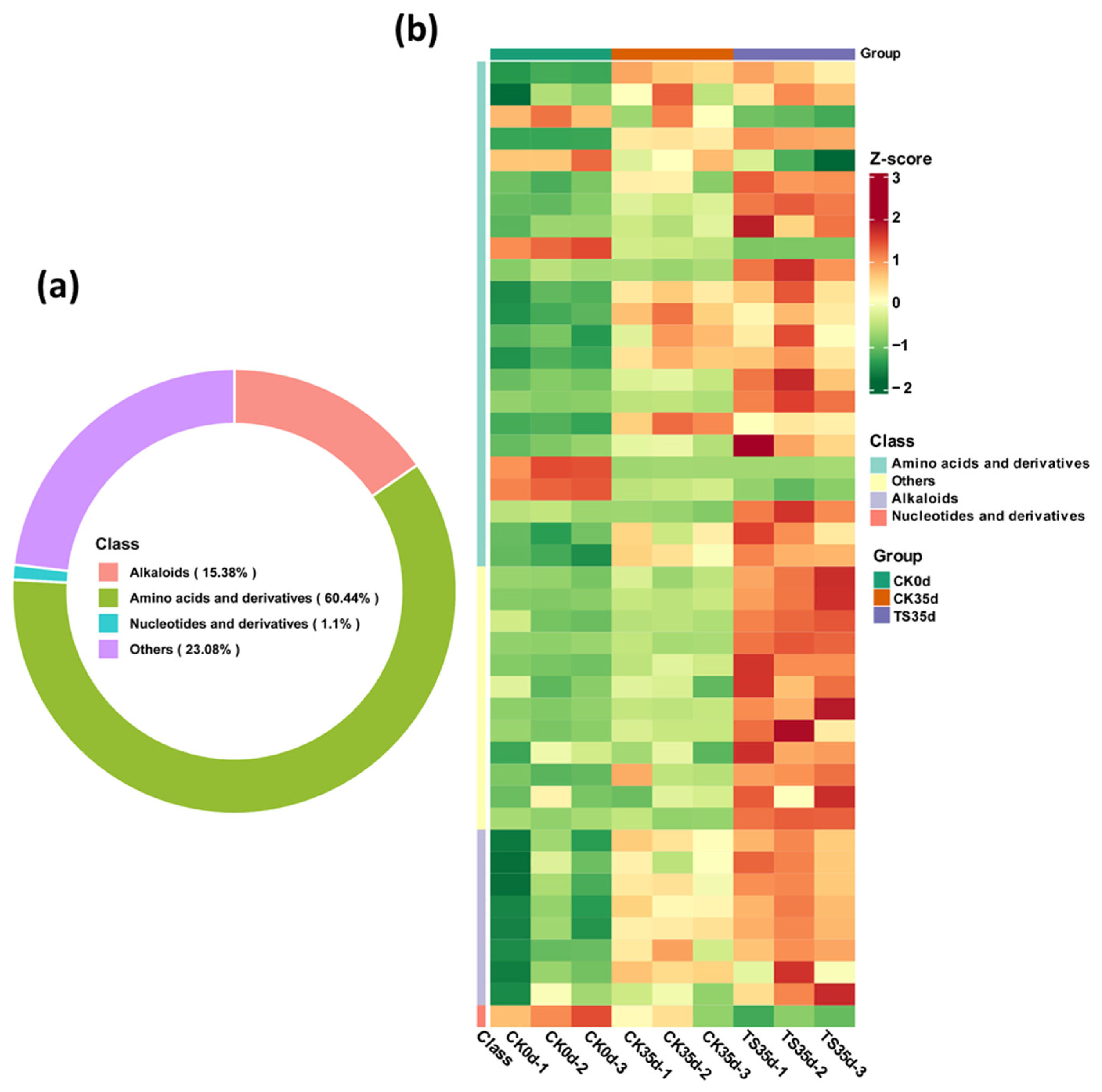
2.2.1. Composition and Clustering Results of Sulfur-Containing Metabolites in Each Treatment Group
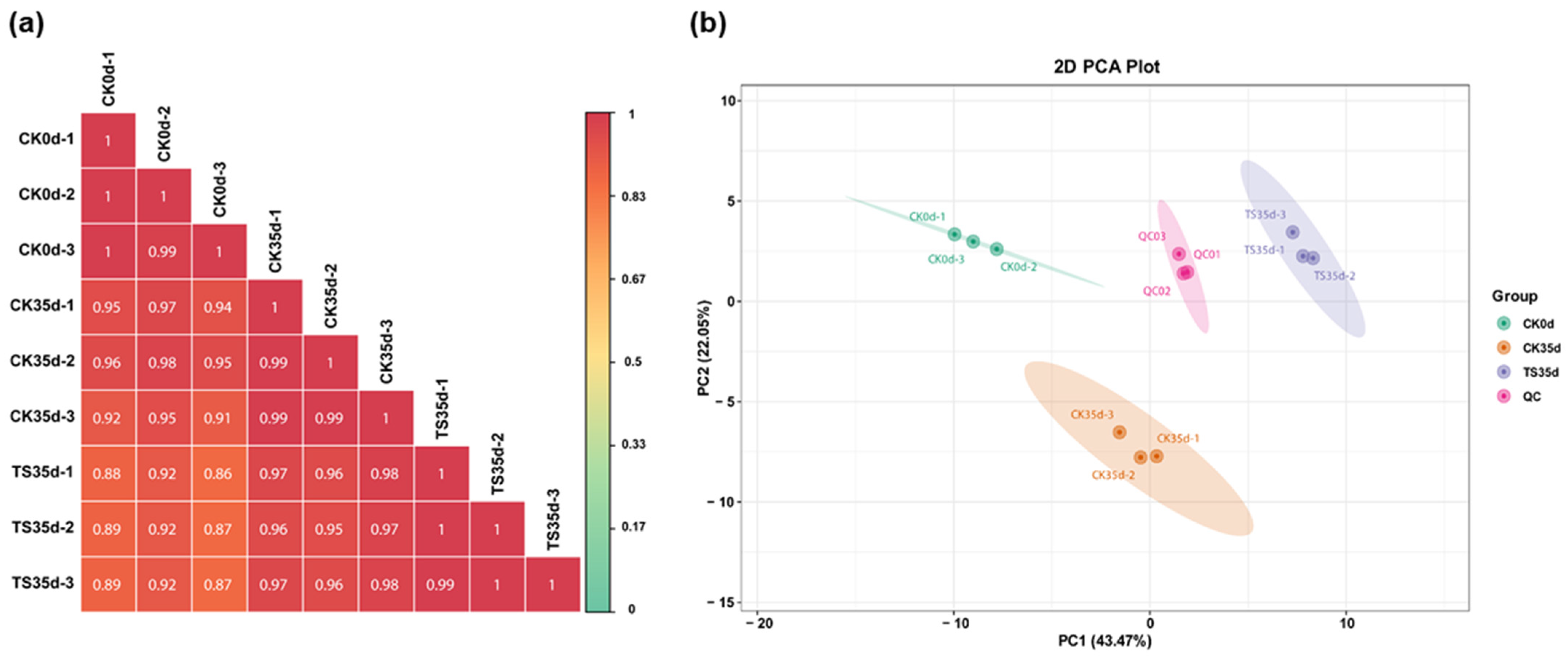
2.2.2. Correlation and Variability Analysis Between Treatment Groups and Replicate Samples
2.3. Screening of Differential Metabolite Between Treatment Groups
2.3.1. Screening of Differential Metabolites of the CK0d_vs_CK35d Treatment Combination
2.3.2. Screening of Differential Metabolites of the CK0d_vs_TS35d Treatment Combination
2.3.3. Screening of Differential Metabolites of the CK35d_vs_TS35d Treatment Combination
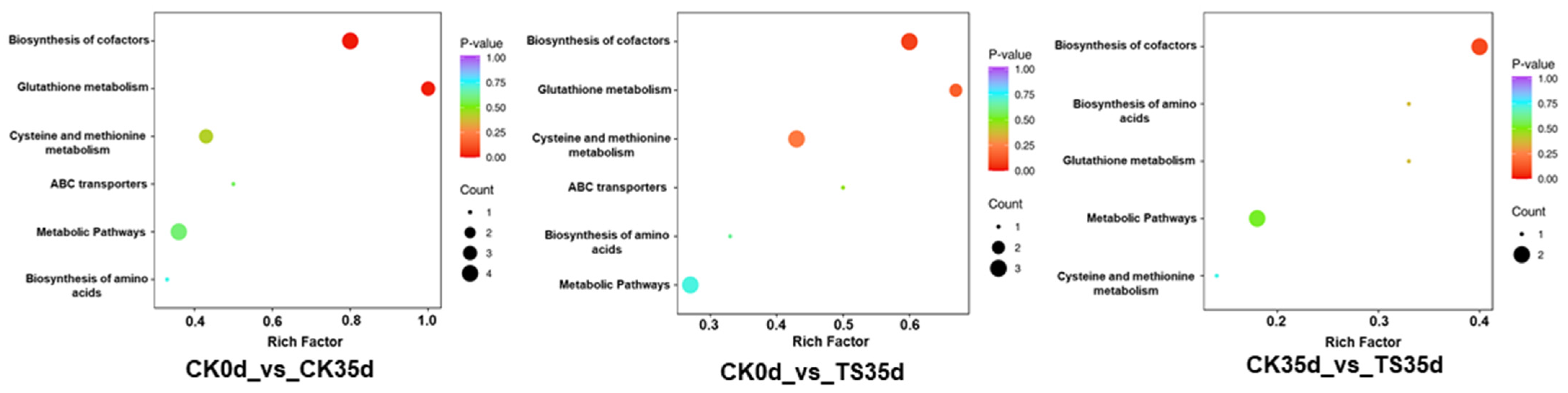
2.3.4. Comparison of the Enrichment Pathways of Differential Metabolites in Each Treatment Combination
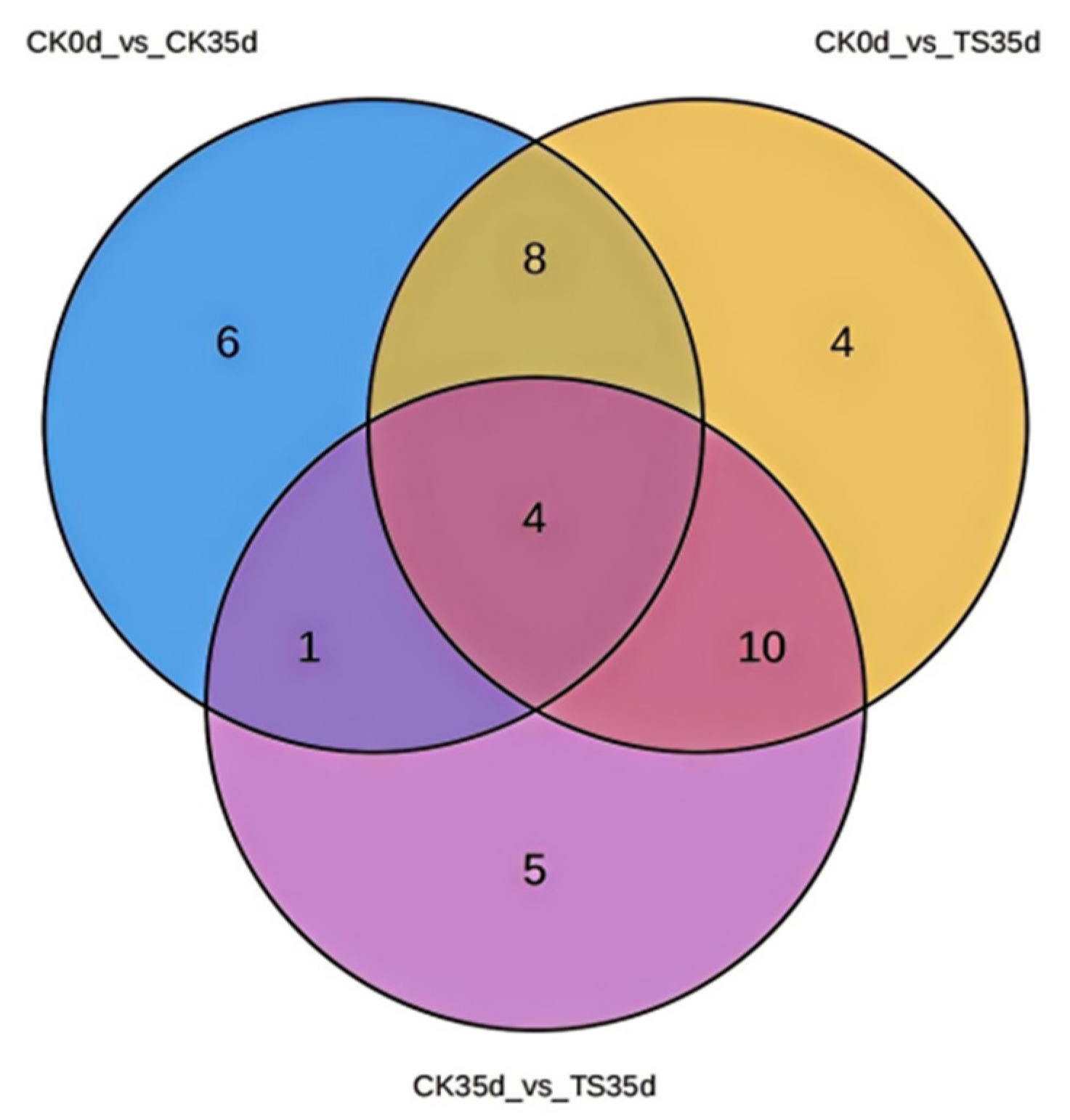
2.3.5. Screening of Differential Metabolite Among Different Combinations
2.3.6. Screening of Differential Metabolites Among Different Combinations from SO2-Treated Fruits
3. Discussion
3.1. Effects of Postharvest SO2 Treatment on the Flavor of Longan Aril and Glucosinolate-Related Substances After Storage
3.2. Effect of Glucosinolate-Related Substances on the Flavor of Longan Aril
4. Materials and Methods
4.1. Materials
4.2. Processing
4.3. Reagents and Equipment
4.3.1. Reagents
4.3.2. Equipment
4.4. Methods
4.4.1. Edible Fruit Rate and Commodity Rate
4.4.2. SO2 Content (in Terms of Sulfite)
- X—Sulfur dioxide content in the sample (in terms of SO2), in milligrams per kilogram (mg/kg);
- m1—The mass of SO2 in the test solution for determination as determined from the standard curve, measured in micrograms (μg);
- m0—The mass of SO2 in the blank solution for determination, as determined from the standard curve, measured in micrograms (μg);
- V1—The constant volume of the sample extraction solution, in milliliters (mL);
- m2—The mass of the sample, in grams (g);
- V2—The volume of the sample extraction solution for measurement in milliliters (mL).
4.4.3. Detection of Glucosinolate and Other Sulfur-Containing Metabolites
4.4.4. Sample Extraction
4.4.5. Chromatography and Mass Spectrometry Acquisition Conditions
4.4.6. The Mass Spectrometry Conditions
4.5. Data Processing
4.5.1. Qualitative and Quantitative Analysis of Metabolites
4.5.2. Differential Metabolite Screening
4.5.3. Drawing of Heatmap
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, B.; Liang, Z.; Zang, Y.; Zhu, Z.; Yang, J. Diversity of glucosinolates among common Brassicaceae vegetables in China. Hortic. Plant J. 2023, 9, 365–380. [Google Scholar] [CrossRef]
- Cheng, K.; Yang, L.; Fang, Z. Research progress on the synthesis and regulation of major glucosinolates in cruciferous plants. China Veg. 2010, 12, 1–6. [Google Scholar]
- Ren, J. Research progress on anticancer activity and cancer prevention of dietary glucosinolate derivatives. J. Chongqing Technol. Busi. Univer. 2019, 36, 1–10. [Google Scholar]
- Lee, Y.R.; Chen, M.; Lee, J.D. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 2019, 364, 159. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Huang, Y.; Jiao, Z. Research progress on glucosinolate in radish. China Melon 2021, 34, 1–7. [Google Scholar]
- Tortorella, S.M.; Royce, S.G.; Licciardi, P.V. Dietary sulforaphane in cancer chemoprevention: The role of epigenetic regulation and HDAC inhibition. Antioxid Redox Signal. 2015, 22, 1382–1424. [Google Scholar] [CrossRef]
- Eugui, D.; Velasco, P.; Abril-Urías, P.; Escobar, C.; Gómez-Torres, Ó.; Caballero, S.; Poveda, J. Glucosinolate-extracts from residues of conventional and organic cultivated broccoli leaves (Brassica oleracea var. italica) as potential industrially-scalable efficient biopesticides against fungi, oomycetes and plant parasitic nematodes. Ind. Crop. Prod. 2023, 200, 116841. [Google Scholar] [CrossRef]
- Abdelshafeek, K.A.; El-Shamy, A.M. Review on glucosinolates: Unveiling their potential applications as drug discovery leads in extraction, isolation, biosynthesis, biological activity, and corrosion protection. Food Biosci. 2023, 56, 103071. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Yang, Y.; Tao, H.; Mustafa, G.; Meng, F.; Wang, Q. Biofortification of health-promoting glucosinolates in cruciferous sprouts along the whole agro-food chain. Trends Food Sci. 2023, 140, 104164. [Google Scholar] [CrossRef]
- Beran, F.; Pauchet, Y.; Kunert, G.; Reichelt, M.; Wielsch, N.; Vogel, H.; Heckel, D.G. Phyllotreta striolata flea beetles use host plant defense compounds to create their own glucosinolate-myrosinase system. Proc. Natl. Acad. Sci. USA 2014, 111, 7349–7354. [Google Scholar] [CrossRef]
- Buxdorf, K.; Yaffe, H.; Barda, O.; Levy, M. The effects of glucosinolates and their breakdown products on necrotrophic fungi. PLoS ONE 2013, 8, 70771. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Chen, X.; Yang, J. Research progress on the effects of environmental factors on glucosinolated. Chin. J. Plant. Physil. 2020, 56, 1765–1772. [Google Scholar]
- Casajús, V.; Civello, P.; Martinez, G.; Howe, K.; Fish, T.; Yang, Y.; Lobato, M.G. Effect of continuous white light illumination on glucosinolate metabolism during postharvest storage of broccoli. LWT 2021, 145, 111302. [Google Scholar] [CrossRef]
- Andernach, L.; Witzel, K.; Hanschen, F.S. Effect of long-term storage on glucosinolate and S-methyl-l-cysteine sulfoxide hydrolysis in cabbage (Brassica oleracea var. capitata). Food Chem. 2024, 430, 136969. [Google Scholar] [CrossRef]
- Wu, S.M.; Wu, C.P.; Lin, Y.H.; Wu, Y.H.; Huang, B.C.; Wang, C.Y. Effect of high pressure pretreatment on myrosinase-glucosinolate system, physicochemical and bacterial properties during fermentation of brine-pickled radishes. Int. Food Res. 2022, 162, 112018. [Google Scholar] [CrossRef]
- Yang, E.Y.; Han, Y.S.; Sim, K.H. Characterization of nutritional, physiochemical, and mineral compositions of aril and seed of longan fruit (Dimocarpus longan L.). Int. Food Res. 2021, 28, 91–101. [Google Scholar] [CrossRef]
- Han, D.; Luo, T.; Zhang, L.; Wu, J.; Wu, H.; Wu, Z.; Pan, X. Optimized precooling combined with SO2-released paper treatment improves the storability of longan (Dimocarpus longan Lour.) fruits stored at room temperature. Food Sci Nutr. 2020, 8, 2827–2838. [Google Scholar] [CrossRef]
- Han, D.; Zhu, C.; Zhang, L. Effect of SO2 plastic wrap combined with cold storage pre-cooling on low-temperature storage effect of longan fruit. J. Preserv. Process. 2020, 20, 1–11. [Google Scholar]
- Han, D.; Zhu, C.; Zheng, Y.; Wu, J.; Zhang, L.; Luo, T.; Wu, Z.; Li, J.; Guo, D. Effect of low temperature pre-cooling on mild and mass loss of longan fruit in the treatment of SO2 plastic wrap at room temperature. Guangdong Agric. Sci. 2018, 45, 38–47. [Google Scholar]
- NY 1440-2007; Residual Limit of Sulfur Dioxide in Tropical Fruits. China Standards Press: Beijing, China, 2007.
- Kim, S.-H.; Ochar, K.; Iwar, K.; Lee, Y.-J.; Kang, H.J.; Na, Y.-W. Variations of Major Glucosinolates in Diverse Chinese Cabbage (Brassica rapa ssp. pekinensis) Germplasm as Analyzed by UPLC-ESI-MS/MS. Int. J. Mol. Sci. 2024, 25, 4829. [Google Scholar] [CrossRef]
- Lee, M.-K.; Chun, J.-H.; Byeon, D.H.; Chung, S.-O.; Park, S.U.; Park, S.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, Y.-P.; Kim, S.-J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. LWT-Food Sci. Technol. 2014, 58, 93–101. [Google Scholar] [CrossRef]
- Iwasaki, R.; Bito, T.; Ishihara, A.; Watanabe, F.; Yabuta, Y. NAD+ enhances the activity and thermostability of S-adenosyl-L-homocysteine hydrolase from Pyrococcus horikoshii OT3. Biosci. Biotechnol. Biochem. 2023, 87, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. Glutathione and glutathione disulfide–their biomedical and pharmaceutical applications. Med. Chem. Res. 2023, 32, 1972–1994. [Google Scholar] [CrossRef]
- Mitreiter, S.; Gigolashvili, T. Regulation of Glucosinolate Biosynthesis. J. Exp. Bot. 2021, 72, 70–91. [Google Scholar] [CrossRef]
- Lin, Y.; Xian, S.; Fan, J.; Sun, X.; Geng, Z.; Du, C. The role of glucosinolates and study on anabolism in Brassica napus L. Biotechnology 2018, 28, 397–402. [Google Scholar]
- Zheng, C.; Yang, Y.; Wei, F.; Lv, X.; Xia, Z.; Qi, M.; Zhou, Q. Widely targeted metabolomics reveal the glucosinolate profile and odor-active compounds in flowering Chinese cabbage powder. Int. Food Res. 2023, 172, 113121. [Google Scholar] [CrossRef]
- Sharma, S.; Rani, H.; Kaur, G.; Kumar, S.; Sheikh, S.; Samota, M.K. Comprehensive overview of glucosinolates in crucifers: Occurrence, roles, metabolism, and transport mechanisms—A review. Phytochem. Rev. 2024, 1–28. [Google Scholar] [CrossRef]
- Yang, Q. Green Synthesis and Activity of Novel Indospiron Alkaloid Analogues; College of Food and Biological Engineering, Chengdu University: Chengdu, China, 2024. [Google Scholar]
- Shiga, H.; Yoshii, H.; Ohe, H.; Yasuda, M.; Furuta, T.; Kuwahara, H.; Linko, P. Encapsulation of shiitake (Lenthinus edodes) flavors by spray drying. Biosci. Biotechnol. Biochem. 2004, 68, 66–71. [Google Scholar] [CrossRef]
- Wu, Z.; Han, D. An Application of a Longan SO2-Released Paper. ZL Patent No. 201610227848.7, 16 March 2018. [Google Scholar]
- Huang, L.; Lai, L.; Zhang, X.; Lin, S.; Jin, G.; Li, D. Practical assay for determining residual sulfite of the wine in rapid detection or quantitative analysis. LWT 2023, 189, 115503. [Google Scholar] [CrossRef]
- GB 5009.34-2022; National Food Safety Standard—Determination of Sulfur Dioxide in Food. National Health Commission of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography− mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.J.; Deese, A.J.; Robertson, D.G. The application of metabonomics as an early in vivo toxicity screen. In Metabonomics in Toxicity Assessment; CRC Press: Boca Raton, FL, USA, 2005; pp. 211–240. [Google Scholar]
- Chen, Y.; Zhang, R.; Song, Y.; He, J.; Sun, J.; Bai, J.; Abliz, Z. RRLC-MS/MS-based metabonomics combined with in-depth analysis of metabolic correlation network: Finding potential biomarkers for breast cancer. Analyst 2009, 134, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]

| Sample | Edible Fruit Rate (%) | Commercial Fruit Rate (%) | Browning Fruit Rate (%) | SO2 Content in Aril mg/kg |
|---|---|---|---|---|
| CK35d | 75.43 ± 1.41 A | 0 A | 100 A | 4.93 ± 0.25 A |
| ST35d | 99.44 ± 0.36 B | 99.44 ± 0.36 B | 0.56 ± 0.36 B | 8.67 ± 0.34 B |
| Compounds Name | Class | Type of Difference |
|---|---|---|
| Cys-Asn-Ser-Ala-Arg | Amino acids and their derivatives | up |
| S-Allyl-L-cysteine | Amino acids and their derivatives | up |
| S-(5′-Adenosy)-L-homocysteine | Amino acids and their derivatives | up |
| Oxiglutatione | Amino acids and their derivatives | down |
| Phe-Met-Tyr | Amino acids and their derivatives | up |
| γ-Glutamylmethionine | Amino acids and their derivatives | up |
| Thr-Glu-Met | Amino acids and their derivatives | up |
| γ-Glu-Cys | Amino acids and their derivatives | up |
| Heptyl glucosinolate | Glucosinolates | up |
| 6-Heptyl Glucosinolate | Glucosinolates | up |
| Indole-5-carboxylic acid * | Indole alkaloids | up |
| Indole-3-carboxylic acid * | Indole alkaloids | up |
| Met-Thr-Ile | Amino acids and their derivatives | up |
| Fall-with-Typr | Amino acids and their derivatives | up |
| Thr-With-Leu | Amino acids and their derivatives | up |
| Glutathione reduced form | Amino acids and their derivatives | down |
| Tryptophan N-rutinoside | Indole alkaloids | up |
| Lys-Ser-Asp-Glu-Met | Amino acids and their derivatives | up |
| L-Homocystine | Amino acids and their derivatives | up |
| Compounds Name | Class | Type of Difference |
|---|---|---|
| Cys-Asn-Ser-Ala-Arg | Amino acids and their derivatives | up |
| S-Allyl-L-cysteine | Amino acids and their derivatives | up |
| 1-Hydroxymethyl glucosinolate * | Glucosinolates | up |
| 4-(Methylsulfonyl)butyl glucosinolate * | Glucosinolates | up |
| S-(5′-Adenosy)-L-homocysteine | Amino acids and their derivatives | up |
| γ-L-Glutamyl-S-methyl-L-cysteine | Amino acids and their derivatives | up |
| Oxiglutatione | Amino acids and their derivatives | down |
| His-Asn-Cys | Amino acids and their derivatives | up |
| Phe-Met-Tyr | Amino acids and their derivatives | up |
| γ-Glutamylmethionine | Amino acids and their derivatives | up |
| 2(R)-Hydroxy-3-butenyl glucosinolate | Glucosinolates | up |
| Met-Val-His-Leu-Thr | Amino acids and their derivatives | up |
| N,N-Dimethyl-5-methoxytryptamine | Indole alkaloids | up |
| 3-Hydroxypropyl glucosinolate | Glucosinolates | up |
| 4-Methylsulfonyl-3-butenyl glucosinolate * | Glucosinolates | up |
| 4-Hydroxybutylthioside * | Glucosinolates | up |
| Met-Thr-Ile | Amino acids and their derivatives | up |
| 2-Propenyl glucosinolate (Sinigrin) | Glucosinolates | up |
| Fall-with-Typr | Amino acids and their derivatives | up |
| Thr-with-Leu | Amino acids and their derivatives | up |
| Glutathione reduced form | Amino acids and their derivatives | down |
| 2-Hydroxybutyl glucosinolate * | Glucosinolates | up |
| 4-Methylsulfinylbutyl glucosinolate (Glucoraphanin) | Glucosinolates | up |
| Lys-Ser-Asp-Glu-Met | Amino acids and their derivatives | up |
| 2-Hydroxy-2-methylpropyl glucosinolate * | Glucosinolates | up |
| L-Homocystine | Amino acids and their derivatives | up |
| Compounds Name | Class | Type of Difference |
|---|---|---|
| 1-Hydroxymethyl glucosinolate * | Glucosinolates | up |
| 4-(Methylsulfonyl)butyl glucosinolate * | Glucosinolates | up |
| 4-Methylsulfinyl-3-Butenyl glucosinolate | Glucosinolates | up |
| Ala-Ser-Leu-Cys-Cys | Amino acids and their derivatives | down |
| S-(5′-Adenosy)-L-homocysteine | Amino acids and their derivatives | up |
| γ-L-Glutamyl-S-methyl-L-cysteine | Amino acids and their derivatives | up |
| Oxiglutatione | Amino acids and their derivatives | down |
| N-Acetyl-L-cysteine | Amino acids and their derivatives | up |
| His-Asn-Cys | Amino acids and their derivatives | up |
| Thr-Glu-Met | Amino acids and their derivatives | down |
| 2(R)-Hydroxy-3-butenyl glucosinolate | Glucosinolates | up |
| S-(Methyl)glutathione | Amino acids and their derivatives | up |
| 3-Hydroxypropyl glucosinolate | Glucosinolates | up |
| 4-Hydroxybutylthioside * | Glucosinolates | up |
| Met-Thr-Ile | Amino acids and their derivatives | up |
| 2-Propenyl glucosinolate (Sinigrin) | Glucosinolates | up |
| Thr-With-Leu | Amino acids and their derivatives | up |
| 2-Hydroxybutyl glucosinolate * | Glucosinolates | up |
| Leu-Leu-Met | Amino acids and their derivatives | up |
| 2-Hydroxy-2-methylpropyl glucosinolate * | Glucosinolates | up |
| Molecular Formula | Substance Name | Secondary Classification | CK0d _vs_ CK35d | FC Value | CK0d _vs_ ST35d | FC Value | CK35d _vs_ ST35d | FC Value | |
|---|---|---|---|---|---|---|---|---|---|
| Effective differential metabolites | C11H21NO10S2 | 1-Hydroxymethyl glucosinolate * | Glucosinolates | FALSE | 1.52 | TRUE | 4.22 | TRUE | 2.78 |
| C12H23NO11S3 | 4-(Methylsulfonyl)butyl glucosinolate * | Glucosinolates | FALSE | 1.40 | TRUE | 3.06 | TRUE | 2.18 | |
| C9H16N2O5S | γ-L-Glutamyl-S-methyl-L-cysteine | Amino acids and their derivatives | FALSE | 1.46 | TRUE | 2.97 | TRUE | 2.03 | |
| C13H20N6O5S | His-Asn-Cys | Amino acids and their derivatives | FALSE | 1.02 | TRUE | 2.24 | TRUE | 2.20 | |
| C11H19NO10S2 | 2(R)-Hydroxy-3-butenyl glucosinolate | Glucosinolates | FALSE | 1.16 | TRUE | 2.43 | TRUE | 2.08 | |
| C10H19NO10S2 | 3-hydroxypropyl glucosinolate | Glucosinolates | FALSE | 1.25 | TRUE | 3.52 | TRUE | 2.82 | |
| C11H21NO10S2 | 4-Hydroxybutylthioside * | Glucosinolates | FALSE | 1.13 | TRUE | 2.29 | TRUE | 2.02 | |
| C10H17NO9S2 | 2-Propenyl glucosinolate (Sinigrin) | Glucosinolates | FALSE | 1.99 | TRUE | 6.37 | TRUE | 3.21 | |
| C11H21NO10S2 | 2-hydroxybutyl glucosinolate * | Glucosinolates | FALSE | 1.96 | TRUE | 5.18 | TRUE | 2.65 | |
| C11H21NO10S2 | 2-Hydroxy-2-methylpropyl glucosinolate * | Glucosinolates | FALSE | 1.05 | TRUE | 4.23 | TRUE | 4.04 | |
| Pre-active differential metabolites | C26H45N7O7S | Met-Val-His-Leu-Thr | Amino acids and their derivatives | FALSE | 1.40 | TRUE | 2.29 | FALSE | 1.63 |
| C13H18N2O | N,N-Dimethyl-5-methoxytryptamine | Indole alkaloids | FALSE | 1.74 | TRUE | 2.02 | FALSE | 1.16 | |
| C12H20NO11S3 | 4-Methylsulfonyl-3-butenyl glucosinolate * | Glucosinolates | FALSE | 1.52 | TRUE | 2.90 | FALSE | 1.91 | |
| C12H23NO10S3 | 4-Methylsulfinylbutyl glucosinolate (Glucoraphanin) | Glucosinolates | FALSE | 1.94 | TRUE | 3.01 | FALSE | 1.55 | |
| Post active differential metabolites | C12H21NO10S3 | 4-Methylsulfinyl-3-Butenyl glucosinolate | Glucosinolates | FALSE | 0.58 | FALSE | 1.30 | TRUE | 2.23 |
| C18H33N5O7S2 | Ala-Ser-Leu-Cys-Cys | Amino acids and their derivatives | FALSE | 1.82 | FALSE | 0.78 | TRUE | 0.43 | |
| C5H9NO3S | N-Acetyl-L-Cysteine | Amino acids and their derivatives | FALSE | 0.59 | FALSE | 1.50 | TRUE | 2.53 | |
| C11H19N3O6S | S-(Methyl)glutathione | Amino acids and their derivatives | FALSE | 0.62 | FALSE | 2.12 ** | TRUE | 3.43 | |
| C17H33N3O4S | Leu-Leu-Met | Amino acids and their derivatives | FALSE | 0.91 | FALSE | 1.82 | TRUE | 2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahfuzur, R.M.; Han, D.; Xu, J.; Lin, Y.; Guo, X.; Luo, T.; Wu, Z.; Huang, S.; Lv, X.; Wei, J. Effects of Postharvest SO2 Treatment on Longan Aril Flavor and Glucosinolate Metabolites. Plants 2024, 13, 3061. https://doi.org/10.3390/plants13213061
Mahfuzur RM, Han D, Xu J, Lin Y, Guo X, Luo T, Wu Z, Huang S, Lv X, Wei J. Effects of Postharvest SO2 Treatment on Longan Aril Flavor and Glucosinolate Metabolites. Plants. 2024; 13(21):3061. https://doi.org/10.3390/plants13213061
Chicago/Turabian StyleMahfuzur, Rob Md, Dongmei Han, Jianhang Xu, Yuqiong Lin, Xiaomeng Guo, Tao Luo, Zhenxian Wu, Shilian Huang, Xinmin Lv, and Junbin Wei. 2024. "Effects of Postharvest SO2 Treatment on Longan Aril Flavor and Glucosinolate Metabolites" Plants 13, no. 21: 3061. https://doi.org/10.3390/plants13213061
APA StyleMahfuzur, R. M., Han, D., Xu, J., Lin, Y., Guo, X., Luo, T., Wu, Z., Huang, S., Lv, X., & Wei, J. (2024). Effects of Postharvest SO2 Treatment on Longan Aril Flavor and Glucosinolate Metabolites. Plants, 13(21), 3061. https://doi.org/10.3390/plants13213061








