Overexpression of Cassava MeSTP7 Promotes Arabidopsis Seedling Development
Abstract
1. Introduction
2. Results
2.1. Expression Patterns of MeSTP7 in Cassava Tissues
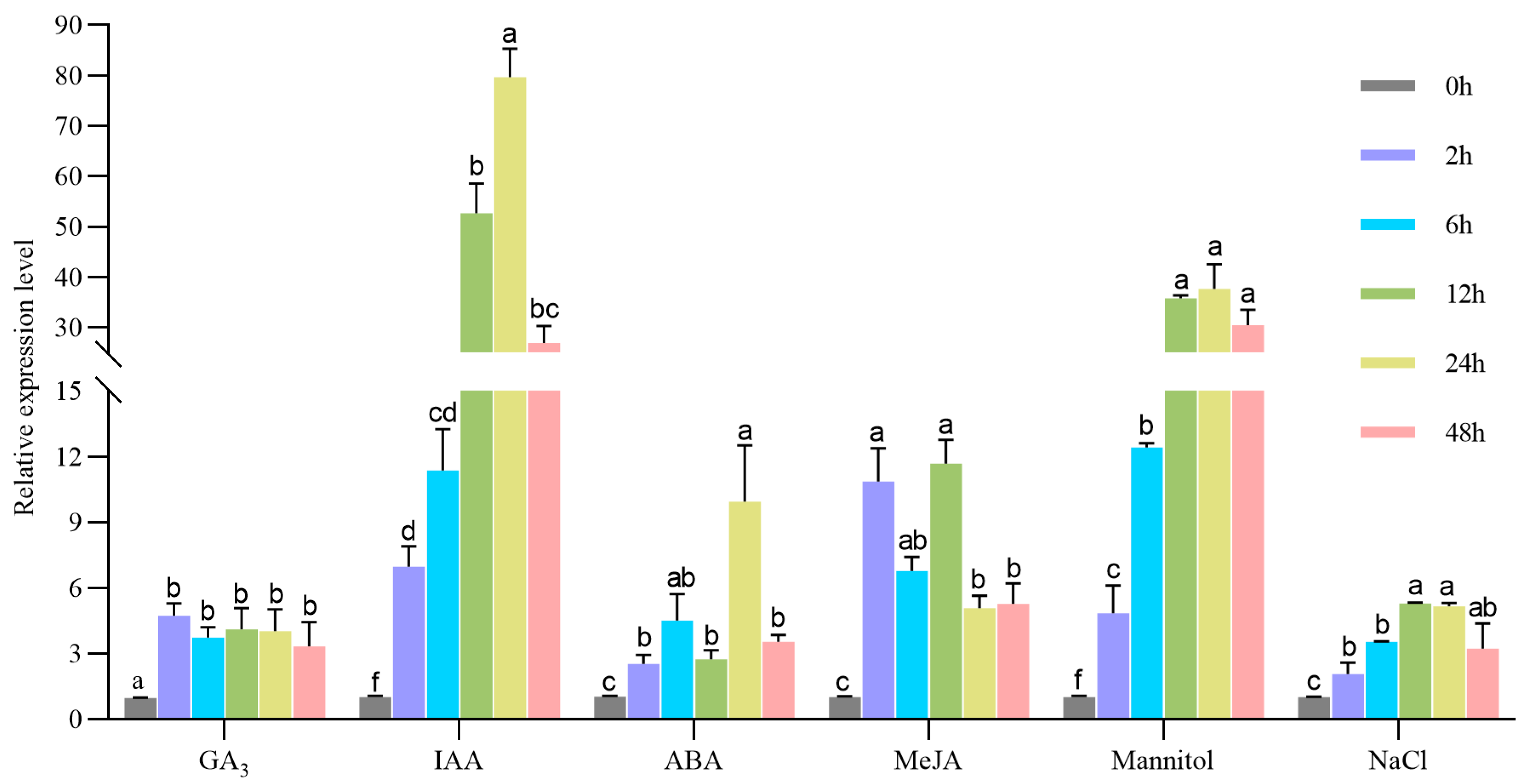
2.2. The Expression Pattern of MeSTP7Gene Under Hormone and Abiotic Stressor Treatments
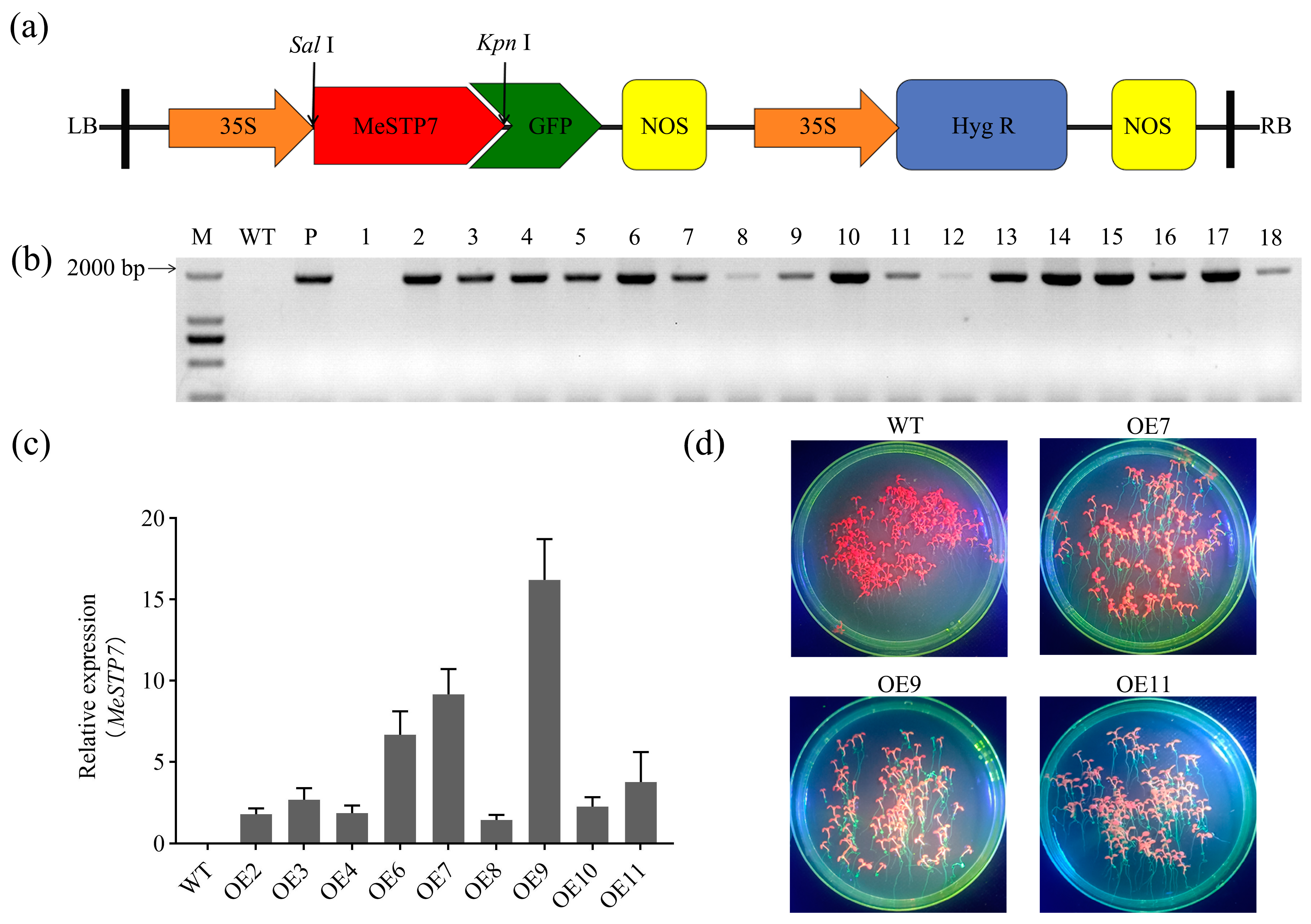
2.3. Transformation of MeSTP7 in Arabidopsis
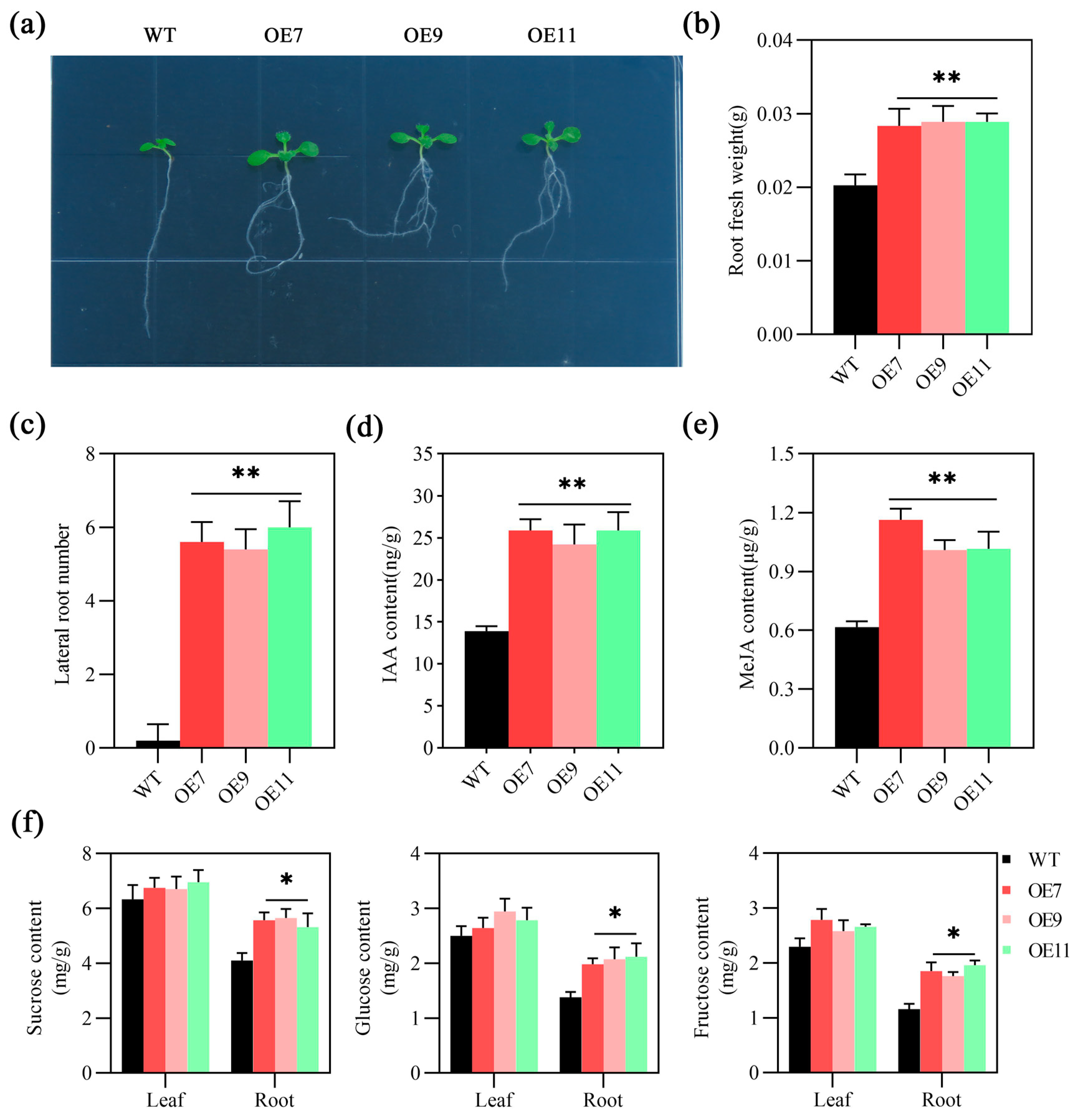
2.4. MeSTP7 Promotes the Early Growth of Arabidopsis
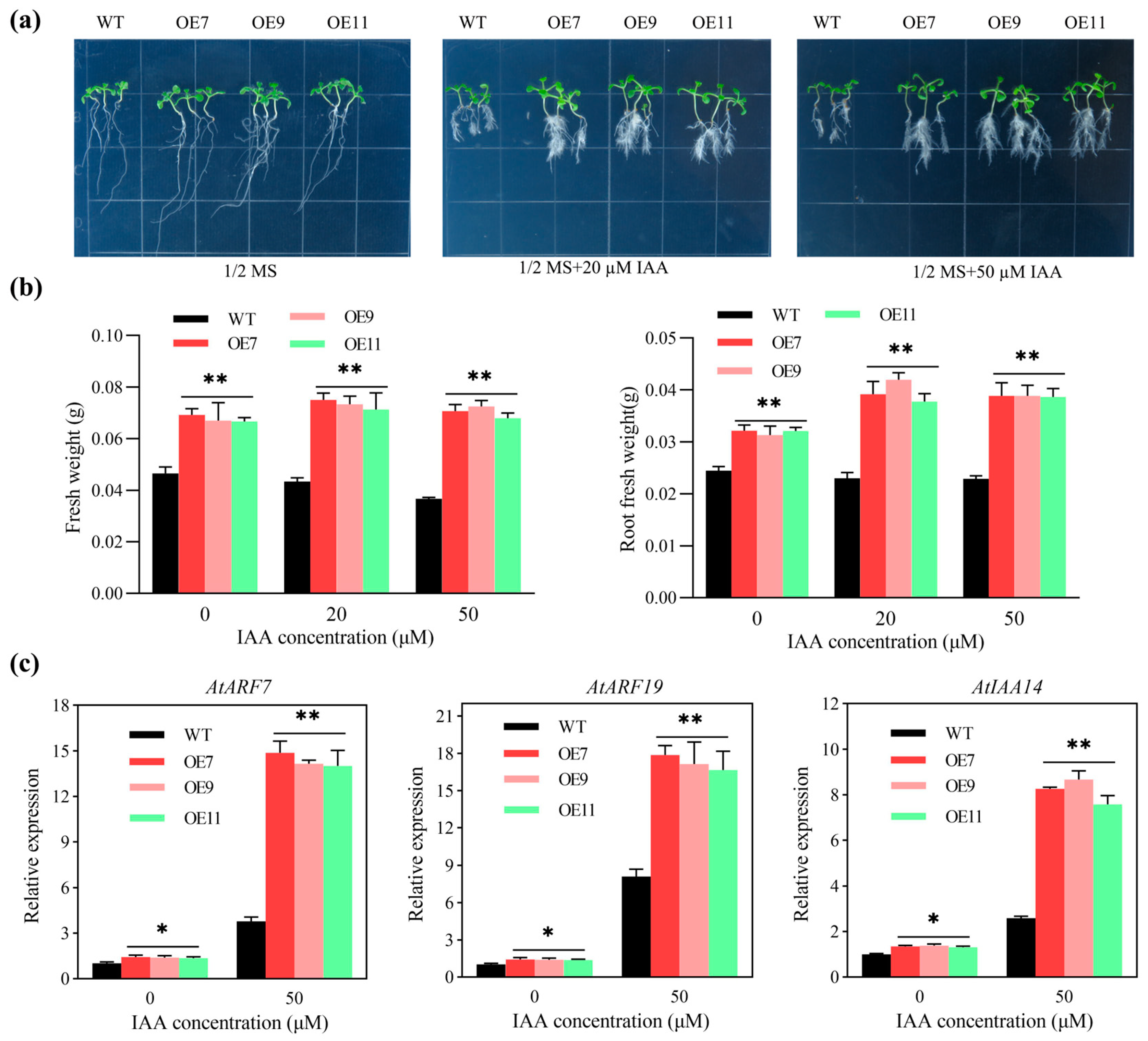
2.5. The Number of Lateral Roots of Transgenic Arabidopsis Increased Under IAA Treatment
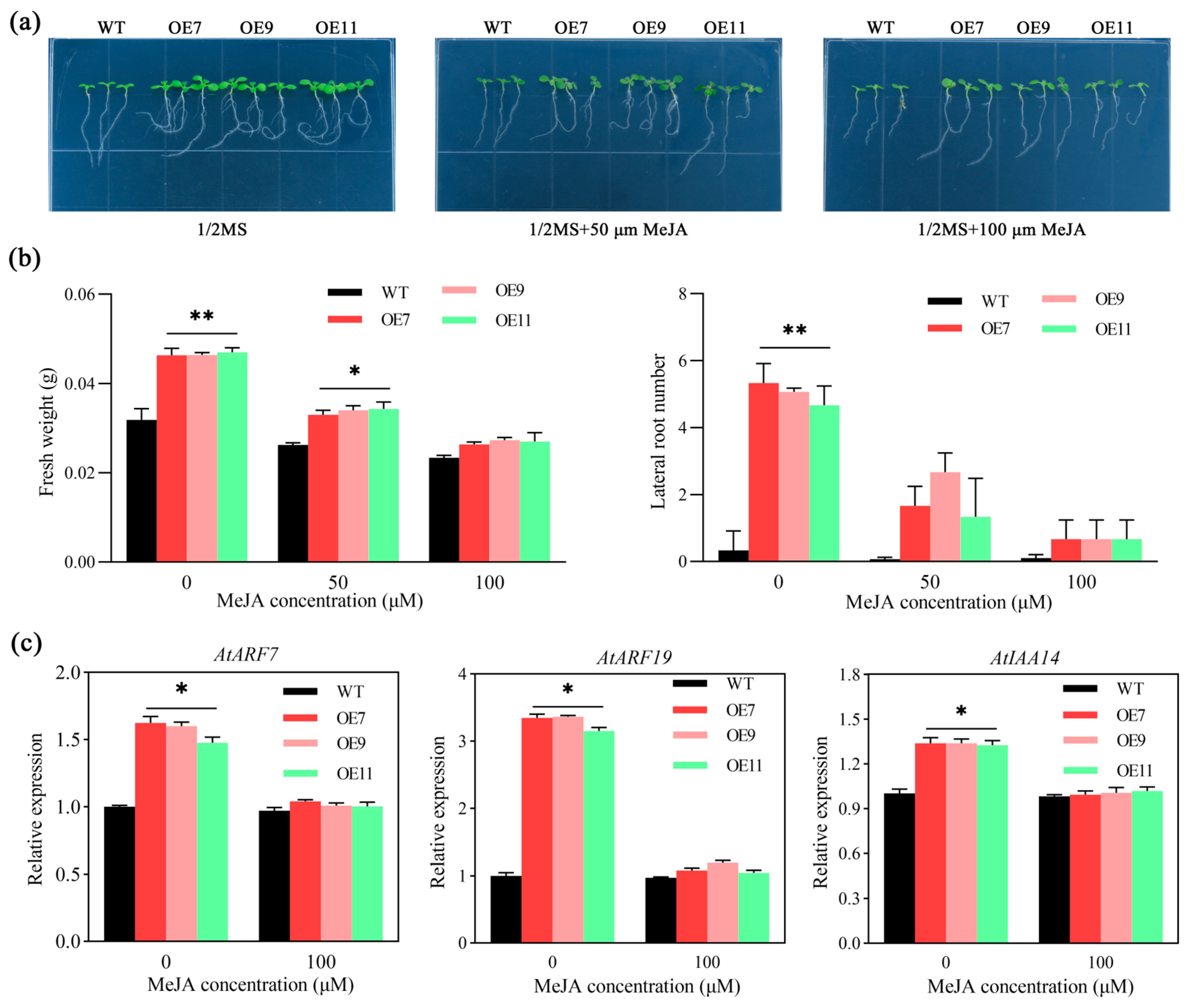
2.6. The Number of Lateral Roots of Transgenic Arabidopsis Decreased under MeJA Treatment
3. Discussion
4. Materials and Methods
4.1. Analysis of the Tissue-Specific Expression Pattern of MeSTP7 in Cassava
4.2. Hormonal and Abiotic Stressors Treatments for Cassava
4.3. Construction of MeSTP7 Plant Overexpression Vector
4.4. Genetic Transformation and Identification of MeSTP7-Overexpression Arabidopsis Transgenic Lines
4.5. Phenotypic Observation of Transgenic Arabidopsis at Different Growth Stages
4.6. Hormonal Treatment of Transgenic Arabidopsis
4.7. Determination of Endogenous Hormone Content in Transgenic Arabidopsis
4.8. RNA Extraction and qRT-PCR Analysis of Related Genes
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nassar, N.; Ortiz, R. Breeding cassava to feed the poor. Sci. Am. 2010, 302, 78–82, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cui, Y.; Zhou, Y.; Luo, Z.; Liu, J.; Zhao, M. The industrial applications of cassava: Current status, opportunities and prospects. J. Sci. Food Agric. 2017, 97, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Cadena, P.; Hoogenboom, G.; Cock, J.H.; Ramirez-Villegas, J.; Pypers, P.; Kreye, C.; Tariku, M.; Ezui, K.S.; Becerra Lopez-Lavalle, L.A.; Asseng, S. Modeling growth, development and yield of cassava: A review. Field Crops Res. 2021, 267, 108140. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef] [PubMed]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef]
- Deng, X.; An, B.; Zhong, H.; Yang, J.; Kong, W.; Li, Y. A Novel Insight into Functional Divergence of the MST Gene Family in Rice Based on Comprehensive Expression Patterns. Genes 2019, 10, 239. [Google Scholar] [CrossRef]
- Slewinski, T.L. Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: A physiological perspective. Mol. Plant 2011, 4, 641–662. [Google Scholar] [CrossRef]
- Büttner, M.; Sauer, N. Monosaccharide transporters in plants: Structure, function and physiology. Biochim. Biophys. Acta 2000, 1465, 263–274. [Google Scholar] [CrossRef]
- Aoki, N.; Scofield, G.N.; Wang, X.D.; Offler, C.E.; Patrick, J.W.; Furbank, R.T. Pathway of sugar transport in germinating wheat seeds. Plant Physiol. 2006, 141, 1255–1263. [Google Scholar] [CrossRef]
- Gibson, S.I. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef]
- Schofield, R.A.; Bi, Y.M.; Kant, S.; Rothstein, S.J. Over-expression of STP13, a hexose transporter, improves plant growth and nitrogen use in Arabidopsis thaliana seedlings. Plant Cell Environ. 2009, 32, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Flütsch, S.; Nigro, A.; Conci, F.; Fajkus, J.; Thalmann, M.; Trtílek, M.; Panzarová, K.; Santelia, D. Glucose uptake to guard cells via STP transporters provides carbon sources for stomatal opening and plant growth. EMBO Rep. 2020, 21, e49719. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulos, V.; Gilbert, M.J.; Pittman, J.K.; Marvier, A.C.; Buchanan, A.J.; Sauer, N.; Hall, J.L.; Williams, L.E. The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atbetafruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol. 2003, 132, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.N.; Dennis, E.S.; Dolferus, R. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiol. 2007, 48, 1319–1330. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Y.; Jin, Y.; Tong, X.; Wei, X.; Ma, F.; Ma, B.; Li, M. Ectopic expression of apple hexose transporter MdHT2.2 reduced the salt tolerance of tomato seedlings with decreased ROS-scavenging ability. Plant Physiol. Biochem. PPB 2020, 156, 504–513. [Google Scholar] [CrossRef]
- Büttner, M. The Arabidopsis sugar transporter (AtSTP) family: An update. Plant Biol. 2010, 12 (Suppl. S1), 35–41. [Google Scholar] [CrossRef]
- Rottmann, T.; Klebl, F.; Schneider, S.; Kischka, D.; Rüscher, D.; Sauer, N.; Stadler, R. Sugar Transporter STP7 Specificity for l-Arabinose and d-Xylose Contrasts with the Typical Hexose Transporters STP8 and STP12. Plant Physiol. 2018, 176, 2330–2350. [Google Scholar] [CrossRef]
- Truernit, E.; Stadler, R.; Baier, K.; Sauer, N. A male gametophyte-specific monosaccharide transporter in Arabidopsis. Plant J. 1999, 17, 191–201. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Büttner, M.; Sauer, N. AtSTP6, a new pollen-specific H+-monosaccharide symporter from Arabidopsis. Plant Physiol. 2003, 131, 70–77. [Google Scholar] [CrossRef]
- Cordoba, E.; Aceves-Zamudio, D.L.; Hernández-Bernal, A.F.; Ramos-Vega, M.; León, P. Sugar regulation of SUGAR TRANSPORTER PROTEIN 1 (STP1) expression in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 147–159. [Google Scholar] [CrossRef]
- Otori, K.; Tanabe, N.; Tamoi, M.; Shigeoka, S. Sugar Transporter Protein 1 (STP1) contributes to regulation of the genes involved in shoot branching via carbon partitioning in Arabidopsis. Biosci. Biotechnol. Biochem. 2019, 83, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, H.; Zhu, Q.; Liu, D.; Li, Z.; Chen, H.; Luo, J.; Gong, P.; Ismail, A.M.; Zhang, Z. Knockout of the sugar transporter OsSTP15 enhances grain yield by improving tiller number due to increased sugar content in the shoot base of rice (Oryza sativa L.). New Phytol. 2024, 241, 1250–1265. [Google Scholar] [CrossRef] [PubMed]
- Mamun, E.A.; Alfred, S.; Cantrill, L.C.; Overall, R.L.; Sutton, B.G. Effects of chilling on male gametophyte development in rice. Cell Biol. Int. 2006, 30, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Zhang, Y.; Chai, C.; Wei, G.; Wei, X.; Xu, H.; Wang, M.; Ouwerkerk, P.B.; Zhu, Z. Molecular cloning, functional characterization and expression analysis of a novel monosaccharide transporter gene OsMST6 from rice (Oryza sativa L.). Planta 2008, 228, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, X.; Yang, J.; Li, H.; Ma, B.; Zhang, K.; Zhang, Y.; Cheng, L.; Ma, F.; Li, M. Heterologous expression of the apple hexose transporter MdHT2.2 altered sugar concentration with increasing cell wall invertase activity in tomato fruit. Plant Biotechnol. J. 2020, 18, 540–552. [Google Scholar] [CrossRef]
- Ma, Q.J.; Sun, M.H.; Liu, Y.J.; Lu, J.; Hu, D.G.; Hao, Y.J. Molecular cloning and functional characterization of the apple sucrose transporter gene MdSUT2. Plant Physiol. Biochem. PPB 2016, 109, 442–451. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Zhao, D.; Tang, Z.; Zhang, T.; Zhang, K.; Dong, J.; Zhang, H. Genetic regulation of lateral root development. Plant Signal. Behav. 2023, 18, 2081397. [Google Scholar] [CrossRef]
- Hu, F.; Fang, D.; Zhang, W.; Dong, K.; Ye, Z.; Cao, J. Lateral root primordium: Formation, influencing factors and regulation. Plant Physiol. Biochem. PPB 2024, 207, 108429. [Google Scholar] [CrossRef]
- Fukaki, H.; Tameda, S.; Masuda, H.; Tasaka, M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 2002, 29, 153–168. [Google Scholar] [CrossRef]
- Fukaki, H.; Taniguchi, N.; Tasaka, M. PICKLE is required for SOLITARY-ROOT/IAA14-mediated repression of ARF7 and ARF19 activity during Arabidopsis lateral root initiation. Plant J. 2006, 48, 380–389. [Google Scholar] [CrossRef]
- Huang, K.L.; Ma, G.J.; Zhang, M.L.; Xiong, H.; Wu, H.; Zhao, C.Z.; Liu, C.S.; Jia, H.X.; Chen, L.; Kjorven, J.O.; et al. The ARF7 and ARF19 Transcription Factors Positively Regulate PHOSPHATE STARVATION RESPONSE1 in Arabidopsis Roots. Plant Physiol. 2018, 178, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Ikeyama, Y.; Tasaka, M.; Fukaki, H. RLF, a cytochrome b(5)-like heme/steroid binding domain protein, controls lateral root formation independently of ARF7/19-mediated auxin signaling in Arabidopsis thaliana. Plant J. 2010, 62, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Hayashi, K.; Suzuki, T.; Fukaki, H.; Prusinska, J.; Meester, C.; Quareshy, M.; Egoshi, S.; Matsuura, H.; Takahashi, K.; et al. Jasmonic Acid Inhibits Auxin-Induced Lateral Rooting Independently of the CORONATINE INSENSITIVE1 Receptor. Plant Physiol. 2018, 177, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Raya-González, J.; Pelagio-Flores, R.; López-Bucio, J. The jasmonate receptor COI1 plays a role in jasmonate-induced lateral root formation and lateral root positioning in Arabidopsis thaliana. J. Plant Physiol. 2012, 169, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Hellmann, H.A.; Smeekens, S. Sugar sensing and signaling in plants. Front. Plant Sci. 2014, 5, 113. [Google Scholar] [CrossRef]
- Liu, Q.; Dang, H.; Chen, Z.; Wu, J.; Chen, Y.; Chen, S.; Luo, L. Genome-Wide Identification, Expression, and Functional Analysis of the Sugar Transporter Gene Family in Cassava (Manihot esculenta). Int. J. Mol. Sci. 2018, 19, 987. [Google Scholar] [CrossRef]
- Rottmann, T.; Fritz, C.; Sauer, N.; Stadler, R. Glucose Uptake via STP Transporters Inhibits in Vitro Pollen Tube Growth in a HEXOKINASE1-Dependent Manner in Arabidopsis thaliana. Plant Cell 2018, 30, 2057–2081. [Google Scholar] [CrossRef]
- Kong, W.; An, B.; Zhang, Y.; Yang, J.; Li, S.; Sun, T.; Li, Y. Sugar Transporter Proteins (STPs) in Gramineae Crops: Comparative Analysis, Phylogeny, Evolution, and Expression Profiling. Cells 2019, 8, 560. [Google Scholar] [CrossRef]
- Komaitis, F.; Kalliampakou, K.; Botou, M.; Nikolaidis, M.; Kalloniati, C.; Skliros, D.; Du, B.; Rennenberg, H.; Amoutzias, G.D.; Frillingos, S.; et al. Molecular and physiological characterization of the monosaccharide transporters gene family in Medicago truncatula. J. Exp. Bot. 2020, 71, 3110–3125. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Souza, L.; Yokoyama, R.; Sonnewald, U.; Fernie, A.R. Understanding source–sink interactions: Progress in model plants and translational research to crops. Mol. Plant 2023, 16, 96–121. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Neuhaus, H.E.; Cheng, J.; Bie, Z. Contributions of sugar transporters to crop yield and fruit quality. J. Exp. Bot. 2022, 73, 2275–2289. [Google Scholar] [CrossRef] [PubMed]
- Weschke, W.; Panitz, R.; Gubatz, S.; Wang, Q.; Radchuk, R.; Weber, H.; Wobus, U. The role of invertases and hexose transporters in controlling sugar ratios in maternal and filial tissues of barley caryopses during early development. Plant J. 2003, 33, 395–411. [Google Scholar] [CrossRef]
- Weschke, W.; Panitz, R.; Sauer, N.; Wang, Q.; Neubohn, B.; Weber, H.; Wobus, U. Sucrose transport into barley seeds: Molecular characterization of two transporters and implications for seed development and starch accumulation. Plant J. 2000, 21, 455–467. [Google Scholar] [CrossRef]
- Peng, Q.; Cai, Y.; Lai, E.; Nakamura, M.; Liao, L.; Zheng, B.; Ogutu, C.; Cherono, S.; Han, Y. The sucrose transporter MdSUT4.1 participates in the regulation of fruit sugar accumulation in apple. BMC Plant Biol. 2020, 20, 191. [Google Scholar] [CrossRef]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.D.; Ogé, L.; Hamama, L.; Atanassova, R. The Sugar-Signaling Hub: Overview of Regulators and Interaction with the Hormonal and Metabolic Network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef]
- Iftikhar, J.; Lyu, M.; Liu, Z.; Mehmood, N.; Munir, N.; Ahmed, M.A.A.; Batool, W.; Aslam, M.M.; Yuan, Y.; Wu, B. Sugar and Hormone Dynamics and the Expression Profiles of SUT/SUC and SWEET Sugar Transporters during Flower Development in Petunia axillaris. Plants 2020, 9, 1770. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, M.; Jamsheer, K.M.; Laxmi, A. Jasmonic acid coordinates with light, glucose and auxin signalling in regulating branching angle of Arabidopsis lateral roots. Plant Cell Environ. 2022, 45, 1554–1572. [Google Scholar] [CrossRef] [PubMed]
- Orman-Ligeza, B.; Morris, E.C.; Parizot, B.; Lavigne, T.; Babé, A.; Ligeza, A.; Klein, S.; Sturrock, C.; Xuan, W.; Novák, O.; et al. The Xerobranching Response Represses Lateral Root Formation When Roots Are Not in Contact with Water. Curr. Biol. CB 2018, 28, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, C.; Gan, L.; Ng, D.; Xia, K. GA3 enhances root responsiveness to exogenous IAA by modulating auxin transport and signalling in Arabidopsis. Plant Cell Rep. 2015, 34, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Zhang, Z.; Wang, Y.L.; Zhong, L.Y.; Chao, Z.F.; Gao, Y.Q.; Han, M.L.; Xu, L.; Chao, D.Y. Phytochrome B inhibits darkness-induced hypocotyl adventitious root formation by stabilizing IAA14 and suppressing ARF7 and ARF19. Plant J. 2021, 105, 1689–1702. [Google Scholar] [CrossRef]
- Banda, J.; Bellande, K.; von Wangenheim, D.; Goh, T.; Guyomarc’h, S.; Laplaze, L.; Bennett, M.J. Lateral Root Formation in Arabidopsis: A Well-Ordered LRexit. Trends Plant Sci. 2019, 24, 826–839. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, S.; Wang, X.; Yan, W.; Liu, Q.; Wang, N.; Zhang, J.; Guo, J.; Liu, J.; Luo, L. Overexpression of Cassava MeSTP7 Promotes Arabidopsis Seedling Development. Plants 2024, 13, 3102. https://doi.org/10.3390/plants13213102
Geng S, Wang X, Yan W, Liu Q, Wang N, Zhang J, Guo J, Liu J, Luo L. Overexpression of Cassava MeSTP7 Promotes Arabidopsis Seedling Development. Plants. 2024; 13(21):3102. https://doi.org/10.3390/plants13213102
Chicago/Turabian StyleGeng, Sha, Xiaotong Wang, Wei Yan, Qian Liu, Na Wang, Jianyu Zhang, Jianchun Guo, Jiao Liu, and Lijuan Luo. 2024. "Overexpression of Cassava MeSTP7 Promotes Arabidopsis Seedling Development" Plants 13, no. 21: 3102. https://doi.org/10.3390/plants13213102
APA StyleGeng, S., Wang, X., Yan, W., Liu, Q., Wang, N., Zhang, J., Guo, J., Liu, J., & Luo, L. (2024). Overexpression of Cassava MeSTP7 Promotes Arabidopsis Seedling Development. Plants, 13(21), 3102. https://doi.org/10.3390/plants13213102




