Unlocking the Hidden Potential of Rosemary (Salvia rosmarinus Spenn.): New Insights into Phenolics, Terpenes, and Antioxidants of Mediterranean Cultivars
Abstract
1. Introduction
2. Results
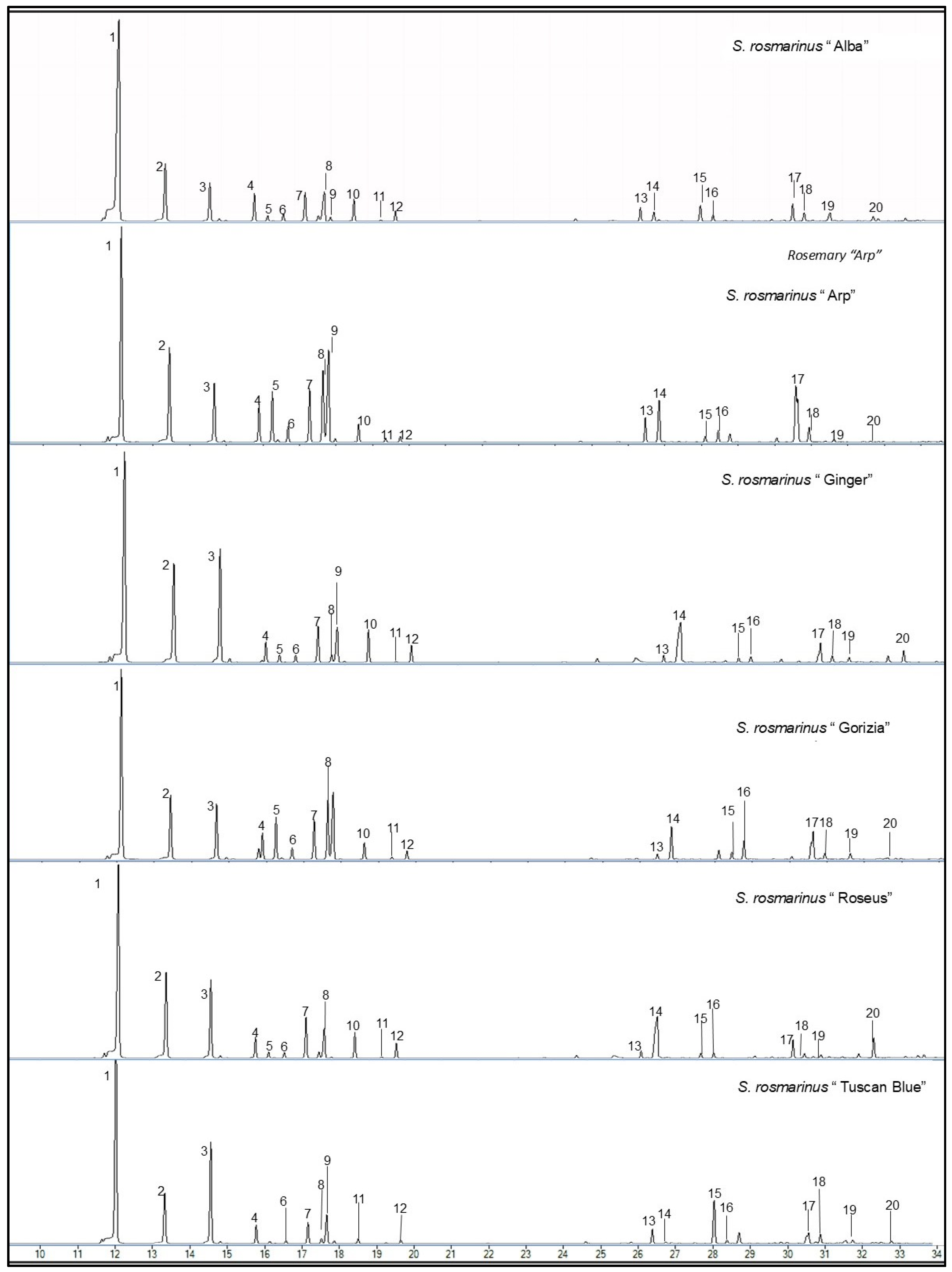
2.1. Analysis of Phenolic Compounds in Rosemary Cultivars
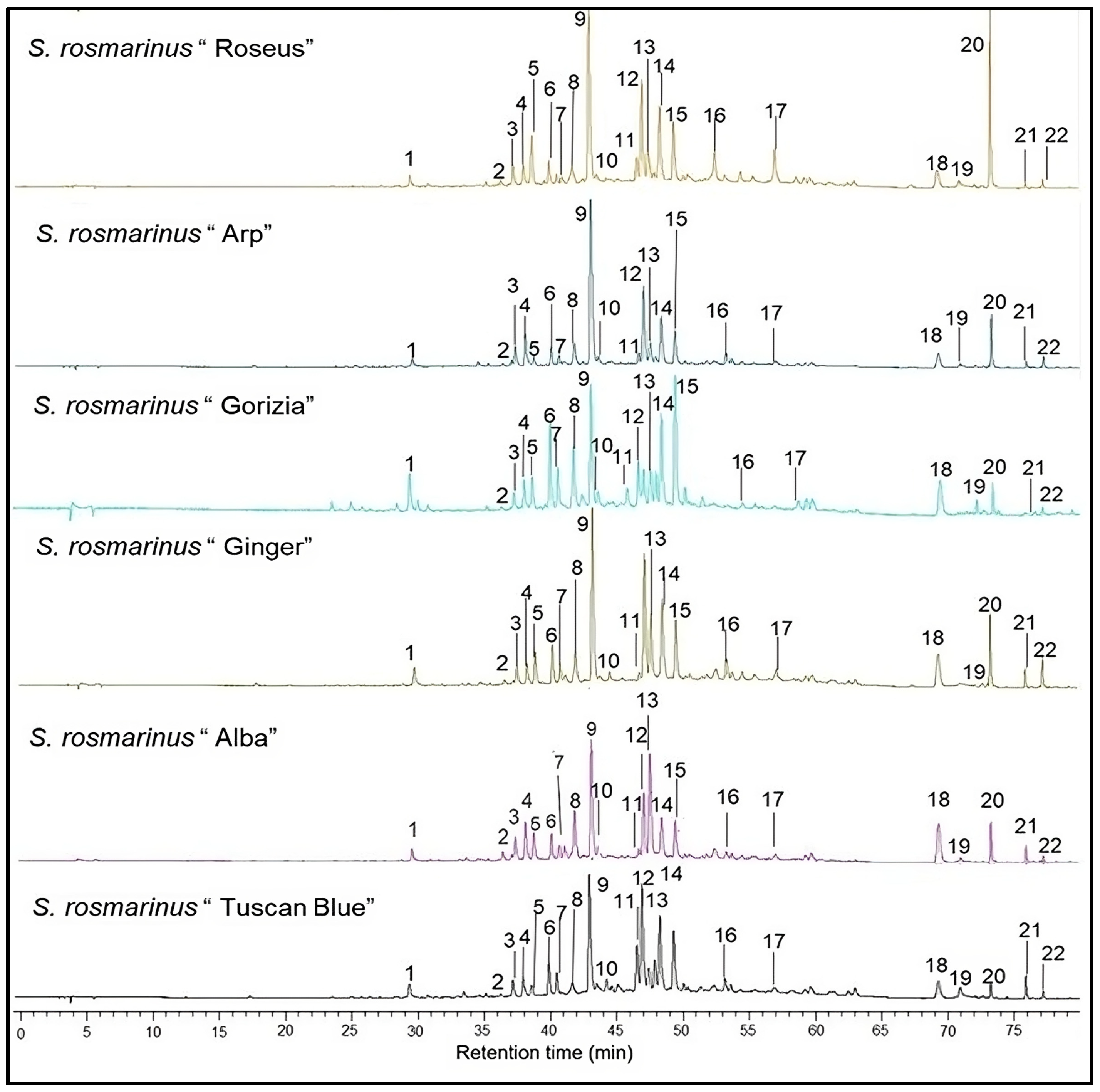
2.2. Analysis of Terpenes in Rosemary Cultivars
2.3. Antioxidant Activity of Rosemary Extracts
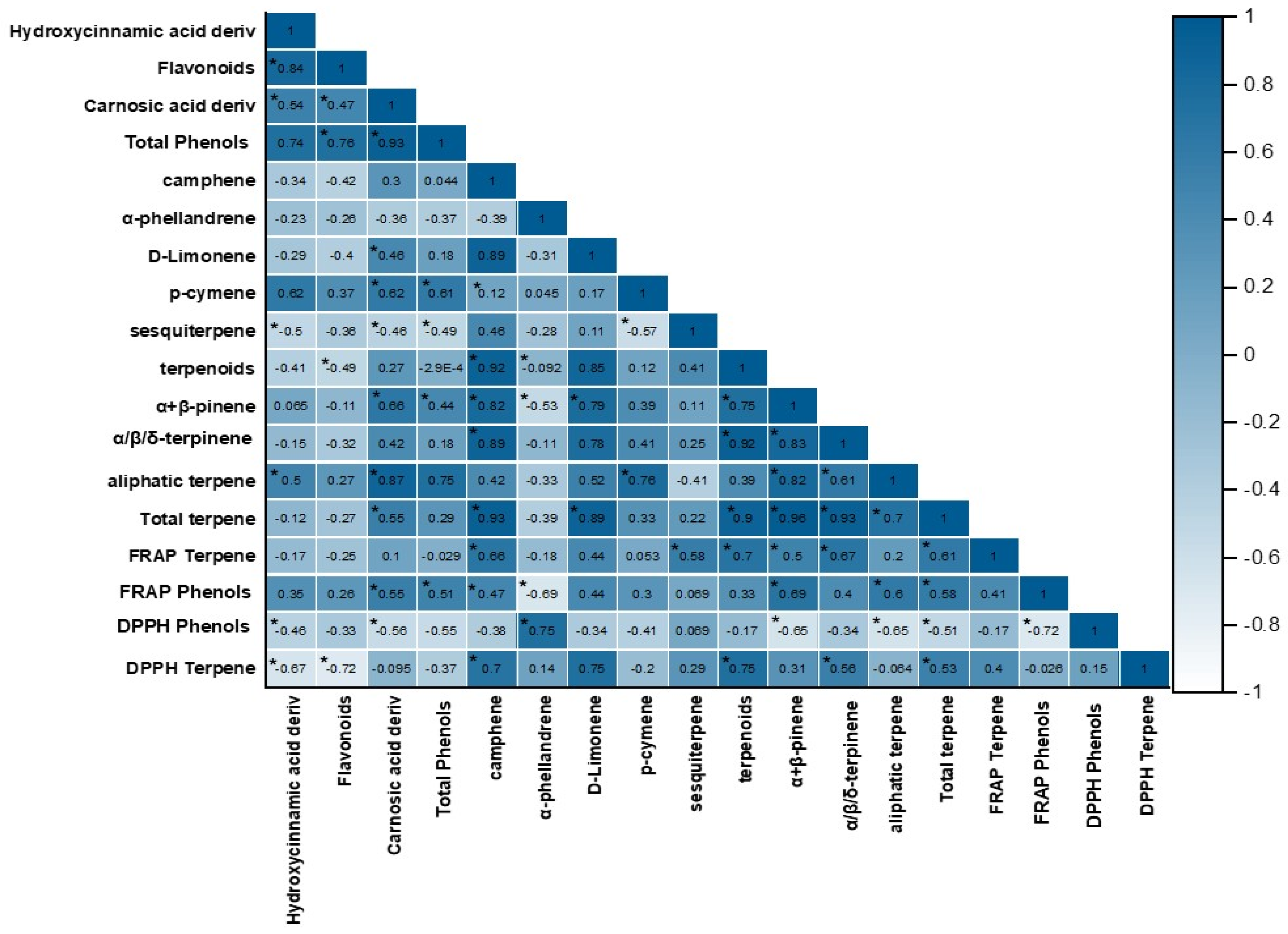
2.4. Pearson’s Correlations Between Total Terpenes, Phenolics, and Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Sampling of Foliar Tissue of Rosemary Cultivars
4.2. Analysis of Phenolic and Phenolic Diterpenes Compounds
4.3. GC-MS Analysis of Terpene Content in Rosemary Cultivars
4.4. Antioxidant Activity Assays of Rosemary Extracts
4.4.1. DPPH Radical-Scavenging Activity Assay
4.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunziata, A.; De Benedetti, L.; Marchioni, I.; Cervelli, C. High resolution melting profiles of 364 genotypes of Salvia rosmarinus in 16 microsatellite loci. Ecol. Evol. 2013, 9, 3728–3739. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A Novel Insight on an Ancient Aromatic Plant: The Rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Marin, M.; Koko, V.; Duletić-Laušević, S.; Marin, P.D.; Rančić, D.; Dajic-Stevanovic, Z. Glandular Trichomes on the Leaves of Rosmarinus officinalis: Morphology, Stereology and Histochemistry. S. Afr. J. Bot. 2006, 72, 378–382. [Google Scholar] [CrossRef]
- Lešnik, S.; Furlan, V.; Bren, U. Rosemary (Rosmarinus officinalis L.): Extraction Techniques, Analytical Methods and Health-Promoting Biological Effects. Phytochem. Rev. 2021, 20, 1273–1328. [Google Scholar] [CrossRef]
- del Baño, M.J.; Lorente, J.; Castillo, J.; Benavente-García, O.; del Río, J.A.; Ortuño, A.; Quirin, K.-W.; Gerard, D. Phenolic Diterpenes, Flavones, and Rosmarinic Acid Distribution during the Development of Leaves, Flowers, Stems, and Roots of Rosmarinus officinalis. Antioxidant activity. J. Agric. Food Chem. 2003, 51, 4247–4253. [Google Scholar] [CrossRef]
- Lamponi, S.; Baratto, M.C.; Miraldi, E.; Baini, G.; Biagi, M. Chemical Profile, Antioxidant, Anti-Proliferative, Anticoagulant and Mutagenic Effects of a Hydroalcoholic Extract of Tuscan Rosmarinus officinalis. Plants 2021, 10, 97. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities–Potentials and challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- de Macedo, L.M.; Santos, É.M.d.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., Syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L.; Ayala-Gómez, A. Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics 2020, 7, 77. [Google Scholar] [CrossRef]
- Science, R.B.G.K. Salvia rosmarinus Spenn. Available online: http://plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:457138-1 (accessed on 24 July 2024).
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant Secondary Metabolites Synthesis and Their Regulations under Biotic and Abiotic Constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Segneanu, A.; Velciov, S.M.; Olariu, S.; Cziple, F.; Damian, D.; Grozescu, I. Bioactive Molecules Profile from Natural Compounds. In Amino Acid—New Insights and Roles in Plant and Animal; InTech: London, UK, 2017. [Google Scholar]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and Antioxidant Properties of Rosemary and Sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) Essential Oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.; Batool, R.; Akhtar, W.; Shahzad, T.; Malik, A.; Shah, M.A.; Iqbal, S.; Rauf, A.; Zengin, G.; Bouyahya, A.; et al. Rosemary Species: A Review of Phytochemicals, Bioactivities and Industrial Applications. S. Afr. J. Bot. 2022, 151, 3–18. [Google Scholar] [CrossRef]
- Gori, A.; Nascimento, L.B.; Ferrini, F.; Centritto, M.; Brunetti, C. Seasonal and Diurnal Variation in Leaf Phenolics of Three Medicinal Mediterranean Wild Species: What Is the Best Harvesting Moment to Obtain the Richest and the Most Antioxidant Extracts? Molecules 2020, 25, 956. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, Y.; Bouzaine, T.; Boussaid, M. Essential Oils Composition in Two Rosmarinus officinalis L. Varieties and Incidence for Antimicrobial and Antioxidant Activities. Food Chem. Toxicol. 2010, 48, 3144–3152. [Google Scholar] [PubMed]
- Farhadi, F.; Baradaran Rahimi, V.; Mohamadi, N.; Askari, V.R. Effects of Rosmarinic Acid, Carnosic Acid, Rosmanol, Carnosol, and Ursolic Acid on the Pathogenesis of Respiratory Diseases. Bifactors 2023, 49, 478–501. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Keita, H.; Ortiz, B.L.S.; dos Santos Sampaio, T.I.; Ferreira, I.M.; Lima, E.S.; de Jesus Amazonas da Silva, M.; Fernandes, C.P.; de Faria Mota Oliveira, A.E.M.; da Conceição, E.C.; et al. Anti-Inflammatory Activity of Nanoemulsions of Essential Oil from Rosmarinus officinalis L.: In Vitro and in Zebrafish Studies. Inflammopharmacology 2018, 26, 1057–1080. [Google Scholar] [CrossRef]
- Bahri, S.; Ben Ali, R.; Gasmi, K.; Mlika, M.; Fazaa, S.; Ksouri, R.; Serairi, R.; Jameleddine, S.; Shlyonsky, V. Prophylactic and Curative Effect of Rosemary Leaves Extract in a Bleomycin Model of Pulmonary Fibrosis. Pharm. Biol. 2017, 55, 462–471. [Google Scholar] [CrossRef]
- Bahri, S.; Ali, R.B.; Abdennabi, R.; Nahdi, A.; Mlika, M.; Jameleddine, S. Industrial Elimination of Essential Oils from Rosmarinus officinalis: In Support of the Synergic Antifibrotic Effect of Rosmarinic and Carnosic Acids in Bleomycin Model of Lung Fibrosis. Nutr. Cancer 2021, 73, 2376–2387. [Google Scholar] [CrossRef]
- Meziane, H.; Zraibi, L.; Albusayr, R.; Bitari, A.; Oussaid, A.; Hammouti, B.; Touzani, R. Rosmarinus officinalis Linn.: Unveiling Its Multifaceted Nature in Nutrition, Diverse Applications, and Advanced Extraction Methods. J. Umm Al-Qura Univ. Appl. Sci. 2024, 2024, 1–29. [Google Scholar] [CrossRef]
- Raffo, A.; Baiamonte, I.; De Benedetti, L.; Lupotto, E.; Marchioni, I.; Nardo, N.; Cervelli, C. Exploring Volatile Aroma and Non-Volatile Bioactive Compounds Diversity in Wild Populations of Rosemary (Salvia rosmarinus Spenn.). Food Chem. 2023, 404, 134532. [Google Scholar] [CrossRef] [PubMed]
- Mira-Sánchez, M.D.; Castillo-Sánchez, J.; Morillas-Ruiz, J.M. Comparative Study of Rosemary Extracts and Several Synthetic and Natural Food Antioxidants. Relevance of Carnosic Acid/Carnosol Ratio. Food Chem. 2020, 309, 125688. [Google Scholar] [CrossRef] [PubMed]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic Acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Artali, R.; Facino, R.M.; Gelmini, F. An Analytical and Theoretical Approach for the Profiling of the Antioxidant Activity of Essential Oils: The Case of Rosmarinus officinalis L. J. Pharm. Biomed. Anal. 2011, 55, 1255–1264. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Hendel, N.; Sarri, D.; Sarri, M.; Napoli, E.; Palumbo Piccionello, A.; Ruberto, G. Phytochemical Analysis and Antioxidant and Antifungal Activities of Powders, Methanol Extracts, and Essential Oils from Rosmarinus officinalis L. and Thymus ciliatus Desf. Benth. Int. J. Mol. Sci. 2024, 25, 7989. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Ezzat, S.M.; El Bishbishy, M.H.; Mnayer, D.; Sharopov, F.; Kılıç, C.S.; Neagu, M.; Constantin, C.; Sharifi-Rad, M.; Atanassova, M.; et al. Rosmarinus Plants: Key Farm Concepts towards Food Applications. Phytother. Res. 2020, 34, 1474–1518. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Fang, X.; Wada, S. Enhancing the Antioxidant Effect of α-Tocopherol with Rosemary in Inhibiting Catalyzed Oxidation Caused by Fe2+ and Hemoprotein. Food Res. Int. 1993, 26, 405–411. [Google Scholar]
- Ebrahimi, E.; Haghjou, M.; Nematollahi, A.; Goudarzian, F. Effects of Rosemary Essential Oil on Growth Performance and Hematological Parameters of Young Great Sturgeon (Huso Huso). Aquaculture 2020, 521, 734909. [Google Scholar] [CrossRef]
- Žegura, B.; Dobnik, D.; Niderl, M.H.; Filipič, M. Antioxidant and Antigenotoxic Effects of Rosemary (Rosmarinus officinalis L.) Extracts in Salmonella Typhimurium TA98 and HepG2 Cells. Environ. Toxicol. Pharmacol. 2011, 32, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. Subcellular Compartmentation of the Diterpene Carnosic Acid and Its Derivatives in the Leaves of Rosemary. Plant Physiol. 2001, 125, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Wenkert, E.; Fuchs, A.; McChesney, J.D.; Brieskorn, H.; Fuchs, A.; Bredenberg, J.B.; McChesney, J.D.; Wenkert, E.; Beak, P.; Carney, J.R.W.; et al. Chemical Artifacts from the Family Labiatae. J. Org. Chem. 1965, 30, 2931–2934. [Google Scholar] [CrossRef]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Green Processes for the Extraction of Bioactives from Rosemary: Chemical and Functional Characterization via Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry and in-Vitro Assays. J. Chromatogr. A 2010, 1217, 2512–2520. [Google Scholar] [CrossRef]
- Brückner, K.; Božić, D.; Manzano, D.; Papaefthimiou, D.; Pateraki, I.; Scheler, U.; Ferrer, A.; de Vos, R.C.H.; Kanellis, A.K.; Tissier, A. Characterization of Two Genes for the Biosynthesis of Abietane-Type Diterpenes in Rosemary (Rosmarinus officinalis) Glandular Trichomes. Phytochemistry 2014, 101, 52–64. [Google Scholar] [CrossRef]
- Tounekti, T.; Munné-Bosch, S. Enhanced phenolic diterpenes antioxidant levels through non-transgenic approaches. Crit. Rev. Plant Sci. 2012, 31, 505–519. [Google Scholar] [CrossRef]
- Jing, Z.; Wang, C.; Yang, Q.; Wei, X.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Ma, X.; Liu, K.; et al. Luteolin Attenuates Glucocorticoid-induced Osteoporosis by Regulating ERK/Lrp-5/GSK-3β Signaling Pathway in Vivo and in Vitro. J. Cell. Physiol. 2019, 234, 4472–4490. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Zhang, J.; Zhao, C.; Lu, S.; Qiao, J.; Han, M. The Combinatory Effects of Natural Products and Chemotherapy Drugs and Their Mechanisms in Breast Cancer Treatment. Phytochem. Rev. 2020, 19, 1179–1197. [Google Scholar] [CrossRef]
- Cuvelier, M.; Richard, H.; Berset, C. Antioxidative Activity and Phenolic Composition of Pilot-plant and Commercial Extracts of Sage and Rosemary. J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Ben Farhat, M.; Jordán, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in Essential Oil, Phenolic Compounds, and Antioxidant Activity of Tunisian Cultivated Salvia officinalis L. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.B.; Landoulsi, A.; Chaouch-Hamada, R.; Sotomayor, J.A.; Jordán, M.J. Characterization and Quantification of Phenolic Compounds and Antioxidant Properties of Salvia Species Growing in Different Habitats. Ind. Crops Prod. 2013, 49, 904–914. [Google Scholar] [CrossRef]
- Kim, M.-G.; Kim, S.-M.; Min, J.-H.; Kwon, O.-K.; Park, M.-H.; Park, J.-W.; Ahn, H.I.; Hwang, J.-Y.; Oh, S.-R.; Lee, J.-W.; et al. Anti-Inflammatory Effects of Linalool on Ovalbumin-Induced Pulmonary Inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural Phenolic Compounds From Medicinal Herbs and Dietary Plants: Potential Use for Cancer Prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef]
- Xavier, V.; Spréa, R.; Finimundy, T.C.; Heleno, S.A.; Amaral, J.S.; Barros, L.; Ferreira, I.C.F.R. Terpenes. In Natural Secondary Metabolites; Springer International Publishing: Cham, Switzerland, 2023; pp. 107–156. [Google Scholar]
- Melero-Bravo, E.; Ortiz de Elguea-Culebras, G.; Sánchez-Vioque, R.; Fernández-Sestelo, M.; Herraiz-Peñalver, D.; Sánchez-Vioque, R. Variability of Essential Oil in Cultivated Populations of Rosmarinus officinalis L. in Spain. Euphytica 2022, 218, 65. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.; Zhang, R.-W.; Fan, X.-E.; Chen, H.-J. Quantitation of the Hydroxycinnamic Acid Derivatives and the Glycosides of Flavonols and Flavones by UV Absorbance after Identification by LC-MS. J. Agric. Food Chem. 2012, 60, 544–553. [Google Scholar] [CrossRef]
- Che Zain, M.S.; Osman, M.F.; Lee, S.Y.; Shaari, K. UHPLC-UV/PDA Method Validation for Simultaneous Quantification of Luteolin and Apigenin Derivatives from Elaeis Guineensis Leaf Extracts: An Application for Antioxidant Herbal Preparation. Molecules 2021, 26, 1084. [Google Scholar] [CrossRef]
- Software Workstation Mass Hunter. Available online: https://www.agilent.com/en/product/software-informatics/mass-spectrometry-software/data-analysis/qualitative-analysis (accessed on 2 December 2024).
- Kandi, S.; Charles, A.L. Statistical Comparative Study between the Conventional DPPH Spectrophotometric and Dropping DPPH Analytical Method without Spectrophotometer: Evaluation for the Advancement of Antioxidant Activity Analysis. Food Chem. 2019, 287, 338–345. [Google Scholar] [CrossRef]
- IBM Software IBM SPSS Statistics. Available online: https://www.ibm.com/es-es/products/spss-statistics (accessed on 16 August 2023).

| S. rosmarinus Cultivars | ||||||
|---|---|---|---|---|---|---|
| Class | ‘T. Blue’ | ‘Gorizia’ | ‘Alba’ | ‘Ginger’ | ‘Roseus’ | ‘Arp’ |
| Hydroxycinnamic acid derivatives | 1.08 ± 0.28 b | 0.38 ± 0.045 c | 0.82 ± 0.22 b | 0.23 ± 0.09 c | 0.64 ± 0.078 b | 1.83 ± 0.10 a |
| Flavonoids | 6.36 ± 1.43 a | 2.92 ± 0.92 b | 5.32 ± 1.51 a | 1.72 ± 0.68 b | 1.89 ± 0.67 b | 4.98 ± 1.07 a |
| Phenolic diterpenes | 14.91 ± 1.47 b | 12.00 ± 2.57 c | 24.85 ± 2.72 a | 14.55 ± 1.49 b | 17.13 ± 3.32 b | 15.44 ± 5.35 b |
| Total polyphenols | 22.35 ± 3.18 b | 15.30 ± 3.54 b | 30.99 ± 4.45 a | 17.35 ± 2.35 b | 19.65 ± 4.60 b | 22.25 ± 6.51 ab |
| Carnosic acid derivatives/Carnosol | 2.35 ± 0.14 | 4.62 ± 0.21 | 4.38 ± 0.89 | 93.47 ± 3.01 | 9.82 ± 1.65 | 3.79 ± 0.38 |
| S. rosmarinus Cultivars | ||||||
|---|---|---|---|---|---|---|
| Compounds | ‘T. Blue’ | Gorizia’ | ‘Alba’ | ‘Ginger’ | ‘Roseus’ | ‘Arp’ |
| Camphene | 0.988 ± 0.115 bc | 0.640 ± 0.095 c | 2.359 ± 0.099 ab | 3.097 ± 0.029 a | 2.438 ± 0.173 ab | 0.794 ± 0.019 bc |
| α-Phellandrene | - | 0.224 ± 0.018 a | 0.092 ± 0.003 c | 0.089 ± 0.003 c | 0.053 ± 0.011 d | 0.152 ± 0.009 b |
| D-Limonene | 0.373 ± 0.028 c | 0.410 ± 0.060 c | 1.140 ± 0.053 a | 0.958 ± 0.086 b | 1.208 ± 0.050 a | 0.374 ± 0.066 c |
| p-Cymene | 0.070 ± 0.009 d | 0.024 ± 0.005 e | 0.323 ± 0.027 a | 0.109 ± 0.011 c | 0.025 ± 0.004 e | 0.237 ± 0.022 b |
| sesquiterpenes | 0.030 ± 0.004 b | 0.022 ± 0.002 c | 0.006 ± 0.001 e | 0.055 ± 0.004 a | 0.029 ± 0.003 b | 0.013 ± 0.002 d |
| α/β-Pinene | 4.579 ± 0.432 d | 1.668 ± 0.207 e | 9.127 ± 0.338 a | 6.854 ± 0.109 b | 5.318 ± 0.193 c | 1.458 ± 0.080 e |
| α/γ/δ-Terpinene | 0.099 ± 0.005 e | 0.181 ± 0.034 d | 0.770 ± 0.101 b | 0.819 ± 0.007 a | 0.386 ± 0.072 c | 0.190 ± 0.022 d |
| Aliphatic terpenes | 0.273 ± 0.018 b | 0.083 ± 0.004 d | 0.683 ± 0.062 a | 0.244 ± 0.023 bc | 0.204 ± 0.006 c | 0.197 ± 0.018 c |
| Terpenoids | 2.286 ± 0.372 e | 3.079 ± 0.170 d | 4.527 ± 0.176 b | 5.210 ± 0.346 a | 4.119 ± 0.188 c | 2.339 ± 0.099 e |
| Total terpenes | 8.708 ± 0.983 d | 6.331 ± 0.595 d | 19.028 ± 0.877 a | 17.434 ± 0.740 b | 13.779 ± 0.744 c | 5.753 ± 0.429 e |
| Cultivar | Phenolic Extracts | Terpene Extracts | ||
|---|---|---|---|---|
| FRAP (mM Fe(II)/mg) | DPPH (EC50, µg/mL) | FRAP (mM Fe(II)/mg) | DPPH (EC50, µg/mL) | |
| ‘Tuscan Blue’ | 9.374 ± 1.554 b | 0.660 ± 0.010 c | 11.699 ± 1.561 c | 0.210 ± 0.002 e |
| ‘Gorizia’ | 6.469 ± 0.691 d | 0.940 ± 0.0130 a | 11.260 ± 1.513 c | 0.529 ± 0.010 c |
| ‘Alba’ | 9.766 ± 1.028 b | 0.630 ± 0.060 c | 13.185 ± 1.762 c | 0.528 ± 0.066 c |
| ‘Ginger’ | 8.659 ± 0.823 bc | 0.750 ± 0.0100 bc | 16.677 ± 2.580 b | 0.690 ± 0.067 b |
| ‘Roseus’ | 8.485 ± 0.739 bc | 0.750 ± 0.020 bc | 12.325 ± 1.645 c | 0.784 ± 0.025 a |
| ‘Arp’ | 7.166 ± 0.761 c | 0.800 ± 0.030 bc | 11.072 ± 1.513 c | 0.393 ± 0.034 d |
| Positive control | 61.258 ± 5.923 a | 0.100 ± 0.012 d | 61.258 ± 5.923 a | 0.100 ± 0.012 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, A.; Menicucci, F.; Brunetti, C.; dos Santos Nascimento, L.B.; Pasquini, D.; Alderotti, F.; Detti, C.; Ferrini, F.; Gori, A. Unlocking the Hidden Potential of Rosemary (Salvia rosmarinus Spenn.): New Insights into Phenolics, Terpenes, and Antioxidants of Mediterranean Cultivars. Plants 2024, 13, 3395. https://doi.org/10.3390/plants13233395
Baptista A, Menicucci F, Brunetti C, dos Santos Nascimento LB, Pasquini D, Alderotti F, Detti C, Ferrini F, Gori A. Unlocking the Hidden Potential of Rosemary (Salvia rosmarinus Spenn.): New Insights into Phenolics, Terpenes, and Antioxidants of Mediterranean Cultivars. Plants. 2024; 13(23):3395. https://doi.org/10.3390/plants13233395
Chicago/Turabian StyleBaptista, Andrea, Felicia Menicucci, Cecilia Brunetti, Luana Beatriz dos Santos Nascimento, Dalila Pasquini, Francesca Alderotti, Cassandra Detti, Francesco Ferrini, and Antonella Gori. 2024. "Unlocking the Hidden Potential of Rosemary (Salvia rosmarinus Spenn.): New Insights into Phenolics, Terpenes, and Antioxidants of Mediterranean Cultivars" Plants 13, no. 23: 3395. https://doi.org/10.3390/plants13233395
APA StyleBaptista, A., Menicucci, F., Brunetti, C., dos Santos Nascimento, L. B., Pasquini, D., Alderotti, F., Detti, C., Ferrini, F., & Gori, A. (2024). Unlocking the Hidden Potential of Rosemary (Salvia rosmarinus Spenn.): New Insights into Phenolics, Terpenes, and Antioxidants of Mediterranean Cultivars. Plants, 13(23), 3395. https://doi.org/10.3390/plants13233395










