Identification of Fungus GZ in Buckwheat Rhizosphere and Its Promoting Effect in Buckwheat Seed Germination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Culture Medium
2.2. Isolation of Test Strains
2.3. Identification of Test Strains
2.3.1. Morphological Identification
2.3.2. Molecular Biology Identification
2.4. Impact of Test Strains on the Germination of Buckwheat Seeds
2.5. Data Processing and Analysis
3. Results and Analysis
3.1. Morphological Analysis of Buckwheat Rhizosphere Fungi
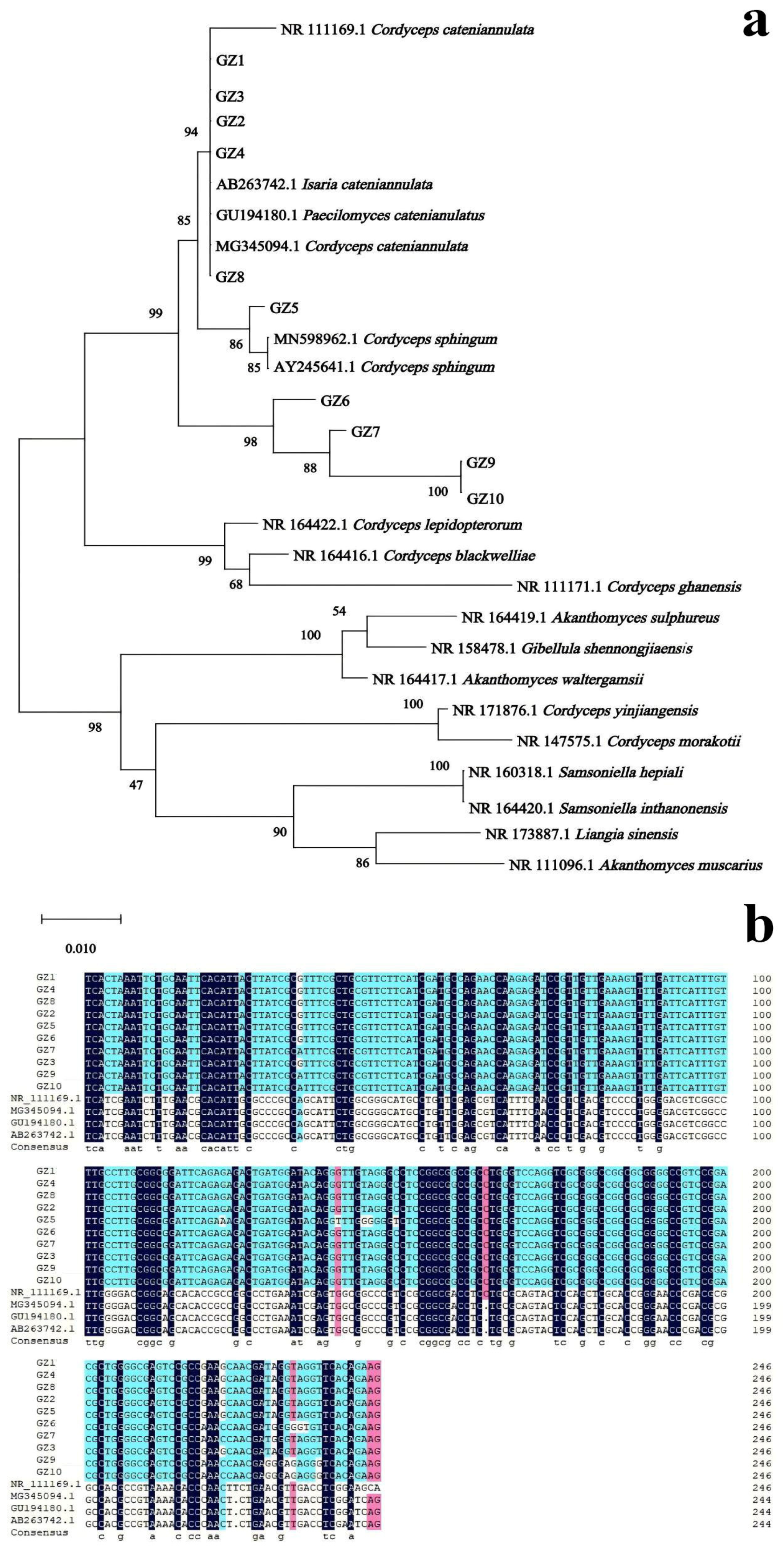
3.2. Molecular Biological Analysis of Buckwheat Rhizosphere Fungus GZ
3.3. Analysis of Promoting Buckwheat Seed Germination by I. cateniannulata
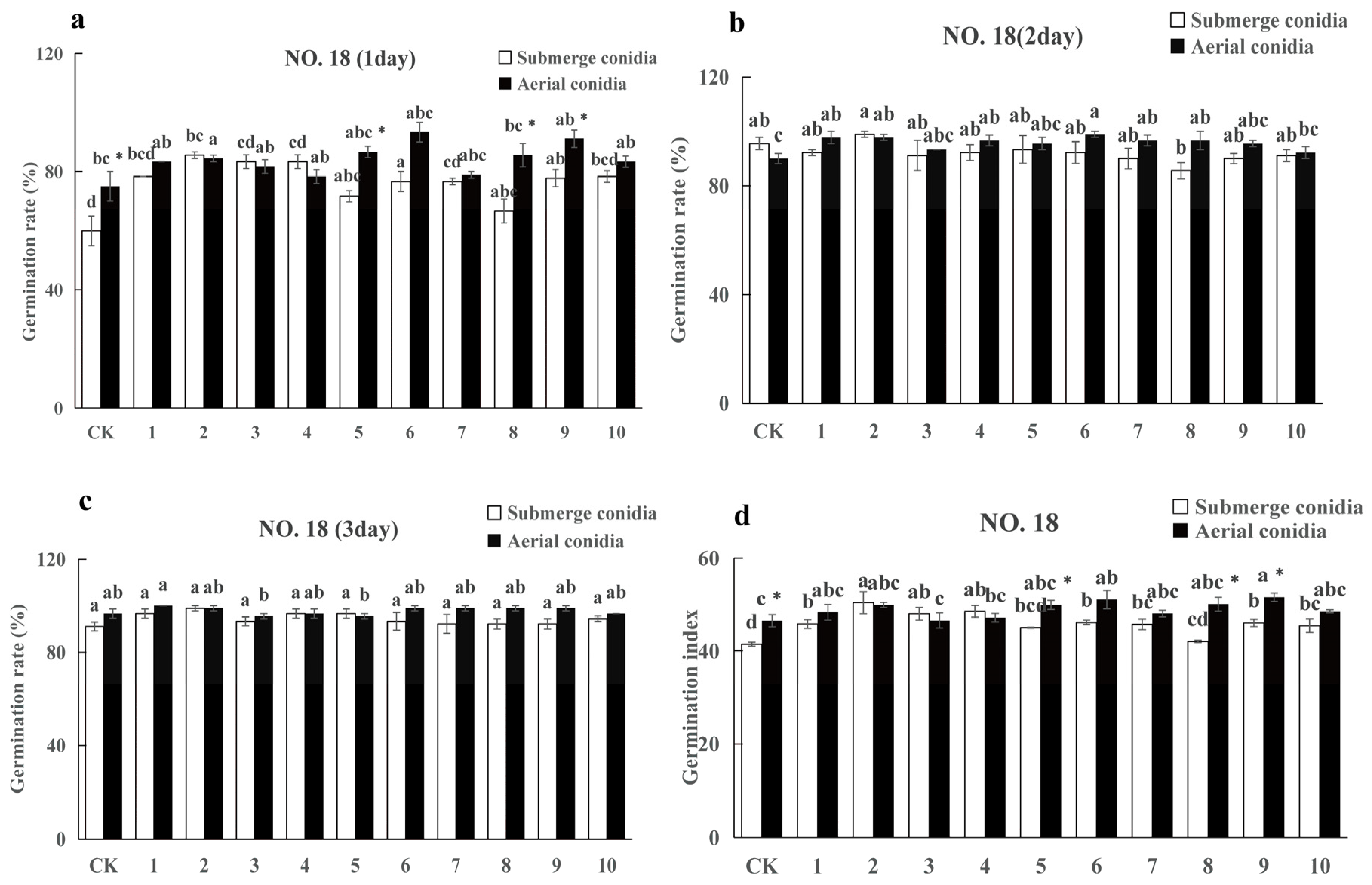
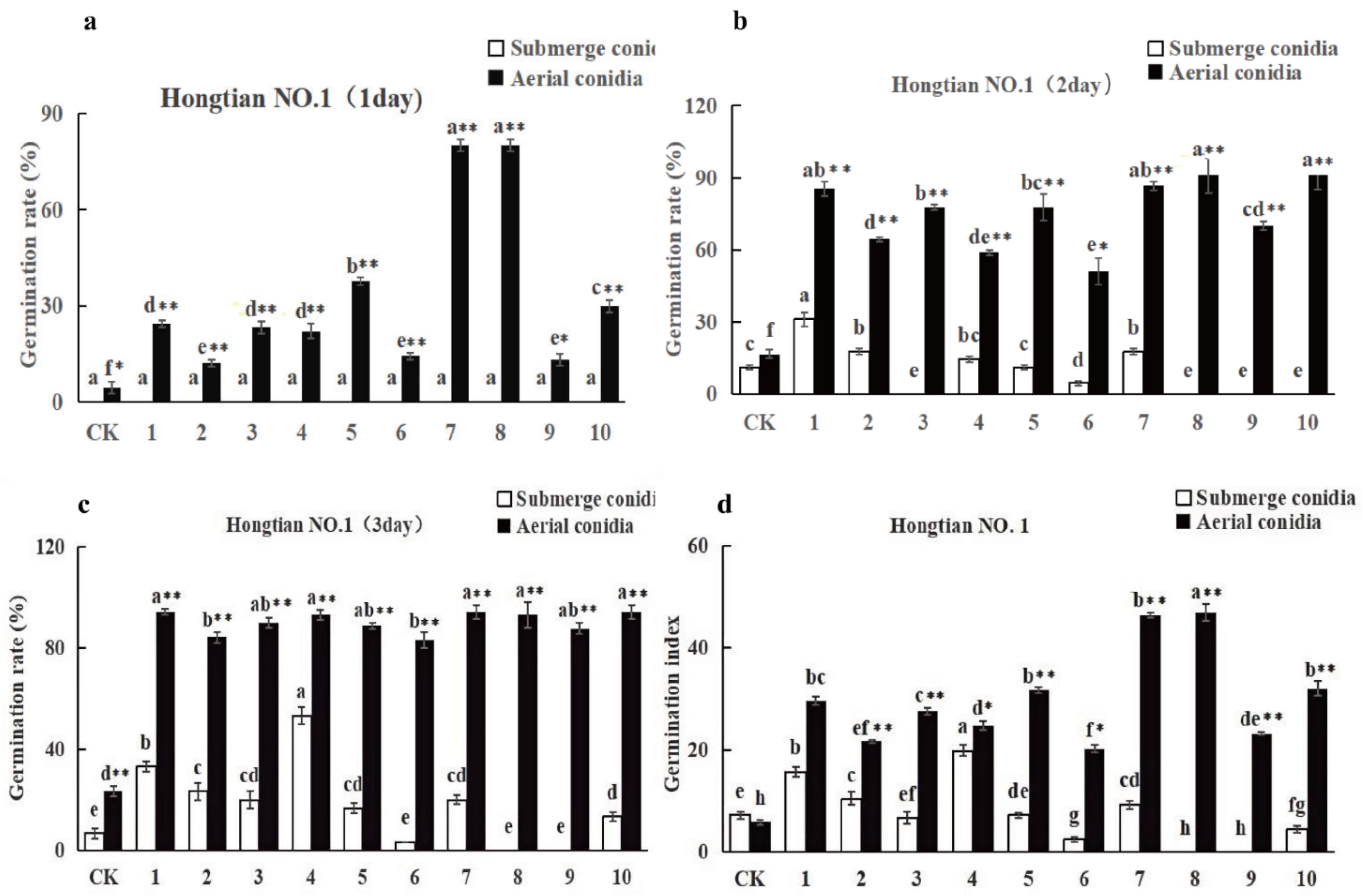
3.3.1. The Influence of Spore Suspension of I. cateniannulata on Germination Process of Buckwheat Seeds
3.3.2. The Influence of Spore Suspension of I. cateniannulata on Buckwheat Seed Germination Rate
3.3.3. The Influence of Spore Suspension of I. cateniannulata on Bud Length of Buckwheat Seeds
3.3.4. The Influence of Spore Suspension of I. cateniannulata on Bud Diameter of Buckwheat Seeds
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y. The tartary buckwheat genome provides insights into rutin biosynthesis and abiotic stress tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Su, W. Research progress on nutritional value, biological function and application in animal production of golden buckwheat. Feed Res. 2022, 45, 157–160. [Google Scholar] [CrossRef]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, D.M.S.B.; Nishida, S.; Tawaraya, K.; Wasaki, J. Organ-specific allocation pattern of acquired phosphorus and dry matter in two rice genotypes with contrasting tolerance to phosphorus deficiency. Soil Sci. Plant Nutr. 2018, 64, 282–290. [Google Scholar] [CrossRef]
- Hou, Y.; Deng, Y. Seedling test of Acacia mangium cultivated by rhizobium seed dressing. Guangxi For. Sci. 2002, 4, 204–205. [Google Scholar] [CrossRef]
- Wang, Z.; Du, J.; Niu, Y.; Yao, T. Screening and Growth-promoting characteristics of Plant Growth-Promoting Rhizobacteria of Oat Rhizosphere in Alpine Reseeding Grassland of Zoige. Acta Agrestia Sin. 2023, 31, 1406–1413. [Google Scholar] [CrossRef]
- TWei, Z.; Wen, Y.; Wang, C.; Wang, S.; Chen, Y.; Zhang, Z.; Fan, Q.; Hu, W.; Long, Z.; Jiang, S. The salt tolerance and growth-promoting effect of an IAA-producing Bacillus ST37 on rape. Jiangsu Agric. Sci. 2023, 51, 210–215. [Google Scholar] [CrossRef]
- Zhao, G.; Tang, Y.; Wang, A.H. Present Situation and Prospect of Buckwheat Breeding in China. Seed World 2002, 4, 3–4. [Google Scholar]
- Qi, Y.J. Main Fungal Diseases and Fungicide Screening of Buckwheat in Northwestern. Bachelor’s Thesis, Lanzhou University, Lanzhou, China, 2020. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, M.K.; Fang, Z.Y.; Chen, Y.N.; Du, H.M.; Wang, A.; Zhou, Y.; Wu, D.D. Evaluation on Cold Resistance of Buckwheat Germplasm during Germination Stage in Sichuan Province. Agric. Meteorol. China 2023, 44, 795–804. [Google Scholar] [CrossRef]
- Li, X.M.; He, Y.; Zhou, Y.P.; Tian, S.J.; Long, Q.; Tang, Z.F. Study on Stress Resistance of Buckwheat Seed Germination. Feed Res. 2022, 45, 76–79. [Google Scholar] [CrossRef]
- Wang, J.W.; Yang, X.Y.; Liu, H.; Yu, S. Effects of Salt and Alkali Stress on Seed Germination of Lolium perenne. J. Northeast Agric. Sci. 2023, 48, 60–62+74. [Google Scholar] [CrossRef]
- Yu, S.H.; Zheng, Y.; Meng, F.J.; Liu, H. Effects of Salt-alkali Sresson Seed Germination and Growth of Picea pungens var. glauca. Seed 2022, 41, 31–36. [Google Scholar] [CrossRef]
- Li, C.H.; Sun, M.K.; Wu, H.; JiaYang, D.L.; Tian, J.; Li, H.; Wang, Y.Q.; Ren, C.Z. Effects of Saline-alkali Stresson Seed Germination and Seedling Growth of Fagopyrum esculentum. Seed 2023, 42, 54–60. [Google Scholar] [CrossRef]
- Lei, X.H.; Wan, C.Q.; Tao, J.C.; Leng, J.J.; Wu, X.Y.; Wang, J.L.; Wang, P.K.; Yang, Q.H.; Feng, B.L.; Gao, J.F. Effects of soaking seeds with MT and EBR on germination and seedling growth in buckwheat under salt stress. J. Crops 2022, 48, 1210–1221. [Google Scholar] [CrossRef]
- Li, X.D. Study on Tartary Buckwheat Germination in Stress and Its Functioral Components. Bachelor’s Thesis, Jiangnan University, Wuxi, China, 2013. [Google Scholar]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.L.; Wang, Q.K.; Mao, X.Q.; Lu, Y.Z.; Zhang, X.D.; Liu, Q.L. Effects of Different Plant Growth-promoting Bacteria on Seed Germination and Seedling Growth of Sesbania. Mod. Agric. Sci. Technol. 2023, 146–149. [Google Scholar] [CrossRef]
- Adesina, A.A.; Babalola, O.O. Fungi that promote plant growth in the rhizosphere boost crop growth. J. Fungi 2023, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Hyakumachi, M. Plant-growth-promoting fungi from turfgrass rhizosphere with potential for disease suppression. Soil Microorg. 1994, 44, 53–68. [Google Scholar] [CrossRef]
- Hyakumachi, M.; Kubota, M. Fungi as plant growth promoter and disease suppressor. Fungal Biotechnol. Agric. Food Environ. Appl. 2004, 21, 101–110. [Google Scholar]
- Murali, M.; Amruthesh, K.N.; Sudisha, J.; Niranjana, S.R.; Shetty, H.S. Screening for plant growth promoting fungi and their ability for growth promotion and induction of resistance in pearl millet against downy mildew disease. J. Phytol. 2012, 4, 30–36. [Google Scholar]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant growth-promoting fungi (PGPF): Phytostimulation and induced systemic resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; pp. 135–191. [Google Scholar] [CrossRef]
- Naziya, B.; Murali, M.; Amruthesh, K.N. Plant Growth-Promoting Fungi (PGPF) Instigate Plant Growth and Induce Disease Resistance in Capsicum annuum L. upon Infection with Colletotrichum capsici (Syd.). Butl. Bisby Biomol. 2020, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Li, D. Community Structure and Growth-Promoting Characteristics of Seed Germination Associated Microbiome in Astragalus Membranaceus. Bachelor’s Thesis, Northwest A & F University, Xianyang, China, 2022. [Google Scholar] [CrossRef]
- Jiang, X.L. Symbiotic Fungi Diversity of Germination Stage and Symbiotic Germination Mechanism of Gymnadenia Conopsea Seeds. Bachelor’s Thesis, Peking Union Medical College, Beijing, China, 2022. [Google Scholar] [CrossRef]
- Zhao, W.J.; Wang, X.X.; Yang, Y.; Zhang, Y.L.; Dang, S.; He, J.Q. Selection of Rhizotrophic Bacteria from Rhizosphere and Its Effect on Seed Germination of Black Barley. Seed 2018, 37, 1–5+10. [Google Scholar] [CrossRef]
- Leelavathy, K.M. Effect of rhizosphere fungi on seed germination. Plant Soil 1969, 30, 473–476. [Google Scholar] [CrossRef]
- Angel, C.-C.H.; Lourdes, M.R.; Carlos, C.P.; José, L.B. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Li, J.H.; Ling, J.C. Effect of Gibberellin Treatment on Seed Germination of Syringa reticulata ssp. amurensis. Hebei For. Sci. Technol. 2015, 8, 1–2. [Google Scholar] [CrossRef]
- Peng, X.; Wu, Y.; Zhang, X.N.; Cheng, W.H.; Zhu, L.W.; Chen, Q.F.; Deng, J. Screening of High Germination Rate Strains of Entomopathogenic Fungi against Buckwheat Seeds. J. Shandong Agric. Univ. (Nat. Sci. Ed.) 2022, 53, 393–400. [Google Scholar] [CrossRef]
- Dai, S.L.; Lin, J.; Gao, L. A brief report on the antibacterial effect of penicillin and streptomycin in PDA culture medium. Edible Fungi China 2007, 4, 53–54. [Google Scholar] [CrossRef]
- Wu, Q.F.; Fu, L.; Lu, Z.F. Experimental study on isolation, purification and molecular identification of soil microorganisms. J. Anyang Inst. Technol. 2016, 15, 27–29. [Google Scholar] [CrossRef]
- Yang, M. Bioscience Q000057. Chinese Fungi Vol.13 Entomophthorales; Science Press: Beijing, China, 2001. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar] [CrossRef]
- Clewley, J.P.; Arnold, C. Megalign: The multiple alignment module of LASERGENE. In Sequence Data Analysis Guidebook; Methods in Molecular Biology; Humana Press: Clifton, NJ, USA, 1997; Volume 70, pp. 119–129. [Google Scholar] [CrossRef]
- Xie, Z.H.; Yu, M.F.; Gu, W.J.; Zhou, S.Z.; Ma, Y. Isolation, identification and antibacterial activity analysis of new Bacillus species. J. Tradit. Chin. Vet. Med. 2024, 43, 54–58. [Google Scholar] [CrossRef]
- Liang, Z.Q. Two new species of Paecilomyces. Acta Microbiol. Sin. 1981, 21, 31–34+132. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- He, J.W. Optimization of Germination Process of Tartary Buckwheat and Analysis of Related Metabolomics. Bachelor’s Thesis, Foshan University, Foshan, China, 2020. [Google Scholar] [CrossRef]
- Thevenot, C.; Thomas, F. Physiology of apple-embryo cotyledons in relation to dormancy. 1. Influence of germination conditions on their morphological development. Bull Soc. Bot. Fr. Actual. Bot. 1983, 130, 89–100. [Google Scholar]
- Schopfer, P. Biomechanics of plant growth. Am. J. Bot. 2006, 93, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.M.; Liu, C.; Gu, L.D.; Chen, Q.F.; Zhang, X.N. Studies on the Phosphorus-Solubilizing Ability of Isaria cateinannulata and Its Influence on the Growth of Fagopyrum tataricum Plants. Plants 2024, 13, 1694. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, W.H.; Zhang, X.N.; Meng, Z.Y.; Zhu, L.W.; Deng, J.C.; Chen, Q.F. Determination of the growth curve of Isaria cateinannulata. J. Guizhou Norm. Univ. (Nat. Sci.) 2023, 41, 102–106. [Google Scholar] [CrossRef]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Mora, J.P.; Smith-Ramírez, C.; Zúñiga-Feest, A. The role of fleshy pericarp in seed germination and dispersal under flooded conditions in three wetland forest species. Acta Oecologica 2013, 46, 10–16. [Google Scholar] [CrossRef]
- Xu, H.H.; Li, N.; Liu, S.J.; Wang, W.Q.; Wang, W.P.; Zhang, H.; Cheng, H.Y.; Song, S.Q. Research Progress in Seed Germination and Its Control. Acta Agron. Sin. 2014, 40, 1141–1156. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The Multifarious PGPR Serratia marcescens CDP-13 Augments Induced Systemic Resistance and Enhanced Salinity Tolerance of Wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Fu, L.; Li, X.; Gao, L.; Jiang, J.X.; Sun, J.Z. Endophytic bacteria can mitigate salinity stress on seed germination and physiology in hybrid Pennisetum. Pratac. Sci. 2017, 34, 2099–2108. [Google Scholar] [CrossRef]
- Li, P.; Chen, Q.J.; Shi, T.X.; Meng, Z.Y.; Liang, C.G.; Wang, Y.; Chen, Q.F. Cracking types of common buckwheat pericarp and its effect on early germination traits. Guihaia 2020, 40, 954–962. [Google Scholar] [CrossRef]
- Shasmita; Swain, B.B.; Mohapatra, P.K.; Naik, S.K.; Mukherjee, A.K. Biopriming for induction of disease resistance against pathogens in rice. Planta 2022, 255, 113. [Google Scholar] [CrossRef] [PubMed]
- Song, Y. Screening of Plant Growth Promoting Bacteria Under Saline-Alkali Stress and Its Growth Promoting Mechanism. Bachelor’s Thesis, Northeast Forestry University, Harbin, China, 2021. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, P.Y.; Li, X.L.; Wang, J.F.; Yang, J.L. Distribution of soil fungal diversity and community structure in different vegetation types on the eastern slopes of Helan Mountains. Ecol. Environ. Sci. 2022, 31, 239–247. [Google Scholar] [CrossRef]
- Wang, D.F.; Yang, G.; Wang, Q.S.; Zeng, M.S.; Wu, G.Y. Identification of two Isaria isolates and bioassay of their pathogenicity against tea tortrix Homona coffearia and smaller tea tortrix Adoxophyes honmai. Acta Phytophylacica Sin. 2014, 41, 531–539. [Google Scholar] [CrossRef]
- Zhang, X.N.; Guo, J.J.; Zou, X.; Jin, D.C. Pathogenic differences of the entomopathogenic fungus Isaria cateniannulata to the spider mite Tetranychus urticae (Trombidiformes: Tetranychidae) and its predator Euseius nicholsi (Mesostigmata: Phytoseiidae). Exp. Appl. Acarol. 2018, 75, 69–84. [Google Scholar] [CrossRef]
- Zhang, X.N.; Jin, D.C.; Zou, X.; Guo, J.J. Laboratory and field evaluation of an entomopathogenic fungus, Isaria cateniannulata strain 08XS-1, against Tetranychus urticae (Koch). Pest Manag. Sci. 2016, 72, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.Y.; Xie, W.W.; Zhang, J.; Hu, Q.B. Biodiversity of entomopathogenic fungi in the soils of South China. Microorganisms 2019, 7, 311. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, T.W.; Liang, J.D.; Zhou, X.; Liang, Z.Q. Culture characteristics, pathogenicity, and genetic variation of Isaria cateniannulata isolates. J. Fungi 2012, 31, 341–349. [Google Scholar] [CrossRef]
- Zhu, X.Y. Study on Biological Characteristics and Geneticdiversity of Paecilomyces Cateniannulatus in a Massions Pine Plantations Evosystem. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2008. [Google Scholar]
- Zhu, X.Y.; Li, Z.Z.; Fan, M.Z.; Bao, S.M.; Yang, Z.Y. Solid Culture conditions of Three Strains of Paecilomyces catenlannuus. J. Anhui Agric. Univ. 2008, 1, 38–41. [Google Scholar] [CrossRef]
- Shimazu, M. Paecilomyces cateniannulatus Liang, a commonly found, but an unrecorded entomogenous fungus in Japan. Appl. Entomol. Zool. 2001, 36, 283–288. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Liu, A.Y.; Cheng, Y.B. Study on entomopathogenic fungi II. Solid production and pathogenicity of Paecilomyces cateniannulatus strain 2401. Guizhou Agric. Sci. 1984, 2, 39–42. [Google Scholar]
- Wang, C.S.; Huang, B.; Wang, S.B.; Li, Z.Z.; Fan, M.Z. Diversity of culture characteristics of Beauveria bassiana and the analysis of strain association with insect host and geographic origin. Biodiversity 2002, 10, 196–201. [Google Scholar]
- Wang, C.S.; Li, Z.Z.; Fan, M.Z.; Han, B.Y. Near relation characterization of Beauveria bassiana strains isolated from different hosts and geographic origins. Appl. Ecol. 1998, 189–194. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, L. Screening of Plant Growth Promoting Rhizobacteria of Xiang Lotus (Nelumbo nucifera Gaertn.) and Their Promoting Growth Effects. Hunan Agric. Sci. 2023, 8, 50–53+57. [Google Scholar] [CrossRef]
- Li, B. Evaluation of Growth-Promotion Effects of PGPRs from Different Crops on Wheat, Rice and Cotton. Bachelor’s Thesis, Anhui Agricultural University, Hefei, China, 2014. [Google Scholar]
- Gillespie-Sasse, L.M.J.; Almassi, F.; Ghisalberti, E.L.; Sivasithamparam, K. Use of a clean seedling assay to test plant growth promotion by exudates from a sterile red fungus. Soil Biol. Biochem. 1991, 23, 95–97. [Google Scholar] [CrossRef]
- Yao, B.L. Breeding of Oreorchis patens (Lindl.) Lindl. and Rhizosphere Growth-Promoting Bacteria. Bachelor’s Thesis, Changchun University, Changchun, China, 2021. [Google Scholar] [CrossRef]
- Peng, X. Effects of Colonization by Isaria cateinannulata on Enzyme Activity and Metabolites of Buckwheat Seeds During Germination. Bachelor’s Thesis, Guizhou Normal University, Guiyang, China, 2023. [Google Scholar]
- Buchanan, B.B.; Balmer, Y. REDOX REGULATION: A Broadening Horizon. Annu. Rev. Plant Biol. 2005, 56, 187–220. [Google Scholar] [CrossRef]
- Shi, Y. Effects of Endophytic Fungi on Growth and Development of Plants. Mod. Agric. Sci. Technol. 2010, 6, 36–38. [Google Scholar]
- Qayyum, M.A.; Wakil, W.; Arif, M.J.; Sahi, S.T.; Dunlap, C.A. Infection of Helicoverpa armigera by endophytic Beauveria bassiana colonizing tomato plants. Biol. Control. 2015, 90, 200–207. [Google Scholar] [CrossRef]
- Wang, H.F.; Wei, Y.; Ji, L.L.; Fu, J.L.; Bai, J. Colonization and rice growth-promoting effects of different Metarhizium strains. Microbiol. China 2024, 51, 1–19. [Google Scholar] [CrossRef]
- Liao, X.G.; Hu, M.Y.; Meng, Z.L.; Luo, Q.; Bai, W.Q. Effects of Different Metarhizium Strains on hizospheric Colonization and Growth of Corn. Southwest China J. Agric. Sci. 2021, 34, 1657–1662. [Google Scholar] [CrossRef]
- Zhao, F.J.; Deng, B.W.; Xie, X.C.; Chang, L.; Ling, J.; Yang, X.Y. Relationship between extracellular enzyme activity of high temperature resistant germination bacteria and germination of Gastrodia elata seeds. Jiangsu Agric. Sci. 2022, 50, 205–210. [Google Scholar] [CrossRef]
- Fu, K. Effects of artificial aging on seed vigor of rice. Agric. Sci. Eng. China 2024, 36, 65–69. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Liu, J.M.; Huang, X.L.; Chen, J.Z.; Wang, D.; Tong, B.L. torage Substance Content and Corresponding Enzyme Activity During the Stratification of Cinnamomum migao Seeds. Mol. Plant Breed. 2022, 20, 1317–1324. [Google Scholar] [CrossRef]
- Hu, X.W.; Wang, Y.R.; Wu, Y.P. Effects of the pericarp on imbibition, seed germination, and seedling establishmentin seeds of Hedysarum scoparium Fisch. Et Mey. Ecol. Res. 2009, 24, 559–564. [Google Scholar] [CrossRef]
- Holder, D.J.; Keyhani, N.O. Adhesion of the Entomopathogenic Fungus Beauveria (Cordyceps) bassiana to Substrata. Appl. Environ. Microbiol. 2005, 71, 5260–5266. [Google Scholar] [CrossRef] [PubMed]
- Wanchoo, A.; Lewis, M.W.; Keyhani, N.O. Lectin mapping reveals stage-specific display of surface carbohydrates in in vitro and haemolymph-derived cells of the entomopathogenic fungus Beauveria bassiana. Microbiology 2009, 155, 3121–3133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Peng, X.; Yang, G.M.; Chen, Q.F.; Jin, D.C. The Colonization and Effect of Isaria cateinannulata on Buckwheat Sprouts. Plants 2023, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Lu, Y.; Zhu, H.; Wan, T.; Li, Q.; Zhang, Z.K. Endophytic blastospores of Beauveria bassiana provide high resistance against plant disease caused by Botrytis cinerea. Fungal Biol. 2022, 126, 528–533. [Google Scholar] [CrossRef]
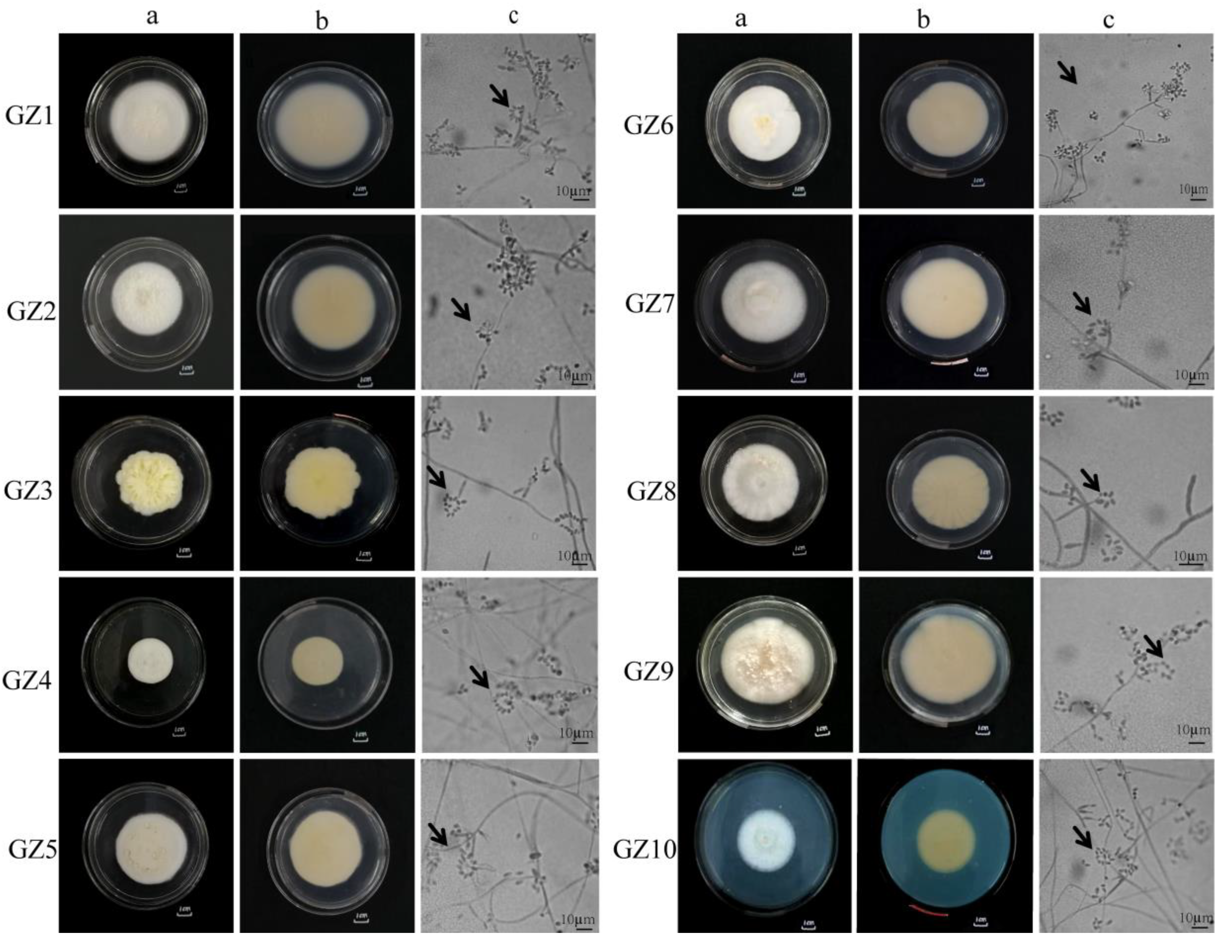


| Strain | Strain ID | Source Habitat | Collection Date |
|---|---|---|---|
| 1 | GZ1 | Duyun, Guizhou | 31 October 2019 |
| 2 | GZ2 | Weining, Guizhou | 13 September 2020 |
| 3 | GZ3 | Duyun, Guizhou | 31 October 2019 |
| 4 | GZ4 | Kaiyang, Guizhou | 4 December 2020 |
| 5 | GZ5 | Duyun, Guizhou | 24 October 2019 |
| 6 | GZ6 | Kaiyang, Guizhou | 6 December 2020 |
| 7 | GZ7 | Kaiyang, Guizhou | 6 December 2020 |
| 8 | GZ8 | Duyun, Guizhou | 31 October 2019 |
| 9 | GZ9 | Weining, Guizhou | 12 September 2020 |
| 10 | GZ10 | Duyun, Guizhou | 31 October 2019 |
| Strain | Morphological Characteristics |
|---|---|
| GZ1 | The colony diameter was 70.00 ± 1.00 mm, with both sides being white. The mycelium was fluffy, and the entire colony was dense with wrinkles on the reverse side. The hyphae were smooth, branched, and septate, with a diameter of 2.3–5.7 µm. The conidiophore was 14–23 µm long, bearing 2–4 phialides, with the base of the phialides exhibiting spherical expansion. Conidia were hyaline, round, oval, or ovoid, 3.8–10.9 × 3.2–6.2 µm in size, and produced in imbricate chains (Figure 1—GZ1a, b, c). |
| GZ2 | The colony diameter was 60.66 ± 1.52 mm. The front side was white, and the reverse side was pale yellow. The mycelium was fluffy, with the whole colony was being dense. The reverse side of the colony was circular, with a flat edge, a raised center, and a neat margin. Yellow droplets were present in the center. The hyphae were smooth, branched, and septate, with a diameter of 1.8–3.4 µm. The conidiophore was produced in 2 whorls, bearing phialides. The base of the phialides was spherical and expanded. The conidia were unicellular, hyaline, and oval to nearly spherical, measuring 5.4–9.1 × 2.1–5.6 µm in size, and usually produced in imbricate chains (Figure 1—GZ2a, b, c). |
| GZ3 | The colony diameter was 57.00 ± 2.00 mm, with both sides of the colony being white and containing light yellow pigments. The colony was white and fluffy, with no fascicled hyphae. The colony was flat, with a fluffy edge. The hyphae were smooth, branched, and septate, with a diameter of 1.2–3.1 µm. The sporulation axis was 14.3–15.3 µm long and 1.2–1.4 µm wide, usually produced in 2 whorls, with phialides typically in 2-4 whorls. The phialides were long, awl-shaped, with slimy heads at the apex. Conidia were 2.6–6.9 × 1.1–1.6 µm in size, round or oval, and produced in imbricate chains (Figure 1—GZ3a, b, c). |
| GZ4 | The colony diameter was 51.33 ± 2.23 mm, with the front side being white and the reverse side light yellow. The colony was fluffy, with dense mycelium and a raised center. The hyphae were hyaline, smooth, branched, and septate, with a diameter of 1.8–4.2 µm. The conidiophore was 12.6–14.4 µm long. The conidia were hyaline, round or oval, 3.8–7.6 × 3.2–6.3 µm in size, and produced in imbricate chains (Figure 1—GZ4a, b, c). |
| GZ5 | The colony diameter was 59.33 ± 3.51 mm, with both sides of the colony light yellow, and the lower part of the front side was white and fluffy. The upper part was light yellow and branched, with a raised center and flat edges. The colony’s edge was petal-shaped and fluffy. The hyphae were 2.1–3.9 µm in diameter, septate, hyaline, and smooth. The conidiophores were short or absent, about 15.3–13.6 µm long and 1.8–1.6 µm wide. The conidium were 2.1–3.9 µm × 2.2–4.3 µm in size, hyaline, smooth, oval or nearly spherical, and produced in imbricate chains (Figure 1—GZ5a, b, c). |
| GZ6 | The colony diameter was 64.00 ± 3.00 mm, with the front side being white and the reverse side light yellow. Yellow pigments were secreted. The hyphae were cottony, with radial grooves on the surface and a raised center, while the colony’s edge was petal-shaped. The hyphae were 2.1–4.2 µm in diameter, septate, hyaline, and smooth. The conidiophores were short, about 24.5–27.4 µm long and 2.3–2.6 µm wide, bearing 2–4 phialides. Most phialides had a base that was spherical and swollen, abruptly tapering at the upper half. The conidia were 3.9–6.1 × 2.2–4.3 µm in size, hyaline, smooth, mostly oval or nearly spherical, and produced in imbricate chains (Figure 1—GZ6a, b, c). |
| GZ7 | The colony diameter was 56.00 ± 2.00 mm, with both sides of the colony being white and fluffy. The hyphae were hyaline, septate, and branched, with a diameter of 1.8–4.7 µm. The conidiophores were 12.1–16.3 µm long, bearing 2–4 phialides with a spherical and swollen base. The conidia were hyaline, mostly round, oval or ovoid, and produced in imbricate chains (Figure 1—GZ7a, b, c). |
| GZ8 | The colony diameter was 68.00 ± 2.00 mm, with the front side being white and the reverse side light yellow. The colony was downy to fluffy, with dense mycelium and fascicled hyphae. The surface of the hyphae was wrinkled, with an irregularly raised center and flat edges. The mycelium was hyaline, septate, and 2.3–3.8 µm in diameter. The conidiogenous cells were long phialide-shaped, 9.0–26.0 µm long, and arose directly from the hyphae or from the conidiophores. The conidia were oval or ovoid, unicellular, hyaline, and 4.3–7.8 µm × 2.1–3.7 µm in size, commonly produced in imbricate chains (Figure 1—GZ8a, b, c). |
| GZ9 | The colony diameter was 59.33 ± 3.05 mm, with both sides of the colony being white and containing light yellow pigments. The colony was white and fluffy, flat, and with a fluffy edge. The mycelium was hyaline, septate, branched, and 1.34–3.43 µm in diameter. The conidiogenous cells were long phialide-shaped, 12.2–24 µm long, arising directly from the hyphae or from the conidiophores. The conidia were oval or round, unicellular, hyaline, and 1.4–5.1 × 2.4–3.5 µm in size, commonly produced in imbricate chains (Figure 1—GZ9a, b, c). |
| GZ10 | The colony diameter was 43.33 ± 2.51 mm, with the colony being white, fluffy, and flat and showing indistinct concentric rings. The front side was white, while the reverse side was light yellow, with a rounded, fluffy edge. The hyphae were hyaline, smooth, septate, and branched, with a diameter of 0.8–1.7 µm. The conidiophores were 7.9–29.6 µm long. The conidia were unicellular, hyaline, smooth, mostly oval or ovoid, measuring 2.4–4.3 × 1.9–2.9 µm, and commonly produced in imbricate chains (Figure 1—GZ10a, b, c). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, G.; Gu, L.; Liu, C.; Chen, Q. Identification of Fungus GZ in Buckwheat Rhizosphere and Its Promoting Effect in Buckwheat Seed Germination. Plants 2024, 13, 3360. https://doi.org/10.3390/plants13233360
Zhang X, Yang G, Gu L, Liu C, Chen Q. Identification of Fungus GZ in Buckwheat Rhizosphere and Its Promoting Effect in Buckwheat Seed Germination. Plants. 2024; 13(23):3360. https://doi.org/10.3390/plants13233360
Chicago/Turabian StyleZhang, Xiaona, Guimin Yang, Lingdi Gu, Can Liu, and Qingfu Chen. 2024. "Identification of Fungus GZ in Buckwheat Rhizosphere and Its Promoting Effect in Buckwheat Seed Germination" Plants 13, no. 23: 3360. https://doi.org/10.3390/plants13233360
APA StyleZhang, X., Yang, G., Gu, L., Liu, C., & Chen, Q. (2024). Identification of Fungus GZ in Buckwheat Rhizosphere and Its Promoting Effect in Buckwheat Seed Germination. Plants, 13(23), 3360. https://doi.org/10.3390/plants13233360





