Hydroethanolic Extract of Polygonum aviculare L. Mediates the Anti-Inflammatory Activity in RAW 264.7 Murine Macrophages Through Induction of Heme Oxygenase-1 and Inhibition of Inducible Nitric Oxide Synthase
Abstract
:1. Introduction
2. Results
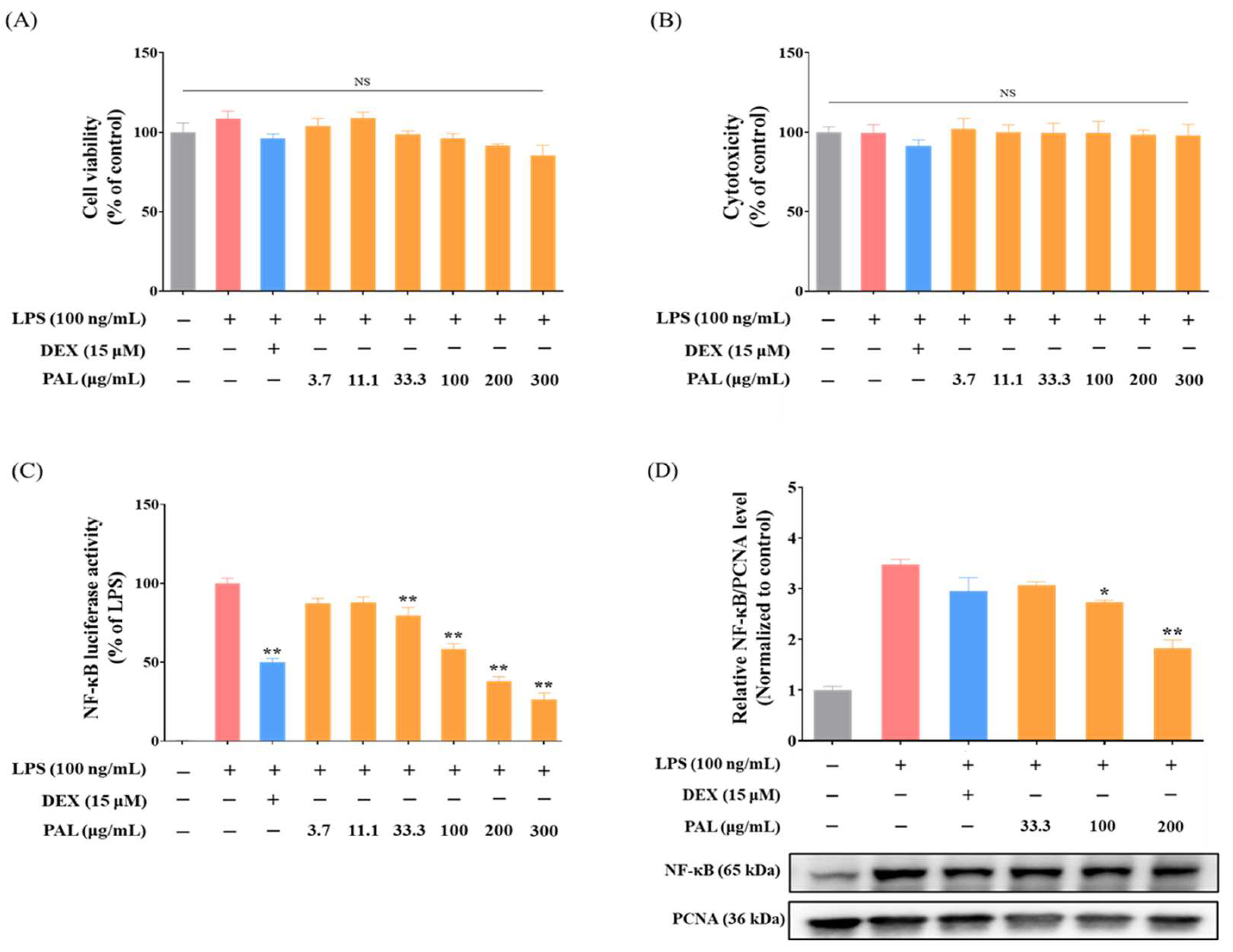
2.1. PAL Suppresses NF-κB Transcriptional Activity in LPS-Activated RAW 264.7 Macrophages
2.2. PAL Down-Regulates COX-2 and iNOS Expression in LPS-Activated RAW 264.7 Macrophages
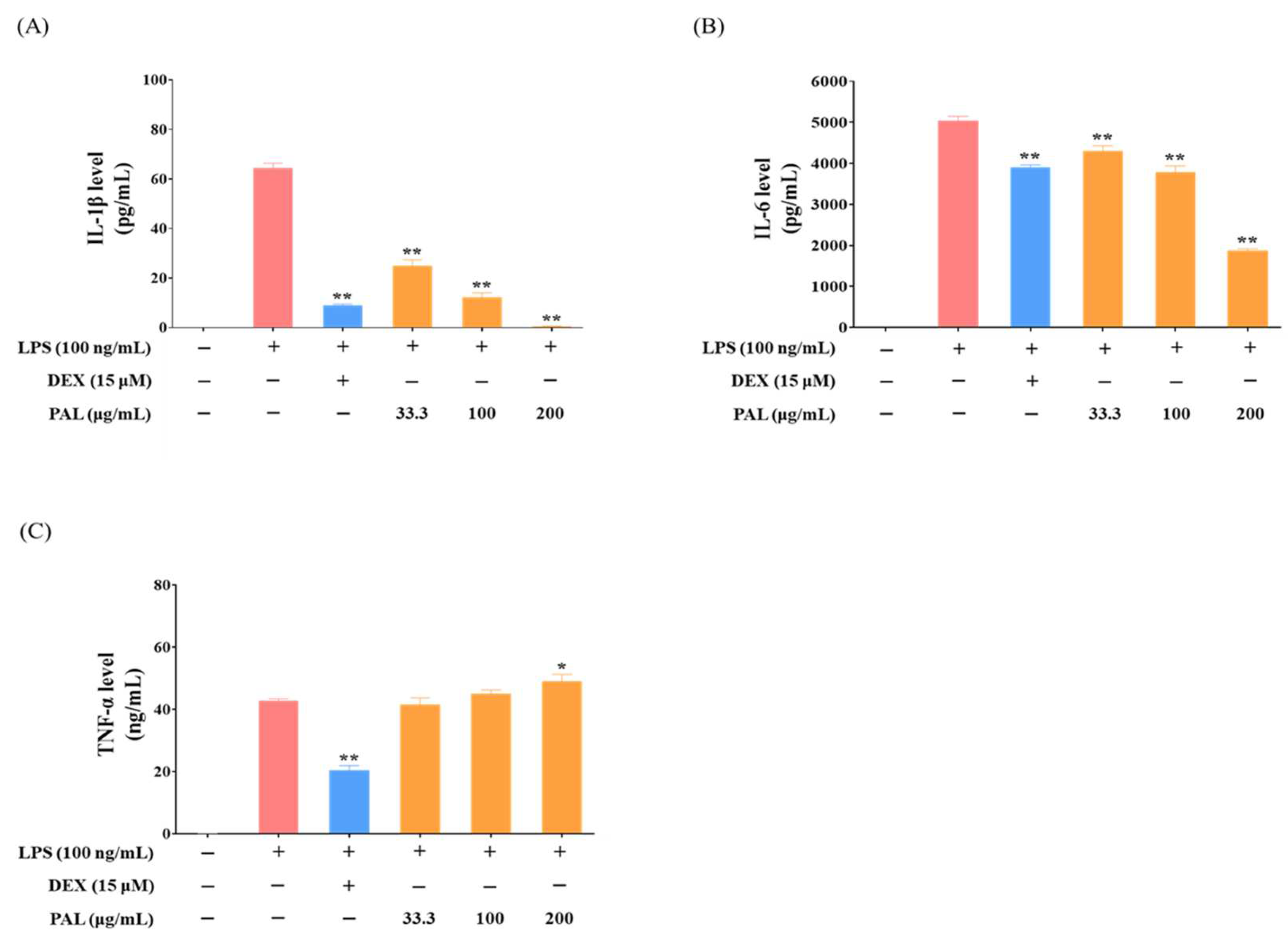
2.3. PAL Reduces Pro-Inflammatory Cytokines in LPS-Activated RAW 264.7 Macrophages
2.4. PAL Induces HO-1 by Nrf2 Activation
2.5. PAL Suppresses iNOS Expression by Inducing HO-1
2.6. Seven Phytochemicals Were Detected in PAL
2.7. Inhibition of HO-1 Expression Nullified the Reduction in NF-κB Activity and NO Production Induced by KAE and QUE
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Hydroethanolic Extract of PAL
4.3. Cell Lines and Culture
4.4. NF-κB Luciferase Activity Assay
4.5. Determination of NO Production (Griess Assay)
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. ARE-Luciferase Activty Assay
4.8. Measurement of Intracellular ROS Level
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, U.; Seo, S.; Wibowo, A.E.; Pongtuluran, O.B.; Lee, K.; Han, S.B.; Cho, S. Anti-inflammatory and antioxidant activities of methanol extract of Piper betle Linn. (Piper betle L.) leaves and stems by inhibiting NF-κB/MAPK/Nrf2 signaling pathways in RAW 264.7 macrophages. Biomed. Pharmacother. 2022, 155, 113734. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014, 73, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Martins, S.G.; Zilhão, R.; Thorsteinsdóttir, S.; Carlos, A.R. Linking Oxidative Stress and DNA Damage to Changes in the Expression of Extracellular Matrix Components. Front. Genet. 2021, 12, 673002. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, Q.; Zou, K.; Huang, H.; Chen, J.; Wang, P.; Yuan, W.; Xiao, L.; Tong, P.; Jin, H. Glucocorticoid-induced activation of NOX/ROS/NF-κB signaling in MSCs contributes to the development of GONFH. Apoptosis 2023, 23, 1332–1345. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Curr. Signal Transduct. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Noreen, H.; Rehman, S.; Gul, S.; Amjad Kamal, M.; Paul Kamdem, J.; Zaman, B.; da Rocha, J.B.T. Oxidative stress and antioxidant potential of one hundred medicinal plants. Curr. Top. Med. Chem. 2017, 17, 1336–1370. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Xie, Y.; Luo, J.; Gong, Q.; Li, C. Natural flavones from edible and medicinal plants exhibit enormous potential to treat ulcerative colitis. Front. Pharmacol. 2023, 14, 1168990. [Google Scholar] [CrossRef]
- Mazzocchi, A.; De Cosmi, V.; Risé, P.; Milani, G.P.; Turolo, S.; Syrén, M.-L.; Sala, A.; Agostoni, C. Bioactive compounds in edible oils and their role in oxidative stress and inflammation. Front. Physiol. 2021, 12, 659551. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.-K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-κB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2022, 9, 809952. [Google Scholar] [CrossRef] [PubMed]
- Kupczyński, R.; Szumny, A.; Bednarski, M.; Piasecki, T.; Śpitalniak-Bajerska, K.; Roman, A. Application of Pontentilla Anserine, Polygonum aviculare and Rumex Crispus Mixture Extracts in A Rabbit Model with Experimentally Induced E. coli Infection. Animals 2019, 9, 774. [Google Scholar] [CrossRef]
- Russell, G. List of Species of Southern African Plants, 2nd ed.; Botanical Research Institute: Lucknow, India, 1984. [Google Scholar]
- Salama, H.M.; Marraiki, N. Antimicrobial activity and phytochemical analyses of Polygonum aviculare L. (Polygonaceae), naturally growing in Egypt. Saudi J. Biol. Sci. 2010, 17, 57–63. [Google Scholar] [CrossRef]
- Seo, S.H.; Lee, S.; Cha, P.; Kim, M.; Min, D.S.; Choi, K. Polygonum aviculare L. and its active compounds, quercitrin hydrate, caffeic acid, and rutin, activate the Wnt/β-catenin pathway and induce cutaneous wound healing. Phytother. Res. 2016, 30, 848–854. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Żyżyńska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Howard, M. Traditional Folk Remedies: A Comprehensive Herbal; Century: Salt Lake, UT, USA, 1987. [Google Scholar]
- Marković, M.; Pljevljakušić, D.; Matejić, J.; Nikolić, B.; Smiljić, M.; Ðelić, G.; Papović, O.; Ðokić, M.; Stankov-Jovanović, V. The plants traditionally used for the treatment of respiratory infections in the Balkan Peninsula (Southeast Europe). Lek. Sirov. 2022, 42, 68–88. [Google Scholar] [CrossRef]
- Matejić, J.S.; Stefanović, N.; Ivković, M.; Živanović, N.; Marin, P.D.; Džamić, A.M. Traditional uses of autochthonous medicinal and ritual plants and other remedies for health in Eastern and South-Eastern Serbia. J. Ethnopharmacol. 2020, 261, 113186. [Google Scholar] [CrossRef]
- Robu, T.; Robu, B.; Robu, S. Valorification of herbs in phytotherapy-an alternative of chemical treatments in agriculture. Environ. Eng. Manag. J. 2008, 7, 579–588. [Google Scholar] [CrossRef]
- Park, S.H.; Jang, S.; Son, E.; Lee, S.W.; Sung, Y.-Y.; Kim, H.K. Polygonum aviculare L. extract reduces fatigue by inhibiting neuroinflammation in restraint-stressed mice. Phytomedicine 2018, 42, 180–189. [Google Scholar] [CrossRef]
- Park, S.H.; Sung, Y.-Y.; Nho, K.J.; Kim, H.K. Anti-atherosclerotic effects of Polygonum aviculare L. ethanol extract in ApoE knock-out mice fed a Western diet mediated via the MAPK pathway. J. Ethnopharmacol. 2014, 151, 1109–1115. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Yoon, T.; Yang, W.-K.; Kim, S.J.; Kim, D.-S.; Kim, H.K. The Antiobesity Effect of Polygonum aviculare L. Ethanol Extract in High-Fat Diet-Induced Obese Mice. Evid. Based Complement. Alternat. Med. 2013, 2013, 626397. [Google Scholar] [CrossRef]
- Begné, M.G.; Yslas, N.; Reyes, E.; Quiroz, V.; Santana, J.; Jimenez, G. Clinical effect of a Mexican sanguinaria extract (Polygonum aviculare L.) on gingivitis. J. Ethnopharmacol. 2001, 74, 45–51. [Google Scholar] [CrossRef]
- Fan, D.; Zhou, X.; Zhao, C.; Chen, H.; Zhao, Y.; Gong, X. Anti-inflammatory, antiviral and quantitative study of quercetin-3-O-β-D-glucuronide in Polygonum perfoliatum L. Fitoterapia 2011, 82, 805–810. [Google Scholar] [CrossRef]
- Ilari, S.; Dagostino, C.; Malafoglia, V.; Lauro, F.; Giancotti, L.A.; Spila, A.; Proietti, S.; Ventrice, D.; Rizzo, M.; Gliozzi, M.; et al. Protective Effect of Antioxidants in Nitric Oxide/COX-2 Interaction During Inflammatory Pain: The Role of Nitration. Antioxidants 2020, 9, 1284. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Chun, K.-S.; Cha, H.-H.; Han, S.S.; Keum, Y.-S.; Park, K.-K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 480–481, 243–268. [Google Scholar] [CrossRef] [PubMed]
- Taki, N.; Tatro, J.M.; Lowe, R.; Goldberg, V.M.; Greenfield, E.M. Comparison of the roles of IL-1, IL-6, and TNFα in cell culture and murine models of aseptic loosening. Bone 2007, 40, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Cha, H.-J.; Choi, E.O.; Kim, C.H.; Kim, G.-Y.; Yoo, Y.H.; Hwang, H.-J.; Park, H.T.; Yoon, H.M.; Choi, Y.H. Activation of the Nrf2/HO-1 signaling pathway contributes to the protective effects of baicalein against oxidative stress-induced DNA damage and apoptosis in HEI193 Schwann cells. Int. J. Med. Sci. 2019, 16, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2017, 29, 1727–1745. [Google Scholar] [CrossRef]
- Li, N.; Venkatesan, M.I.; Miguel, A.; Kaplan, R.; Gujuluva, C.; Alam, J.; Nel, A. Induction of Heme Oxygenase-1 Expression in Macrophages by Diesel Exhaust Particle Chemicals and Quinones via the Antioxidant-Responsive Element. J. Immun. 2000, 165, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.-G.; Yang, S.-L.; Hu, J.-L.; Li, P.-D.; Chen, Y.-S.; Wang, Q.-S. Evaluation of HO-1 expression, cellular ROS production, cellular proliferation and cellular apoptosis in human esophageal squamous cell carcinoma tumors and cell lines. Oncol. Rep. 2016, 35, 2270–2276. [Google Scholar] [CrossRef]
- Jang, S.; Lee, A.; Hwang, Y.-H. Chemical Profile Determination and Quantitative Analysis of Components in Oryeong-San Using UHPLC-Q-Orbitrap-MS and UPLC-TQ-MS/MS. Molecules 2023, 28, 3685. [Google Scholar] [CrossRef]
- Wang, D.-D.; Liang, J.; Yang, W.-Z.; Hou, J.-J.; Yang, M.; Da, J.; Wang, Y.; Jiang, B.-H.; Liu, X.; Wu, W.-Y.; et al. HPLC/qTOF-MS-oriented characteristic components data set and chemometric analysis for the holistic quality control of complex TCM preparations: Niuhuang Shangqing pill as an example. J. Pharm. Biomed. Anal. 2014, 89, 130–141. [Google Scholar] [CrossRef]
- Pawłowska, K.A.; Kryżman, M.; Zidorn, C.; Pagitz, K.; Popowski, D.; Granica, S. HPLC-DAD-MS3 fingerprints of phenolics of selected Polygonum taxa and their chemometric analysis. Phytochemistry 2023, 208, 113605. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Chang, H.-M.; Li, C.-Y.; Cui, Y.; Lee, C.-L.; Chen, C.-S. Reactive Oxygen Species and Inflammatory Responses of Macrophages to Substrates with Physiological Stiffness. ACS Appl. Mater. Interfaces. 2020, 12, 48432–48441. [Google Scholar] [CrossRef]
- El-Kenawi, A.; Ruffell, B. Inflammation, ROS, and Mutagenesis. Cancer Cell 2017, 32, 727–729. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef]
- Mata-Ramírez, D.; Serna-Saldívar, S.O.; Antunes-Ricardo, M. Enhancement of anti-inflammatory and antioxidant metabolites in soybean (Glycine max) calluses subjected to selenium or UV-light stresses. Sci. Hortic. 2019, 257, 108669. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Y.; Wang, M. Bioactive Substances of Plant Origin. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 967–1008. [Google Scholar]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.-H.; Lee, C.-K.; Ha, N.-J.; Yim, D.; et al. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-κB pathways. J. Inflamm. 2011, 8, 16. [Google Scholar] [CrossRef]
- Needleman, P.; Manning, P. Interactions between the inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) pathways: Implications for therapeutic intervention in osteoarthritis. Osteoarthr. Cartil. 1999, 7, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Prajapati, R.; Seong, S.H.; Jung, H.A.; Choi, J.S. Antioxidant and Antineuroinflammatory Mechanisms of Kaempferol-3-O-β-d-Glucuronate on Lipopolysaccharide-Stimulated BV2 Microglial Cells Through the Nrf2/HO-1 Signaling Cascade and MAPK/NF-κB Pathway. ACS Omega 2023, 8, 6538–6549. [Google Scholar] [CrossRef]
- Khajuria, V.; Gupta, S.; Sharma, N.; Tiwari, H.; Bhardwaj, S.; Dutt, P.; Satti, N.; Nargotra, A.; Bhagat, A.; Ahmed, Z. Kaempferol-3-o-β-d-glucuronate exhibit potential anti-inflammatory effect in LPS stimulated RAW 264.7 cells and mice model. Int. Immunopharmacol. 2018, 57, 62–71. [Google Scholar] [CrossRef]
- Jiang, T.; Tian, F.; Zheng, H.; Whitman, S.A.; Lin, Y.; Zhang, Z.; Zhang, N.; Zhang, D.D. Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 2014, 85, 333–343. [Google Scholar] [CrossRef]
- Kim, J.-E.; You, D.-J.; Lee, C.; Ahn, C.; Seong, J.Y.; Hwang, J.-I. Suppression of NF-κB signaling by KEAP1 regulation of IKKβ activity through autophagic degradation and inhibition of phosphorylation. Cell. Signal. 2010, 22, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Kim, B.N.; Lim, S.; Lee, J.; Kim, J.; Jeong, J.-G.; Kim, S. Effective suppression of nitric oxide production by HX106N through transcriptional control of heme oxygenase-1. Exp. Biol. Med. 2015, 240, 1136–1146. [Google Scholar] [CrossRef]
- Bonelli, M.; Savitskaya, A.; Steiner, C.-W.; Rath, E.; Bilban, M.; Wagner, O.; Bach, F.H.; Smolen, J.S.; Scheinecker, C. Heme oxygenase-1 end-products carbon monoxide and biliverdin ameliorate murine collagen induced arthritis. Clin. Exp. Rheumatol. 2012, 30, 73–78. [Google Scholar]
- Chiang, S.-K.; Chen, S.-E.; Chang, L.-C. The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival. Cells 2021, 10, 2401. [Google Scholar] [CrossRef]
- Pae, H.-O.; Chung, H.-T. Heme oxygenase-1: Its therapeutic roles in inflammatory diseases. Immune Netw. 2009, 9, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-R.; Hu, R.; Keum, Y.-S.; Hebbar, V.; Shen, G.; Nair, S.S.; Kong, A.-N.T. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003, 63, 7520–7525. [Google Scholar] [PubMed]
- Woo, Y.; Oh, J.; Kim, J.-S. Suppression of Nrf2 activity by chestnut leaf extract increases chemosensitivity of breast cancer stem cells to paclitaxel. Nutrients 2017, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Jang, S.A.; Kim, T.; Ha, H. Anti-osteoporotic and Anti-adipogenic Effects of 55 Rhus chinensis Nutgalls in Ovariectomized Mice Fed with a High-fat Diet. Planta Med. 2019, 56, 1128–1135. [Google Scholar] [CrossRef]




| No | Rt (min) | Calculated (m/z) | Measured (m/z) | Error (ppm) | Adduct | Formula | MS/MS Fragments (m/z) | Identification |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.93 | 483.0780 | 483.0789 | 1.804 | [M-H]− | C20H20O14 | 331, 313, 271 | Digalloylhexoside [23] |
| 2 | 6.68 | 463.0882 | 463.0891 | 1.968 | [M-H]− | C21H20O12 | - | Myricetrin * |
| 3 | 7.31 | 433.0776 | 433.0781 | 1.143 | [M-H]− | C20H18O11 | - | Avicularin * |
| 4 | 7.54 | 447.0933 | 447.0939 | 1.324 | [M-H]− | C21H20O11 | 447, 301 | Quercitrin * |
| 5 | 8.05 | 317.0303 | 317.0305 | 0.718 | [M-H]− | C15H10O8 | - | Myricetin * |
| 6 | 9.59 | 301.0354 | 301.0359 | 1.588 | [M-H]− | C15H10O7 | - | Quercetin * |
| 7 | 11.05 | 285.0405 | 285.041 | 1.842 | [M-H]− | C15H10O6 | - | Kaempferol * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, C.H.; Chung, Y.C.; Lee, A.; Hwang, Y.-H. Hydroethanolic Extract of Polygonum aviculare L. Mediates the Anti-Inflammatory Activity in RAW 264.7 Murine Macrophages Through Induction of Heme Oxygenase-1 and Inhibition of Inducible Nitric Oxide Synthase. Plants 2024, 13, 3314. https://doi.org/10.3390/plants13233314
Jang CH, Chung YC, Lee A, Hwang Y-H. Hydroethanolic Extract of Polygonum aviculare L. Mediates the Anti-Inflammatory Activity in RAW 264.7 Murine Macrophages Through Induction of Heme Oxygenase-1 and Inhibition of Inducible Nitric Oxide Synthase. Plants. 2024; 13(23):3314. https://doi.org/10.3390/plants13233314
Chicago/Turabian StyleJang, Chan Ho, You Chul Chung, Ami Lee, and Youn-Hwan Hwang. 2024. "Hydroethanolic Extract of Polygonum aviculare L. Mediates the Anti-Inflammatory Activity in RAW 264.7 Murine Macrophages Through Induction of Heme Oxygenase-1 and Inhibition of Inducible Nitric Oxide Synthase" Plants 13, no. 23: 3314. https://doi.org/10.3390/plants13233314
APA StyleJang, C. H., Chung, Y. C., Lee, A., & Hwang, Y.-H. (2024). Hydroethanolic Extract of Polygonum aviculare L. Mediates the Anti-Inflammatory Activity in RAW 264.7 Murine Macrophages Through Induction of Heme Oxygenase-1 and Inhibition of Inducible Nitric Oxide Synthase. Plants, 13(23), 3314. https://doi.org/10.3390/plants13233314








