Understanding the Influence of Secondary Metabolites in Plant Invasion Strategies: A Comprehensive Review
Abstract
:1. Introduction
1.1. Invasion Mechanism
1.1.1. The Enemy Release Hypothesis
1.1.2. The Novel Weapons Hypothesis
1.1.3. Resistance Against Herbivores
1.1.4. Secondary Metabolites
1.1.5. Antimicrobial Abilities
1.1.6. Mutualistic Interactions
2. Secondary Metabolites in Invasive Plants
2.1. Phenolic Compounds
2.2. Alkaloids
2.3. Terpenes
2.4. Volatile Organic Compounds
2.5. Phytochemicals Reported in Invasion Mechanisms
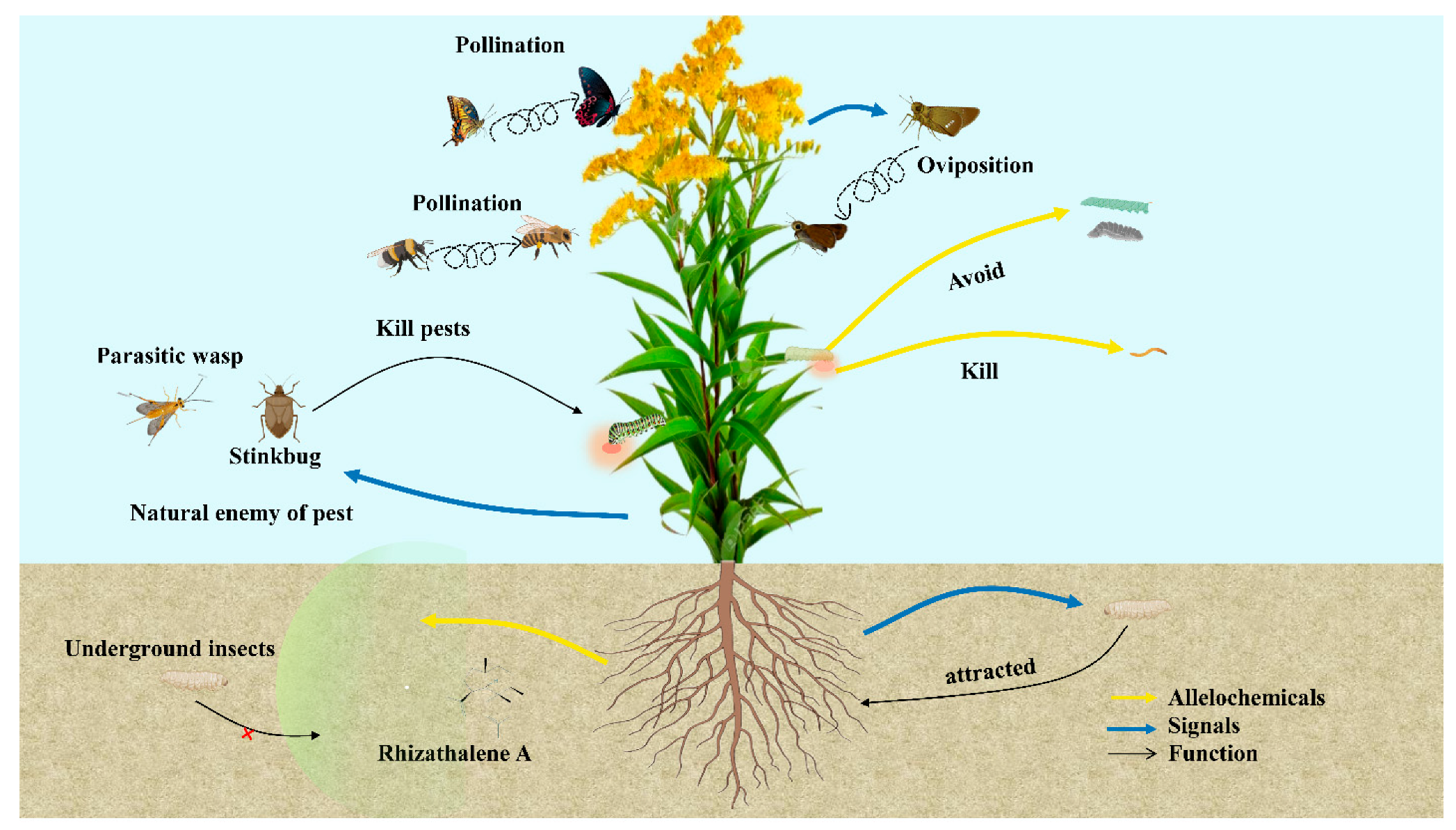
3. Functions of Secondary Metabolites
3.1. Allelopathy of Invasive Plants
3.1.1. Plants Interspecific Allelochemicals
3.1.2. Plants Intraspecific Allelochemicals
3.2. Herbivory and Invasive Plant Species Interactions
3.2.1. Insects
| Invasive Plant | Extract | Phytochemical | Target Insect | Mode of Action | References |
|---|---|---|---|---|---|
| Ageratina adenophora (Spreng). | Aqueous | Epifriedelinol, stigmasterol, octacosanoic acid, 8-daucos tero1, 2- isopropeny1-5- acetyl-6-hydrxybenzofuran aceate and o-hydroxy einnamic acid | Rice weevil, maize weevil, Chinese bean weevil and European bean weevil | Toxicity | [161] |
| Alternanthera brasiliana (L.) Kuntze | Ethanolic extract | Kaempferol and kaempferol analogs, quercetin and quercetin analogs, stigmasterol, β-sitosterol, spinasterol and ferulic acid | Drosophila melanogaster | Toxicity | [184] |
| Ageratina adenophora (Spreng). | Ethyl acetate | Cadinene sesquiterpenes, 5,6-dihydroxycadinan-3-ene-2,7-dione | Meloidogyne incognita | Antinemic activity | [174] |
| Ageratum conyzoides L. Lemmon grass | Crude extracts | PONNEEM | Aedes, Anopheles, Culex spp. | Affects the oviposition rate and increases the deterrence percentage | [176,185] |
| Methanol extracts | 6-demethyoxyageratochromene (precocene I) and ageratochromene (precocene II) | Preris rapae and Plutella xyloaella | Antifeeding effects | [125] | |
| Mikania micrantha Kunth. | Methonal extract | Mikanin, eupalitin, eupafolin, (3,4′,5,7-tetra-hydroxy 6- methoxyflavone 3-O-β-D-glucopyranoside, luteolin, 3,5-di-O-caffeoylquinic acid n-butyl ester and 3,4-di-O-caffeoylquinic acid n-butyl ester were identified from M. micrantha | Oriental fruit fly | Repellent effects | [105] |
| EOs | β-cubebene, terpinolene, β-caryophyllene, 1imonene, β-farnesene, ocimene, δcadino1, γ-terpinene, ethylnaphthalene, a-caryophy11ene, | Plutella xylostella, Phyllotretast riolata and Phaedon brassicae | Oviposition deterrent | [179,180] | |
| Chromolaena odoratum L. | Alcohol extracts | Chalcones and flavonols | Plutella xylostella | Repellent | [183] |
| Crude extracts | Helicoverpa armigera | Antifeeding effects | [181] | ||
| EOs | Trans-caryophyllene, β-cadinene, a-copaene, caryophyllene oxide, germacrene-D and n-humuhne | Phyllotreta striolata | Oviposition deterrent | [186] | |
| Parthenium hysterophorus L. | Flower, leaf stem powders | Parthenin ageratochromene, precocene I, and precocene II have strong insecticidal effects, endo-borneol, farnesol, quercetin, kaempferol, and its glucosides | Callosobruchus chinensis | Repellency, inhibit cholinesterase | [187] |
| Aqueous leaf and stem | Aedes aegypti, Sitophious oryzae | Toxic and oviposition deterrent | [188,189] | ||
| Melia azedarach L. | Aqueous extract Fruits | Azadirachtin | Callosobruchus maculatus | Toxicity and repellency | [190] |
3.2.2. Soil Microorganism
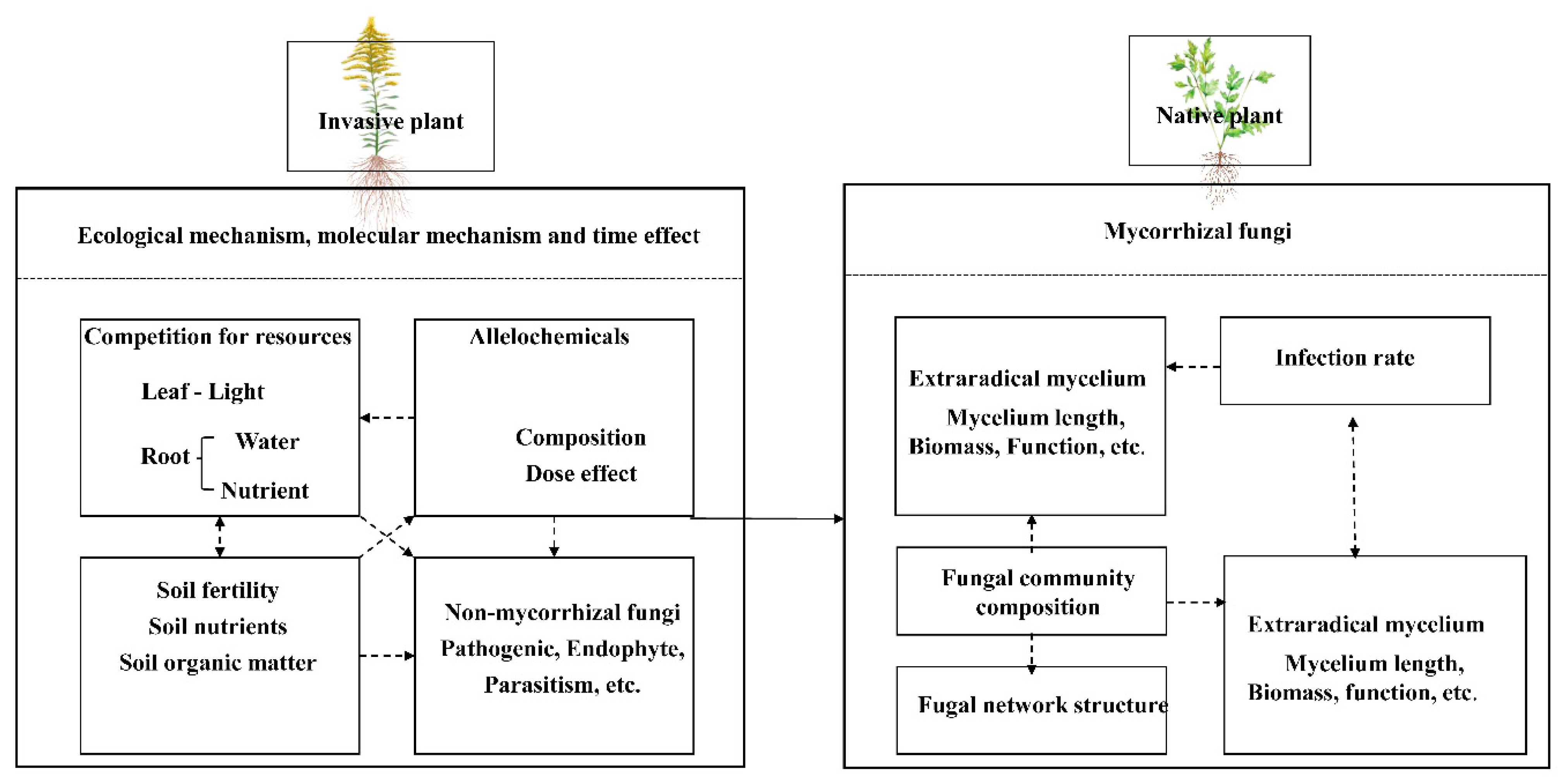
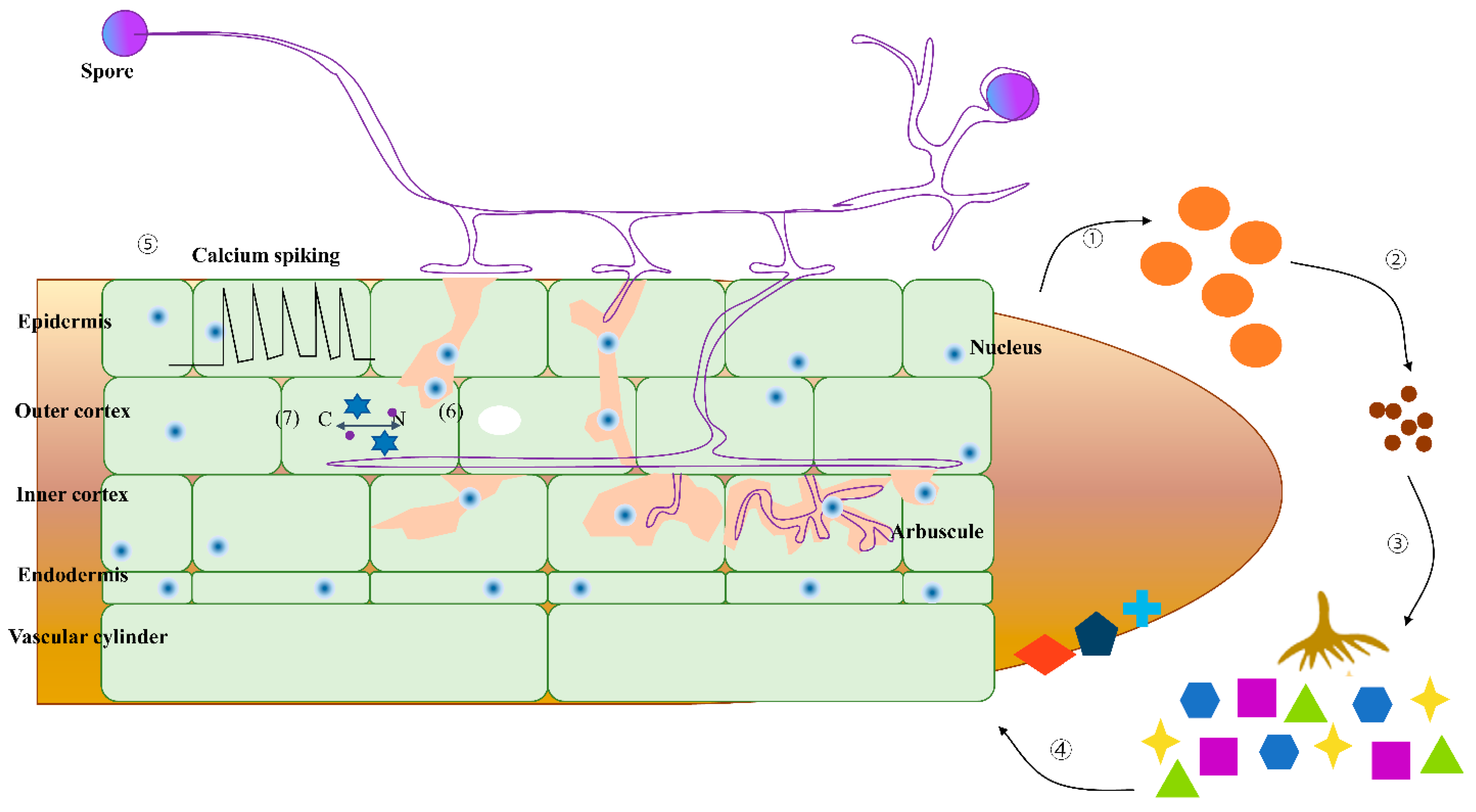
3.3. Arbuscular Mycorrhizal Fungi (AMF)
3.3.1. Symbiotic Relationship Between Invasive/Native Plants and AMF Communities
3.3.2. Mechanisms by Which Invasive Plants Affect Native Plant Mycorrhizal Fungi
Ecological Mechanisms
Molecular Mechanism
4. Management of Invasive Plant Species
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Zhu, M.; Chen, X.Q.B. Review on Allelopathy of Exotic Invasive Plants. In Procedia Engineering; Elsevier: Amsterdam, The Netherlands, 2011; Volume 18, pp. 240–246. [Google Scholar]
- Krogsgaard Svendsen, I. The Effects That the Current Climate Crisis Have on the Biogeography and Environment, Needed Adaptations and Conservation. Am. J. Biosci. 2020, 8, 20. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.M.; Gemmell, N.J.; Rand, T.A.; Ewers, R.M. Interactive Effects of Habitat Modification and Species Invasion on Native Species Decline. Trends Ecol. Evol. 2007, 22, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, G.; Mammola, S.; Verbrugge, L.; Isaia, M. Aliens in Caves: The Global Dimension of Biological Invasions in Subterranean Ecosystems. Biol. Rev. 2023, 98, 849–867. [Google Scholar] [CrossRef]
- Wu, J.-W.; Li, F.-L.; Yao, S.-K.; Zhao, Z.-Y.; Feng, X.; Chen, R.-Z.; Xu, Y.-Q. Iva xanthiifolia Leaf Extract Reduced the Diversity of Indigenous Plant Rhizosphere Bacteria. BMC Plant Biol. 2023, 23, 297. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Wang, W.; Wang, J.; Yu, F. Effects of Invasive Plant Diversity on Soil Microbial Communities. Diversity 2022, 14, 992. [Google Scholar] [CrossRef]
- Yang, B.; Li, J. Phytotoxicity of Root Exudates of Invasive Solidago canadensis on Co-occurring Native and Invasive Plant Species. Pakistan J. Bot. 2022, 54, 1019–1024. [Google Scholar] [CrossRef]
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why Are Invasive Plants Successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef] [PubMed]
- Costan, C.A.; Godsoe, W.K.; Bufford, J.L.; Marris, J.W.M.; Hulme, P.E. Can the Enemy Release Hypothesis Explain the Success of Rumex (Polygonaceae) Species in an Introduced Range? Biol. Invasions 2022, 24, 2665–2677. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L. Life and Death in the Soil Microbiome: How Ecological Processes Influence Biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Fischbein, D.; Corley, J.C. Population Ecology and Classical Biological Control of Forest Insect Pests in a Changing World. For. Ecol. Manag. 2022, 520, 120400. [Google Scholar] [CrossRef]
- Sanderson, C.H.; Zonneveld, R.; Smith, M.C.; Minteer, C.R.; Purcell, M.F. Life History of the Leaf-Feeding Beetle Calomela intemerata, a Potential Biocontrol Agent against Acacia auriculiformis. Entomol. Exp. Appl. 2023, 171, 902–912. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.-L.; Jahn, L.V.; Burns, J.H. Invaders Responded More Positively to Soil Biota than Native or Noninvasive Introduced Species, Consistent with Enemy Escape. Biol. Invasions 2023, 25, 351–364. [Google Scholar] [CrossRef]
- Liu, L.; Fang, X.; Ren, S.; Jia, R.; Liu, Q.; Liu, H.; Xiu, L.; Yaqoob, S.; Cai, D.; Liu, J. Targeted Metabolic Reveals Different Part of Maize in Polyphenolic Metabolites during Germination and Hypoglycemic Activity Analysis. Food Chem. X 2023, 19, 100848. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Gurusinghe, S.; Weston, L.A. The Potential Role of Allelopathy in the Persistence of Invasive Weeds. In Persistence Strategies of Weeds; Wiley: New York, NY, USA, 2022; pp. 271–301. [Google Scholar]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy and Its Application as a Weed Management Tool: A Review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Bukovinszky, T.; van der Putten, W.H. Interactions between Invasive Plants and Insect Herbivores: A Plea for a Multitrophic Perspective. Biol. Conserv. 2010, 143, 2251–2259. [Google Scholar] [CrossRef]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance Comparisons of Co-Occurring Native and Alien Invasive Plants: Implications for Conservation and Restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Chauhan, B.S.; Farooq, M.; Shabbir, A.; Adkins, S.W. What Do We Really Know about Alien Plant Invasion? A Review of the Invasion Mechanism of One of the World’s Worst Weeds. Planta 2016, 244, 39–57. [Google Scholar] [CrossRef]
- Savoia, D. Plant-Derived Antimicrobial Compounds: Alternatives to Antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef]
- Shahrtash, M.; Brown, S.P. A Path Forward: Promoting Microbial-Based Methods in the Control of Invasive Plant Species. Plants 2021, 10, 943. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones Stimulate Arbuscular Mycorrhizal Fungi by Activating Mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D. Speak, Friend, and Enter: Signalling Systems That Promote Beneficial Symbiotic Associations in Plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Khattak, W.A.; Sun, J.; Hameed, R.; Zaman, F.; Abbas, A.; Khan, K.A.; Elboughdiri, N.; Akbar, R.; He, F.; Ullah, M.W.; et al. Unveiling the Resistance of Native Weed Communities: Insights for Managing Invasive Weed Species in Disturbed Environments. Biol. Rev. 2024, 99, 753–777. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, E.A.E.; El-Keblawy, A.; Mosa, K.A.; Okoh, A.I.; Saadoun, I. Role of Endophytes and Rhizosphere Microbes in Promoting the Invasion of Exotic Plants in Arid and Semi-Arid Areas: A Review. Sustainability 2021, 13, 13081. [Google Scholar] [CrossRef]
- Inderjit; Callaway, R.M. Experimental Designs for the Study of Allelopathy. Plant Soil 2003, 256, 1–11. [Google Scholar]
- Bais, H.P.; Walker, T.S.; Kennan, A.J.; Stermitz, F.R.; Vivanco, J.M. Structure-Dependent Phytotoxicity of Catechins and Other Flavonoids: Flavonoid Conversions by Cell-Free Protein Extracts of Centaurea maculosa (Spotted Knapweed) Roots. J. Agric. Food Chem. 2003, 51, 897–901. [Google Scholar] [CrossRef]
- Castaldi, S.; Carfora, A.; Fiorentino, A.; Natale, A.; Messere, A.; Miglietta, F.; Cotrufo, M.F. Inhibition of Net Nitrification Activity in a Mediterranean Woodland: Possible Role of Chemicals Produced by Arbutus Unedo. Plant Soil 2009, 315, 273–283. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Kushwaha, A.; Hans, N.; Giri, B.S.; Rene, E.R.; Rani, R. Uncovering the Phytochemicals of Root Exudates and Extracts of Lead (Pb) Tolerant Chrysopogon zizanioides (L.) Roberty in Response to Lead Contamination and Their Effect on the Chemotactic Behavior of Rhizospheric Bacteria. Environ. Sci. Pollut. Res. 2022, 29, 44998–45012. [Google Scholar] [CrossRef]
- Khan, A.A.; Wang, T.; Hussain, T.; Amna Ali, F.; Shi, F.; Chaudhary, H.J. Halotolerant-Koccuria rhizophila (14asp)-induced amendment of salt stress in pea plants by limiting Na+ uptake and elevating production of antioxidants. Agronomy 2021, 11, 1907. [Google Scholar] [CrossRef]
- Graff, P.; Gundel, P.E.; Salvat, A.; Cristos, D.; Chaneton, E.J. Protection Offered by Leaf Fungal Endophytes to an Invasive Species against Native Herbivores Depends on Soil Nutrients. J. Ecol. 2020, 108, 1592–1604. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, B.; Zhang, S.; Tang, J.; Tu, C.; Hu, S.; Yong, J.W.H.; Chen, X. Enhanced Allelopathy and Competitive Ability of Invasive Plant Solidago canadensis in Its Introduced. J. Plant Ecol. 2013, 6, 253–263. [Google Scholar] [CrossRef]
- Ens, E.J.; Bremner, J.B.; French, K.; Korth, J. Identification of Volatile Compounds Released by Roots of an Invasive Plant, Bitou Bush (Chrysanthemoides monilifera Spp. Rotundata), and Their Inhibition of Native Seedling Growth. Biol. Invasions 2009, 11, 275–287. [Google Scholar] [CrossRef]
- Thorpe, A.S.; Thelen, G.C.; Diaconu, A.; Callaway, R.M. Root Exudate Is Allelopathic in Invaded Community but Not in Native Community: Field Evidence for the Novel Weapons Hypothesis. J. Ecol. 2009, 97, 641–645. [Google Scholar] [CrossRef]
- Cappuccino, N.; Arnason, J.T. Novel Chemistry of Invasive Exotic Plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef]
- Afzal, M.R.; Naz, M.; Ashraf, W.; Du, D. The Legacy of Plant Invasion: Impacts on Soil Nitrification and Management Implications. Plants 2023, 12, 2980. [Google Scholar] [CrossRef]
- Liu, S.; Wu, F.; Wen, X. Allelopathic Effects of Root Exudates of Chinese Onion on Tomato Growth and the Pathogen Fusarium oxysporum(Sch1) f.Sp. Lycopersici. Allelopath. J. 2013, 31, 387–404. [Google Scholar]
- Singh, A.A.; Ghosh, A.; Agrawal, M.; Agrawal, S.B. Secondary Metabolites Responses of Plants Exposed to Ozone: An Update. Environ. Sci. Pollut. Res. 2023, 30, 88281–88312. [Google Scholar] [CrossRef] [PubMed]
- Ramawat, K.G.; Goyal, S. Co-Evolution of Secondary Metabolites During Biological Competition for Survival and Advantage: An Overview; Reference Series in Phytochemistry; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–17. [Google Scholar]
- Melzig, M.F. Plant Polyphenols as Inhibitors of Hydrolases Are Regulators of Digestion. Complement. Med. Res. 2023, 30, 453–459. [Google Scholar] [CrossRef]
- Zhang, R.; Qu, S.; Zhang, B.; Gao, Y.; Xing, F. Interactive Effects between the Invasive Weed Stellera chamaejasme and Grass: Can Arbuscular Mycorrhizal Fungi and Fungal Pathogens Coregulate Interspecific Relationships? Front. Microbiol. 2023, 14, 1236891. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.; Nie, C.; Wang, T.; Qin, X.; Yang, J.; Chang, Y.; Nie, S.; Fu, Y. Comprehensive Phytochemical Analysis of Lingonberry (Vaccinium vitisidaea L.) from Different Regions of China and Their Potential Antioxidant and Antiproliferative Activities. RSC Adv. 2023, 13, 29438–29449. [Google Scholar] [CrossRef] [PubMed]
- Košćak, L.; Lamovšek, J.; Đermić, E.; Prgomet, I.; Godena, S. Microbial and Plant-Based Compounds as Alternatives for the Control of Phytopathogenic Bacteria. Horticulturae 2023, 9, 1124. [Google Scholar] [CrossRef]
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a Source of Bioherbicides: Challenges and Prospects for Sustainable Agriculture. Rev. Environ. Sci. Biotechnol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Yu, J.W.; Lee, J.H.; Song, M.H.; Keum, Y.S. Metabolomic Responses of Lettuce (Lactuca sativa) to Allelopathic Benzoquinones from Iris sanguinea Seeds. J. Agric. Food Chem. 2023, 71, 5143–5153. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, A.; Ostrowska, P. Essential Oils and Their Components in Combating Fungal Pathogens of Animal and Human Skin. J. Med. Mycol. 2021, 31, 101118. [Google Scholar] [CrossRef]
- Suwitchayanon, P.; Kunasakdakul, K.; Kato-Noguchi, H. Screening the Allelopathic Activity of 14 Medicinal Plants from Northern Thailand. Environ. Control Biol. 2017, 55, 143–145. [Google Scholar] [CrossRef]
- Ulbrich, T.C.; Rivas-Ubach, A.; Tiemann, L.K.; Friesen, M.L.; Evans, S.E. Plant Root Exudates and Rhizosphere Bacterial Communities Shift with Neighbor Context. Soil Biol. Biochem. 2022, 172, 108753. [Google Scholar] [CrossRef]
- Shan, Z.; Zhou, S.; Shah, A.; Arafat, Y.; Arif Hussain Rizvi, S.; Shao, H. Plant Allelopathy in Response to Biotic and Abiotic Factors. Agronomy 2023, 13, 2358. [Google Scholar] [CrossRef]
- Reiss, A.; Fomsgaard, I.S.; Mathiassen, S.K.; Kudsk, P. Weed Suppressive Traits of Winter Cereals: Allelopathy and Competition. Biochem. Syst. Ecol. 2018, 76, 35–41. [Google Scholar] [CrossRef]
- Han, M.; Yang, H.; Huang, H.; Du, J.; Zhang, S.; Fu, Y. Allelopathy and Allelobiosis: Efficient and Economical Alternatives in Agroecosystems. Plant Biol. 2023, 26, 11–27. [Google Scholar] [CrossRef]
- Bachheti, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant Allelochemicals and Their Various Applications BT—Co-Evolution of Secondary Metabolites. In Co-Evolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2020; pp. 441–465. [Google Scholar]
- Hussain, M.I.; Araniti, F.; Schulz, M.; Baerson, S.; Vieites-Álvarez, Y.; Rempelos, L.; Bilsborrow, P.; Chinchilla, N.; Macías, F.A.; Weston, L.A.; et al. Benzoxazinoids in Wheat Allelopathy—From Discovery to Application for Sustainable Weed Management. Environ. Exp. Bot. 2022, 202, 104997. [Google Scholar] [CrossRef]
- Pedrol, N.; González, L.; Reigosa, M.J. Allelopathy and Abiotic Stress. In Allelopathy: A Physiological Process with Ecological Implicartion; Reigosa, M.J., Pedrol, N., Gonzales, L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 211–227. [Google Scholar]
- Berdy, J. Contribution on the Study of Allelopathic Interactions Between Amaranthus retroflexus L. and Secale cereale L. Master’s Thesis, University of Liège, Gembloux, Belgium, 2023. [Google Scholar]
- Rai, P.K.; Singh, J.S. Ecological Insights and Environmental Threats of Invasive Alien Plant Chromolaena odorata: Prospects for Sustainable Management. Weed Biol. Manag. 2024, 24, 15–37. [Google Scholar] [CrossRef]
- Yadav, S.P.S.; Mehata, D.K.; Pokhrel, S.; Ghimire, N.P. A Comprehensive Study of Banmara (Invasive Alien Plant Species): Understanding the Invasive Potential and Ecological Consequences for Biodiversity. J. Agric. 2024, 15, 101030. [Google Scholar]
- Ali, J.; Chen, R.Z. Chemical Ecology: Insect-Plant Interactions; CRC Press: Boca Raton, FL, USA, 2024; pp. 1–182. [Google Scholar]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; Perreau, F.; Rajjou, L.; Ben Malek, R.; Lepiniec, L.; Mouille, G. Specialized Metabolites in Seeds. Adv. Bot. Res. 2021, 98, 35–70. [Google Scholar]
- Ait Elallem, K.; Sobeh, M.; Boularbah, A.; Yasri, A. Chemically Degraded Soil Rehabilitation Process Using Medicinal and Aromatic Plants: Review. Environ. Sci. Pollut. Res. 2021, 28, 73–93. [Google Scholar] [CrossRef]
- Adams, B.; Yusuf, A.A.; Torto, B.; Khamis, F.M. Non-Host Plant Odors Influence the Tritrophic Interaction between Tomato, Its Foliar Herbivore Tuta absoluta and Mirid Predator Nesidiocoris tenuis. Front. Plant Sci. 2023, 14, 1014865. [Google Scholar] [CrossRef]
- Guo, X.; Hu, Y.; Ma, J.Y.; Wang, H.; Wang, K.L.; Wang, T.; Jiang, S.Y.; Jiao, J.B.; Sun, Y.K.; Jiang, X.L.; et al. Nitrogen Deposition Effects on Invasive and Native Plant Competition: Implications for Future Invasions. Ecotoxicol. Environ. Saf. 2023, 259, 1150299. [Google Scholar] [CrossRef]
- Korpelainen, H.; Pietiläinen, M. What Makes a Good Plant Invader? Life 2023, 13, 1596. [Google Scholar] [CrossRef]
- Poljuha, D.; Sladonja, B.; Uzelac Božac, M.; Šola, I.; Damijanić, D.; Weber, T. The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy. Plants 2024, 13, 1745. [Google Scholar] [CrossRef]
- Abbas, F.; O’Neill Rothenberg, D.; Zhou, Y.; Ke, Y.; Wang, H.C. Volatile Organic Compounds as Mediators of Plant Communication and Adaptation to Climate Change. Physiol. Plant. 2022, 174, e13840. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Liu, J.; Zhou, W.; Ma, C.; Luo, S. A New Perspective on Plant Defense against Foliar Gall-Forming Aphids through Activation of the Fruit Abscission Pathway. Plant Physiol. Biochem. 2023, 196, 1046–1054. [Google Scholar] [CrossRef]
- Asif, A.; Baig, M.A.; Siddiqui, M.B. Role of Jasmonates and Salicylates in Plant Allelopathy; Springer: Berlin/Heidelberg, Germany, 2021; pp. 115–127. [Google Scholar]
- Reglinski, T.; Wurms, K.V.; Vanneste, J.L.; Ah Chee, A.; Schipper, M.; Cornish, D.; Yu, J.; McAlinden, J.; Hedderley, D. Kiwifruit Resistance to Sclerotinia sclerotiorum and Pseudomonas syringae Pv. Actinidiae and Defence Induction by Acibenzolar-S-Methyl and Methyl Jasmonate Are Cultivar Dependent. Int. J. Mol. Sci. 2023, 24, 15952. [Google Scholar] [CrossRef]
- Okosun, O.O.; George, J.; Reddy, G.V.P. Role of Kairomones in Biological Control of Pests: Commercial Potential. In Development and Commercialization of Biopesticides; Elsevier: Amsterdam, The Netherlands, 2023; pp. 57–80. [Google Scholar]
- Lankau, R.A. Coevolution between Invasive and Native Plants Driven by Chemical Competition and Soil Biota. Proc. Natl. Acad. Sci. USA 2012, 109, 11240–11245. [Google Scholar] [CrossRef]
- Batish, D.R.; Kaur, S.; Singh, H.P.; Kohli, R.K. Role of Root-Mediated Interactions in Phytotoxic Interference of Ageratum conyzoides with Rice (Oryza sativa). Flora Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 388–395. [Google Scholar] [CrossRef]
- Hazrati, H.; Fomsgaard, I.S.; Kudsk, P. Root-Exuded Benzoxazinoids: Uptake and Translocation in Neighboring Plants. J. Agric. Food Chem. 2020, 68, 10609–10617. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.L.; Cantrell, C.L.; Dayan, F.E.; Byrd, J.D.; Duke, S.O. Tabanone, a New Phytotoxic Constituent of Cogongrass (Imperata cylindrica). Weed Sci. 2012, 60, 212–218. [Google Scholar] [CrossRef]
- Fraser, L.H.; Carlyle, C.N. Is Spotted Knapweed (Centaurea stoebe L.) Patch Size Related to the Effect on Soil and Vegetation Properties? Plant Ecol. 2011, 212, 975–983. [Google Scholar] [CrossRef]
- Yadav, V.; Singh, N.B.; Singh, H.; Singh, A.; Hussain, I. Allelopathic Invasion of Alien Plant Species in India and Their Management Strategies: A Review. Trop. plant Res. 2016, 3, 87–101. [Google Scholar]
- Vivanco, J.M.; Bais, H.P.; Stermitz, F.R.; Thelen, G.C.; Callaway, R.M. Biogeographical Variation in Community Response to Root Allelochemistry: Novel Weapons and Exotic Invasion. Ecol. Lett. 2004, 7, 285–292. [Google Scholar] [CrossRef]
- Irimia, R.E.; Lopes, S.M.M.; Sotes, G.; Cavieres, L.A.; Eren, Ö.; Lortie, C.J.; French, K.; Hierro, J.L.; Rosche, C.; Callaway, R.M.; et al. Biogeographic Differences in the Allelopathy of Leaf Surface Extracts of an Invasive Weed. Biol. Invasions 2019, 21, 3151–3168. [Google Scholar] [CrossRef]
- Landau, I.; Müller-Schärer, H.; Ward, P.I. Influence of Cnicin, a Sesquiterpene Lactone of Centaurea maculosa (Asteraceae), on Specialist and Generalist Insect Herbivores. J. Chem. Ecol. 1994, 20, 929–942. [Google Scholar] [CrossRef]
- Liu, B.; Yan, J.; Li, W.; Yin, L.; Li, P.; Yu, H.; Xing, L.; Cai, M.; Wang, H.; Zhao, M.; et al. Mikania micrantha Genome Provides Insights into the Molecular Mechanism of Rapid Growth. Nat. Commun. 2020, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ye, W.; Wei, X.; Zhang, C. Allelopathic Potential of Sesquiterpene Lactones and Phenolic Constituents from Mikania micrantha H. B. K. Biochem. Syst. Ecol. 2008, 36, 867–871. [Google Scholar] [CrossRef]
- López, M.L.; Bonzani, N.E.; Zygadlo, J.A. Allelopathic Potential of Tagetes Minuta Terpenes by a Chemical, Anatomical and Phytotoxic Approach. Biochem. Syst. Ecol. 2008, 36, 882–890. [Google Scholar] [CrossRef]
- Zhou, S.; Zokir, T.; Mei, Y.; Lei, L.; Shi, K.; Zou, T.; Zhang, C.; Shao, H. Allelopathic Effect of Serphidium kaschgaricum (Krasch.) Poljak. Volatiles on Selected Species. Plants 2021, 10, 495. [Google Scholar] [CrossRef]
- Preston, C.A.; Betts, H.; Baldwin, I.T. Methyl Jasmonate as an Allelopathic Agent: Sagebrush Inhibits Germination of a Neighboring Tobacco, Nicotiana attenuata. J. Chem. Ecol. 2002, 28, 2343–2369. [Google Scholar] [CrossRef]
- Berillo, D.; Kozhahmetova, M.; Lebedeva, L. Overview of the Biological Activity of Anthraquinons and Flavanoids of the Plant Rumex Species. Molecules 2022, 27, 1204. [Google Scholar] [CrossRef]
- Bashar, H.M.K.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Uddin, M.K.; Asib, N.; Anwar, M.P.; Rahaman, F. A Mystic Weed, Parthenium hysterophorus: Threats, Potentials and Management. Agronomy 2021, 11, 1514. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, S.; Singh, H.P.; Batish, D.R. Alterations in Phytotoxicity and Allelochemistry in Response to Intraspecific Variation in Parthenium hysterophorus. Ecol. Complex. 2022, 50, 100999. [Google Scholar] [CrossRef]
- Nimal Chandrasena, N.C.; Rao, A.N. Parthenium Weed: Uses and Abuses. In Parthenium Weed: Biology, Ecology and Management; CABI Digital Library: Wallingford, UK, 2018; pp. 190–211. [Google Scholar]
- Kaur, L.; Malhi, D.S.; Cooper, R.; Kaur, M.; Sohal, H.S.; Mutreja, V.; Sharma, A. Comprehensive Review on Ethnobotanical Uses, Phytochemistry, Biological Potential and Toxicology of Parthenium hysterophorus L.: A Journey from Noxious Weed to a Therapeutic Medicinal Plant. J. Ethnopharmacol. 2021, 281, 114525. [Google Scholar] [CrossRef] [PubMed]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant Allelochemicals: Agronomic, Nutritional and Ecological Relevance in the Soil System. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Pan, L.; He, F.; Liang, Q.; Bo, Y.; Lin, X.; Javed, Q.; Sun, J. Allelopathic Effects of Caffeic Acid and Its Derivatives on Seed Germination and Growth Competitiveness of Native Plants (Lantana indica) and Invasive Plants (Solidago canadensis). Agriculture 2023, 13, 1719. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Zhu, X.; Zhang, Z.; Huang, X. Identify Potential Allelochemicals from Humulus scandens (Lour.) Merr. Root Extracts That Induce Allelopathy on Alternanthera philoxeroides (Mart.) Griseb. Sci. Rep. 2021, 11, 7068. [Google Scholar] [CrossRef]
- Šoln, K.; Žnidaršič, N.; Dolenc Koce, J. Root Growth Inhibition and Ultrastructural Changes in Radish Root Tips after Treatment with Aqueous Extracts of Fallopia japonica and F. ×bohemica Rhizomes. Protoplasma 2022, 259, 343–355. [Google Scholar] [CrossRef]
- Nakamura, N.; Nemoto, M. Allelopathic Potential of Eupatorium odoratum in Abandoned Shifting Cultivation Fields in the Tropics. J. Weed Sci. Technol. 1993, 38, 103–108. [Google Scholar] [CrossRef]
- Sahid, I.; Yusoff, N. Allelopathic Effects of Chromolaena odorata (L.) King and Robinson and Mikania micrantha H.B.K. on Three Selected Weed Species. Aust. J. Crop Sci. 2014, 8, 1024–1028. [Google Scholar]
- Poonpaiboonpipat, T.; Krumsri, R.; Kato-noguchi, H. Allelopathic and Herbicidal Effects of Crude Extract from Chromolaena odorata (L.) R.M.King and H.Rob. on Echinochloa crus-galli and Amaranthus viridis. Plants 2021, 10, 1609. [Google Scholar] [CrossRef]
- Erida, G.; Saidi, N.; Syafruddin, H. Allelopathic Screening of Several Weed Species as Potential Bioherbicides. IOP Conf. Ser. Earth Environ. Sci. 2019, 334, 012034. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy and Allelochemicals of Imperata cylindrica as an Invasive Plant Species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Ahmad-Hamdani, M.S.; Hasan, M. Phytochemical Constituents and Allelopathic Potential of Parthenium hysterophorus L. in Comparison to Commercial Herbicides to Control Weeds. Plants 2021, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, A.A.; Weston, P.A.; Gurusinghe, S.; Latif, S.; Adkins, S.W.; Weston, L.A. Toxic Potential and Metabolic Profiling of Two Australian Biotypes of the Invasive Plant Parthenium Weed (Parthenium hysterophorus L.). Toxins 2020, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Bora, A.R.; Babu, D.S.; Kalita, S.; Chetry, S. Harmful Effect of the Invasive Weed Mikania Micrantha with Special Reference to India: A Review. Agric. Rev. 2021, 44, 380–384. [Google Scholar] [CrossRef]
- Shao, H.; Peng, S.; Wei, X.; Zhang, D.; Zhang, C. Potential Allelochemicals from an Invasive Weed Mikania micrantha H.B.K. J. Chem. Ecol. 2005, 31, 1657–1668. [Google Scholar] [CrossRef]
- Syngkli, R.B.L.; Lallianpuii, S.; Rai, P.K. Microcosm Investigation on the Allelochemical Potential of Mikania micrantha to the Selected Food Crop. Int. J. Plant Environ. 2022, 8, 137–142. [Google Scholar] [CrossRef]
- Rashid, M.H.; Asaeda, T.; Uddin, M.N. The Allelopathic Potential of Kudzu (Pueraria montana). Weed Sci. 2010, 58, 47–55. [Google Scholar] [CrossRef]
- Erida, G.; Saidi, N.; Hasanuddin, H.; Syafruddin, S. Herbicidal Effects of Ethyl Acetate Extracts of Billygoat Weed (Ageratum conyzoides L.) on Spiny Amaranth (Amaranthus spinosus L.) Growth. Agronomy 2021, 11, 1991. [Google Scholar] [CrossRef]
- Kong, C.; Liang, W.; Hu, F.; Xu, X.; Wang, P.; Jiang, Y.; Xing, B. Allelochemicals and Their Transformations in the Ageratum conyzoides Intercropped Citrus Orchard Soils. Plant Soil 2004, 264, 149–157. [Google Scholar] [CrossRef]
- Darji, T.B.; Adhikari, B.; Pathak, S.; Neupane, S.; Thapa, L.B.; Bhatt, T.D.; Pant, R.R.; Pant, G.; Pal, K.B.; Bishwakarma, K. Phytotoxic Effects of Invasive Ageratina adenophora on Two Native Subtropical Shrubs in Nepal. Sci. Rep. 2021, 11, 13663. [Google Scholar] [CrossRef]
- Wan, F.H.; Liu, W.X.; Guo, J.Y.; Qiang, S.; Li, B.P.; Wang, J.J.; Yang, G.Q.; Niu, H.B.; Gui, F.R.; Huang, W.K.; et al. Invasive Mechanism and Control Strategy of Ageratina adenophora (Sprengel). Sci. China Life Sci. 2010, 53, 1291–1298. [Google Scholar] [CrossRef]
- Khan, I.U.; Qi, S.S.; Gul, F.; Manan, S.; Rono, J.K.; Naz, M.; Shi, X.N. A Green Approach Used for Heavy Metals ‘Phytoremediation’ Via Invasive Plant Species to Mitigate Environmental Pollution: A Review. Plants 2023, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.M.; Wagentristl, H.; Renner-Martin, K.; Urban, B.; Durec, N.; Kaul, H.P. Extracts and Residues of Common Ragweed (Ambrosia artemisiifolia L.) Cause Alterations in Root and Shoot Growth of Crops. Plants 2023, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Javaid, N.; Javaid, A.; Shah, M.H.; Khan, I.H.; Waleed, S.M. Herbicidal activity of Ageratum conyzoides against Parthenium. J. Weed Sci. Res. 2020, 27, 137–146. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Soriano, G.; Masi, M.; Carretero, P.; Vilariño-Rodríguez, S.; Cimmino, A. (4Z)-Lachnophyllum Lactone, an Acetylenic Furanone from Conyza bonariensis, Identified for the First Time with Allelopathic Activity against Cuscuta campestris. Agric. 2022, 12, 790. [Google Scholar] [CrossRef]
- Puig, C.G.; Reigosa, M.J.; Valentão, P.; Andrade, P.B.; Pedrol, N. Unravelling the Bioherbicide Potential of Eucalyptus globulus Labill: Biochemistry and Effects of Its Aqueous Extract. PLoS ONE 2018, 13, e0192872. [Google Scholar] [CrossRef]
- Das, M.; Sharma, R.; Nath, N. Invasive Alien Herbaceous Species in Terrestrial and Swampland Habitats in India: A Review. Int. J. Bot. Stud. 2021, 6, 661–668. [Google Scholar]
- Suzuki, M.; Chozin, M.A.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Activity of Chinese Violet (Asystasia gangetica (L.) T. Anderson) and Two Phytotoxic Substances. Weed Biol. Manag. 2019, 19, 3–8. [Google Scholar] [CrossRef]
- Maurya, P.; Mazeed, A.; Kumar, D.; Ahmad, I.Z.; Suryavanshi, P. Medicinal and Aromatic Plants as an Emerging Source of Bioherbicides. Curr. Sci. 2022, 122, 258–266. [Google Scholar] [CrossRef]
- Daniela Lopes, A.; Graciela Iecher Faria Nunes, M.; Paulo Francisco, J.; Henrique dos Santos, E. Potential Allelopathic Effect of Species of the Asteraceae Family and Its Use in Agriculture. In Vegetation Dynamics, Changing Ecosystems and Human Responsibility; IntechOpen: London, UK, 2023. [Google Scholar]
- Motmainna, M.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Hasan, M.; Yeasmin, S.; Anwar, M.P.; Islam, A.K.M.M. Allelopathic Potential of Tropical Plants—A Review. Agronomy 2023, 13, 2063. [Google Scholar] [CrossRef]
- Bezerra, J.J.; do Nascimento, T.G.; Kamiya, R.U.; do Nascimento Prata, A.P.; de Medeiros, P.M.; de MendonÃ, C.N. Phytochemical Screening, Chromatographic Profile and Evaluation of Antimicrobial and Antioxidant Activities of Three Species of the Cyperaceae Juss. Family. J. Med. Plants Res. 2019, 13, 312–320. [Google Scholar]
- Silva, M.P.; Piazza, L.A.; López, D.; López Rivilli, M.J.; Turco, M.D.; Cantero, J.J.; Tourn, M.G.; Scopel, A.L. Phytotoxic Activity in Flourensia campestris and Isolation of (-)-Hamanasic Acid A as Its Active Principle Compound. Phytochemistry 2012, 77, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Nyasha, S.; Innocent, P.; Simbarashe, M.; Ronald, M.; Kasirayi, M. Herbicidal Effects of Datura Stramonium (L.) Leaf Extracts on Amaranthus hybridus (L.) and Tagetes minuta (L.). African J. Agric. Res. 2018, 13, 1754–1760. [Google Scholar] [CrossRef]
- Webb, M. Biological Control of Weeds. A World Catalogue of Agents and Their Target Weeds. Plant Pathol. 1999, 48, 836–837. [Google Scholar] [CrossRef]
- Kumar, D.; Thakur, C.; Bhardwaj, D.R.; Sharma, H.; Sharma, N.; Sharma, P.; Sharma, A. Key Factor of Invasion and Control Measures of Major Exotic Weed Species in Subtropical and Temperate Forest of North Western Himalaya. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 2529–2541. [Google Scholar] [CrossRef]
- Hodgins, K.A.; Bock, D.G.; Rieseberg, L.H. Trait Evolution in Invasive Species. Annu. Plant Rev. Online 2018, 1, 459–496. [Google Scholar]
- Ehlers, B.K.; Berg, M.P.; Staudt, M.; Holmstrup, M.; Glasius, M.; Ellers, J.; Tomiolo, S.; Madsen, R.B.; Slotsbo, S.; Penuelas, J. Plant Secondary Compounds in Soil and Their Role in Belowground Species Interactions. Trends Ecol. Evol. 2020, 35, 716–730. [Google Scholar] [CrossRef]
- Adomako, M.O.; Xue, W.; Du, D.L.; Yu, F.H. Soil biota and soil substrates influence responses of the rhizomatous clonal grass Leymus chinensis to nutrient heterogeneity. Plant Soil 2021, 465, 19–29. [Google Scholar] [CrossRef]
- Xia, Z.; Yu, L.; He, Y.; Korpelainen, H.; Li, C. Broadleaf Trees Mediate Chemically the Growth of Chinese Fir through Root Exudates. Biol. Fertil. Soils 2019, 55, 737–749. [Google Scholar] [CrossRef]
- Clarke, C.R.; Timko, M.P.; Yoder, J.I.; Axtell, M.J.; Westwood, J.H. Molecular Dialog between Parasitic Plants and Their Hosts. Annu. Rev. Phytopathol. 2019, 57, 279–299. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Goncalves, P.; Copeland, E.; Qi, S.S.; Dai, Z.C.; Li, G.L.; Thomas, T. Invasion by the weed Conyza canadensis alters soil nutrient supply and shifts microbiota structure. Soil Biol. Biochem. 2020, 143, 107739. [Google Scholar] [CrossRef]
- Thapa, L.B.; Kaewchumnong, K.; Sinkkonen, A.; Sridith, K. Airborne and Belowground Phytotoxicity of Invasive Ageratina adenophora on Native Species in Nepal. Plant Ecol. 2020, 221, 883–892. [Google Scholar] [CrossRef]
- Fu, D.; Wu, X.; Huang, N.; Duan, C. Effects of the Invasive Herb Ageratina adenophora on Understory Plant Communities and Tree Seedling Growth in Pinus yunnanensis Forests in Yunnan, China. J. For. Res. 2018, 23, 112–119. [Google Scholar] [CrossRef]
- Lithashabin, P.K.; Kumar, P.N.S.; Rajagopal, P.L.; Arthi, I.; Anjana, A.K.; Yamuna, C.V. An Updated Review on Various Pharmacological Activity of Biophytum sensitivum. Lithashabinal. World J. Pharm. Res. 2020, 9, 2510–2520. [Google Scholar]
- Akbar, R.; Khan, I.A. Toxicity of Five Plant Extracts against Callosobruchus maculatus Fab. (Coleoptera Bruchidae) a Major Insect Pest of Stored Pulses. Fresenius Environ. Bull. 2021, 30, 5098–5107. [Google Scholar]
- Adomako, M.O.; Ning, L.; Tang, M.; Du, D.L.; van Kleunen, M.Y.F.H. Diversity-and density-mediated allelopathic effects of resident plant communities on invasion by an exotic plant. Plant Soil 2019, 440, 581–592. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Ding, L.; Kong, C.H. Allelopathy and Allelochemicals in Grasslands and Forests. Forests 2023, 14, 562. [Google Scholar] [CrossRef]
- Aguilera, N.; Guedes, L.M.; Alvarado, U.; Sáez-Carrillo, K. Teline Monspessulana Can Harm the Chilean Native Tree Nothofagus obliqua: Effects on Germination and Initial Growth. Plants 2023, 12, 3419. [Google Scholar] [CrossRef]
- McNichol, B.H.; Russo, S.E. Plant Species’ Capacity for Range Shifts at the Habitat and Geographic Scales: A Trade-Off-Based Framework. Plants 2023, 12, 1248. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Crop Allelopathy for Sustainable Weed Management in Agroecosystems: Knowing the Present with a View to the Future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Zhou, N.; Mu, M.; Yang, M.; Zhou, Y.; Ma, M. The Effect of Microbial Fertilizer on the Growth, Rhizospheric Environment and Medicinal Quality of Fritillaria taipaiensis. Horticulturae 2021, 7, 500. [Google Scholar] [CrossRef]
- Kong, C.H.; Xuan, T.D.; Khanh, T.D.; Tran, H.D.; Trung, N.T. Allelochemicals and Signaling Chemicals in Plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef] [PubMed]
- Chautá, A.; Kessler, A. Metabolic Integration of Spectral and Chemical Cues Mediating Plant Responses to Competitors and Herbivores. Plants 2022, 11, 2768. [Google Scholar] [CrossRef] [PubMed]
- Anten, N.P.R.; Chen, B.J.W. Detect Thy Family: Mechanisms, Ecology and Agricultural Aspects of Kin Recognition in Plants. Plant Cell Environ. 2021, 44, 1059–1071. [Google Scholar] [CrossRef]
- Mofikoya, A.O.; Bui, T.N.T.; Kivimäenpää, M.; Holopainen, J.K.; Himanen, S.J.; Blande, J.D. Foliar Behaviour of Biogenic Semi-Volatiles: Potential Applications in Sustainable Pest Management. Arthropod. Plant. Interact. 2019, 13, 193–212. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Z.; Zhao, S.W.; Wang, X.; Li, Y.S.; Liu, J.N.; Wang, S.; Xi, J.H. The Herbivore-Induced Plant Volatile Tetradecane Enhances Plant Resistance to Holotrichia parallela Larvae in Maize Roots. Pest Manag. Sci. 2022, 78, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefèvre, F.; Pierman, B.; Lynch, J.H.S.; Porter, J.A.; Yanagisawa, M.; Wetzstein, H.Y.; Morgan, J.A.; et al. Emission of Volatile Organic Compounds from Petunia Flowers Is Facilitated by an ABC Transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole Is an Essential Herbivore-Induced Volatile Priming Signal in Maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef]
- Gfeller, V.; Huber, M.; Förster, C.; Huang, W.; Köllner, T.G.; Erb, M. Root Volatiles in Plant–Plant Interactions I: High Root Sesquiterpene Release Is Associated with Increased Germination and Growth of Plant Neighbours. Plant Cell Environ. 2019, 42, 1950–1963. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and Exotic Plant Invasion: From Molecules and Genes to Species Interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef]
- Rezaie, N.; Pallozzi, E.; Ciccioli, P.; Calfapietra, C.; Fares, S. Temperature Dependence of Emission of Volatile Organic Compounds (VOC) from Litters Collected in Two Mediterranean Ecosystems Determined before the Flaming Phase of Biomass Burning. Environ. Pollut. 2023, 338, 122703. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, L.; Chen, C.; Zhou, Y.; Xiao, F.; Wang, Y.; Li, Q. Effect of Plant VOCs and Light Intensity on Growth and Reproduction Performance of an Invasive and a Native Phytolacca Species in China. Ecol. Evol. 2022, 12, e8522. [Google Scholar] [CrossRef] [PubMed]
- Halarewicz, A.; Szumny, A.; Bączek, P. Effect of Prunus Serotina Ehrh. Volatile Compounds on Germination and Seedling Growth of Pinus Sylvestris L. Forests 2021, 12, 846. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Y.; Chen, J.; Ji, J.; He, H. Identification and Comparison of Allelopathic Effects from Leaf and Flower Volatiles of the Invasive Plants Mikania micrantha. Chemoecology 2021, 31, 355–365. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; González, L.; López-Nogueira, A.; Cavaleiro, C.; Pedrol, N. Volatile organic compounds of Acacia longifolia and Their Effects on Germination and Early Growth of Species from Invaded Habitats. Chem. Ecol. 2018, 34, 126–145. [Google Scholar] [CrossRef]
- Akbar, R.; Khan, I.A. Population dynamics of insect pests on six okra varieties in Peshawar. J. Entomol. Zool. Stud. 2015, 3, 91–94. [Google Scholar]
- Akbar, R.; Khan, I.A.; Alajmi, R.A.; Ali, A.; Faheem, B.; Usman, A.; Aboul-Soud, M.A. Evaluation of insecticidal potentials of five plant extracts against the stored grain pest, Callosobruchus maculatus (Coleoptera: Bruchidae). Insects 2022, 13, 1047. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Y.; Jing, Y.; Segar, S.T.; Zhang, Y.; Wang, G.; Chen, J.; Liu, Q.F.; Chen, S.; Chen, Y.; et al. Molecular Mechanisms of Mutualistic and Antagonistic Interactions in a Plant–Pollinator Association. Nat. Ecol. Evol. 2021, 5, 974–986. [Google Scholar] [CrossRef]
- Khan, I.A.; Rasheed, A. Population Dynamics of Natural Enemies Ladybird Beetle Coccinella septumpunctata L. (Coccinellidae: Coleoptera) and Syrphid Fly Episyrphus balteatus Degeer (Syrphidae: Diptera) on Six Okra Cultivars in Peshawar. J. Entomol. Zool. Stud. 2015, 3, 24–26. [Google Scholar]
- Xie, L.J.; Zeng, R.S.; Bi, H.H.; Song, Y.Y.; Wang, R.L.; Su, Y.J.; Chen, M.; Chen, S.; Liu, Y.H. Allelochemical Mediated Invasion of Exotic Plants in China. Allelopath. J. 2010, 25, 31–50. [Google Scholar]
- Saeed, R.; Hassan, M.W.U.; Jaleel, W.; Ikhlaq, M.; Ali Shah, S.I.; Niaz, S.; Azad, R.; Akbar, R.; Mahmood, Z.; Mukhtar, A.; et al. Influence of Natural and Non-Natural Diets on the Fitness and Rearing of Pectinophora gossypiella Saunders. Sci. Rep. 2023, 13, 13666. [Google Scholar] [CrossRef]
- Zhou, T.X.; Yang, M.L.; Gu, F. Antifeeding Activity Determination of Eupatorium Adenophorum Extracts on Pieris Rapae Larvae. J. Yunnan Agric. Univ. 2003, 18, 259–263. [Google Scholar]
- Sintim, H.O.; Ansah, K.D. Effects of Biopesticides Extracted with a Homemade Solvent on Stored Maize Protection. Agric. Trop. Subtrop. 2023, 56, 125–142. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, G.; Li, G. Studies on the Chemical Constituents of Eupatorium Adenophorum Spreng. Nat. Prod. Res. Dev. 1997, 9, 35–39. [Google Scholar]
- Coutinho, H.D.M.; de Morais Oliveira-Tintino, C.D.; dos Santos, J.F.S. Toxicity against Drosophila melanogaster and Antiedematogenic and Antimicrobial Activities of Alternanthera brasiliana (L.) Kuntze (Amaranthaceae). Environ. Sci. Pollut. Res. 2018, 25, 10353–10361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fan, Z.; Xue, W.; Sun, F.; Zhu, H.; Huang, D.; Wang, Z.; Dong, L. Vitexin Regulates Epac and Nlrp3 and Ameliorates Chronic Cerebral Hypoperfusion Injury. Can. J. Physiol. Pharmacol. 2021, 99, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, H.A.; Saad, A.S.; Mourad, A.K.; Taman, F.A.; Mohamed, I.B. Joint Action of Quercetin with Four Insecticides on the Cotton Leaf-Worm Larvae, Spodoptera littoralis Boisd. (Lep.: Noctuidae) in Egypt. Commun. Agric. Appl. Biol. Sci. 2007, 72, 445–457. [Google Scholar]
- Gade, S.; Rajamanikyam, M.; Vadlapudi, V.; Nukala, K.M.; Aluvala, R.; Upadhyayula, S.M. Acetylcholinesterase Inhibitory Activity of Stigmasterol & Hexacosanol Is Responsible for Larvicidal and Repellent Properties of Chromolaena odorata. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 541–550. [Google Scholar]
- Zolotar’, R.M.; Bykhovets, A.I.; Sokolov, S.N.; Kovganko, N.V. Structure-Activity Relationship of Insecticidal Steroids. IV. 3β-Chlorosubstituted Derivatives of Cholesterol and β-Sitosterol. Chem. Nat. Compd. 2002, 38, 70–73. [Google Scholar] [CrossRef]
- Ahmed, M.; Qin, P.; Ji, M.; An, R.; Guo, H.; Shafi, J. Spinasterol, 22,23-Dihydrospinasterol and Fernenol from Citrullus Colocynthis L. With Aphicidal Activity against Cabbage Aphid Brevicoryne brassicae L. Molecules 2020, 25, 2184. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.Q.; Yan, S.Y.; Pan, W.J.; Zhang, M.X.; Cai, Q.N. Interaction of Ferulic Acid with Glutathione S-Transferase and Carboxylesterase Genes in the Brown Planthopper, Nilaparvata Lugens. J. Chem. Ecol. 2017, 43, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Dong, B.; Yuan, X.; Guo, Y.; Zhang, L.; Zhao, B. Simultaneous Detoxification and Preparative Separation of Chlorogenic Acid from Eupatorium adenophorum by Combined Column Chromatography. Sep. Sci. Technol. 2017, 52, 1114–1121. [Google Scholar] [CrossRef]
- Kundu, A.; Saha, S.; Walia, S.; Dutta, T.K. Antinemic Potentiality of Chemical Constituents of Eupatorium adenophorum Spreng Leaves Against Meloidogyne incognita. Natl. Acad. Sci. Lett. 2016, 39, 145–149. [Google Scholar] [CrossRef]
- Okunade, A.L. Ageratum Conyzoides, L. (Asteraceae). Fitoterapia 2002, 73, 1–16. [Google Scholar] [CrossRef]
- Ramasamy, V.; Karthi, S.; Ganesan, R.; Prakash, P.; Senthil-Nathan, S.; Umavathi, S.; Krutmuang, P.; Vasantha-Srinivasan, P. Chemical Characterization of Billy Goat Weed Extracts Ageratum conyzoides (Asteraceae) and Their Mosquitocidal Activity against Three Blood-Sucking Pests and Their Non-Toxicity against Aquatic Predators. Environ. Sci. Pollut. Res. 2021, 28, 28456–28469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A.K.; Kumar, B.; Shakya, M.; Patel, J.A.; Kumar, B.; Bisht, N.; Chigure, G.M.; Singh, K.; Kumar, R.; et al. Characterization of Deltamethrin, Cypermethrin, Coumaphos and Ivermectin Resistance in Populations of Rhipicephalus Microplus in India and Efficacy of an Antitick Natural Formulation Prepared from Ageratum conyzoides. Ticks Tick. Borne. Dis. 2021, 12, 101818. [Google Scholar] [CrossRef]
- Kathare, J.M.; Mbaria, J.M.; Nguta, J.M.; Moriasi, G.A.; Mainga, A.O. Antimicrobial Efficacy, Cytotoxicity, Acute Oral Toxicity, and Phytochemical Investigation of the Aqueous and Methanolic Stem Bark Extracts of Bridellia Micrantha (Hochst.) Baill. Pharmacogn. J. 2021, 13, 1248–1256. [Google Scholar] [CrossRef]
- Zhang, M.; Ling, B.; Kong, C.; Pang, X.; Liang, G. Chemical Components of Volatile Oil from Mikania Micrantha and Its Biological Activity on Insects. Chin. J. Appl. Ecol. 2003, 14, 93–96. [Google Scholar] [CrossRef]
- Feng, H.L.; Yang, C.J.; Zhang, X.; Ye, W.H. Preliminary Studies on the Bioactivity of Crude Extract from Mikania Micrantha on Insect and Plant, Pathogen. Acta Sci. Nat. Univ. Sunyatseni 2004, 43, 82–85. [Google Scholar]
- Gorawade, V.; Attar, U.; Shiragave, P. Bioefficacy and GC-MS Analysis of Chromolaena Odorata and Leonotis Nepetifolia Leaf Extracts against Spodoptera Litura. J. Crop Prot. 2022, 11, 361–375. [Google Scholar]
- Qasim, M.; Islam, W.; Ashraf, H.J.; Ali, I.; Wang, L. Saponins in Insect Pest Control BT—Co-Evolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2020; pp. 897–924. [Google Scholar]
- Olawale, F.; Olofinsan, K.; Iwaloye, O. Biological Activities of Chromolaena Odorata: A Mechanistic Review. S. Afr. J. Bot. 2022, 144, 44–57. [Google Scholar] [CrossRef]
- Gomes, L.d.C.M.; Prado, J.C.S. Survey of Species Cultivated in Brazil and Their Biological Applications: A Review. S. Asian J. Res. Microbiol. 2023, 16, 38–53. [Google Scholar] [CrossRef]
- Ali Khan, I.; Khan, H.; Khan, I.; Akbar, R.; Alam, M.; Saeed, M.; Farid, A.; Ali, I.; Habib, K.; Fayaz, W. Efficacy of Some Plant Extracts on Larval Mortality of Culex quinquefasciatus (Say) (Diptera: Culicidae) in Peshawar. J. Entomol. Zool. Stud. 2015, 3, 331–333. [Google Scholar]
- Gorawade, V.B.; Attar, U.A.; Shiragave, P.D. Insecticidal Potential and Thin Layer Chromatographic Profiling of Chromolaena odorata L. and Leonotis nepetifolia (L.) R.Br. Leaf Extracts against Helicoverpa armigera (Hubner). Int. J. Entomol. Res. 2021, 6, 46–51. [Google Scholar]
- Gautam, S.; Khanal, S.; Khanal, D.; Mishra, S.R.; Ghimire, S. Phytochemical Screening of Selected Botanicals and Their Effectiveness Against Maize Weevil (Sitophilus zeamais Motsch.) at Paklihawa, Rupandehi, Nepal. Adv. J. Grad. Res. 2021, 11, 34–44. [Google Scholar] [CrossRef]
- Chinnathambi, A.; Ali Alharbi, S.; Lavarti, R.; Jhanani, G.K.; On-uma, R.; Jutamas, K.; Anupong, W. Larvicidal and Pupicidal Activity of Phyto-Synthesized Zinc Oxide Nanoparticles against Dengue Vector Aedes aegypti. Environ. Res. 2023, 216, 114574. [Google Scholar] [CrossRef]
- Akbar, R.; Afzal, S.; Sun, J.; Faheem, B.; Bibi, R.; Azad, R.; Farid, A.; Ahmad, H.I.; Ataya, F.S.; Khan, M.A.; et al. Efficacy of Various Plant Extracts and Synergism Against Domestic Species of Rice Weevil Sitophilous oryzae (Curculionidae: Coleoptera). Polish J. Environ. Stud. 2024, 33, 3033–3044. [Google Scholar] [CrossRef]
- Akbar, R.; Faheem, B.; Aziz, T.; Ali, A.; Ullah, A.; Khan, I.A.; Sun, J. Evaluating the Efficacy of Plant Extracts in Managing the Bruchid Beetle, Callosobruchus maculatus (Coleoptera: Bruchidae). Insects 2024, 15, 691. [Google Scholar] [CrossRef]
- Kardol, P.; Wardle, D.A. How Understanding Aboveground-Belowground Linkages Can Assist Restoration Ecology. Trends Ecol. Evol. 2010, 25, 670–679. [Google Scholar] [CrossRef]
- Mandle, L.; Bufford, J.L.; Schmidt, I.B.; Daehler, C.C. Woody Exotic Plant Invasions and Fire: Reciprocal Impacts and Consequences for Native Ecosystems. Biol. Invasions 2011, 13, 1815–1827. [Google Scholar] [CrossRef]
- More, S.; Shinde, S.; Kasture, M. Root Exudates a Key Factor for Soil and Plant: An Overview. Pharma Innov. J. 2019, 8, 449–459. [Google Scholar]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and Allelochemicals of Leucaena leucocephala as an Invasive Plant Species. Plants 2022, 11, 1672. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, N.; Benin, B.M.; Mudarmah, K.; Pant, B.D.; Chen, G.; Shin, W.S.; Kim, M.H.; Huang, S.D. Harnessing the Dual Antimicrobial Mechanism of Action with Fe(8-Hydroxyquinoline)3 to Develop a Topical Ointment for Mupirocin-Resistant MRSA Infections. Antibiotics 2023, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- McLeod, M.L.; Bullington, L.; Cleveland, C.C.; Rousk, J.; Lekberg, Y. Invasive Plant-Derived Dissolved Organic Matter Alters Microbial Communities and Carbon Cycling in Soils. Soil Biol. Biochem. 2021, 156, 108191. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Klironomos, J.N. Breaking New Ground: Soil Communities and Exotic Plant Invasion. Bioscience 2005, 55, 477–487. [Google Scholar] [CrossRef]
- Yu, L.Q.; Fu, Y.; Zhou, Y.J.; Zhang, J.P.; Lu, Y.L.; Xuan, S.N. Comparison of Allelopathy Potential between an Exotic Invasive Weed Alternanthera philoxeroides and a Local Weed Alternanthera Sessilis. Chin. J. Rice Sci. 2007, 21, 84–89. [Google Scholar]
- Vishwakarma, K.; Sharma, S.; Kumar, V.; Upadhyay, N.; Kumar, N.; Mishra, R.; Yadav, G.; Verma, R.K.; Tripathi, D.K. Current Scenario of Root Exudate-Mediated Plant-Microbe Interaction and Promotion of Plant Growth. In Probiotics Agroecosystem; Springer: Berlin/Heidelberg, Germany, 2017; pp. 349–369. [Google Scholar]
- El-Kamali, H.H. Acute Toxicity of Lantana camara Leaves in Goats. Small Rumin. Res. 2005, 58, 107–110. [Google Scholar]
- Caplan, J.S.; Yeakley, J.A. Rubus armeniacus (Himalayan Blackberry) Occurrence and Growth in Relation to Soil and Light Conditions in Western Oregon. Northwest Sci. 2006, 80, 9–17. [Google Scholar]
- Frost, C.M.; Callaway, R.M. The Palatability and Competition of a Toxic Plant, Centaurea maculosa, to Generalist and Specialist Herbivores. Ecology 2007, 88, 943–950. [Google Scholar]
- Fletcher, R.A.; Callaway, R.M.; Atwater, D.Z. An Exotic Invasive Plant Selects for Increased Competitive Tolerance, but Not Competitive Suppression, in a Native Grass. Oecologia 2016, 181, 499–505. [Google Scholar] [CrossRef]
- Li, W.; Zheng, Y.; Zhang, L.; Lei, Y.; Li, Y.; Liao, Z.; Li, Z.; Feng, Y. Postintroduction Evolution Contributes to the Successful Invasion of Chromolaena odorata. Ecol. Evol. 2020, 10, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Moreno Jiménez, E.; Ferrol, N.; Corradi, N.; Peñalosa, J.M.; Rillig, M.C. The Potential of Arbuscular Mycorrhizal Fungi to Enhance Metallic Micronutrient Uptake and Mitigate Food Contamination in Agriculture: Prospects and Challenges. New Phytol. 2023, 242, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, H.; He, Z.; Zhang, F.; Li, L.; Wang, N.; Mao, D. Medical Therapy of Hearing Impairment and Tinnitus with Chinese Medicine: An Overview. Chin. J. Integr. Med. 2023, 29, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, Q.; Dai, S.; Meng, L.; He, M.; Chen, S.; Zhao, C.; Dan, X.; Cai, Z.; Zhang, J.; et al. Effects of Solidago canadensis L. on Mineralization-Immobilization Turnover Enhance Its Nitrogen Competitiveness and Invasiveness. Sci. Total Environ. 2023, 882, 163641. [Google Scholar] [CrossRef]
- Roche, M.D.; Pearse, I.S.; Sofaer, H.R.; Kivlin, S.N.; Spyreas, G.; Zaya, D.N.; Kalisz, S. Invasion-Mediated Mutualism Disruption Is Evident across Heterogeneous Environmental Conditions and Varying Invasion Intensities. Ecography 2023, 2023, e06434. [Google Scholar] [CrossRef]
- Honor, R.; Marcellus, M.; Colautti, R.I. Direct and Indirect Fitness Effects of Competition Limit Evolution of Allelopathy in an Invading Plant. bioRxiv 2023, 6, 2023-06. [Google Scholar]
- Mettler, C.A.; Carlson, B.E. Direction of Alliaria petiolata (Garlic Mustard) Leachate’s Effect on Early Litter Mass Loss Is Dependent upon Saprotrophic Community Composition. Bios 2023, 94, 20–29. [Google Scholar] [CrossRef]
- Kaur, S.; Campbell, B.J.; Suseela, V. Root Metabolome of Plant–Arbuscular Mycorrhizal Symbiosis Mirrors the Mutualistic or Parasitic Mycorrhizal Phenotype. New Phytol. 2022, 234, 672–687. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef]
- Liao, D.; Wang, S.; Cui, M.; Liu, J.; Chen, A.; Xu, G. Phytohormones Regulate the Development of Arbuscular Mycorrhizal Symbiosis. Int. J. Mol. Sci. 2018, 19, 3146. [Google Scholar] [CrossRef]
- Wu, F.; Gao, Y.; Yang, W.; Sui, N.; Zhu, J. Biological Functions of Strigolactones and Their Crosstalk with Other Phytohormones. Front. Plant Sci. 2022, 13, 821563. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Mingtao, T.; Shuai, W.; Lin, Z.; Qing, W.; Guirong, W.; Shanchun, Y. Defense Responses of Arbuscular Mycorrhizal Fungus-Colonized Poplar Seedlings against Gypsy Moth Larvae: A Multiomics Study. Hortic. Res. 2021, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Nihranz, C.T.; Kolstrom, R.L.; Kariyat, R.R.; Mescher, M.C.; De Moraes, C.M.; Stephenson, A.G. Herbivory and Inbreeding Affect Growth, Reproduction, and Resistance in the Rhizomatous Offshoots of Solanum carolinense (Solanaceae). Evol. Ecol. 2019, 33, 499–520. [Google Scholar] [CrossRef]
- Enebe, M.C.; Erasmus, M. Symbiosis—A Perspective on the Effects of Host Traits and Environmental Parameters in Arbuscular Mycorrhizal Fungal Richness, Colonization and Ecological Functions. Agriculture 2023, 13, 1899. [Google Scholar] [CrossRef]
- Zhang, M.; Otsuki, K.; Li, W. Molecular Networking as a Natural Products Discovery Strategy. Acta Mater. Medica 2023, 2, 126–141. [Google Scholar] [CrossRef]
- Afreen, T.; Kumari, S.; Bhadouria, R.; Devi, R.S.; Singh, S.; Tripathi, S. Plant Invasion and Soil Processes: A Mechanistic Understanding. In Plant Invasions and Global Climate Change; Springer: Singapore, 2023; pp. 227–246. [Google Scholar]
- Miles, J.; Allen, E.B. The Reconstruction of Disturbed Arid Lands. An Ecological Approach. In The Journal of Applied Ecology; Routledge: London, UK, 1989; Volume 26, p. 1089. [Google Scholar]
- Cipollini, K.; Greenawalt Bohrer, M. Comparison of Allelopathic Effects of Five Invasive Species on Two Native Species. J. Torrey Bot. Soc. 2016, 143, 427–436. [Google Scholar] [CrossRef]
- Munakata, R.; Larbat, R.; Duriot, L.; Olry, A.; Gavira, C.; Mignard, B.; Hehn, A.; Bourgaud, F. Polyphenols from Plant Roots: An Expanding Biological Frontier. Recent Adv. Polyphen. Res. 2019, 6, 207–236. [Google Scholar]
- Qin, F.; Yu, S. Arbuscular Mycorrhizal Fungi Protect Native Woody Species from Novel Weapons. Plant Soil 2019, 440, 39–52. [Google Scholar] [CrossRef]
- Bunn, R.A.; Ramsey, P.W.; Lekberg, Y. Do Native and Invasive Plants Differ in Their Interactions with Arbuscular Mycorrhizal Fungi? A Meta-Analysis. J. Ecol. 2015, 103, 1547–1556. [Google Scholar] [CrossRef]
- Funk, J.L. The Physiology of Invasive Plants in Low-Resource Environments. Conserv. Physiol. 2013, 1, cot026. [Google Scholar] [CrossRef]
- Huang, K.; Kong, D.L.; Lu, X.R.; Feng, W.W.; Liu, M.C.; Feng, Y.L. Lesser Leaf Herbivore Damage and Structural Defense and Greater Nutrient Concentrations for Invasive Alien Plants: Evidence from 47 Pairs of Invasive and Non-Invasive Plants. Sci. Total Environ. 2020, 723, 137829. [Google Scholar] [CrossRef] [PubMed]
- Van Kleunen, M.; Weber, E.; Fischer, M. A Meta-Analysis of Trait Differences between Invasive and Non-Invasive Plant Species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, H.; Wang, S.; Wei, M.; Du, D. Plant Community and the Influence of Plant Taxonomic Diversity on Community Stability and Invasibility: A Case Study Based on Solidago Canadensis L. Sci. Total Environ. 2021, 768, 144518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular Mycorrhizal Fungi Conducting the Hyphosphere Bacterial Orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef]
- Greer, M.J.; Wilson, G.W.T.; Hickman, K.R.; Wilson, S.M. Experimental Evidence That Invasive Grasses Use Allelopathic Biochemicals as a Potential Mechanism for Invasion: Chemical Warfare in Nature. Plant Soil 2014, 385, 165–179. [Google Scholar] [CrossRef]
- Tian, B.; Pei, Y.; Huang, W.; Ding, J.; Siemann, E. Increasing Flavonoid Concentrations in Root Exudates Enhance Associations between Arbuscular Mycorrhizal Fungi and an Invasive Plant. ISME J. 2021, 15, 1919–1930. [Google Scholar] [CrossRef]
- Ozturk, M.; Bhat, R.A.; Ashraf, M.; Tonelli, F.M.P.; Unal, B.T.; Dar, G.H. Phytohormones and Stress Responsive Secondary Metabolites; Nikki Levy: Boca Raton, FL, USA, 2023; pp. 1–291. [Google Scholar]
- Plett, J.M.; Plett, K.L.; Wong-Bajracharya, J.; de Freitas Pereira, M.; Costa, M.D.; Kohler, A.; Martin, F.; Anderson, I.C. Mycorrhizal Effector PaMiSSP10b Alters Polyamine Biosynthesis in Eucalyptus Root Cells and Promotes Root Colonization. New Phytol. 2020, 228, 712–727. [Google Scholar] [CrossRef]
- He, J.; Zhang, C.; Dai, H.; Liu, H.; Zhang, X.; Yang, J.; Wang, E. A LysM Receptor Heteromer Mediates Perception of Arbuscular Mycorrhizal Symbiotic Signal in Rice. Mol. Plant 2019, 12, 1561–1576. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Feng, Y.L.; Zhang, L.K.; Callaway, R.M.; Valiente-Banuet, A.; Luo, D.Q. Integrating Novel Chemical Weapons and Evolutionarily Increased Competitive Ability in Success of a Tropical Invader. New Phytol. 2015, 205, 1350–1359. [Google Scholar] [CrossRef]
- Martin, F.M.; Uroz, S.; Barker, D.G. Ancestral alliances: Plant mutualistic symbioses with fungi and bacteria. Science 2017, 356, eaad4501. [Google Scholar] [CrossRef]
- Hickman, D.T.; Rasmussen, A.; Ritz, K.; Birkett, M.A.; Neve, P. Review: Allelochemicals as Multi-Kingdom Plant Defence Compounds: Towards an Integrated Approach. Pest Manag. Sci. 2021, 77, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.P.; Fowler, S.V.; Subbaraj, A.K.; Groenteman, R.; Clavijo-McCormick, A. Metabolomic Analysis of Host Plant Biochemistry Could Improve the Effectiveness and Safety of Classical Weed Biocontrol. Biol. Control 2021, 160, 104663. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.R.; Molinillo, J.M.G. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef] [PubMed]
- Ghidoli, M.; Pesenti, M.; Colombo, F.; Nocito, F.F.; Pilu, R.; Araniti, F. Camelina sativa (L.) Crantz as a Promising Cover Crop Species with Allelopathic Potential. Agronomy 2023, 13, 2187. [Google Scholar] [CrossRef]
- Lenda, M.; Steudel, B.; Skórka, P.; Zagrodzka, Z.B.; Moroń, D.; Bączek-Kwinta, R. Multiple Invasive Species Affect Germination, Growth, and Photosynthesis of Native Weeds and Crops in Experiments. Sci. Rep. 2023, 13, 22146. [Google Scholar] [CrossRef]
- Rogers, J.G.D.; Plagányi, É.E.; Babcock, R.C.; Fletcher, C.S.; Westcott, D.A. Improving Coral Cover Using an Integrated Pest Management Framework. Ecol. Appl. 2023, 33, e2913. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Fan, R.; Naz, H.; Bamisile, B.S.; Hafeez, M.; Ghani, M.I.; Wei, Y.; Xu, Y.; Chen, X. Insights into Insecticide-Resistance Mechanisms in Invasive Species: Challenges and Control Strategies. Front. Physiol. 2023, 13, 1112278. [Google Scholar] [CrossRef]

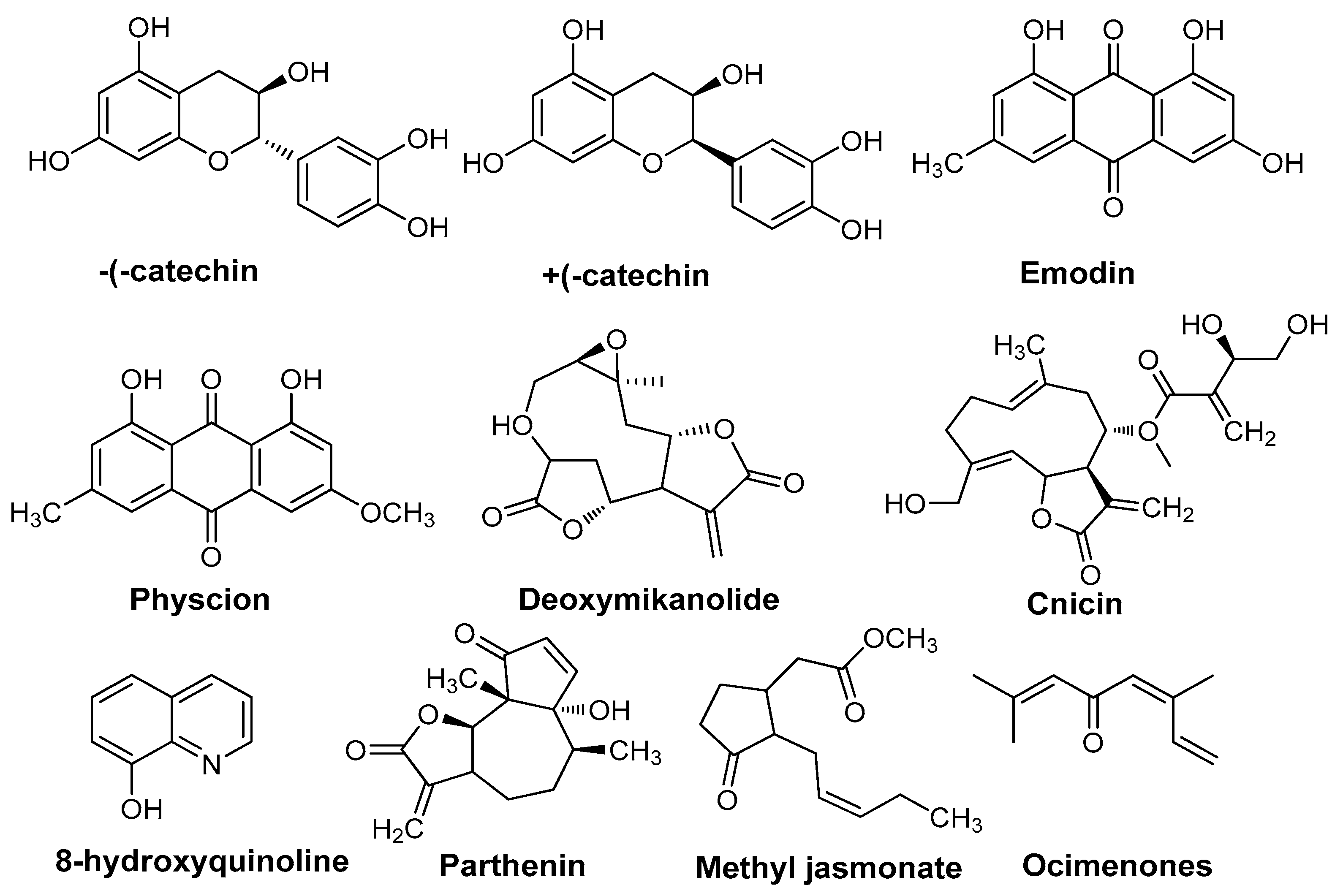


| Plant Species | Category | Compounds | Mechanism | References |
|---|---|---|---|---|
| Artemisia tridentata Nutt. | Volatile organic compounds | Methyl jasmonate | Activates expression of defense genes | [70] |
| Alliaria petiolate (M.Bieb.) Cavara & Grande | Phenolic compounds | Glucosinolates (sinigrin) | Mycorrhiza are suppressed by sinigrin, which breaks their mutualistic relationships with native plants. | [73] |
| Ageratum conyzoides L. | P-coumaric acid, gallic acid, ferulic acid, p-hydroxybenzoic acid, and anisic aci | Rice growth was adversely influenced by phytotoxins released into the soil rhizosphere by A. conyzoides residues and root exudates. | [74] | |
| Cymbopogon nardus (L.) Rendle | N-Octanoyl tyramine | Inhibits ripening of Lepidium sativum, L. sativa, Echinochloa crusgalli, Lolium multiflorum | [49] | |
| Juglans nigra L. | Juglone | Inhibitor of the essential enzyme for the formation of plastoquinone, hydroxyphenylpyruvate dioxygenase (HPPD), as well as other plants’ photosynthetic and respiratory electron transport systems | [47] | |
| Secale cereale L. | Alkaloid compounds | Benzoxazinoid | Boosts benzoxazinoids’ synthesis and exudation from roots in reaction to nearby plants | [75] |
| Echium plantagineum L. | Pyrrolidine and Naphthoquinones | Provide a competitive edge over weeds and protect against livestock and insect herbivory. | [75] | |
| Senecio jacobaea L. | Pyrrolizidine | Increased alkaloids produced in non-native range compared to native range; protection against generalists | [75] | |
| Imperata cylindrica (L.) P. Beauv. | Tarpenes | Tabanone, 4-(2-butenylidene)-3,5,5-trimethyl-2-cyclohexen-1-one; cogongrass, | Impeded the growth of the garden onion’s roots, the lesser duckweed’s frond area, and the garden lettuce’s fresh weight gain. | [76] |
| Invasive Plant Species | Allelochemicals | Mode of Action | Effected Plants | References |
|---|---|---|---|---|
| Solidago canadensis L. | Kaempferol-3-O-d-glucoside | Growth | Arabidopsis thaliana (L.) Heynh., Echinochloa colona L. | [93] |
| Ageratina adenophora (Spreng). | Propan-2-ylidene (4,7-dimethyl-1-) tetrahydronaphthalene-1,4,4a, 8a, 2(1H, 7H) DTD and 6-hydroxy-5-isopropyl-3 | Growth and development | Osbeckia stellate buch. HAM. EX D. DON and Elsholtzia blanda Benth.) Benth. | [109] |
| Polygonum cuspidatum Sieb. et Zucc | (−)-catechin, (−)-epicatechin, resveratroloside, and piceatannol | Growth | Lepidium sativum L. | [110] |
| Chromolaena odoratum L. | Globulol, α-cadinal, 1-hexadecanol, caryophyllene, (−)-spathulenol, and caryphyllene oxide hexadecane | Growth | Eleusine indica (L.) Gaertn, Cyperus iria L., and Ageratum conyzoides L. | [111] |
| Ambrosia artemisiifolia L. | α-pinent, β-pinene, cineole, camphene, spanthueol | Germinations and root growth | Zea mays L. (Corn), Triticum aestivum L. and Oryza sativa L. | [112] |
| Ageratum conyzoides L. | Precocenes, sesquiterpenes, Gallic acid, proteocatechins acid and coumaric acid, | Germination up to 89% | Parthenium hysterophorus L. | [113] |
| Conyza bonariensis (L.) Cronquist | (4Z)-lachnophyllum lactone | Suppression of growth | Cuscuta campestris L. | [114] |
| Eucalyptus camaldulensis Dehnh. | Syringic acid, vanillic acid, gentisic, gallic, p-coumaric, p-hydroxybenzoic, and catechol | Suppression of germination and growth | Portulaca oleracea L. | [46] |
| Eichhornia colona L. | Tricin | Inhibit germination and seedling growth | Glycine max L. and Oryzae sativa L. | [16] |
| Eucalyptus globulus Labill. | Kaempferol 3-O-glucoside, hyperoside, and shikimic-succinic acids | Inhibit germination, growth and physiological parameters | Agrostis stolonifera L. | [115] |
| Mikania micrantha Kunth. | Dihydromikanolide, deoxymikanolide, 2,3-epoxy-1-hydroxy4,9-germacradiene12, 8:15,6-diolide. | Limit the length of the radicle and shoot. | Trifolium repens L., Raphanus sativus L., and Lolium perenne L. | [104] |
| Parthenium hysterophorus L. | Caffeic acid, parthenin | Suppress the growth of seedlings and germination | Digitaria sanguinalis (L.) Scop. and Eleusine indica (L.) Gaertn | [116] |
| Asystasia gangetica L. | (6R,9S)-3-oxo-α-ionol and indole-3-carboxaldehyde | Cause 10% yield reduction | Cucumis sativus L. | [117] |
| Artemisia annuas L. | Artemisinin | Prevent development and expansion of the roots | Ipomoea lacunose L., Lactuca sativa L., Portulaca oleracea L. | [118] |
| Bidens pilosa L. | Terpenes, phenolic acids, polyacetylenes, flavonoids, and fatty acids | Inhibit the growth | Zea mays L., Sorghum bicolor (L.) Moench., Lactuca sativa L, and Vigna radiate (L.) R. Wilczek | [119] |
| Brachiaria mutica (Forssk.) Stapf | Tannin, saponin | Germination and growth suppression | Mimosa pudica L. | [120] |
| Cyperus rotundus L. | Quercetin, luteolin, chrysin, rutin, myricitrin, catechin, apigenin, and chlorogenic acid | Lowers yield by 93% and 86% | Oryza sativa L. | [121] |
| Pueraria montana (Lour.) Merr. | 12(13)-dien-bisabolene, 7-carboxy-8-hydroxy-1(2), and (-)-hamanasic acid A | Germination and Growth | Lactuca sativa L. and Raphanus sativa L., Bidens pilosa L. and Lolium perenne L. | [122] |
| Datura stramonium L. | Tropane alkaloids, Scopolamine, Hyoscyamine | Germination and growth | Tagetes minuta L. and Amaranthus hybridus L. | [123] |
| Juglans nigra L. | Juglone | Herbicidal activities | Sonchus arvensis L., Cirsium arvense L, Papaver rhoeas L., Lamium amplexicaule L. | [124] |
| Invasive Plants Species | Negative Effect on Receiver Plant Species | Receiver Plants Species | References |
|---|---|---|---|
| Phytolacca americana | Adverse effects on reproductive and morphological features | Phytolacca acinosa | [153] |
| Prunus serotina | Prevented the elongation of the roots, shoots, and germination | Pinus sylvestris | [154] |
| Mikania micrantha | Decreased rate of germination reduced levels of chlorophyll and reduced levels of malondialdehyde and reduced activity of superoxide dismutase | Abutilon theophrasti, Bidens pilosa, Chrysanthemum coronarium and Lactuca sativa | [155] |
| Ageratina adenophora | Reduced germination rate and limited height of seedlings reduced biomass of the shoots and roots | Schima wallichii | [132] |
| Acacia longifolia | Reduced biomass, shoot length, and root length | Lolium multiflorum, Plantago lanceolata and Trifolium subterraneum | [156] |
| Invasive Plant Species | Novel Compounds | Impact on Soil Microbe | References |
|---|---|---|---|
| Solidago gigantean Aiton. | Sesquiterpene lactones | Affect soil microbial communities and inhibit microbial activity. | [151] |
| Lantana camara L. | Lantadene A | Disrupt microbial symbioses and alter soil microbial communities. | [200] |
| Rubus armeniacus Focke. | Ellagic acid | Allelopathic and antimicrobial effects on soil microbial populations. | [201] |
| Centaurea maculosa L. | Cnicin | Antifungal and antibacterial properties, affecting soil microbial composition. | [202] |
| Alliaria petiolate (M.Bieb.) Cavara & Grande | Glucosinolates (sinigrin) | Sinigrin suppresses mycorrhiza, therefore disrupting their mutualistic associations with native plants | [73] |
| Phragmites australis (Cav.) Trin. ex Steud. | Catechins | Influence microbial decomposition processes and soil nutrient cycling. | [203] |
| Chromolaena odorata L. | Acutellerin-40, 6,7-trimethy ether, 40, 5,6,7- tetramethoxyflavone, isosakuranetin | Greater amounts of flavonoids in the non-native range provide competitive advantages and better defense against soil borne pathogens | [204] |
| Sr. No | Examples | Mechanism | References |
|---|---|---|---|
| 1 | Parthenium hysterophorus L., an invasive plant, may develop far more quickly than crops like Sorghum bicolor L. Moench) and Zea mays L. | Species competition | [20] |
| 2 | When 19 paired invasive and native plants in Hawaii were compared for resource usage efficiency, it was found that invasive plants had better rates of carbon absorption, light use, immediate nitrogen, and energy use. | [225] | |
| 3 | Invasive plants have larger leaf nitrogen contents are less damaged by herbivores, according to comparisons between 47 paired invasive and non-invasive species’ leaf herbivore resistance and nutrient content. | [226] | |
| 4 | When 125 invasive plants and 196 non-invasive plants are compared physiologically, that invasive plants are more advantageous in terms of growth rate, resource allocation, and stress resistance. | [227] | |
| 5 | Plantanum carolinense L., Solanum carolinense L. is an exotic plant with great cold resistance and asexual reproduction. | [228] | |
| 6 | Solidago canadensis L. is an invasive plant that can benefit from increasing nitrogen deposition and climate warming by acquiring more leaf resources. | [229] | |
| 7 | Leachate of the invasive plant Bothriochloa ischaemum L. Keng prevents native species Schizachyrium scoparium (Michx.) Nash and Andropogon gerardii L. from germinating and growing | Allelochemicals | [230] |
| 8 | Lactuca sativa L., a native plant, seed germination and seedling growth inhibited by allelochemicals released by S. canadensis L. invasion | [155] | |
| 9 | Crystals of solanine and oxalate are found in the exotic plant Solanum carolinense L. | [104] | |
| 10 | To aid in its invasion, P. hysterophorus L. can release parthenin, vanillic acid, caffeic acid, and other allelochemicals | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, R.; Sun, J.; Bo, Y.; Khattak, W.A.; Khan, A.A.; Jin, C.; Zeb, U.; Ullah, N.; Abbas, A.; Liu, W.; et al. Understanding the Influence of Secondary Metabolites in Plant Invasion Strategies: A Comprehensive Review. Plants 2024, 13, 3162. https://doi.org/10.3390/plants13223162
Akbar R, Sun J, Bo Y, Khattak WA, Khan AA, Jin C, Zeb U, Ullah N, Abbas A, Liu W, et al. Understanding the Influence of Secondary Metabolites in Plant Invasion Strategies: A Comprehensive Review. Plants. 2024; 13(22):3162. https://doi.org/10.3390/plants13223162
Chicago/Turabian StyleAkbar, Rasheed, Jianfan Sun, Yanwen Bo, Wajid Ali Khattak, Amir Abdullah Khan, Cheng Jin, Umar Zeb, Najeeb Ullah, Adeel Abbas, Wei Liu, and et al. 2024. "Understanding the Influence of Secondary Metabolites in Plant Invasion Strategies: A Comprehensive Review" Plants 13, no. 22: 3162. https://doi.org/10.3390/plants13223162
APA StyleAkbar, R., Sun, J., Bo, Y., Khattak, W. A., Khan, A. A., Jin, C., Zeb, U., Ullah, N., Abbas, A., Liu, W., Wang, X., Khan, S. M., & Du, D. (2024). Understanding the Influence of Secondary Metabolites in Plant Invasion Strategies: A Comprehensive Review. Plants, 13(22), 3162. https://doi.org/10.3390/plants13223162










