Comprehensive In Vitro Evaluation of Antibacterial, Antioxidant, and Computational Insights into Blepharis ciliaris (L.) B. L. Burtt from Hail Mountains, Saudi Arabia
Abstract
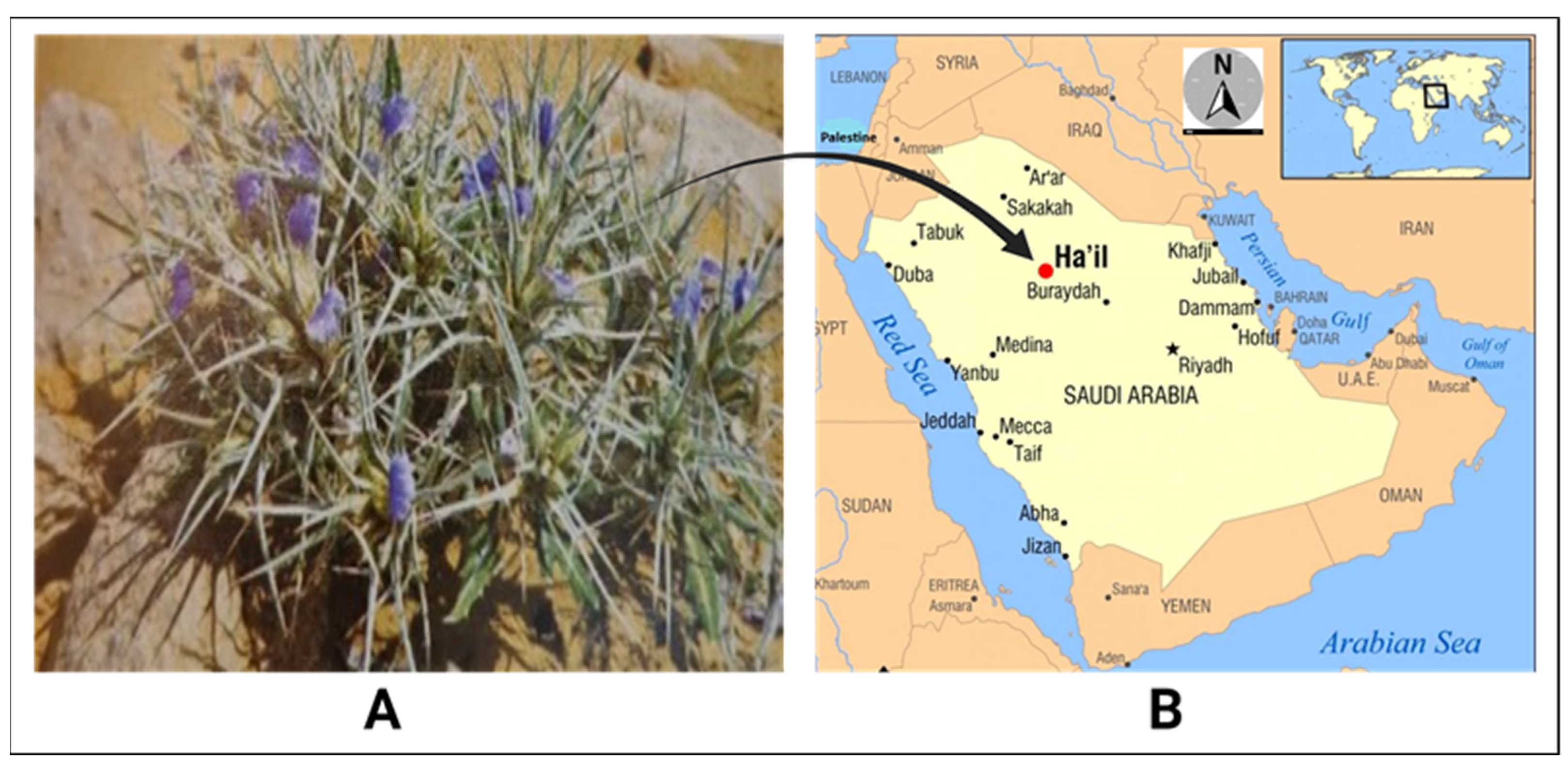
1. Introduction
2. Results and Discussion
2.1. GC-MS Findings
2.2. Antioxidants Activity
2.3. Antibacterial Activity
2.4. Computational Findings
2.4.1. In Silico Identification of Non-Toxic Compounds from Blepharis ciliaris
2.4.2. Drug-Likeness for Blepharis ciliaris Compounds
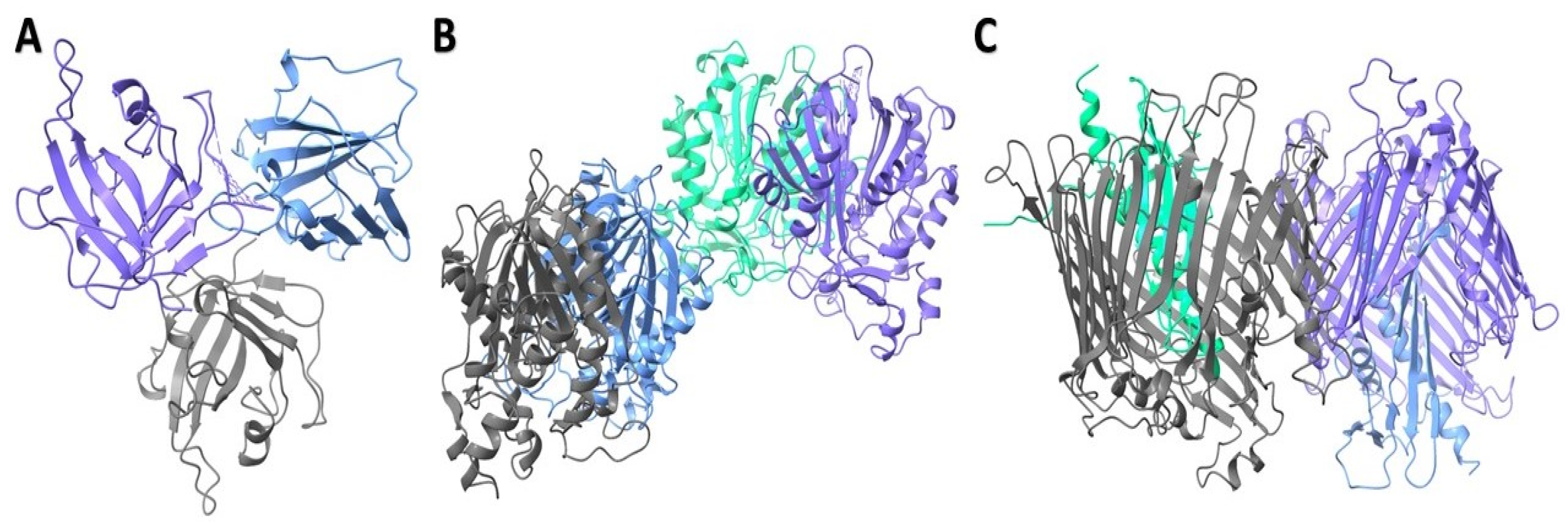
2.4.3. Structural Characterization of Modeled Proteins
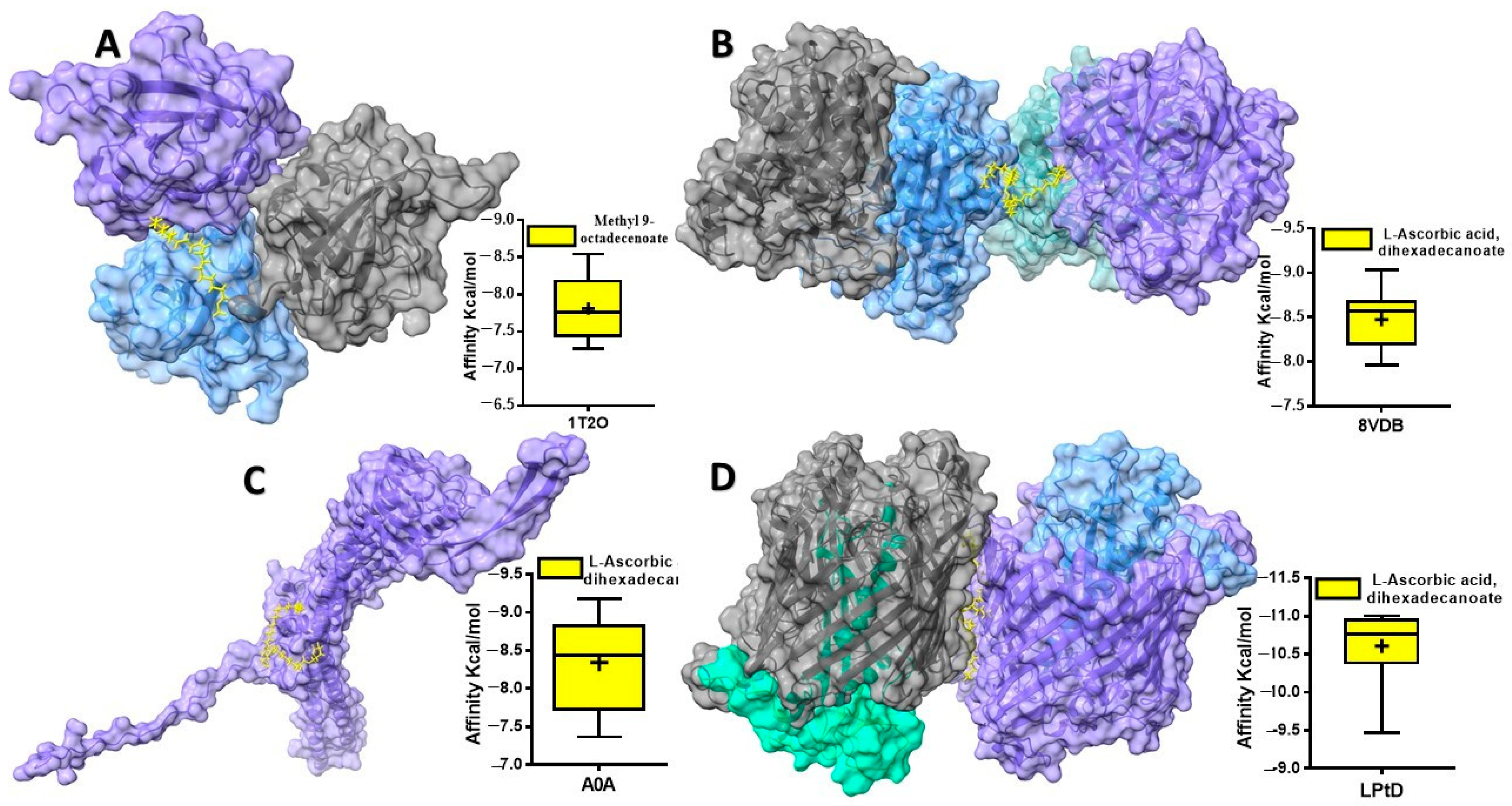
2.4.4. Protein–Ligand Interaction Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Plant Samples and Extraction
3.3. Bacterial Samples
3.4. GC-MS Analysis
3.4.1. Test Conditions
3.4.2. The Experiment of GC-MS
3.5. DPPH Radical Scavenging
3.6. Disc Diffusion Test
3.7. Determination of MIC
3.8. Toxicity Prediction of Blepharis Ciliaris-Derived Compounds
3.9. Physicochemical Properties for Blepharis ciliaris Compounds
3.10. Retrieval of Targeted Receptor Proteins
3.11. Proteins Refinement and Validation for S. marcescens
3.12. Ligand Preparation
3.13. Protein–Ligand Docking
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdallah, E.M. Plants: An alternative source for antimicrobials. J. Appl. Pharm. Sci. 2011, 1, 16–20. [Google Scholar]
- Hacker, K. The burden of chronic disease. Mayo Clin. Proc. Innov. Qual. Outcomes 2024, 8, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef]
- Cook, M.A.; Wright, G.D. The past, present, and future of antibiotics. Sci. Transl. Med. 2022, 14, eabo7793. [Google Scholar] [CrossRef]
- Venkatasubramanian, P.; Balasubramani, S.P.; Kukkupuni, S.K. Reverse Pharmacognosy: Traditional Knowledge Guided Assessment of Medicinal Plant Quality and Efficacy. In Medicinal Agroecology; CRC Press: Boca Raton, FL, USA, 2023; pp. 225–247. [Google Scholar]
- Saganuwan, A.S. Some medicinal plants of Arabian Pennisula. J. Med. Plants Res. 2010, 4, 766–788. [Google Scholar]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182. [Google Scholar]
- Sadeek, A.; Abdallah, E.M. Phytochemical compounds as antibacterial agents: A mini review. Glob. J. Pharmaceut. Sci. 2019, 7, 555720. [Google Scholar]
- Sulieman, A.M.E.; Alanaizy, E.; Alanaizy, N.A.; Abdallah, E.M.; Idriss, H.; Salih, Z.A.; Ibrahim, N.A.; Ali, N.A.; Ibrahim, S.E.; Abd El Hakeem, B.S. Unveiling Chemical, Antioxidant and Antibacterial Properties of Fagonia indica Grown in the Hail Mountains, Saudi Arabia. Plants 2023, 12, 1354. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mossa, J.S.; Al-Said, M.S.; Al-Yahya, M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: A report on seven plant families. Fitoterapia 2004, 75, 149–161. [Google Scholar] [CrossRef]
- Heneidy, S.Z.; Halmy, M.W.A.; Bidak, L.M. The ethnobotanical importance and conservation value of native plants in eastern Arabian Peninsula. Feddes Repert. 2017, 128, 105–128. [Google Scholar] [CrossRef]
- Gutterman, Y. Blepharis. In Handbook of Flowering; CRC Press: Boca Raton, FL, USA, 2019; pp. 108–116. [Google Scholar]
- El-Shanawany, M.A.; Sayed, H.M.; Ibrahim, S.R.; Fayed, M.A.; Radwan, M.M.; Ross, S.A. A new isoflavone from Blepharis ciliaris of an Egyptian origin. Med. Chem. Res. 2013, 22, 2346–2350. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.; Elkhayat, E.S.; Ross, S.A.; Sayed, H.M.; El-Moghazy, S.A.; El-Shanawany, M.A. Blepharisides A and B, new flavonol glycosides from Blepharis ciliaris growing in Saudi Arabia. Phytochem. Lett. 2015, 11, 177–182. [Google Scholar] [CrossRef]
- Phondani, P.C.; Bhatt, I.D.; Negi, V.S.; Kothyari, B.P.; Bhatt, A.; Maikhuri, R.K. Promoting medicinal plants cultivation as a tool for biodiversity conservation and livelihood enhancement in Indian Himalaya. J. Asia-Pac. Biodivers. 2016, 9, 39–46. [Google Scholar] [CrossRef]
- Dirar, A.I.; Wada, M.; Watanabe, T.; Devkota, H.P. Phenolic compounds from the aerial parts of Blepharis linariifolia Pers. and their free radical scavenging and enzyme inhibitory activities. Medicines 2019, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Rutherford, S.T.; Silhavy, T.J.; Huang, K.C. Physical properties of the bacterial outer membrane. Nat. Rev. Microbiol. 2022, 20, 236–248. [Google Scholar] [CrossRef]
- Saidijam, M.; Psakis, G.; Clough, J.L.; Meuller, J.; Suzuki, S.i.; Hoyle, C.J.; Palmer, S.L.; Morrison, S.M.; Pos, M.K.; Essenberg, R.C. Collection and characterisation of bacterial membrane proteins. FEBS Lett. 2003, 555, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Malojčić, G.; Andres, D.; Grabowicz, M.; George, A.H.; Ruiz, N.; Silhavy, T.J.; Kahne, D. LptE binds to and alters the physical state of LPS to catalyze its assembly at the cell surface. Proc. Natl. Acad. Sci. USA 2014, 111, 9467–9472. [Google Scholar] [CrossRef] [PubMed]
- Wallock-Richards, D.J.; Marles-Wright, J.; Clarke, D.J.; Maitra, A.; Dodds, M.; Hanley, B.; Campopiano, D.J. Molecular basis of Streptococcus mutans sortase A inhibition by the flavonoid natural product trans-chalcone. Chem. Commun. 2015, 51, 10483–10485. [Google Scholar] [CrossRef] [PubMed]
- Frankel, B.A.; Bentley, M.; Kruger, R.G.; McCafferty, D.G. Vinyl sulfones: Inhibitors of SrtA, a transpeptidase required for cell wall protein anchoring and virulence in Staphylococcus aureus. J. Am. Chem. Soc. 2004, 126, 3404–3405. [Google Scholar] [CrossRef]
- Clancy, K.W.; Melvin, J.A.; McCafferty, D.G. Sortase transpeptidases: Insights into mechanism, substrate specificity, and inhibition. Pept. Sci. 2010, 94, 385–396. [Google Scholar] [CrossRef]
- Kingston, A.W.; Subramanian, C.; Rock, C.O.; Helmann, J.D. A σW-dependent stress response in Bacillus subtilis that reduces membrane fluidity. Mol. Microbiol. 2011, 81, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-H.; Heath, R.J.; Rock, C.O. β-Ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J. Bacteriol. 2000, 182, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for molecular docking: A review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of Techniques and Solvents on the Antimicrobial and Antioxidant Potential of Extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Talib, F.; Aftab, T. FTIR, HPLC, GC-MS analysis and investigation of hypoglycemic effects of leaves extracts of Fagonia indica. Pharmacogn. Commun. 2021, 11, 109–118. [Google Scholar]
- El-Shanawany, M.; Sayed, H.; Ibrahim, S.; Fayed, M. Stigmasterol tetracosanoate, a new stigmasterol ester from the Egyptian Blepharis ciliaris. Drug Res. 2015, 65, 347–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmad, I.; Sherwani, M.; Hasan, S.; Ahmad, M., Jr.; Osman, S. 9-Hydroxydodecanoic acid, an acid from Blepharis sindica seed oil. Phytochemistry 1983, 22, 493–494. [Google Scholar] [CrossRef]
- Aghaabbasi, K.; Askari, N.; Hassani Kumleh, H.; Torkzadeh-Mahani, M.; Ramzani-Ghara, A. The Blepharis persica seed hydroalcoholic extract synergistically enhances the apoptotic effect of doxorubicin in human colon cancer and gastric cancer cells. Mol. Biol. Rep. 2020, 47, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, S.; Kripa, K. Therapeutic uses of plants of genus Blepharis—A systematic review. Int. J. Pharma Bio Sci. 2016, 7, B236–B243. [Google Scholar]
- Ifeanyi, O.E. A review on free radicals and antioxidants. Int. J. Curr. Res. Med. Sci 2018, 4, 123–133. [Google Scholar]
- Piątkowska, E.; Biel, W.; Witkowicz, R.; Kępińska-Pacelik, J. Chemical composition and antioxidant activity of Asteraceae family plants. Appl. Sci. 2022, 12, 12293. [Google Scholar] [CrossRef]
- Mahboubi, M.; Haghi, G.; Kazempour, N.; Hatemi, A.R. Total phenolic content, antioxidant and antimicrobial activities of Blepharis edulis extracts. Songklanakarin J. Sci. Technol. 2013, 35, 11–16. [Google Scholar]
- Hossain, M.B.; Ahmed, L.; Martin-Diana, A.B.; Brunton, N.P.; Barry-Ryan, C. Individual and combined antioxidant activity of spices and spice phenolics. Antioxidants 2023, 12, 308. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil–A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Ertem, H.; Çakmakçı, S. Shelf life and quality of probiotic yogurt produced with Lactobacillus acidophilus and Gobdin. Int. J. Food Sci. Technol. 2018, 53, 776–783. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2007; pp. 105–125. [Google Scholar]
- Srinivasan, K. Fenugreek (Trigonella foenum-graecum): A review of health beneficial physiological effects. Food Rev. Int. 2006, 22, 203–224. [Google Scholar] [CrossRef]
- Abdallah Emad, M.; Gamal, E.-G.E. Screening for antimicrobial activity of some plants from Saudi folk medicine. Glob. J. Res. Med. Plants Indigen. Med. 2013, 2, 210–218. [Google Scholar]
- Dirar, A.I.; Adhikari-Devkota, A.; Kunwar, R.M.; Paudel, K.R.; Belwal, T.; Gupta, G.; Chellappan, D.K.; Hansbro, P.M.; Dua, K.; Devkota, H.P. Genus Blepharis (Acanthaceae): A review of ethnomedicinally used species, and their phytochemistry and pharmacological activities. J. Ethnopharmacol. 2021, 265, 113255. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.; Van Staden, J. Seasonal variation in antimicrobial and phytochemical properties of frequently used medicinal bulbous plants from South Africa. S. Afr. J. Bot. 2011, 77, 387–396. [Google Scholar] [CrossRef]
- Shahat, A.A.; Mahmoud, E.A.; Al-Mishari, A.A.; Alsaid, M.S. Antimicrobial activities of some Saudi Arabian herbal plants. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Elkahoui, S.; Ghoniem, A.E.M.; Snoussi, M.; Barhoumi, Z.; Badraoui, R. Acacia gerrardii, a desert plant as a sustainable source of natural products with antimicrobial properties. bioRxiv 2024. bioRxiv:2024.2009.2013.612817. [Google Scholar]
- Mohammadipanah, F.; Wink, J. Actinobacteria from arid and desert habitats: Diversity and biological activity. Front. Microbiol. 2016, 6, 1541. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, G.; Bahadur, A.; Xu, Y.; Liu, Y.; Tian, M.; Ding, W.; Chen, T.; Zhang, W.; Liu, G. Genomic investigation of desert Streptomyces huasconensis D23 reveals its environmental adaptability and antimicrobial activity. Microorganisms 2022, 10, 2408. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Koohsari, H.; Ghaemi, E.; Sheshpoli, M.S.; Jahedi, M.; Zahiri, M. The investigation of antibacterial activity of selected native plants from North of Iran. J. Med. Life 2015, 8, 38. [Google Scholar]
- Gutsmann, T.; Seydel, U. Impact of the glycostructure of amphiphilic membrane components on the function of the outer membrane of Gram-negative bacteria as a matrix for incorporated channels and a target for antimicrobial peptides or proteins. Eur. J. Cell Biol. 2010, 89, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.A.; Gruenert, R.; Bednarski, P.; Lindequist, U. Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 260–268. [Google Scholar]
- Sowemimo, A.; Onakoya, M.; Fageyinbo, M.S.; Fadoju, T. Studies on the anti-inflammatory and anti-nociceptive properties of Blepharis maderaspatensis leaves. Rev. Bras. Farmacogn. 2013, 23, 830–835. [Google Scholar] [CrossRef]
- Igwe, O.; Okwunodulu, F. Investigation of bioactive phytochemical compounds from the chloroform extract of the leaves of phyllanthus amarus by GC-MS technique. Int. J. Chem. Pharm. Sci. 2014, 2, 554–560. [Google Scholar]
- Begum, S.F.M.; Priya, S.; Sundararajan, R.; Hemalatha, S. Novel anticancerous compounds from Sargassum wightii: In silico and in vitro approaches to test the antiproliferative efficacy. J. Adv. Pharm. Educ. Res. 2017, 7, 272–277. [Google Scholar]
- Pai, K.; Bodke, Y.D.; Manandhar, S.; Pai, K. In silico-based virtual screening and molecular docking analysis of phytochemicals obtained from methanolic extract of Cleome viscosa Linn. By GC-MS method for its anticancer activity. Asian J. Chem. 2021, 33, 2943. [Google Scholar] [CrossRef]
- Namasivayam, S.; Shankar, K.G.; Vivek, J.; Nizar, M.; Sudarsan, A. In silico and in vitro analysis of quorum quenching active phytochemicals from the ethanolic extract of medicinal plants against quorum sensing mediated virulence factors of Acinetobacter baumannii. Indian J. Biochem. Biophys. IJBB 2019, 56, 276–286. [Google Scholar]
- Ralte, L.; Khiangte, L.; Thangjam, N.M.; Kumar, A.; Singh, Y.T. GC–MS and molecular docking analyses of phytochemicals from the underutilized plant, Parkia timoriana revealed candidate anti-cancerous and anti-inflammatory agents. Sci. Rep. 2022, 12, 3395. [Google Scholar] [CrossRef] [PubMed]
- Gadnayak, A.; Dehury, B.; Nayak, A.; Jena, S.; Sahoo, A.; Panda, P.C.; Ray, A.; Nayak, S. ‘Mechanistic insights into 5-lipoxygenase inhibition by active principles derived from essential oils of Curcuma species: Molecular docking, ADMET analysis and molecular dynamic simulation study. PLoS ONE 2022, 17, e0271956. [Google Scholar] [CrossRef]
- Lipinski, C. Poor aqueous solubility—An industry wide problem in drug discovery. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Amaral, L.; Martins, A.; Spengler, G.; Molnar, J. Efflux pumps of Gram-negative bacteria: What they do, how they do it, with what and how to deal with them. Front. Pharmacol. 2014, 4, 168. [Google Scholar] [CrossRef]
- Pallen, M.J.; Lam, A.C.; Antonio, M.; Dunbar, K. An embarrassment of sortases–a richness of substrates? Trends Microbiol. 2001, 9, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Aulabaugh, A.; Ding, W.; Kapoor, B.; Alksne, L.; Tabei, K.; Ellestad, G. Kinetic mechanism of Staphylococcus aureus sortase SrtA. Biochemistry 2003, 42, 11307–11315. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Nguyen, T.T.T.; Nguyen, H.D.; Nguyen, T.K.; Pham, P.T.V.; Tran, L.T.; Tran, L.T.T.; Tran, M.H. Integrating in Silico and in Vitro studies to screen anti-Staphylococcus aureus activity from Vietnamese Ganoderma multiplicatum and Ganoderma sinense. Nat. Prod. Commun. 2023, 18, 1934578X231167289. [Google Scholar] [CrossRef]
- van Hensbergen, V.P.; Wu, Y.; van Sorge, N.M.; Touqui, L. Type IIA secreted phospholipase A2 in host defense against bacterial infections. Trends Immunol. 2020, 41, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nair, V.; Jain, S.; Gupta, Y. Evaluation of anti-inflammatory activity of plant lipids containing⍺–linolenic acid. Indian J. Exp. Biol. 2008, 46, 453–456. [Google Scholar]
- Rotimi, S.O.; Rotimi, O.A.; Obembe, O.O. In silico analysis of compounds characterized from ethanolic extract of Cucurbita pepo with NF-kB-inhibitory potential. Bangladesh J. Pharmacol. 2014, 9, 551–556. [Google Scholar] [CrossRef]
- Shaheed, K.; AlGaraawi, N.; Alsultany, A.; Abbas, Z.; Khshayyish, I.; Al Khazali, M. Analysis of bioactive phytochemical compound of (Cyperus iria L.) By using gas chromatography–mass spectrometry. In IOP Conference Series: Earth and Environmental Science; IOP: Bristol, UK, 2019; p. 012064. [Google Scholar]
- Reza, A.A.; Haque, M.A.; Sarker, J.; Nasrin, M.S.; Rahman, M.M.; Tareq, A.M.; Khan, Z.; Rashid, M.; Sadik, M.G.; Tsukahara, T. Antiproliferative and antioxidant potentials of bioactive edible vegetable fraction of Achyranthes ferruginea Roxb. in cancer cell line. Food Sci. Nutr. 2021, 9, 3777–3805. [Google Scholar] [CrossRef] [PubMed]
- Manilal, A.; Sujith, S.; Kiran, G.S.; Selvin, J.; Shakir, C. Cytotoxic potentials of red alga, Laurencia brandenii collected from the Indian coast. Glob. J. Pharmacol. 2009, 3, 90–94. [Google Scholar]
- Younis, S.; Taj, S.; Rashid, S. Structural studies of Staphylococcus aureus Sortase inhibiton via Conus venom peptides. Arch. Biochem. Biophys. 2019, 671, 87–102. [Google Scholar] [CrossRef]
- Nickels, J.D.; Poudel, S.; Chatterjee, S.; Farmer, A.; Cordner, D.; Campagna, S.R.; Giannone, R.J.; Hettich, R.L.; Myles, D.A.; Standaert, R.F. Impact of fatty-acid labeling of Bacillus subtilis membranes on the cellular lipidome and proteome. Front. Microbiol. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Pishchany, G.; Mevers, E.; Ndousse-Fetter, S.; Horvath, D.J., Jr.; Paludo, C.R.; Silva-Junior, E.A.; Koren, S.; Skaar, E.P.; Clardy, J.; Kolter, R. Amycomicin is a potent and specific antibiotic discovered with a targeted interaction screen. Proc. Natl. Acad. Sci. USA 2018, 115, 10124–10129. [Google Scholar] [CrossRef]
- Campbell, J.W.; Cronan, J.E., Jr. Bacterial fatty acid biosynthesis: Targets for antibacterial drug discovery. Annu. Rev. Microbiol. 2001, 55, 305–332. [Google Scholar] [CrossRef]
- Chng, S.-S.; Ruiz, N.; Chimalakonda, G.; Silhavy, T.J.; Kahne, D. Characterization of the two-protein complex in Escherichia coli responsible for lipopolysaccharide assembly at the outer membrane. Proc. Natl. Acad. Sci. USA 2010, 107, 5363–5368. [Google Scholar] [CrossRef] [PubMed]
- Freinkman, E.; Chng, S.-S.; Kahne, D. The complex that inserts lipopolysaccharide into the bacterial outer membrane forms a two-protein plug-and-barrel. Proc. Natl. Acad. Sci. USA 2011, 108, 2486–2491. [Google Scholar] [CrossRef]
- Okwu, D.E.; Ighodaro, B.U. GC-MS evaluation of the bioactive compounds and antibacterial activity of the oil fraction from the stem barks of Dacryodes edulis G. Don Lam. Int. J. Drug Dev. Res. 2009, 1, 117–125. [Google Scholar]
- Begic, S.; Worobec, E.A. The role of the Serratia marcescens SdeAB multidrug efflux pump and TolC homologue in fluoroquinolone resistance studied via gene-knockout mutagenesis. Microbiology 2008, 154, 454–461. [Google Scholar] [CrossRef]
- Hejazi, A.; Falkiner, F.R. Serratia marcescens. J. Med. Microbiol. 1997, 46, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Aucken, H.M.; Pitt, T. Antibiotic resistance and putative virulence factors of Serratia marcescens with respect to O and K serotypes. J. Med. Microbiol. 1998, 47, 1105–1113. [Google Scholar] [CrossRef]
- Hornsey, M.; Ellington, M.J.; Doumith, M.; Hudson, S.; Livermore, D.M.; Woodford, N. Tigecycline resistance in Serratia marcescens associated with up-regulation of the SdeXY-HasF efflux system also active against ciprofloxacin and cefpirome. J. Antimicrob. Chemother. 2010, 65, 479–482. [Google Scholar] [CrossRef]
- Colclough, A.L.; Alav, I.; Whittle, E.E.; Pugh, H.L.; Darby, E.M.; Legood, S.W.; McNeil, H.E.; Blair, J.M. RND efflux pumps in Gram-negative bacteria; regulation, structure and role in antibiotic resistance. Future Microbiol. 2020, 15, 143–157. [Google Scholar] [CrossRef]
- Idriss, H.; Siddig, B.; González-Maldonado, P.; Elkhair, H.; Alakhras, A.I.; Abdallah, E.M.; Elzupir, A.O.; Sotelo, P.H. Inhibitory activity of Saussurea costus extract against bacteria, candida, herpes, and SARS-CoV-2. Plants 2023, 12, 460. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Elgaaly, G.A.; Ibrahim, M.M. Identification of bioactive phytochemical from two Punica species using GC–MS and estimation of antioxidant activity of seed extracts. Saudi J. Biol. Sci. 2018, 25, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.; Siddig, B.; Maldonado, P.G.; Elkhair, H.; Alakhras, A.; Abdallah, E.M.; Torres, P.H.S.; Elzupir, A.O. Phytochemical discrimination, biological activity and molecular docking of water-soluble inhibitors from saussurea costus herb against main protease of SARS-CoV-2. Molecules 2022, 27, 4908. [Google Scholar] [CrossRef] [PubMed]
- Al-Aamri, M.S.; Al-Abousi, N.M.; Al-Jabri, S.S.; Alam, T.; Khan, S.A. Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in Eastern Oman. J. Taibah Univ. Med. Sci. 2018, 13, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- El Baz, A.; Mrabti, H.N.; Ashmawy, N.S.; Khan, S.A.; Abdallah, E.M.; Al-Mijalli, S.H.; Alenazy, R.; Alshabrmi, F.M.; Bouyahya, A.; El Hachlafi, N. Phytochemical characterization, antimicrobial properties and in silico modeling perspectives of Anacyclus pyrethrum essential oil. Heliyon 2024, 10, e35079. [Google Scholar] [CrossRef] [PubMed]
- Hamad Al-Mijalli, S.; ELsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of volatile compounds of Mentha piperita and Lavandula multifida and investigation of their antibacterial, antioxidant, and antidiabetic properties. Evid. Based Complement. Altern. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, gkae303. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys. J. 2011, 101, 2525–2534. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
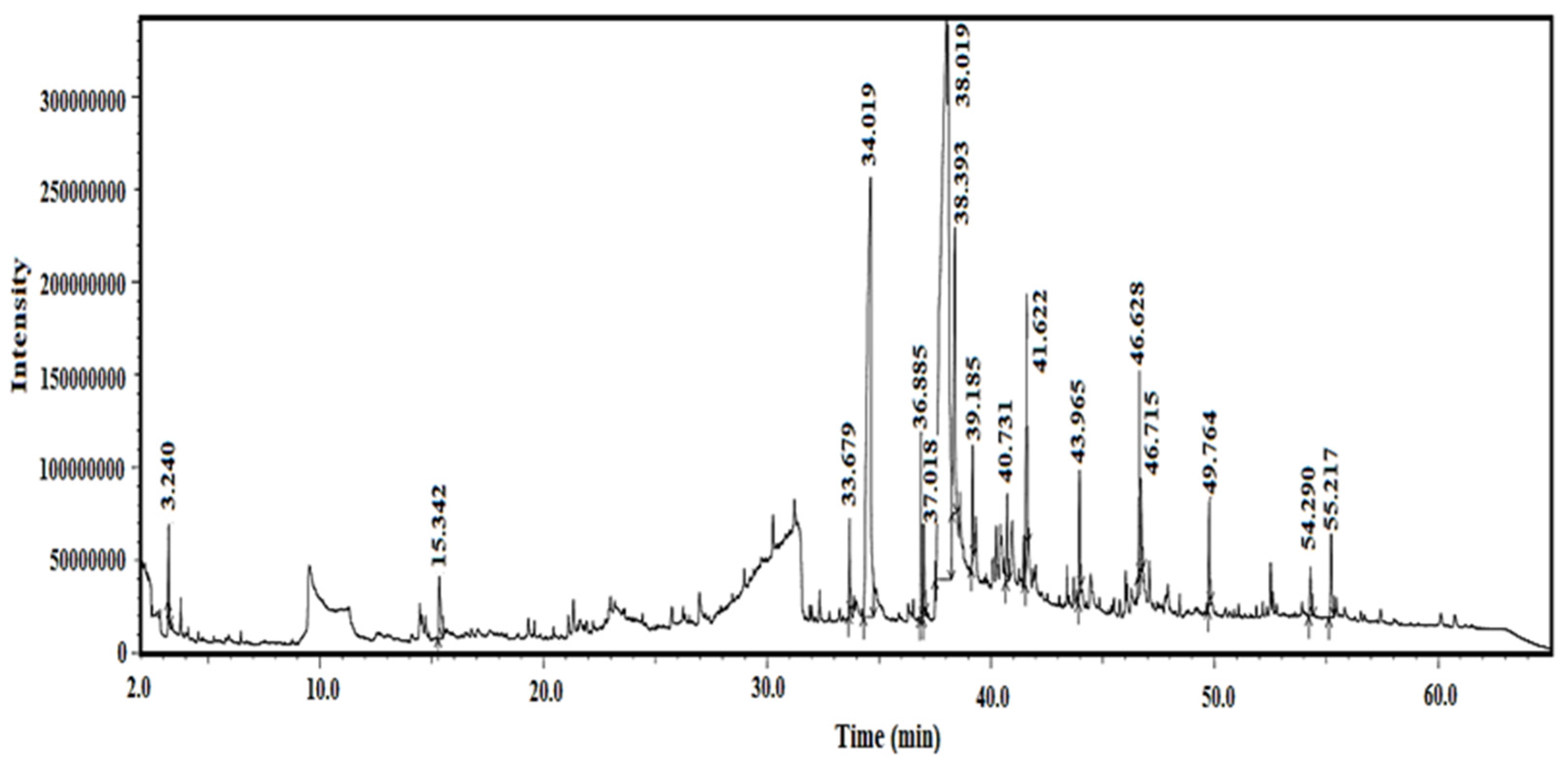

| Peak | Compound Name | Area% | Error% | Ret. Time (min) | Classification |
|---|---|---|---|---|---|
| 1 | 2-Propanol, 1-[(2-hydroxyethyl)thio]- | 1.21 | ±0.05 | 3.240 | Alcohol |
| 2 | Pentadecanoic acid, ethyl methyl ester | 2.63 | ±0.06 | 15.342 | Fatty Acid |
| 3 | Hexadecanoic acid, methyl ester | 1.00 | ±0.04 | 33.679 | Fatty Acid |
| 4 | (+)-Ascorbic acid 2,6-dihexadecanoate | 15.63 | ±0.10 | 34.620 | Vitamin Compound |
| 5 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 2.74 | ±0.03 | 36.885 | Fatty Acid |
| 6 | 9-Octadecenoic acid, methyl ester (E) | 2.78 | ±0.03 | 37.018 | Fatty Acid |
| 7 | 7-Heptadecyn-1-ol | 1.26 | ±0.05 | 38.393 | Fatty Alcohol |
| 8 | Octadecanoic acid | 5.88 | ±0.07 | 39.185 | Fatty Acid |
| 9 | 9-Octadecanoic acid (Z), 2,3-dihydro- | 2.94 | ±0.02 | 40.731 | Fatty Acid |
| 10 | 7-Heptadecenoic acid | 1.90 | ±0.02 | 41.622 | Fatty Acid |
| 11 | 41-Hexadecyn-1-ol | 3.68 | ±0.04 | 46.628 | Fatty Alcohol |
| 12 | Undec-10-ynoic acid, undec-2-en-1-yl ester | 5.67 | ±0.08 | 46.680 | Fatty Acid |
| 13 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 2.78 | ±0.03 | 46.868 | Fatty Acid |
| 14 | Piperine | 3.66 | ±0.06 | 49.764 | Alkaloid |
| 15 | Stigmasterol | 2.24 | ±0.02 | 54.290 | Sterol |
| 16 | gamma-Sitosterol | 1.50 | ±0.01 | 55.217 | Sterol |
| 17 | 9-Tetradecadenoic acid (Z) | 3.22 | ±0.05 | 55.511 | Fatty Acid |
| Concentration of B. ciliaris (µg/mL) | 1 | 2 | 3 | Mean | SD | % Inhibition | p-Value * | Significance (vs. Control) |
|---|---|---|---|---|---|---|---|---|
| 62.5 | 0.364 | 0.386 | 0.367 | 0.3723 | 0.0119 | 16.20 | <0.05 | Significant |
| 125 | 0.264 | 0.286 | 0.267 | 0.2723 | 0.0119 | 38.71 | <0.01 | Highly Significant |
| 250 | 0.19 | 0.198 | 0.189 | 0.1923 | 0.0049 | 56.71 | <0.01 | Highly Significant |
| 500 | 0.185 | 0.184 | 0.145 | 0.1713 | 0.0208 | 61.44 | <0.001 | Highly Significant |
| 1000 | 0.11 | 0.12 | 0.016 | 0.0820 | 0.0574 | 81.55 | <0.001 | Highly Significant |
| Control | 0.42 | 0.449 | 0.464 | 0.4443 | 0.0224 | 0.00 | - | - |
| Bacteria | Methanol Extract (1 mg/disk) | Ampicillin (10 µg/disk) | p-Value | Significance (Methanol vs. Ampicillin) |
|---|---|---|---|---|
| S. aureus | 10.33 ± 1.53 | 25.0 ± 2.0 | <0.001 | Highly Significant |
| B. subtilis | 13.33 ± 1.53 | 23.0 ± 2.5 | <0.001 | Highly Significant |
| E. coli | 10.67 ± 1.53 | 12.0 ± 1.5 | 0.02 | Significant |
| S. marcescens | 10.00 ± 2.00 | 6.0 ± 1.0 | <0.01 | Highly Significant |
| Bacteria | MIC Ratios in (µg/mL) | Fold Difference (Extract/Ampicillin) | |
|---|---|---|---|
| Methanol Extract MIC (µg/mL) | Ampicillin MIC (µg/mL) | ||
| S. aureus | 500 | 1.56 | 320 |
| B. subtilis | 500 | 3.125 | 160 |
| E. coli | 1000 | 25 | 40 |
| S. marcescens | 1000 | 100 | 10 |
| No. | Compound Name | LD50 (mg/kg) | Predicted Toxicity Class * | Average Similarity (%) | Prediction Accuracy (%) |
|---|---|---|---|---|---|
| 1 | 2-Propanol, 1-[(2-hydroxyethyl)thio]- | 300 | 3 | 59.57 | 67.38 |
| 2 | Pentadecanoic acid, ethyl methyl ester | 5000 | 5 | 100 | 100 |
| 3 | Hexadecanoic acid, methyl ester | 5000 | 5 | 100 | 100 |
| 4 | (+)-Ascorbic acid 2,6-dihexadecanoate | 10,000 | 6 | 85.78 | 70.97 |
| 5 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 20,000 | 6 | 85.93 | 70.97 |
| 6 | 9-Octadecenoic acid, methyl ester (E) | 3000 | 5 | 89.64 | 70.97 |
| 7 | 7-Heptadecyn-1-ol | 753 | 4 | 67.58 | 68.07 |
| 8 | Octadecanoic acid | 900 | 4 | 100 | 100 |
| 9 | 9-Octadecanoic acid (Z), 2,3-dihydro- | 48 | 2 | 100 | 100 |
| 10 | 7-Heptadecenoic acid | 48 | 2 | 100 | 100 |
| 11 | 41-Hexadecyn-1-ol | 520 | 4 | 54.95 | 67.38 |
| 12 | Undec-10-ynoic acid, undec-2-en-1-yl ester | 5000 | 5 | 81.82 | 70.97 |
| 13 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 20,000 | 6 | 85.93 | 70.97 |
| 14 | Piperine | 500 | 4 | 53.02 | 67.38 |
| 15 | Stigmasterol | 890 | 4 | 89.38 | 70.97 |
| 16 | gamma-Sitosterol | 890 | 4 | 89.38 | 70.97 |
| 17 | 9-Tetradecadenoic acid (Z) | 48 | 2 | 100 | 100 |
| Compound Name | Molecular Weight | Hydrogen Bonds | Log P * (iLogPo/w) | Molar Refractivity | RO5 Violation ** | |
|---|---|---|---|---|---|---|
| Acceptor Donor | ||||||
| 2-Propanol, 1-[(2-hydroxyethyl)thio]- | 120.21 | 1 | 0 | 2.08 | 35.16 | 0 |
| Pentadecanoic acid, ethyl methyl ester | 270.45 | 2 | 0 | 4.67 | 85.12 | 1 |
| Hexadecanoic acid, methyl ester | 270.45 | 2 | 0 | 4.41 | 85.12 | 1 |
| (+)-Ascorbic acid 2,6-dihexadecanoate | 654.96 | 8 | 2 | 6.91 | 188.84 | 2 |
| 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 294.47 | 2 | 0 | 4.43 | 93.78 | 1 |
| 9-Octadecenoic acid, methyl ester (E) | 310.51 | 2 | 0 | 5.24 | 99.06 | 1 |
| 7-Heptadecyn-1-ol | 238.41 | 1 | 1 | 4.23 | 78.35 | 1 |
| Octadecanoic acid | 284.48 | 2 | 1 | 4.3 | 90.41 | 1 |
| 9-Octadecanoic acid (Z), 2,3-dihydro- | 296.49 | 2 | 1 | 3.68 | 94.74 | 1 |
| 7-Heptadecenoic acid | 268.43 | 2 | 1 | 3.9 | 85.13 | 1 |
| Hexadecynol | 238.41 | 1 | 1 | 4.3 | 78.07 | 1 |
| Undec-10-ynoic acid, undec-2-en-1-yl ester | 320.51 | 2 | 0 | 5.25 | 102.03 | 1 |
| 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 294.47 | 2 | 0 | 4.43 | 93.78 | 1 |
| Piperine | 265.26 | 3 | 1 | 2.3 | 77.35 | 0 |
| Stigmasterol | 412.69 | 1 | 1 | 5.08 | 132.75 | 1 |
| gamma-Sitosterol | 414.71 | 1 | 1 | 5.05 | 133.23 | 1 |
| 9-Tetradecadenoic acid (Z) | 226.36 | 2 | 1 | 3.39 | 70.71 | 0 |
| No. | Compound Name | 1T2O * | 8VDB ** | 4RHB *** | A0A **** |
|---|---|---|---|---|---|
| 1 | Pentadecanoic acid, ethyl methyl ester | −5.979 | −6.696 | −6.112 | −6.194 |
| 2 | Hexadecanoic acid, methyl ester | −7.469 | −7.818 | −7.842 | −7.612 |
| 3 | (+)-Ascorbic acid 2,6-dihexadecanoate | −7.838 | −8.237 | −10.768 | −9.063 |
| 4 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | −7.67 | −7.672 | −8.939 | −7.776 |
| 5 | 9-Octadecenoic acid, methyl ester (E) | −8.543 | −7.927 | −8.945 | −7.839 |
| 6 | 7-Heptadecyn-1-ol | −7.358 | −7.326 | −8.277 | −7.626 |
| 7 | Octadecanoic acid | −7.201 | −7.599 | −8.124 | −6.791 |
| 8 | Hexadecynol | −7.079 | −7.24 | −8.381 | −7.461 |
| 9 | Undec-10-ynoic acid, undec-2-en-1-yl ester | −7.505 | −7.282 | −9.38 | −7.982 |
| 10 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | −7.67 | −7.672 | −8.939 | −7.776 |
| 11 | Piperine | −8.05 | −7.22 | −8.547 | −7.496 |
| 12 | Stigmasterol | −8.316 | −7.094 | −9.77 | −7.949 |
| 13 | gamma-Sitosterol | −8.276 | −7.3 | −9.525 | −8.131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulieman, A.M.E.; Idriss, H.; Alshammari, M.; Almuzaini, N.A.M.; Ibrahim, N.A.; Dahab, M.; Alhudhaibi, A.M.; Alrushud, H.M.A.; Saleh, Z.A.; Abdallah, E.M. Comprehensive In Vitro Evaluation of Antibacterial, Antioxidant, and Computational Insights into Blepharis ciliaris (L.) B. L. Burtt from Hail Mountains, Saudi Arabia. Plants 2024, 13, 3491. https://doi.org/10.3390/plants13243491
Sulieman AME, Idriss H, Alshammari M, Almuzaini NAM, Ibrahim NA, Dahab M, Alhudhaibi AM, Alrushud HMA, Saleh ZA, Abdallah EM. Comprehensive In Vitro Evaluation of Antibacterial, Antioxidant, and Computational Insights into Blepharis ciliaris (L.) B. L. Burtt from Hail Mountains, Saudi Arabia. Plants. 2024; 13(24):3491. https://doi.org/10.3390/plants13243491
Chicago/Turabian StyleSulieman, Abdel Moniem Elhadi, Hajo Idriss, Mamdouh Alshammari, Nujud A. M. Almuzaini, Nosyba A. Ibrahim, Mahmoud Dahab, Abdulrahman Mohammed Alhudhaibi, Hamad Mohammed Abdullah Alrushud, Zakaria Ahmed Saleh, and Emad M. Abdallah. 2024. "Comprehensive In Vitro Evaluation of Antibacterial, Antioxidant, and Computational Insights into Blepharis ciliaris (L.) B. L. Burtt from Hail Mountains, Saudi Arabia" Plants 13, no. 24: 3491. https://doi.org/10.3390/plants13243491
APA StyleSulieman, A. M. E., Idriss, H., Alshammari, M., Almuzaini, N. A. M., Ibrahim, N. A., Dahab, M., Alhudhaibi, A. M., Alrushud, H. M. A., Saleh, Z. A., & Abdallah, E. M. (2024). Comprehensive In Vitro Evaluation of Antibacterial, Antioxidant, and Computational Insights into Blepharis ciliaris (L.) B. L. Burtt from Hail Mountains, Saudi Arabia. Plants, 13(24), 3491. https://doi.org/10.3390/plants13243491







