Transcriptome Analysis Reveals Key Pathways and Genes Involved in Lodging Resistance of Upland Cotton
Abstract
1. Introduction
2. Results
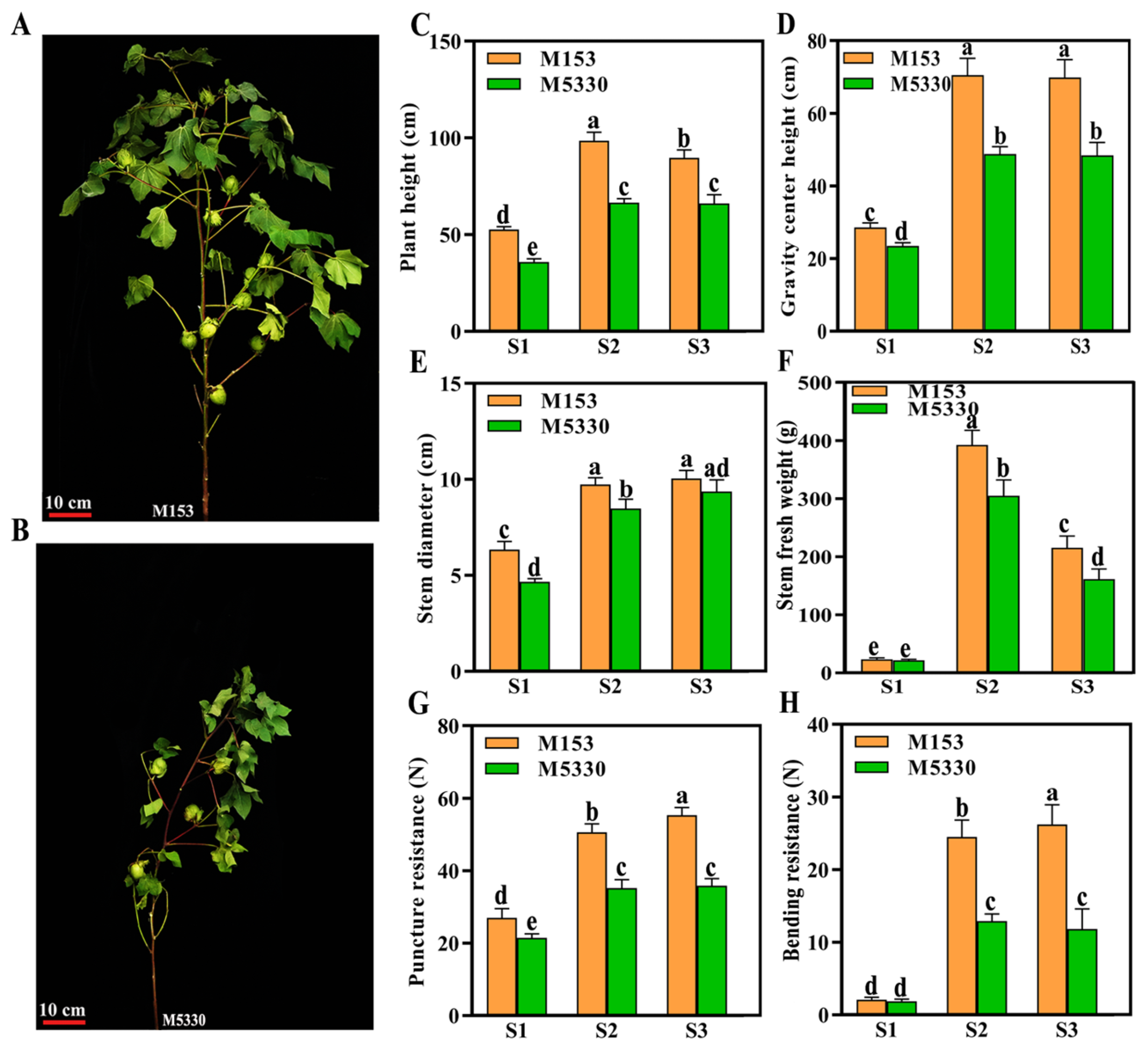
2.1. Stem Morphological Characteristics of Cotton Varieties with Different Lodging Resistance
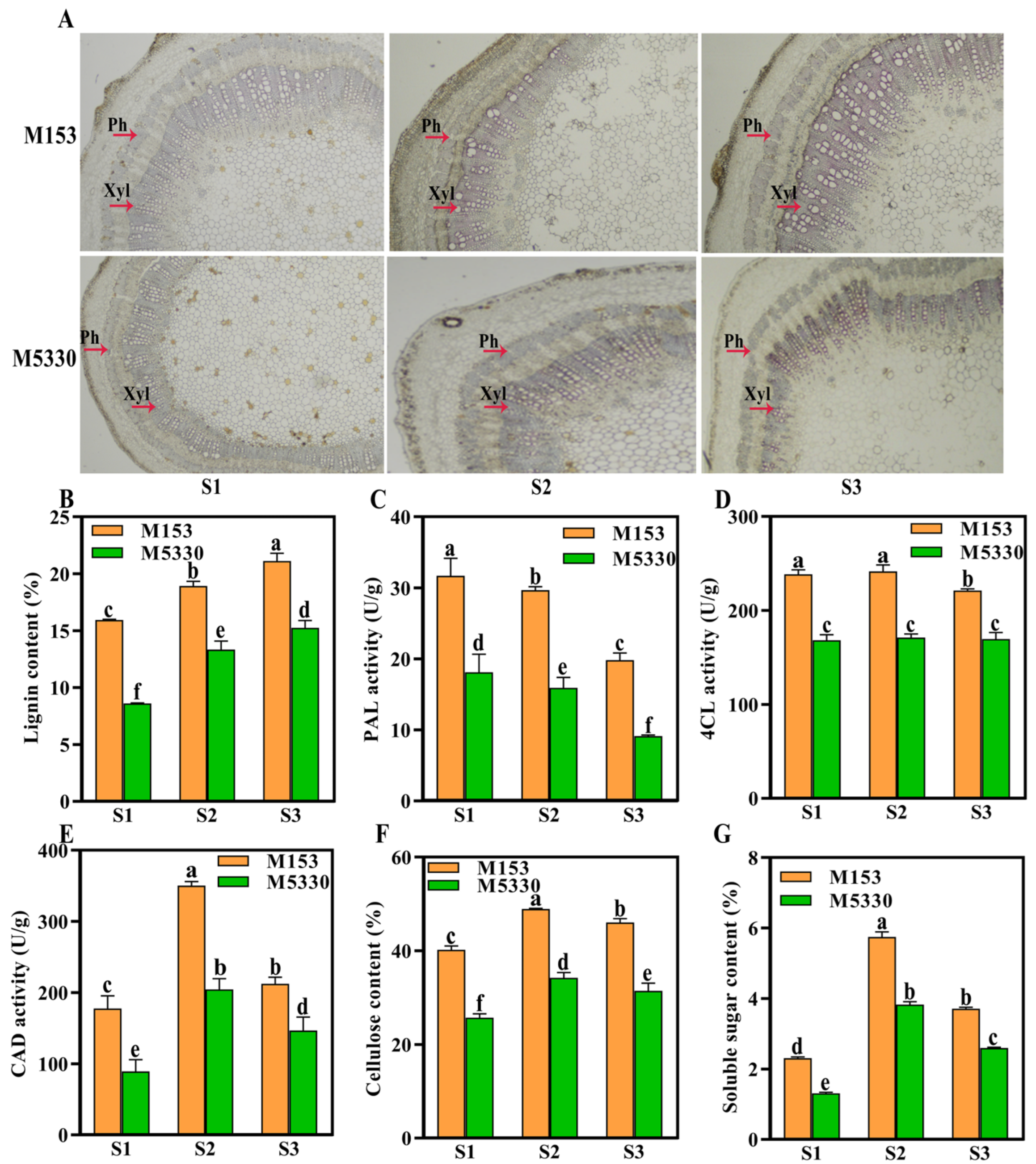
2.2. Lignin Deposition, Lignin Content and Lignin Biosynthesis-Related Enzyme Activity
2.3. Cellulose and Soluble Sugar Content in Cotton
2.4. RNA-Aseq and Differential Expression Analysis
2.5. GO and KEGG Analysis
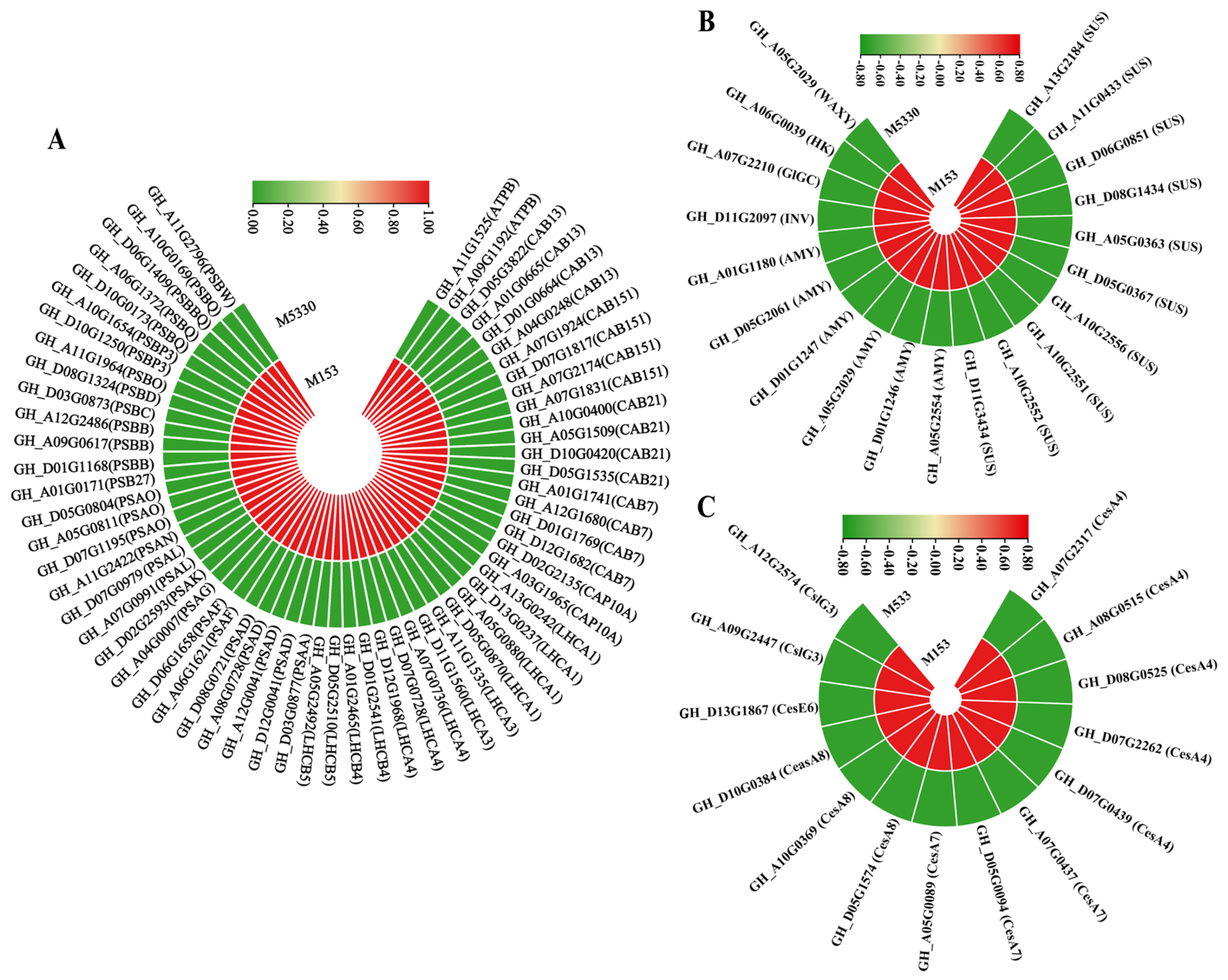
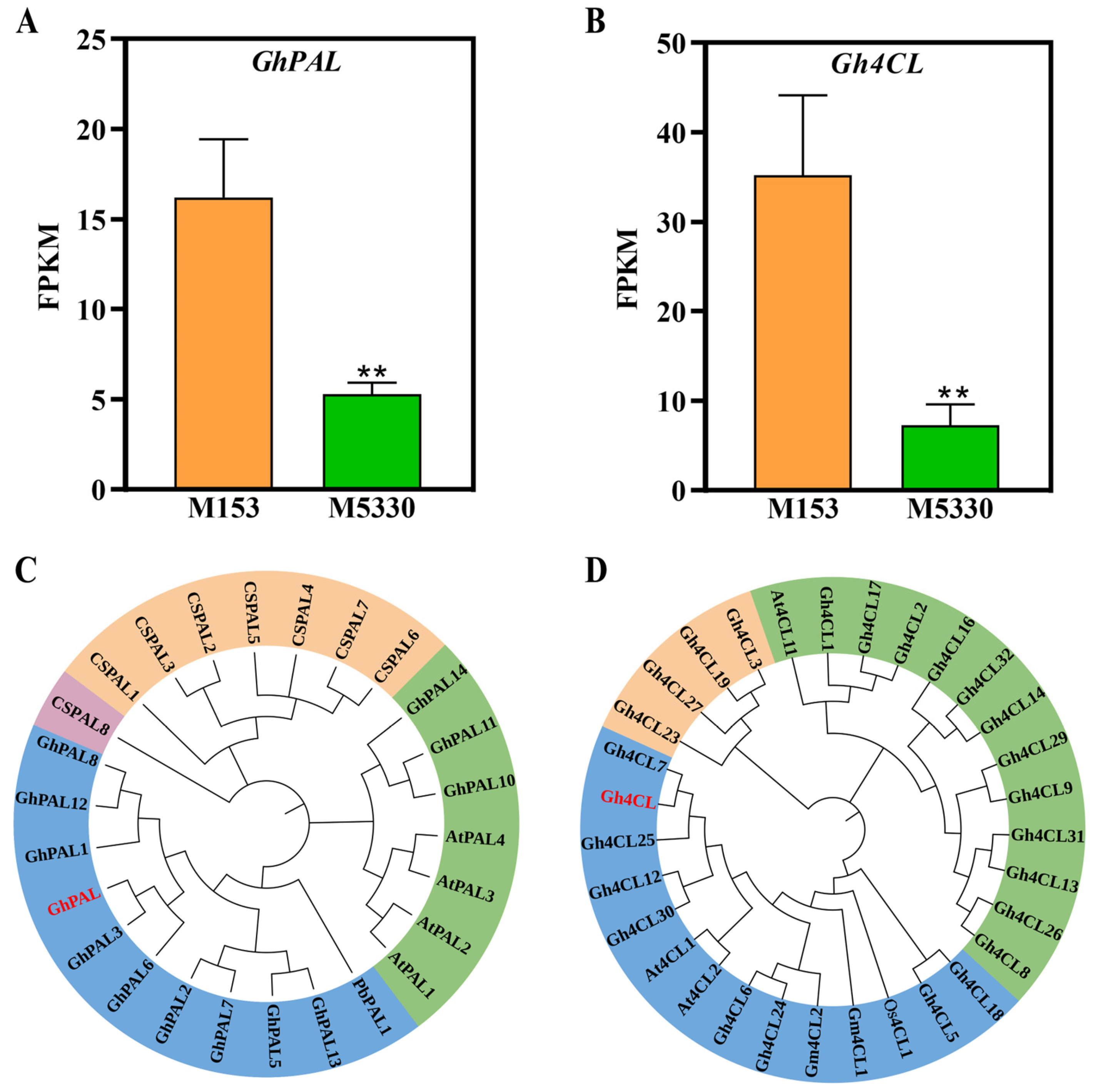
2.6. DEGs Related to Phenylpropanoid Biosynthesis Pathway
2.7. DEGs Related to Photosynthesis and Starch and Sucrose Metabolism Pathways
2.8. DEGs Related to Cellulose Synthesis
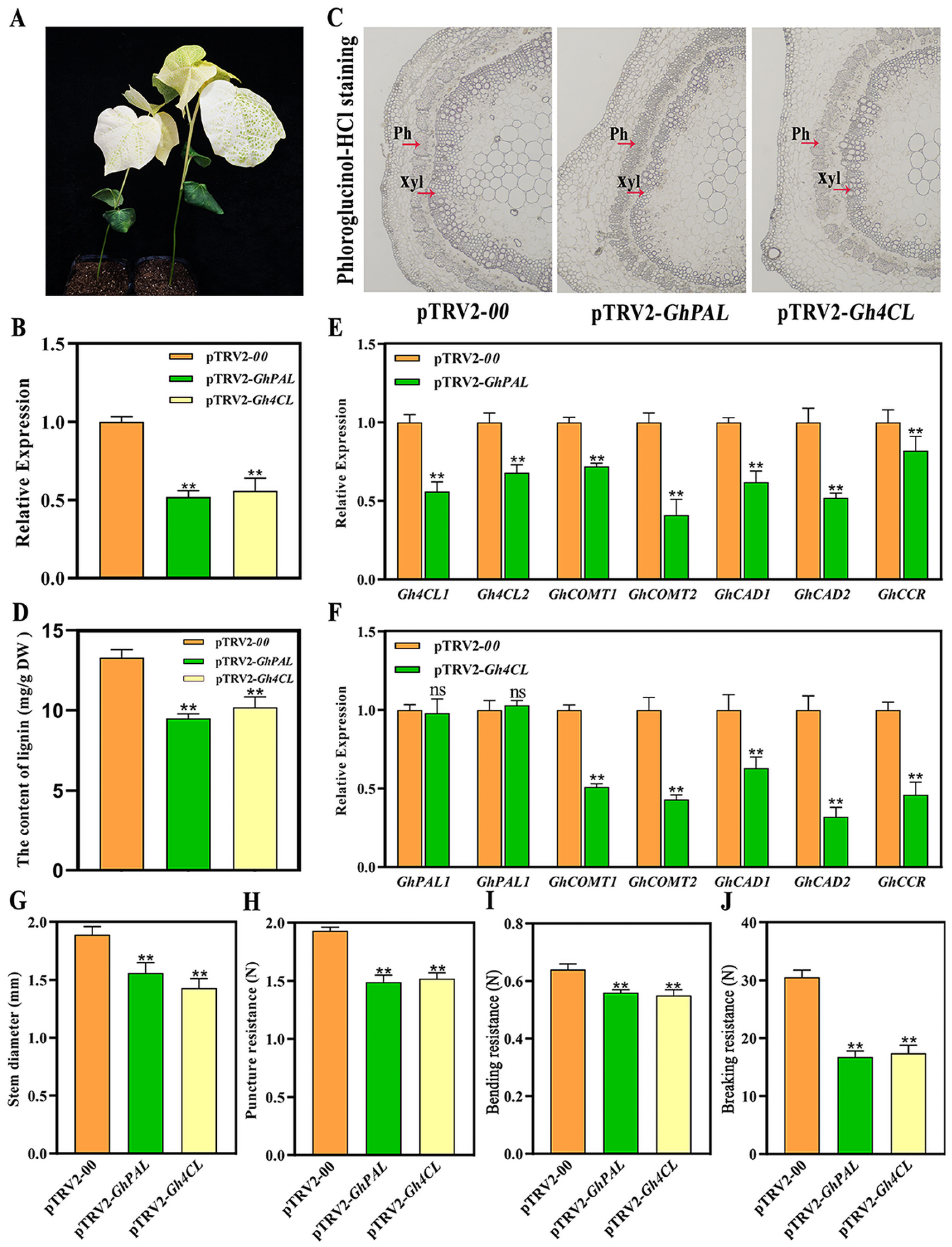
2.9. Functional Confirmation of Two DEGs Related to Lignin Synthesis by VIGS
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Morphological Indices and Mechanical Strength Determination
4.3. Lignin, Cellulose and Soluble Sugar Content Measurements
4.4. Lignin Deposition Observations
4.5. Lignin Biosynthesis-Related Enzyme Activity Measurements
4.6. RNA Sequencing (RNA-Seq)
4.7. TRV (Tobacco Rattle Virus) Treatment
4.8. Gene Expression Analysis
4.9. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Tang, J.; Yu, M.; Zhang, Y.; Abbas, A.; Wang, S.; Bagadeem, S. Sustainable cotton production through increased competitiveness: Analysis of comparative advantage and influencing factors of cotton production in Xinjiang, China. Agronomy 2022, 12, 2239. [Google Scholar] [CrossRef]
- Feng, L.; Wan, S.; Zhang, Y.; Dong, H. Xinjiang cotton: Achieving super-high yield through efficient utilization of light, heat, water, and fertilizer by three generations of cultivation technology systems. Field Crops Res. 2024, 312, 109401. [Google Scholar] [CrossRef]
- Zhao, D.; Luan, Y.; Xia, X.; Shi, W.; Tang, Y.; Tao, J. Lignin provides mechanical support to herbaceous peony (Paeonia lactiflora Pall.) stems. Hortic. Res. 2020, 7, 213. [Google Scholar] [CrossRef]
- Berry, P.M.; Sterling, M.; Spink, J.H.; Baker, C.J.; Sylvester-Bradley, R.; Mooney, S.J.; Tams, A.R.; Ennos, A.R. Understanding and reducing lodging in cereals. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2004; Volume 84, pp. 217–271. [Google Scholar]
- Zhang, J.; Li, G.; Song, Y.; Liu, Z.; Yang, C.; Tang, S.; Zheng, C.; Wang, S.; Ding, Y. Lodging resistance characteristics of high-yielding rice populations. Field Crops Res. 2014, 161, 64–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Wang, H.; Fang, Y.; Dong, H.; Qi, X. Progress in improving stem lodging resistance of Chinese wheat cultivars. Euphytica 2016, 212, 275–286. [Google Scholar] [CrossRef]
- Zhao, L.; Xie, G.; Fan, M.; Anwar, S.; Zhang, Q.; Lu, J.; Zhang, L.; Gao, F.; Wang, C. Mulching and planting density on photosynthesis, lodging resistance, and yield of maize. Int. J. Plant Prod. 2023, 17, 651–665. [Google Scholar] [CrossRef]
- Pinthus, M.J. Lodging in Wheat, Barley, and Oats: The phenomenon, its causes, and preventive measures. Adv. Agron. 1974, 25, 209–263. [Google Scholar]
- Berry, P.M.; Spink, J.H.; Sylvester-Bradley, R.; Pickett, A.; Sterling, M.; Baker, C.; Cameron, N. Lodging Control Through Variety Choice and Management. 2002. Available online: https://www.researchgate.net/profile/Roger-Sylvester-Bradley/publication/237254048_Lodging_control_through_variety_choice_and_management/links/00b7d52cdd4bf24850000000/Lodging-control-through-variety-choice-and-management.pdf (accessed on 12 March 2024).
- Mizuno, H.; Kasuga, S.; Kawahigashi, H. Root lodging is a physical stress that changes gene expression from sucrose accumulation to degradation in sorghum. BMC Plant Biol. 2018, 18, 2. [Google Scholar] [CrossRef]
- Yuldashev, S.K. Lodging in Cotton and Methods for Reducing It. 1966. Available online: https://www.semanticscholar.org/paper/Lodging-in-cotton-and-methods-for-reducing-it.-Yuldashev/8a54d4a752302d1bbd451c296187e9e15c1f98f8 (accessed on 12 March 2024).
- Xiao, S.; Zhang, X.; Yan, C.; Zhang, W.; Hai, L.; Guo, H. Determination of resistance to lodging by stem strength in wheat. Agric. Food Sci. 2002, 1, 7–11. [Google Scholar]
- Qi, D. Study on the architectural characteristics of wheat stalks. J. Shanxi Agric. Univ. 2003, 23, 188–191. [Google Scholar]
- Guo, C.; Gao, Z.; Miao, G. Relationship between lodging and mechanical characteristics of winter wheat stem under different yield levels. Trans. Chin. Soc. Agric. Eng. 2010, 26, 151–155. [Google Scholar]
- Wei, F.Z.; Li, J.C.; Wang, C.Y.; Qu, H.J.; Shen, X.S. Effects of nitrogenous fertilizer application model on culm lodging resistance in winter wheat. Acta Agron. Sin. 2008, 34, 1080–1085. [Google Scholar] [CrossRef]
- Gui, M.Y.; Wang, D.; Xiao, H.H.; Tu, M.; Li, F.L.; Li, W.C.; Ji, S.D.; Wang, T.X.; Li, J.Y. Studies of the relationship between rice stem composition and lodging resistance. J. Agric. Sci. 2018, 3, 387–395. [Google Scholar] [CrossRef]
- Shah, L.; Yahya, M.; Shah, S.M.A.; Nadeem, M.; Ali, A.; Ali, A.; Wang, J.; Riaz, M.W.; Rehman, S.; Wu, W.; et al. Improving lodging resistance: Using wheat and rice as classical examples. Int. J. Mol. Sci. 2019, 20, 4211. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, T.; Zhao, C.; Zhou, M. Lodging prevention in cereals: Morphological, biochemical, anatomical traits and their molecular mechanisms, management and breeding strategies. Field Crops Res. 2022, 289, 108733. [Google Scholar] [CrossRef]
- Hussain, S.; Liu, T.; Iqbal, N.; Brestic, M.; Pang, T.; Mumtaz, M.; Shafiq, I.; Li, S.; Wang, L.; Gao, Y.; et al. Effects of lignin, cellulose, hemicellulose, sucrose and monosaccharide carbohydrates on soybean physical stem strength and yield in intercropping. Photochem. Photobiol. Sci. 2020, 19, 462–472. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Rehman, A.U.; Anjum, S.A.; Iqbal, J.; Ahmad, R. Lodging stress in cereal—Effects and management: An overview. Environ. Sci. Pollut. Res. 2017, 24, 5222–5237. [Google Scholar] [CrossRef]
- Yang, Y.; Mu, J.; Hao, X.; Yang, K.; Cao, Z.; Feng, J.; Li, R.; Zhang, N.; Zhou, G.; Kong, Y.; et al. Identification and analysis of the mechanism of stem mechanical strength enhancement for maize inbred lines QY1. Int. J. Mol. Sci. 2024, 25, 8195. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, C.; Li, X.; He, C.; Ding, Z.; Du, X. Lodging resistance of rice plants studied from the perspective of culm mechanical properties, carbon framework, free volume, and chemical composition. Sci. Rep. 2022, 12, 20026. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.Q.; Li, C.H.; Li, A.F. Studies on the culm quality and anatomy of wheat varieties. Acta Agron. Sin. 1998, 24, 452–458. [Google Scholar]
- Zhang, W.; Zhang, S.; Lu, X.; Li, C.; Liu, X.; Dong, G.; Xia, T. Tissue-specific transcriptome analysis reveals lignocellulose synthesis regulation in elephant grass (Pennisetum purpureum Schum). BMC Plant Biol. 2020, 20, 528. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Luo, Y.; Jin, M.; Chang, Y.; Cui, H.; Sun, S.; Wang, Z. Mixed cropping increases grain yield and lodging resistance by improving the canopy light environment of wheat populations. Eur. J. Agron. 2023, 147, 126849. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book 2011, 9, e152. [Google Scholar] [CrossRef]
- Barik, S.K.; Russell, W.R.; Moar, K.M.; Cruickshank, M.; Scobbie, L.; Duncan, G.; Hoggard, N. The anthocyanins in black currants regulate postprandial hyperglycaemia primarily by inhibiting α-glucosidase while other phenolics modulate salivary α-amylase, glucose uptake and sugar transporters. J. Nutr. Biochem. 2020, 78, 108325. [Google Scholar] [CrossRef]
- Wendt, L.M.; Zhao, H. Review on bioenergy storage systems for preserving and improving feedstock value. Front. Bioeng. Biotechnol. 2020, 8, 370. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Li, G.; Song, C.; Manzoor, M.A.; Li, D.; Cao, Y.; Cai, Y. Functional and kinetics of two efficient phenylalanine ammonia lyase from Pyrus bretschneideri. BMC Plant Biol. 2023, 23, 612. [Google Scholar] [CrossRef]
- Soltani, B.M.; Ehlting, J.; Douglas, C.J. Genetic analysis and epigenetic silencing of At4CL1 and At4CL2 expression in transgenic Arabidopsis. Biotechnol. J. 2006, 1, 1124–1136. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, X.; Ma, J.; Yuan, M.; Zhan, Y.; Li, Y.; Li, H.; Teng, W.; Zhao, X.; Han, Y. Genome-wide identification and expression profiling of 4-coumarate:coenzyme A ligase genes influencing soybean isoflavones at the seedling stage. Crop Pasture Sci. 2023, 75, CP23147. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, L.; Guo, L.; Huang, B.; Li, Y.; Niu, J. Effects of irrigation and nitrogen fertilizer rates on oilseed flax stem lodging resistance and yield. Chin. J. Eco-Agric. 2015, 23, 544–553. [Google Scholar]
- Okuno, A.; Hirano, K.; Asano, K.; Takase, W.; Masuda, R.; Morinaka, Y.; Ueguchi-Tanaka, M.; Kitano, H.; Matsuoka, M. New approach to increasing rice lodging resistance and biomass yield through the use of high gibberellin producing varieties. PLoS ONE 2014, 9, e86870. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Lin, Q.; Li, X.; Teng, N.; Li, Z.; Li, B.; Zhang, A.; Lin, J. Effects of stem structure and cell wall components on bending strength in wheat. Chin. Sci. Bull. 2006, 51, 815–823. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Li, J.; You, Y.; Zhao, H.; Liang, H.; Mao, P. Acid detergent lignin, lodging resistance index, and expression of the caffeic acid O-methyltransferase gene in brown midrib-12 sudangrass. Breed. Sci. 2015, 65, 291–297. [Google Scholar] [CrossRef]
- Lv, G.; Tang, D.; Chen, F.; Sun, Y.; Fang, W.; Guan, Z.; Liu, Z.; Chen, S. The anatomy and physiology of spray cut chrysanthemum pedicels, and expression of a caffeic acid 3-O-methyltransferase homologue. Postharvest Biol. Technol. 2011, 60, 244–250. [Google Scholar] [CrossRef]
- Sreeja, R.; Balaji, S.; Arul, L.; Kumari, A.N.; Bapu, J.R.K.; Subramanian, A. Association of lignin and Flexible Culm 1 (FC1) ortholog in imparting culm strength and lodging resistance in kodo millet (Paspalum scrobiculatum L.). Mol. Breed. 2016, 36, 149. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Yao, J.; Chen, G.; Li, X.; Zhang, Q.; Wu, C. FLEXIBLE CULM 1 encoding a cinnamyl-alcohol dehydrogenase controls culm mechanical strength in rice. Plant Mol. Biol. 2009, 69, 685–697. [Google Scholar] [CrossRef]
- Fang, X.; Liu, X.; Zhang, Y.; Huang, K.; Li, Y.; Nie, J.; She, H.; Ruan, R.; Yuan, X.; Yi, Z. Effects of uniconazole or gibberellic acid application on the lignin metabolism in relation to lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). J. Agron. Crop Sci. 2018, 204, 414–423. [Google Scholar] [CrossRef]
- Sun, S.; Xiong, X.; Zhang, X.; Feng, H.; Zhu, Q.; Sun, J.; Li, Y. Characterization of the Gh4CL gene family reveals a role of Gh4CL7 in drought tolerance. BMC Plant Biol. 2021, 21, 65. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Z.; Zhang, P.; Zhu, X.; Jing, Y.; Wei, J.; Wu, B. Nitric oxide enhances resistance against black spot disease in muskmelon and the possible mechanisms involved. Sci. Hortic. 2019, 256, 108650. [Google Scholar] [CrossRef]
- Li, Q.; Fu, C.; Liang, C.; Ni, X.; Zhao, X.; Chen, M.; Ou, L. Crop lodging and the roles of lignin, cellulose, and hemicellulose in lodging resistance. Agronomy 2022, 12, 1795. [Google Scholar] [CrossRef]
- Liu, W.; Hussain, S.; Liu, T.; Zou, J.; Ren, M.; Zhou, T.; Liu, J.; Yang, F.; Yang, W. Shade stress decreases stem strength of soybean through restraining lignin biosynthesis. J. Integr. Agric. 2019, 18, 43–53. [Google Scholar] [CrossRef]
- Li, F.; Xie, G.; Huang, J.; Zhang, R.; Li, Y.; Zhang, M.; Wang, Y.; Li, A.; Li, X.; Xia, T.; et al. OsCESA9 conserved-site mutation leads to largely enhanced plant lodging resistance and biomass enzymatic saccharification by reducing cellulose DP and crystallinity in rice. Plant Biotechnol. J. 2017, 15, 1093–1104. [Google Scholar] [CrossRef]
- Zhong, R.; Morrison, W.H.; Freshour, G.D.; Hahn, M.G.; Ye, Z.-H. Expression of a mutant form of cellulose synthase AtCesA7 causes dominant negative effect on cellulose biosynthesis. Plant Physiol. 2003, 132, 786–795. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.; Han, Y.; He, Q. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics-A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Xiong, X.; Sun, S.; Zhang, X.; Li, Y.; Liu, F.; Zhu, Q.; Xue, F.; Sun, J. GhWRKY70D13 regulates resistance to Verticillium dahliae in cotton through the ethylene and jasmonic acid signaling pathways. Front. Plant Sci. 2020, 11, 1045. [Google Scholar] [CrossRef]


| Samples | Clean Reads | % ≥ Q30 | Comparison Efficiency | GC Content |
|---|---|---|---|---|
| M153-1 | 21,085,390 | 94.00% | 94.56% | 44.40% |
| M153-2 | 20,148,910 | 93.80% | 92.87% | 44.69% |
| M153-3 | 19,108,469 | 93.94% | 95.68% | 44.04% |
| M5330-1 | 20,972,364 | 93.88% | 93.24% | 44.83% |
| M5330-2 | 19,757,058 | 93.88% | 95.82% | 43.90% |
| M5330-3 | 19,994,421 | 93.73% | 95.87% | 44.04% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Feng, A.; Zhao, C.; Ma, X.; Zhang, X.; Li, Y.; Sun, J. Transcriptome Analysis Reveals Key Pathways and Genes Involved in Lodging Resistance of Upland Cotton. Plants 2024, 13, 3493. https://doi.org/10.3390/plants13243493
Wang Y, Feng A, Zhao C, Ma X, Zhang X, Li Y, Sun J. Transcriptome Analysis Reveals Key Pathways and Genes Involved in Lodging Resistance of Upland Cotton. Plants. 2024; 13(24):3493. https://doi.org/10.3390/plants13243493
Chicago/Turabian StyleWang, Yuan, Ao Feng, Caiwang Zhao, Xiaomei Ma, Xinyu Zhang, Yanjun Li, and Jie Sun. 2024. "Transcriptome Analysis Reveals Key Pathways and Genes Involved in Lodging Resistance of Upland Cotton" Plants 13, no. 24: 3493. https://doi.org/10.3390/plants13243493
APA StyleWang, Y., Feng, A., Zhao, C., Ma, X., Zhang, X., Li, Y., & Sun, J. (2024). Transcriptome Analysis Reveals Key Pathways and Genes Involved in Lodging Resistance of Upland Cotton. Plants, 13(24), 3493. https://doi.org/10.3390/plants13243493





