Abstract
The anti-lung cancer properties of the plant Medicago orbicularis have not been explored yet. Therefore, we identified its phytochemical composition and investigated the antioxidant, anti-hemolytic, and anti-cancerous properties of extracts of this plant in A549 human lung adenocarcinoma cells. The results show that all parts of M. orbicularis (stems, leaves, and fruits) exhibit remarkable hemolytic activities and modest antioxidant capacity. In addition, all extracts showed a dose-dependent anti-cancerous cytotoxic activity against A549 cells, with fruit extracts being the most potent. This cytotoxic effect could be related, at least partly, to the induction of apoptosis, where M. orbicularis fruit extracts reduced the ratio of anti-apoptotic BCL-2/pro-apoptotic BAX, thereby promoting cellular death. Furthermore, the use of M. orbicularis, in combination with a conventional chemotherapeutic agent, cisplatin, was assessed. Indeed, the combination of cisplatin and M. orbicularis fruit extracts was more cytotoxic and induced more aggregation of A549 cells than either treatment alone. GC-MS analysis and total polyphenol and flavonoid content determination indicated that M. orbicularis is rich in compounds that have anti-cancerous effects. We propose M. orbicularis as a potential source of anti-cancerous agents to manage the progression of lung cancer and its resistance to therapy.
Keywords:
Medicago orbicularis; lung cancer; herbal medicine; antioxidants; hemolytic; cisplatin; A549 cells 1. Introduction
Cancer remains the second leading cause of disability and death worldwide, where around one in six deaths are related to cancer. In 2020, around 19 million new cancer cases and almost 10 million cancer-related deaths were recorded globally [1].
Lung cancer has a high incidence rate; it was the second most commonly diagnosed cancer in 2020. Lung cancer is the leading cause of cancer-related deaths (around 1.8 million deaths or 18% of global cancer-related deaths in 2020) [1]. With a 21% 5-year survival rate, lung cancer has one of the most poor prognoses [2]. A significant obstacle in lung cancer therapy is the resistance that it develops to chemo- and radiotherapy. Following therapy, resistance can lead to the relapse of lung cancer around 6 months post-therapy in many patients [3,4]. Cisplatin is a cytotoxic chemotherapeutic agent used to treat several cancers including lung cancer [5]. The standard of treatment for limited-stage lung cancer remains chemotherapy, using four-to-six cycles of cisplatin and etoposide and concurrent radiation therapy. However, 60–70% of patients are at extensive-stage lung cancer and require a different treatment regimen, also involving cisplatin [4,6]. Nevertheless, these treatments can become refractory in patients. When relapse takes place, lung cancer is usually refractory to treatment and has poor prognosis due to the limited availability of therapeutic options [3,4]. As a result, there is an urgent demand for new treatment options and alternative therapeutic modalities that can treat lung cancer and circumvent its chemoresistance. Herbal medicine represents a promising alternative in this regard.
Herbal medicine and phytotherapy, a medicinal treatments based on plants or herbs and their extracts, have played a central role in the development of several anticancer agents such as Paclitaxel, Camptothecin, and Vincristine [7,8,9,10,11]. Herbal medicine can be utilized as an assistant therapeutic modality as well. It can enhance the response rate to chemotherapy as well as radiotherapy, help overcome resistance to therapy, decrease the severity of side effects caused by chemotherapy and radiotherapy, and enhance the quality of life and survival of cancer patients [12,13,14,15]. Herbal preparations and herbal active compounds act as anti-cancerous and anti-metastatic agents through several mechanisms, including scavenging of reactive oxygen species (ROS), modulation of epithelial-to-mesenchymal transition (EMT), impairment of angiogenesis, modification of the expression and activity of matrix metalloproteinases (MMPs), among others [16,17].
The Fabaceae family of plants includes a large number of domesticated species which are harvested as crops for human and animal consumption as well as oils, fuel, fertilizers, and medicinal and agricultural varieties [18]. Medicago is a genus of the Fabaceae family and it comprises more than 83–87 different species of flowering plants [18,19,20]. Twenty of these species are herbaceous perennials, sixty-three are herbaceous annuals, and only two are shrubs [20]. Medicago orbicularis L. Bartal. (common names: black disk, button medick, or button clover) is a species of the Medicago genus indigenous to Eurasia and North Africa [19]. The plant is distributed throughout the Mediterranean basin, mainly in Palestine, Lebanon, Syria, Algeria, Spain, France, Italy, and Greece, as well as in Middle Eastern countries such as Iraq and Iran, and several other countries [18]. M. orbicularis is a winter annual plant which flowers in spring and early summer. Its stems are leafy, its stipules are fimbriate, its leaflets are oval non-hairy with toothed margins, its flowers are orange-yellow, and its seed pods are flat, coiled, and lack spines [19].
M. orbicularis’ phytochemical composition has not yet been investigated. But other Medicago species have been reported to be rich in phytochemicals, including flavonoids, carotenoids, saponins, phenolic acids, and phytoestrogens [21,22,23]. These phytochemicals have been demonstrated to exhibit anti-cancer activities through several mechanisms including apoptosis, antiproliferative, immunomodulatory, and anti-inflammatory activities, as well as their capacity to modulate oxidative stress [24,25,26,27,28]. In addition, close-by species such as M. polymorpha, M. sativa, M. arabica, or M. truncatula were shown to possess antioxidant activities [22,23]. A traditional therapeutic use of M. orbicularis was reported in Turkey for heart diseases [29]. However, there are currently no studies on the antioxidant potential, therapeutic applications, or anti-cancerous properties of M. orbicularis. Therefore, this study aimed to assess the antioxidant capacity, hemolytic properties, phytochemical composition, and the effects of M. orbicularis on cell proliferation and aggregation of A549 human lung cancer cells, as well as the combinatorial effect of cisplatin and M. orbicularis on A549 cells.
2. Materials and Methods
2.1. Cell Culture and Reagents
Human lung adenocarcinoma cell line A549 and primary normal neonatal fibroblast (HDFn) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells were maintained in a humidified chamber at 37 °C and 5% CO2 in DMEM (Dulbecco’s Modified Eagle’s Medium; cat# D0819 Sigma-Aldrich Co., St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) (cat# F9665, Sigma-Aldrich) and 100 U/mL penicillin and 0.1 mg/mL streptomycin (cat# P4333, Sigma-Aldrich). Cells were passaged by trypsinization when they reached 90% confluence.
2.2. Plant Collection and Extraction
Fresh Medicago orbicularis L. Bartal was collected from Almat, Byblos, Lebanon between May and June 2021. A voucher specimen was stored in the herbarium of the Faculty of Pharmacy, Lebanese University, Beirut, Lebanon and was authenticated by Professor George Tohme, an experienced taxonomist and herbalist. Medicago orbicularis L. Bartal: Kingdom Plantae; Phylum Tracheophyta Class Magnoliopsida; Order Fabales; Family Fabaceae; Genus Medicago L. (Index Kewensis; https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:506322-1 (accessed on 20 October 2023)).
Extracts were obtained from leaves, stems, and fruits of M. orbicularis. In brief, the different plant parts were cleaned, air-dried in the shade at room temperature, ground to powder, and kept in plastic containers, away from light, heat, and moisture. Then, 10 g of the powder was mixed with 100 mL of 70% aqueous ethanol, and the mixture was placed in a reciprocating shaker and continuously agitated at 150 rpm for 3 to 4 days. The solutions were filtered and concentrated by a rotary evaporator at 40 °C and then lyophilized using a lyophilizer. The lyophilized extracts were stored at −20 °C until their use. At the time of use, the extracts were dissolved in 70% ethanol.
2.3. DPPH (α, α-Diphenyl-β-picrylhydrazyl) Antioxidant Activity
The antioxidant free radical scavenging ability of the ethanolic extracts of the different parts of M. orbicularis (leaves, fruits, and stems) was measured using the DPPH radical scavenging assay which was performed as described [30]. DPPH changes color from intense purple to pale-yellow due to the donation of a hydrogen atom, if the plant extract has radical scavenging activity. For M. orbicularis ethanolic extracts, 1 mL of different concentrations of the ethanolic plant extracts (50, 150, 250, 350, 500, 1000, 2500, and 4000 μg/mL) was mixed with an equal volume of DPPH (cat# D9312, Sigma-Aldrich Co.) solution (0.15 mM in ethanol). The blank consisted of 1 mL of DPPH solution and 1 mL of 70% ethanol. Mixed samples were then kept in the dark for 30 min and the OD was measured at a wavelength of 515 nm using a spectrophotometer. L-ascorbic acid, a potent antioxidant, was employed as a positive control and reference. The DPPH scavenging activity of each concentration of the extracts was calculated using the following formula:
2.4. In Vitro Cytotoxicity Assay
In vitro cytotoxicity of M. orbicularis extracts was assessed using MTT assay, which is a reduction assay that measures cellular metabolic activity and is reflective of cell viability. A549 and neonatal fibroblast cells were seeded in a 96-well tissue culture plate at a density of 0.7 × 104 cells per well in DMEM culture medium containing 10% FBS and penicillin/streptomycin. After an overnight incubation, cells were treated with different concentrations (50, 100, 150, 200, 300, or 500 μg/mL) of M. orbicularis extracts for 24, 48, and 72 h. As a control, cells were treated with a concentration of ethanol (vehicle-control cells) equal to the concentration of ethanol in the extract-treated cells. Following the treatment, MTT reagent (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide; cat# M5655, Sigma-Aldrich Co.) was added for 3 h until a purple formazan precipitate was formed. Media were removed gently, and an isopropanol-HCl solvent was added to dissolve the formazan precipitates. The optical density (OD) was measured by an ELISA plate reader at a wavelength of 595 nm. The percentage of cell viability of the treated cells was calculated using the following formula: .
The IC50 (half-maximal inhibitory concentration) value was determined by extrapolation from the cell killing curve to determine the concentration of the extract that induced 50% cell death.
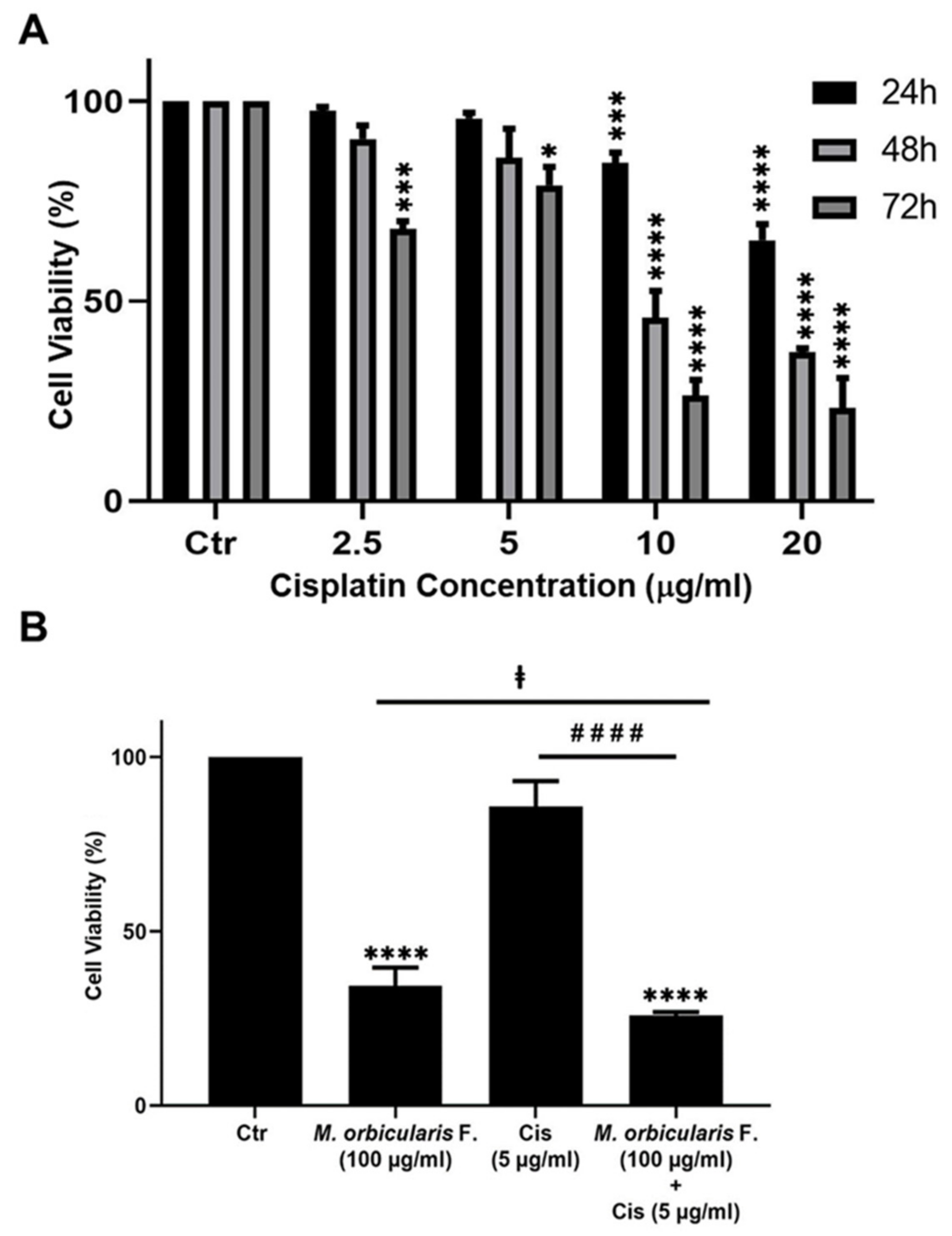
Cisplatin (Sigma-Aldrich Co.), dissolved in DMSO, was used as a positive control of the cytotoxic effect of M. orbicularis’ parts’ extracts on A549 cells. A549 cells were treated for 24, 48, and 72 h with increasing concentrations of cisplatin (2.5, 5, 10, and 20 μg/mL) and compared to the DMSO-treated vehicle-control cells. IC50 values of cisplatin in A549 cells were determined at the three time points. Data are displayed as the mean ± SEM of three independent experiments.
2.5. Cell Aggregation Assay
Seeded A549 cells were detached using 2 mM EDTA PBS (calcium- and magnesium-free). Cells were pelleted and washed with PBS, and then resuspended in 1 mL PBS including or not 100 or 150 μg/mL of M. orbicularis fruit extract alone, 5 μg/mL cisplatin alone, or a combination of both and kept shaking on a rocker for 60 min at 37 °C in a cell culture incubator. Cells were fixed with 1 mL of 1% formaldehyde and imaged using an inverted microscope (Leica Microsystems GmbH, Wetzlar, Germany). Percentage of aggregation was calculated using the following formula: ; where Nt is the number of single cells in treated wells and Nc is number of single cells in the vehicle-control cells.
2.6. Assay of Hemolysis and Anti-Hemolytic Activity
Hemolytic activity assay was performed as previously described [31]. Erythrocytes were obtained after separation from plasma by centrifugation at 2500 rpm for 10 min at 4 °C starting from fresh sheep blood. Briefly, RBCs were washed three times with 1X PBS and then pelleted and resuspended in PBS to obtain a 5% suspension of RBCs. Then, 50 μL of different concentrations (10, 20, 40, 50, and 100 μg/mL) of ethanolic extract of fruits of M. orbicularis were added to 1 mL of the RBC suspension. The mixture was incubated at 37 °C for 1.5 h; then, the suspension was centrifuged at 2500 rpm for 10 min at 4 °C and the OD measured at 540 nm using a spectrophotometer. Ethanol concentrations equal to those present in the fruit extracts were added to blood and used as a negative control, 1% SDS as a positive control of hemolysis of RBCs, and 1X PBS as the blank. Hemolytic levels were calculated as follows:
Anti-hemolytic activity assessment was performed as previously published [32]. Briefly, the same steps were performed as in the hemolysis assay except that H2O2 was added to induce hemolysis. After adding M. orbicularis extracts to the RBC suspension for 20 min, RBCs were incubated with 350 μL of 30% H2O2 at 37 °C for 1.5 h. The suspension was centrifuged at 2500 rpm for 10 min at 4 °C and OD at 540 nm was determined. A total of 30% H2O2 was used as a positive control, and 1X PBS as the blank. Anti-hemolytic levels were calculated according to the following equation:
2.7. Western Blotting Analysis
A549 cells were seeded in a 6-well plate at a density of 2.8 × 105 cells per well and cultured for 24 h. The cells were treated with extracts of M. orbicularis for 48 h and then the cells were washed twice with PBS and lysed using a lysis buffer containing 2% SDS, 60 mM Tris lysis buffer (pH 6.8), and protease inhibitors and centrifuged at 10,000× g for 10 min. The protein concentration of the supernatants was determined using the Bradford protein assay kit (BioRad, Hercules, CA, USA) and 25 μg of protein lysates were resolved on 10% SDS-PAGE before being transferred to a polyvinylidene difluoride membrane (Immobilon PVDF; BioRad, Hercules, CA, USA). The membranes were then blocked for 1 h at room temperature with 5% non-fat dry milk in TBST (TBS and 0.05% Tween 20). Immunodetection was performed by incubating the membrane overnight with specific primary antibodies at 4 °C. Primary antibodies included rabbit anti-human B-cell lymphoma 2 (BCL-2) (Abcam, ab32124; dilution 1/5000), rabbit anti-Bcl-2 associated X protein (BAX) (Abcam, ab32503; dilution 1/2000), and rabbit anti-GAPDH (Abcam, ab181602; dilution 1/10,000). Membranes were washed and incubated with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (Abcam, ab6721; dilution 1/5000) for 1 h followed by washing in TBST. Immunoreactive bands were detected using ECL substrate kit (Thermo Scientific, Rockford, IL, USA), and membranes were scanned using the Chemidoc imaging system (BioRad). The intensity of the obtained bands was quantified using ImageJ software version 1.53t (NIH, MD, USA). All bands were normalized to GAPDH, which was used as a loading control.
2.8. Spectroscopic Determination of Total Phenolic Content
Total phenolic compounds content (TPC) was determined using the Folin–Ciocalteu colorimetric oxidation/reduction-based reaction as previously described [33], with slight modifications. Briefly, 100 µL of diluted fruit extracts of M. orbicularis was added to 500 µL of Folin–Ciocalteu reagent, followed by the addition of 400 µL of saturated sodium carbonate solution (2%). The oxidation products of the reaction show a blue color with a broad light with an absorption maximum of 765 nm. OD 765 of the different mixtures was measured, against a blank, using a spectrophotometer. A calibration curve was prepared using a strong antioxidant, gallic acid, and the results were expressed as gallic acid equivalents (GAEs) in milligrams per gram of the extract.
2.9. Spectroscopic Determination of Total Flavonoids Content
The total flavonoids content (TFC) of M. orbicularis fruit extracts was determined by the aluminum chloride colorimetric method as previously detailed [34]. AlCl3 forms a flavonoid–aluminum complex that has a maximum OD at 510 nm. Briefly, a 100 µL aliquot of appropriately diluted fruit extracts of M. orbicularis was added to 400 µL of distilled water. Then, 400 µL of 5% NaNO2 was added, followed by 300 µL of 10% AlCl3. After 6 min of incubation 200 µL of 1 M NaOH was added to the mixture followed by 240 µL of distilled water. The absorbance of the resulting pink solution was determined at 510 nm versus a blank (water). A calibration curve was prepared using a known flavonoid, namely quercetin. The curve was used to calculate the amount of flavonoids in the plant extracts, which was expressed as quercetin equivalents in mg/g dry weight of the plant part.
2.10. Gas Chromatography/Mass Spectrometry (GC/MS)
The phytochemical compounds of M. orbicularis fruit extracts were analyzed using GC/MS analysis. A Perkin Elmer Clarus 680 (Perkin Elmer, USA) system attached to a triple quadrupole mass spectrometer was used. Chromatography was conducted on a hydrophobic capillary column RTxi-5 Sil MS column (30 m × 0.25 mm ID × 0.25 µm) using an injection volume of 10 µL, a flow rate of 1.5 mL/min, a pressure of 23.1 KPa, and an average velocity of 0.2 sec. The temperatures of the source and the interface were 200 °C and 280 °C, respectively. The initial temperature was set at 80 °C for 2 min, increased to 250 °C at 15 °C/min, and raised to 280 °C at 15 °C/min (held for 12 min). Identification of phytochemicals in extracts was carried out by comparing the obtained retention indices with those of chemical compounds in the database of the National Institute of Standards and Technology (NIST).
2.11. Statistical Analysis
Results were evaluated for statistical difference using one-way ANOVA followed by Bonferroni test to calculate p values. Data are presented as mean ± standard error of the mean (SEM) and a p-value of p < 0.05 was considered as statistically significant. Statistical analysis was performed using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA).
3. Results
3.1. Antioxidant Properties of M. orbicularis Plant Ethanolic Extracts
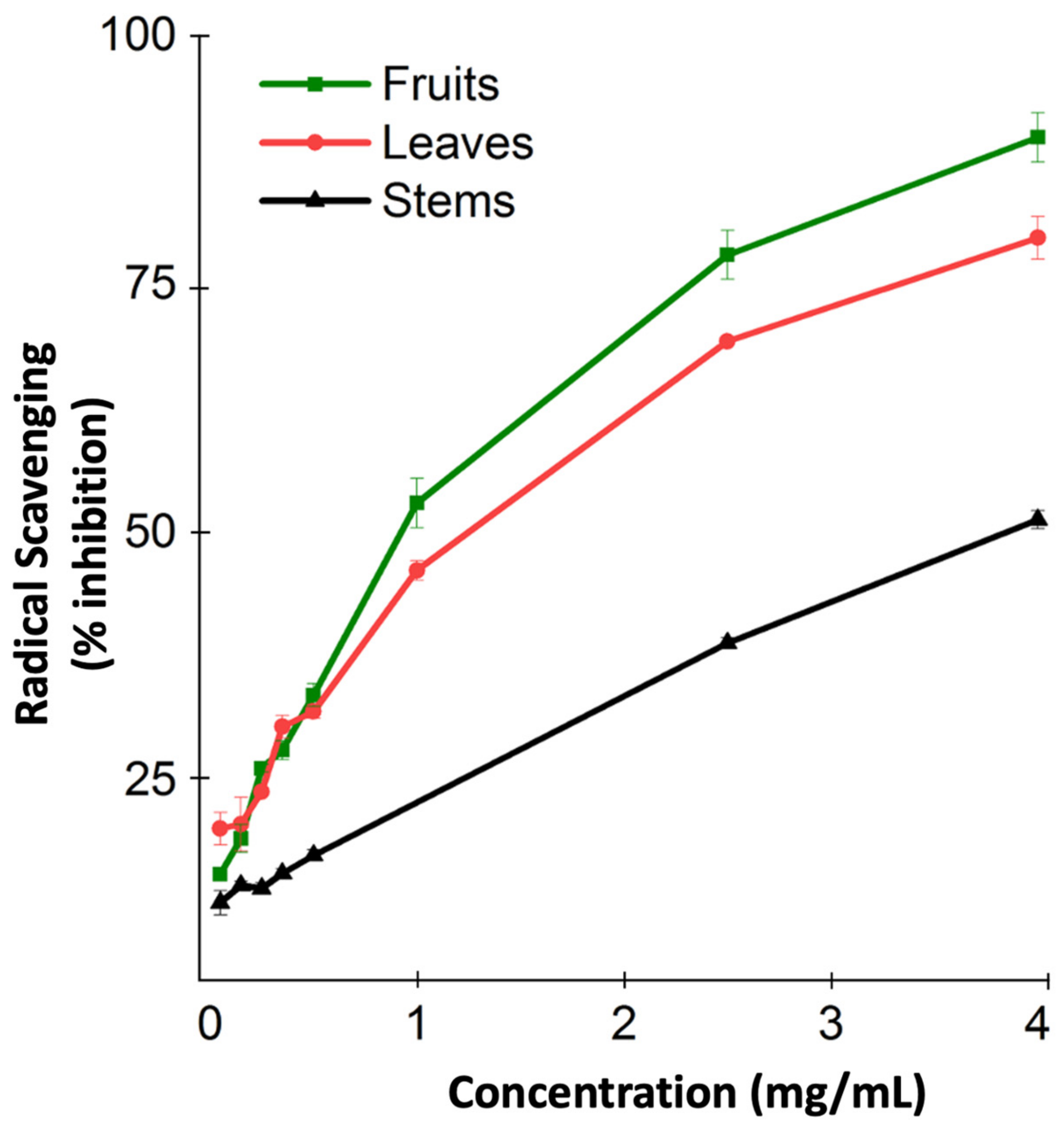
The antioxidant capacity of M. orbicularis was not measured before. Consequently, we used the DPPH radical scavenging assay to measure the antioxidant capacity of the different aerial parts of M. orbicularis. Figure 1 shows the antioxidant capacity of the ethanolic extracts of different parts of M. orbicularis extracts. The leaves’ ethanol extracts had the highest antioxidant potential, followed by the fruits’ ethanol extracts. M. orbicularis’ stems’ ethanol extracts showed the lowest antioxidant activity. The calculation of the IC50 values confirmed these results (Table 1). The IC50 of radical scavenging activity of the strong antioxidant ascorbic acid is also shown in Table 1.
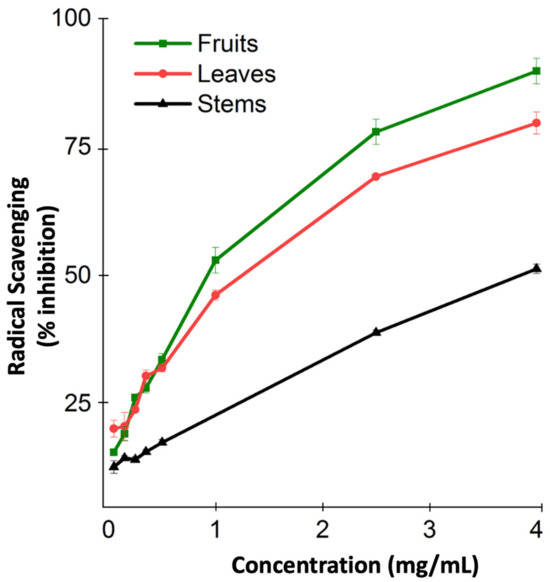
Figure 1.
Ethanolic extracts of M. orbicularis exhibit antioxidant properties. The percentages of radical scavenging activity of different concentrations (50, 150, 250, 350, 500, 1000, 2500, and 4000 μg/mL) of leaves, fruits, and stems of M. orbicularis’ ethanolic extracts were measured by DPPH radical scavenging assay. Data are presented as the mean ± SEM of three independent experiments (n = 3).

Table 1.
Antioxidant activity of ethanolic extracts of M. orbicularis parts.
3.2. M. orbicularis’ Plant Parts’ Ethanolic Extracts Reduce the Viability of A549 Lung Adenocarcinoma Cells
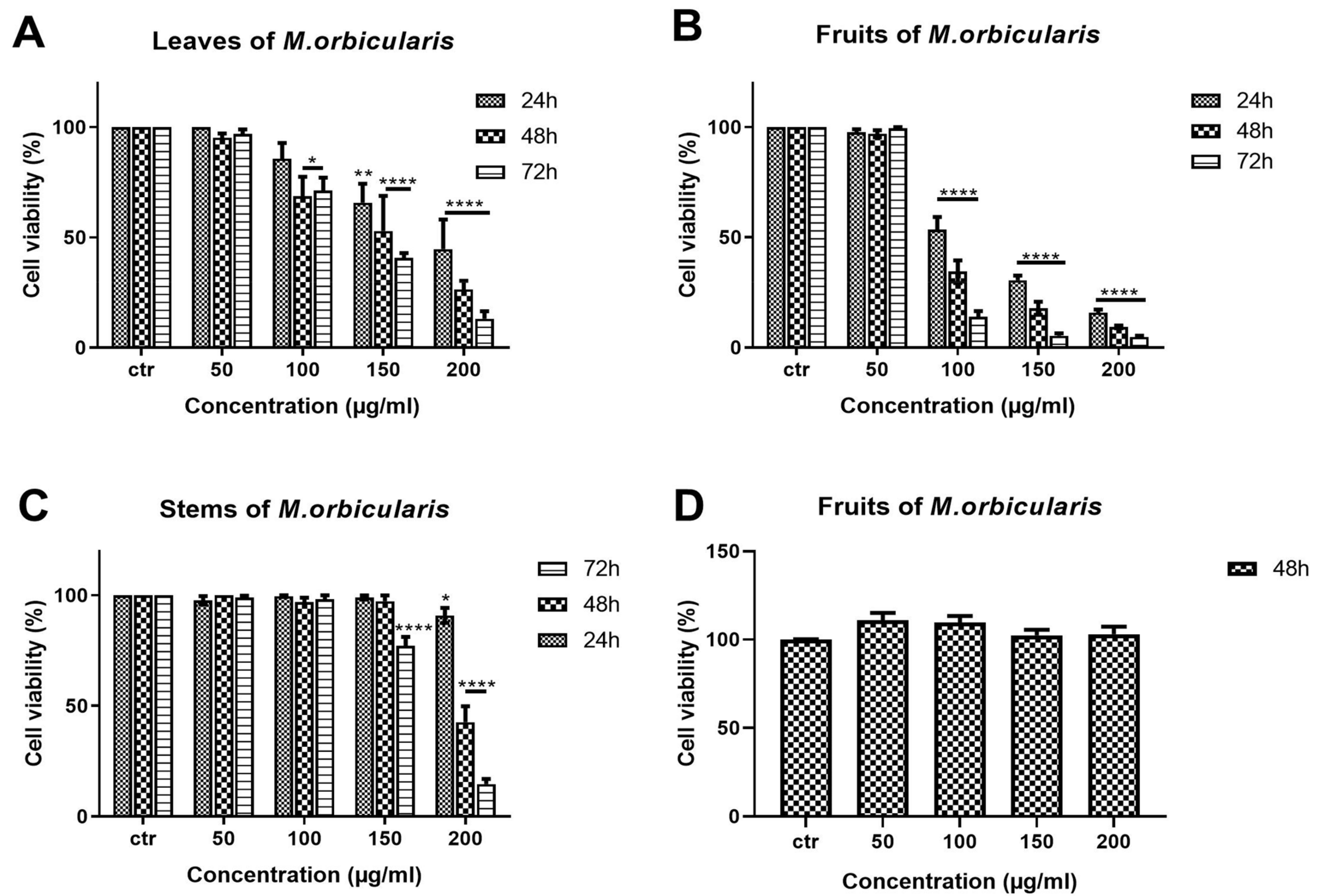
The anti-proliferative effects of different aerial parts of M. orbicularis extracts were tested against A549 human lung adenocarcinoma cells. A549 cells were treated with ethanolic extracts (50, 100, 150, 200 μg/mL) of M. orbicularis plant parts (leaves, fruits, and stems) for 24, 48, and 72 h, and their antiproliferative effects were determined using the metabolism-dye-based MTT assay (Figure 2). All plant extracts reduced the number of metabolically active A549 cells in a concentration- and time-dependent manner (Figure 2). M. orbicularis’ leaves’ extracts at the concentration of 100 μg/mL caused a significant (p < 0.05) decrease in the number of A549 cells starting at 48 h, compared to the control-vehicle-treated cells (Figure 2A). M. orbicularis’ fruits’ extracts, on the other hand, significantly (p < 0.0001) reduced the A549 cell number at the same starting concentration of 100 μg/mL, but at the earlier time point of 24 h (Figure 2B). Figure 2C shows that M. orbicularis’ stems’ ethanolic extracts required a higher concentration (150 μg/mL) to cause a significant (p < 0.0001) decrease in the A549 cell number, at the later time point of 72 h. These data show that all M. orbicularis’ parts’ ethanolic extracts can reduce the viability of A549 cells; M. orbicularis’ fruits’ ethanolic extracts were the most potent at decreasing the viability of A549 cells. The antioxidant ability of M. orbicularis correlated with its anti-proliferative effects as previously reported for other plant extracts (Table 2) [30,35,36]. Table 2 shows that M. orbicularis’ fruits’ ethanol extracts have the lowest IC50 value of A549 cell viability. The IC50 values of A549 cell viability were 116.5 ± 2.06 μg/mL, 91.86 ± 1.96 μg/mL, and 86.18 ± 1.93 μg/mL at 24, 48, and 72 h of treatment.
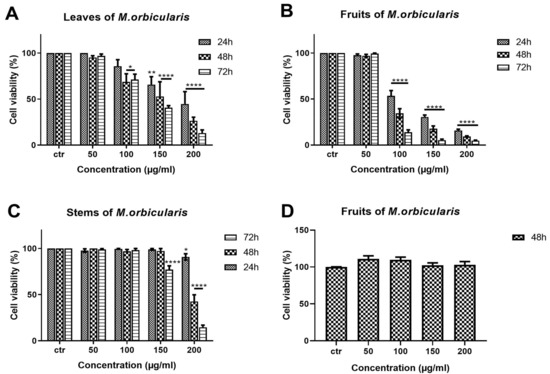
Figure 2.
M. orbicularis’ plant parts’ ethanolic extracts reduce the viability of A549 cells. MTT assay was used to measure cell viability of A549 cells treated for 24, 48, and 72 h with increasing concentrations (50, 100, 150, 200 μg/mL) of M. orbicularis’ plant parts’ ethanolic extracts. (A–C) show the results of MTT cell cytotoxicity assay of A549 cells treated with the indicated concentrations of ethanolic plant extracts of M. orbicularis leaves, fruits, and stems, respectively. (D) shows the cell viability of neonatal fibroblast cells treated with the indicated concentrations of M. orbicularis’ fruits’ ethanolic extract. Viability of treated cells was compared to the control-(ctr) vehicle-treated cells. Data are displayed as the mean ± SEM of three independent experiments (n = 3). * p < 0.05, ** p < 0.01, and **** p < 0.0001.

Table 2.
IC50 values (in μg/mL) of ethanolic extracts of parts of M. orbicularis used to treat A549 cells for 24, 48, and 72 h. The IC50 values of cisplatin in A549 cells are also listed for comparison.
In addition, A549 cells were treated with the chemotherapeutic agent, cisplatin, which potently decreases A549 cell viability (Table 2). M. orbicularis’ fruits’ ethanolic extracts show a decent inhibitory effect when compared with cisplatin (around 10 times higher IC50 at 48 h). Given that M. orbicularis’ fruits’ ethanolic extracts were the most effective at reducing lung cancer cell viability, we decided to perform the next experiments using M. orbicularis’ fruits’ ethanolic extracts.
The antiproliferative effects of M. orbicularis’ ethanolic extracts are specific to cancer cells. M. orbicularis’ fruits’ ethanolic extracts did not decrease the number of normal human neonatal fibroblast cells, even at the highest tested concentration of 200 μg/mL (Figure 2D). In comparison, the same concentration of fruit extracts decreased the number of A549 cells by 90% at 48 h, when compared to control-vehicle-treated cells (Figure 2B).
3.3. M. orbicularis’ Fruits’ Ethanolic Extracts May Induce Apoptosis of A549 Lung Adenocarcinoma Cells
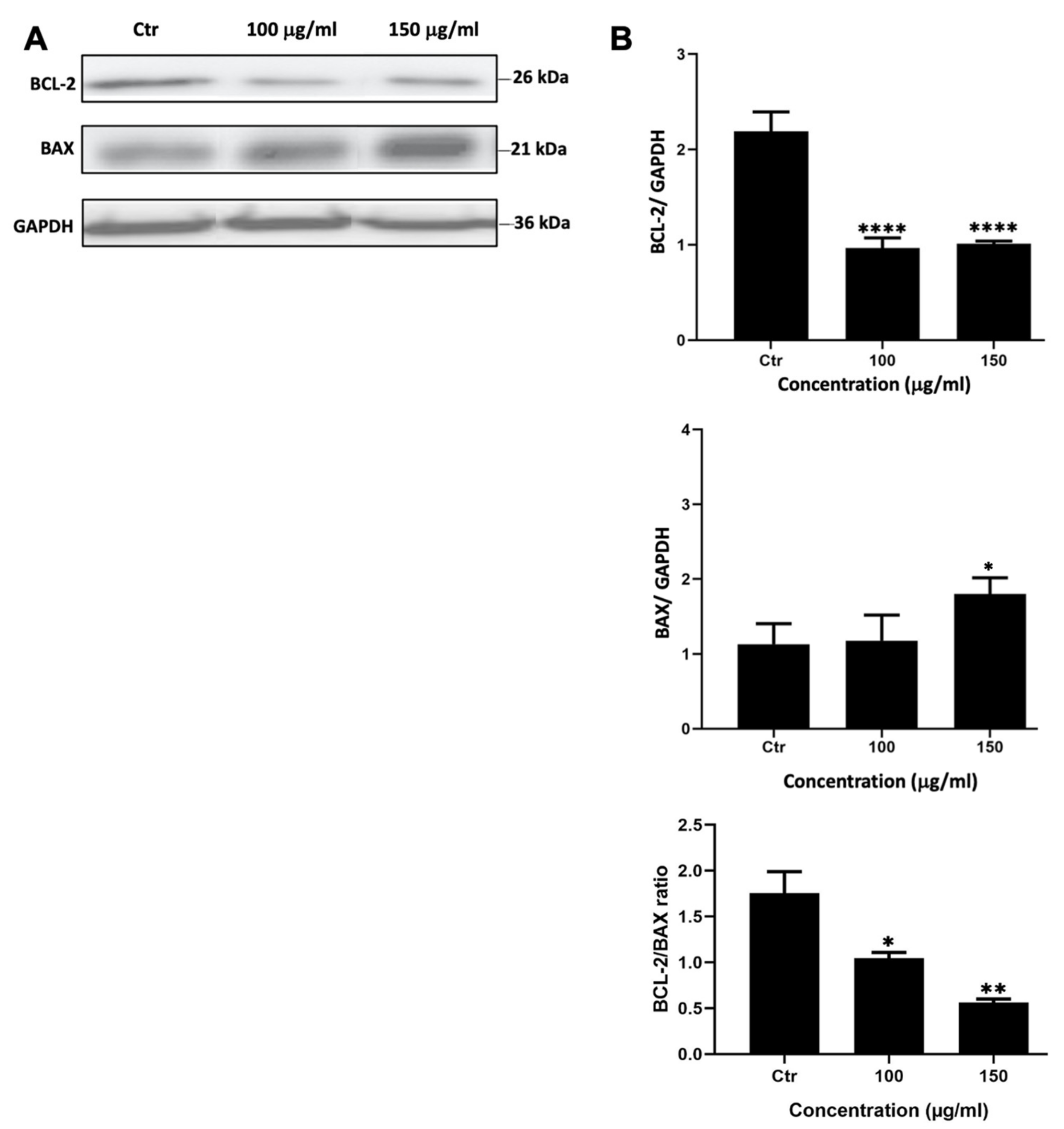
To assess whether M. orbicularis reduced the viability of A549 cells by inducing apoptosis, A549 cells were treated with M. orbicularis fruit extracts for 48 h. Protein lysates were subjected to Western blotting to evaluate the protein levels of the anti-apoptotic protein BCL-2 and the pro-apoptotic protein BAX, hallmark proteins of apoptosis. Figure 3 shows a significant decrease (p < 0.001) in the protein levels of BCL-2 in A549 cells treated with 100 and 150 μg/mL of M. orbicularis fruit extracts. BAX levels decreased after treating A549 cells with both 100 and 150 μg/mL of M. orbicularis fruit extracts, but the decrease was significant (p < 0.05) only at the concentration of 150 μg/mL (Figure 3B). The ratio of BCL-2/BAX, a ratio that determines the balance between anti- versus pro-apoptotic, was dose dependently and significantly (p < 0.05), decreased by treatment of A549 cells with 100 and 150 μg/mL of M. orbicularis fruit extracts. These results indicate that M. orbicularis’ fruit extracts may induce apoptosis in A549 cells and that apoptosis may be responsible, at least in part, for M. orbicularis-induced cell death of A549 cells.
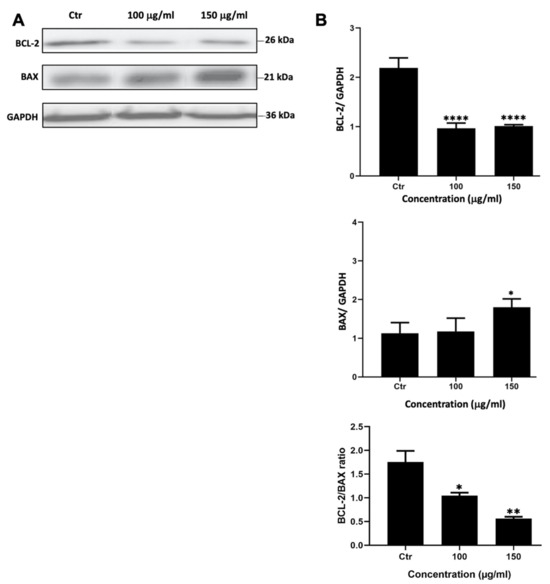
Figure 3.
M. orbicularis’ fruits’ ethanolic extracts induce apoptosis of A549 cells. (A) Representative Western blot of protein lysates from A549 cells treated 100 and 150 μg/mL of M. orbicularis fruit extracts for 48 h. Levels of proteins were normalized to GAPDH protein levels. (B) Quantification of images in (A). Intensity of protein bands were quantified by ImageJ software and normalized to intensity of bands of GAPDH protein. The ratio is expressed in arbitrary units. Data are presented as the mean ± SEM of 3 independent experiments (n = 3). * p < 0.05, ** p < 0.01, and **** p < 0.0001.
3.4. Ethanolic Extracts of M. orbicularis Fruits Exhibit Potent Antihemolytic Properties
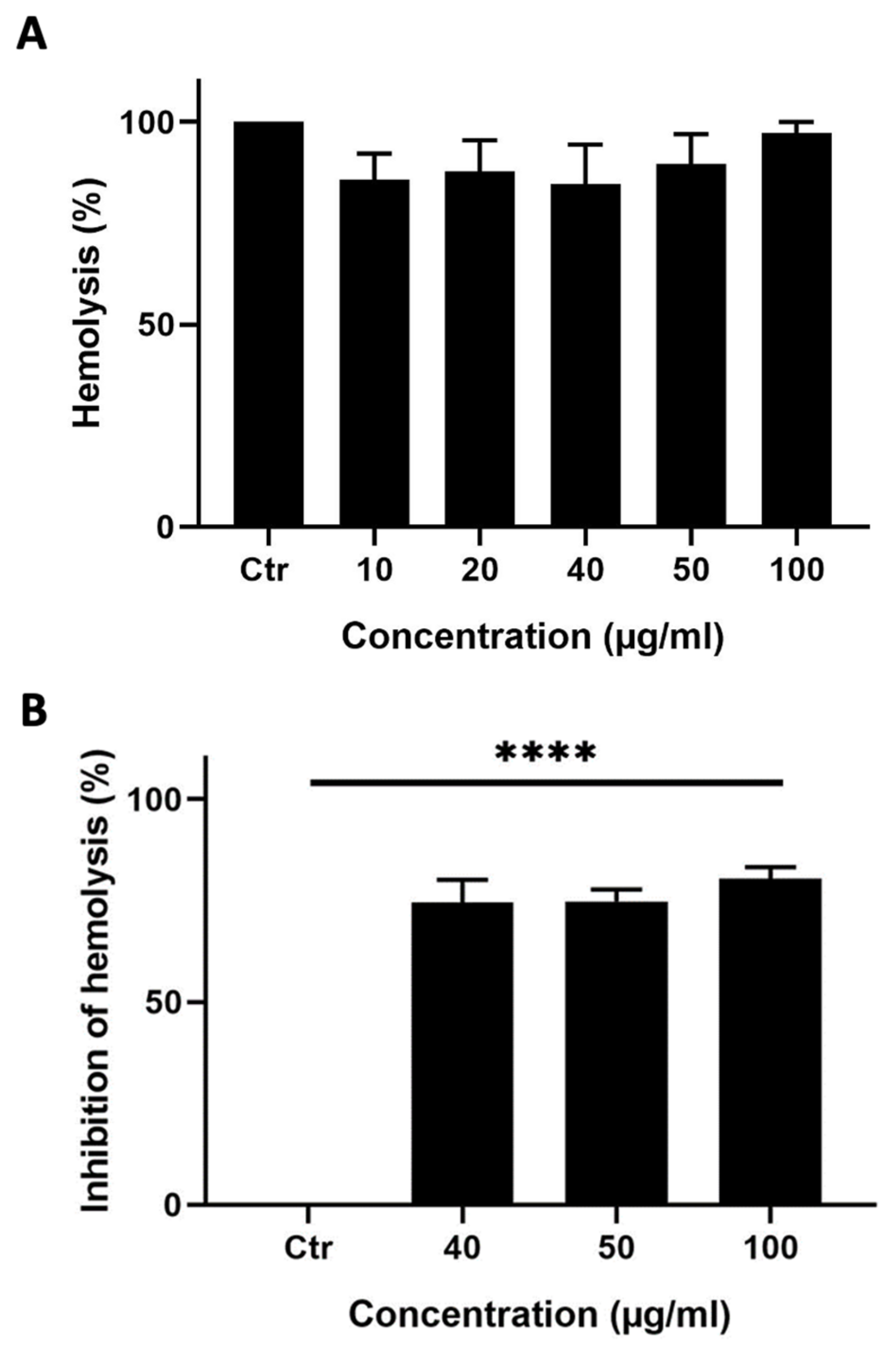
A hemolysis assay was performed to test whether M. orbicularis’ fruits’ ethanolic extracts can induce hemolysis of RBCs. Ethanolic extracts of M. orbicularis fruits did not cause hemolysis of RBCs (Figure 4A).
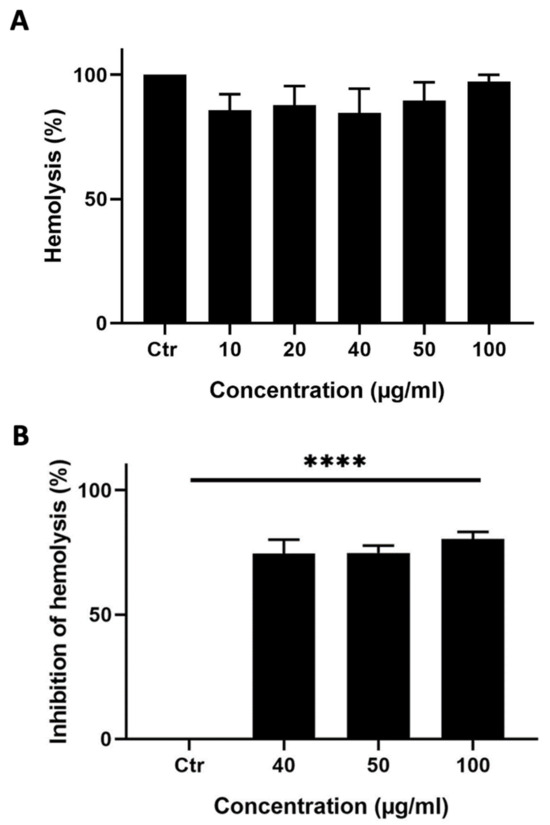
Figure 4.
M. orbicularis’ fruits’ ethanolic extracts show potent anti-hemolytic activity. (A) Hemolytic activity assay. Erythrocytes were treated with different concentrations (10, 20, 40, 50, and 100 μg/mL) of ethanolic extract of fruits of M. orbicularis. Bar graphs represent mean % hemolysis (compared to vehicle-control-treated RBCs) of 3 independent experiments. (B) Erythrocytes were pretreated with different (40, 50, and 100 μg/mL) concentrations of ethanolic extracts from the fruits of M. orbicularis. H2O2 was then added to induce hemolysis. Data represent % inhibition of hemolysis compared to control-(ctr) vehicle-treated cells. Data are displayed as the mean ± SEM of three independent experiments; **** denotes p < 0.0001.
This result prompted us to test the ability of the ethanol extracts from the fruits of M. orbicularis to protect RBCs against hemolysis, which usually results from the use of chemotherapeutic agents in cancer therapy. A hemolysis assay was performed where RBCs were pretreated with different concentrations (40, 50, or 100 μg/mL) of the ethanolic extracts from the fruits of M. orbicularis and then treated with H2O2 to induce hemolysis (Figure 4B). Figure 4B shows that 40, 50, or 100 μg/mL of ethanolic extracts of M. orbicularis fruits significantly inhibited hemolysis of RBCs. For example, a concentration of 100 μg/mL of fruit extracts of M. orbicularis protected 75.65% of RBCs from hemolysis. This result sheds light on the safety of fruit extracts of M. orbicularis and underscores the potential use of these extracts in combination therapy with classical therapeutic agents, known to induce RBC hemolysis.
3.5. Ethanolic Extracts of Fruits of M. orbicularis Enhance Cisplatin-Induced Cytotoxicity in A549 Lung Cancer Cells
Cisplatin has potent anti-proliferative effects against A549 cells [37], and the anti-proliferative effect of cisplatin was confirmed in this study. The IC50 values of the cisplatin-induced reduction in viability of A549 cells were 24.73, 9.56, and 4.72 μg/mL at 24, 48, and 72 h, respectively (Table 1). These values agree with the previously published literature [37,38].
Many types of cancer, including lung cancer, develop resistance to chemotherapy and, as a result, they usually cause relapse following chemotherapy with cisplatin or other chemotherapeutic agents [39]. Combination therapy can help to overcome the chemoresistance that develops against chemotherapeutic agents. When combined with chemotherapy, plant extracts with anti-cancerous properties may offer a way of overcoming chemoresistance and cancer relapse. This is because plant preparations usually have a different mechanism of action than chemotherapeutic agents [16]. Therefore, we assessed the effect of a combination treatment using ethanolic extracts of M. orbicularis fruits on cisplatin-induced death of A549 cells. A549 cells were treated for 48 h with a low-dose of cisplatin (5 μg/mL) in combination with 100 μg/mL of M. orbicularis’ fruits’ extracts. Cell viability assays showed that, at these concentrations of cisplatin alone or M. orbicularis’ fruits’ extracts alone, there were moderate cytotoxicity levels (Table 1, Figure 2B and Figure 5A). In the combination treatment, the cell viability of A549 decreased significantly (p < 0.0001) from 85.9% in A549 cells treated with cisplatin alone to 25.8% in cells treated with the combination of cisplatin and M. orbicularis’ fruits’ extracts (Figure 5B). In addition, the combination treatment enhanced the cytotoxic effect of M. orbicularis’ fruits’ extracts where A549 cell viability decreased from 34.4% in cells treated with M. orbicularis’ fruits’ extracts alone to 25.8% in the combination treatment (Figure 5B). These results show that M. orbicularis’ fruits’ ethanolic extracts sensitized A549 cells to the cytotoxic effects of cisplatin.
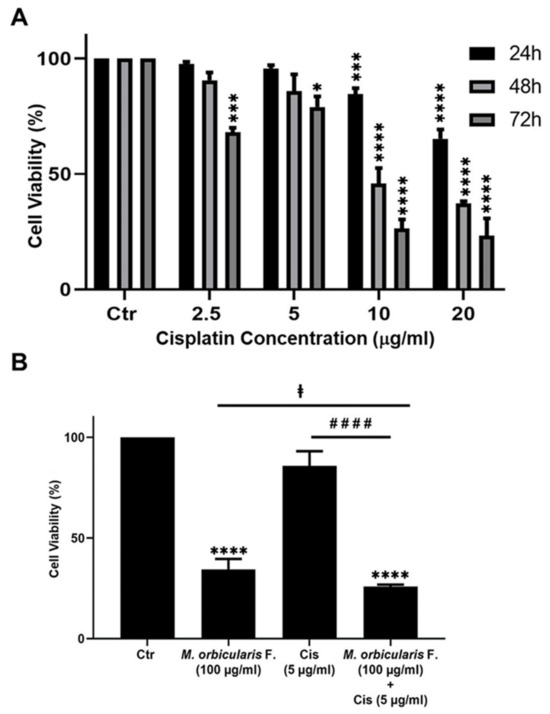
Figure 5.
M. orbicularis enhance cisplatin-induced cytotoxicity in A549 lung cancer cells. (A) Cell viability of A549 cells treated with the indicated concentrations of cisplatin was measured using MTT assay. Data are displayed as % cell viability relative to control (ctr) cells. * p < 0.05, *** p < 0.001, and **** p < 0.0001 (B) A549 cells were treated with fruit extracts of M. orbicularis (100 μg/mL) alone or combined with 5 μg/mL cisplatin (Cis) for 48 h, and then assayed for cell viability using MTT assay. Bar graphs represent % cell viability relative to control (ctr) cells. Data are presented as the mean ± SEM of three independent experiments (n = 3). Significant difference from control: **** p < 0.0001; significant difference from cisplatin treatment alone: #### denotes p < 0.0001; and significant difference from M. orbicularis alone: ‡ denotes p < 0.05. M. orbicularis F. denotes M. orbicularis fruits extracts.
3.6. Ethanolic Extracts of Fruits of M. orbicularis Augment Cisplatin-Induced Aggregation of A549 Lung Cancer Cells
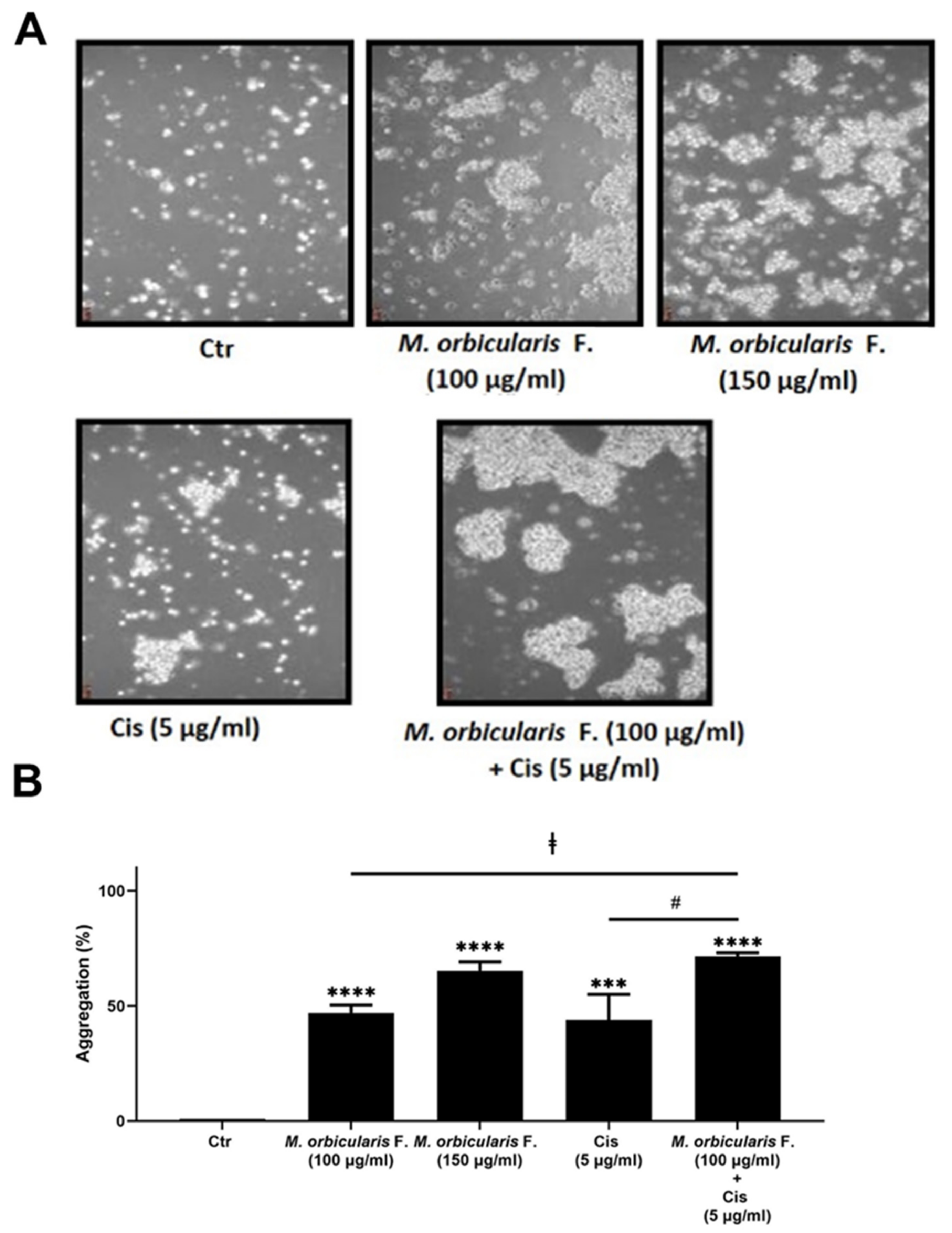
We next sought to examine the effect of M. orbicularis fruit extracts on the cell–cell adhesion of A549 cells using a cell aggregation assay. Figure 6 shows that, after 1 h of treatment of A549 cells with 100 and 150 μg/mL of M. orbicularis fruit extracts, the percentages of cell aggregation significantly (p < 0.001 in comparison to vehicle control cells) increased by 47% and 65%, respectively (Figure 6B). The percentage of cell aggregation of A549 is 44% after treatment with 5 μg/mL cisplatin alone and significantly increased to 71.5% after the co-treatment with cisplatin (5 μg/mL) and M. orbicularis (150 μg/mL) (Figure 6A,B). These results suggest that the M. orbicularis extract significantly augmented the cisplatin-induced aggregation of A549 lung cancer cells.
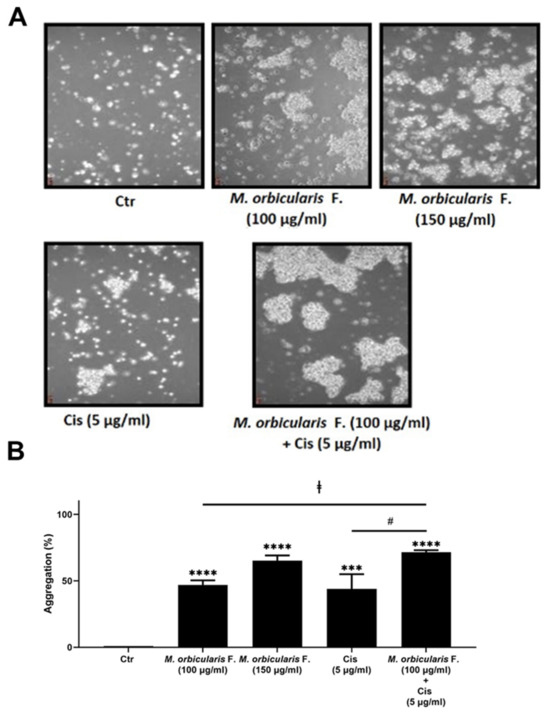
Figure 6.
M. orbicularis augments cisplatin-induced aggregation of A549 lung cancer cells. (A) A549 cells were incubated with M. orbicularis alone (100 or 150 μg/mL) or combined with 5 μg/mL cisplatin (Cis), and then subjected to cell aggregation assay as described in the Materials and Methods Section. (B) Quantification of the data in (A). Micrographs of cells were taken after 1 h of treatment and the percentage of cell–cell aggregations was measured using the following equation: % aggregation = (1 − Nt/Nc) × 100, where Nt is the number of single cells in the control and Nc is the number of single cells in the treated sample. Data represent the mean ± SEM of three independent experiments (n = 3). Significant difference from control: *** p < 0.001, and **** p < 0.0001; significant difference from cisplatin treatment alone: # denotes p < 0.005; and significant difference from M. orbicularis extracts alone: ‡ denotes p < 0.05. M. orbicularis F. denotes M. orbicularis’ fruits’ extracts.
3.7. Total Polyphenol and Flavonoid Contents of M. orbicularis’ Fruits’ Ethanolic Extracts
Since the phytochemicals of M. orbicularis have not been previously identified, the total phenolic and flavonoid contents of M. orbicularis’ fruits’ extracts were assayed. The total phenolics content was 1.1583 ± 0.00005 mg GAE/g of dry matter of Medicago orbicularis fruits. The total flavonoids content was 0.0246 ± 0.00003 mg quercetin equivalents/g of dry matter of Medicago orbicularis fruits. These results show that Medicago orbicularis fruits are rich in phenolics and flavonoids and may explain the anti-cancer properties of Medicago orbicularis fruits against A549 cells. Anti-cancerous properties of plant extracts are often attributed to polyphenols and flavonoids [22,26,40,41,42].
3.8. Identification of Phytochemical Composition of M. orbicularis’ Fruits’ Ethanolic Extracts by GC/MS
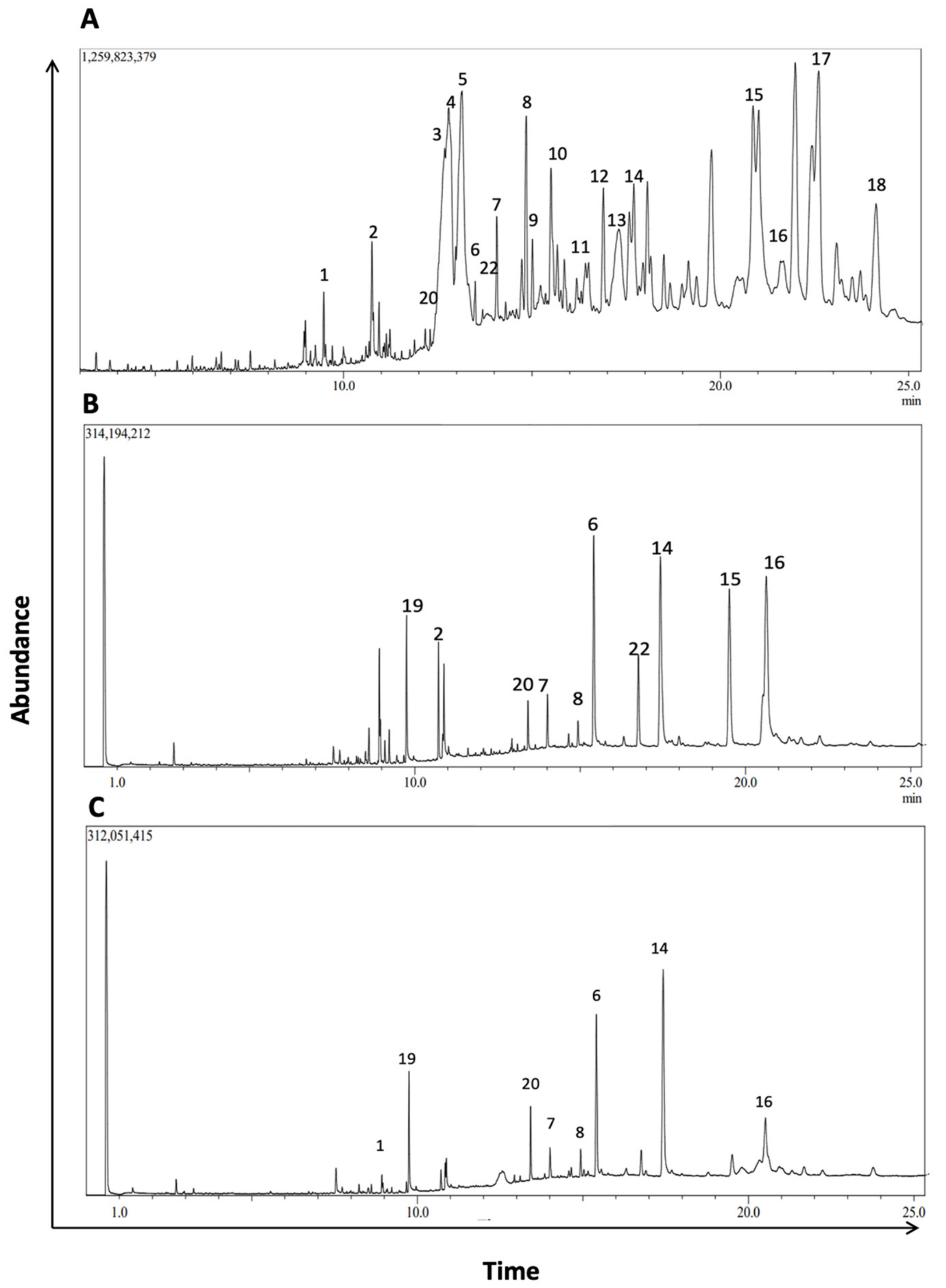
To further characterize the phytochemical contents of M. orbicularis fruits, GC/MS analysis was used (Figure 7). Table 2 lists 18 of the major compounds of M. orbicularis fruits as identified by GC/MS, by comparing their mass spectral fragmentation patterns to those of known compounds listed in the NIST library.
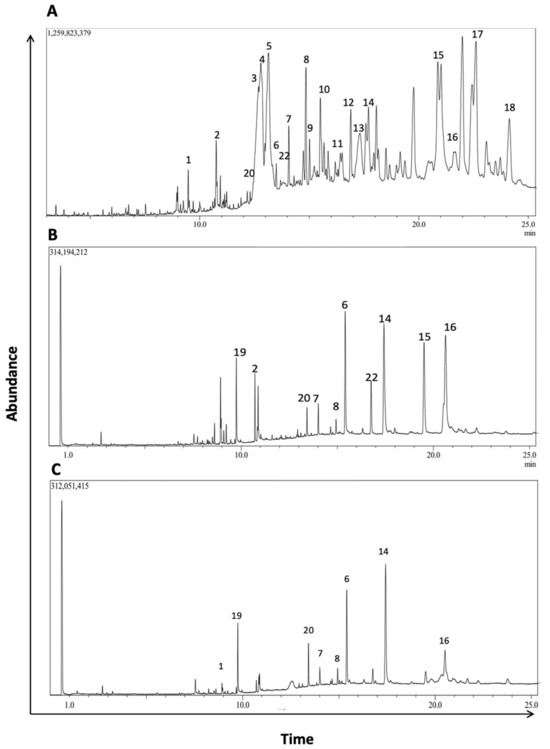
Figure 7.
GC/MS chromatogram of M. orbicularis’ parts’ ethanolic extract displaying the elution profile of phytochemicals listed in Table 3. (A) Fruit extracts, (B) leaf extracts, and (C) stem extracts.
4. Discussion
Cancer is a complex and devastating disease which affects millions of lives worldwide. Lung cancer, particularly non-small cell lung cancer, has the highest mortality rate among malignant tumors across the globe [37]. Current treatments for lung cancer such as chemotherapy and radiotherapy are associated with considerable toxicity and other side effects. In addition, lung cancer patients develop resistance to therapy and suffer from lung cancer recurrence as early as 6 months following therapy [3,4]. In this regard, there is a revived research interest in using herbal- and plant-based therapies as sources of anti-cancer bioactives. Several herbal remedies have been shown to have efficacy as well as minimal toxicity and side effects for disease treatment [12,13]. In addition, plants and their phytochemicals are being used as starting scaffolds for developing more potent anti-cancer agents [15,43]. Historically, plants or herbs and their extracts were used for the development of several important anti-cancer agents such as Paclitaxel, Camptothecin, and Vincristine [7,8,9,10,11,43]. Furthermore, several intricate biochemical pathways are simultaneously dysregulated in cancer [6,15], and herbal-based or herbal-derived remedies can target several molecular pathways and can become an alternative or complementary treatment to conventional cancer therapies [7,12,13,14,15,44,45]. Relatedly, multiple recent in vitro and in vivo studies demonstrated that the concurrent use of natural products with conventional treatments (chemo- and radiotherapy) can synergistically sensitize tumors to therapy, enhancing therapeutic efficacy and reducing toxicities [15]. Many studies have documented the promising use of herbal plant extracts against cancer cell lines in vitro and in vivo in animal models of cancer and are now being tested in clinical trials. These studies have shown that herbal-based remedies have antioxidant, antihemolytic, and apoptosis-inducing effects. They can also impact cell proliferation, aggregation and adhesion, migration, and metastasis [16,17,46].
Medicago orbicularis L. Bartal is an understudied plant species as far as its therapeutic effects are concerned. Except for a study reporting a traditional use of M. orbicularis for heart diseases management in Turkey [29], there are no reports on the therapeutic applications or phytochemical composition of M. orbicularis. Notably, M. orbicularis’ anti-cancerous activities have not been explored yet [18]. It was therefore pertinent to study the phytochemical composition, antioxidant capacity, antihemolytic properties, and the effects of M. orbicularis on cell proliferation, apoptosis, and cell aggregation potential against human lung adenocarcinoma A549 cells, in addition to the combinatorial effect of a treatment of both the chemotherapeutic agent cisplatin and M. orbicularis on A549 cells.
There are no studies currently on the anti-oxidant potential of M. orbicularis, but other species of the genus Medicago such as M. polymorpha, M. sativa, M. arabica, and M. truncatula were shown to have anti-oxidant activities and to be rich in phytochemicals [22,23]. Since anti-oxidant activity is often accompanied by therapeutic effects [22,23,27,28,43,47,48,49,50], this study was initiated with an evaluation of the antioxidant activity of the ethanolic extract of different plant parts of M. orbicularis, including its leaves, fruits and stems. All plant parts of M. orbicularis showed modest anti-oxidant potential and the leaves showed the highest antioxidant activity.
All M. orbicularis plant parts inhibited the proliferation of A549 cells, but M. orbicularis fruits exhibited the highest reduction in viability of A549 cells and were chosen to perform the rest of the experiments of the study. The National Cancer Institute (NCI, USA) considers an IC50 of 30 μg/mL to indicate a strong cytotoxic activity and a promising candidate for the further purification of a crude extract, and an IC50 of 31–200 μg/mL to indicate moderate cytotoxicity [51]. The IC50 of the fruit extracts at 72 h of treatment was 86.18 ± 1.93 μg/mL, indicating that this crude extract has moderate cytotoxicity. To date, no other study has reported the cytotoxicity levels of M. orbicularis against cancerous cells. Importantly, the cytotoxic effects of the fruit extracts showed selectivity to A549 cancerous cells and did not affect human neonatal fibroblast cells even at high concentrations. This indicated that M. orbicularis’ fruits’ extracts may have no side effects when used in vivo.
Plant bioactives can decrease the viability of cancer cells through several mechanisms, including the induction of apoptosis [7,12,13,14,15,44,45]. Cytotoxic agents can cause an increase in the expression of the pro-apoptotic protein BAX that makes pores in the mitochondrial membrane, leading to the release of cytochrome C [52]. The release of cytochrome C initiates the execution phase of apoptosis and activates Caspase 9, which cleaves pro-Caspase 3 into active Caspase 3, the main executer effector caspase [53,54]. Active Caspase 3 can cleave many protein substrates [55], such as caspase-activated DNAse that fragments genomic DNA [56] and poly-adenosine diphosphate (ADP) ribose polymerase-1 (PARP-1), to promote apoptosis [54]. These events are recognized as the intrinsic apoptotic pathway. The anti-apoptotic protein BCL-2 can inhibit the release of cytochrome C into the cytoplasm, thereby attenuating the intrinsic apoptotic pathway [54,57]. Western blotting analysis of lysates of A549 cells treated with 100 and 150 μg/mL M. orbicularis fruit extract indicated that M. orbicularis fruit extracts lowered the expression of BCL-2 and increased the expression of BAX, suggesting the activation of the intrinsic apoptotic pathway. Consequently, there was a dose-dependent decrease in the BCL-2/BAX ratio. This is the first report showing that M. orbicularis-induced death of A549 cells may be mediated, at least partly, by the intrinsic apoptotic machinery. However, confirmation of the induction of the intrinsic apoptosis pathway is warranted in future investigations, particularly the activation of Caspase 3 and PARP-1. Other mechanisms of cell death, such as autophagy, necroptosis, or pyroptosis, may also be induced by M. orbicularis, and need to be tested in future studies.
As part of the safety profile of a cancer drug, its systemic infusion through the blood of a patient should not cause any hemolysis of red blood cells [58]. M. orbicularis fruit extracts did not cause any hemolysis of RBCs. Moreover, the fruits extracts protected RBCs against hemolysis. This attested to the possible safety of these extracts in future in vivo or clinical studies.
Metastasis is the major culprit behind cancer-associated mortality [59,60,61]. For cancer cells to metastasize, they should lose their adhesion to neighboring cells, allowing them to migrate and invade at secondary tumor sites and organs [27,28,60,61,62]. Cancer therapeutics can act by strengthening cell adhesion and aggregation to prevent cell migration and metastasis [63]. In this study, an aggregation assay showed that M. orbicularis fruits significantly enhanced cell aggregation, signifying that the extract enhanced the adhesion of A549 cells. This result attests to the ability of M. orbicularis to attenuate the malignant phenotype of A549 cells. Future studies should focus on elucidating the molecular mechanisms underlying this finding, including the examination of protein kinases involved in cell adhesion and migration such as Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases, cell adhesion proteins, connexins gap junction proteins, and EMT markers such as E-cadherin, N-cadherin, vimentin, and Snail, among others [62,64].
Apart from their direct suppressive effects on carcinogenesis and cancer metastasis, herbal remedies and plant extracts have been used to complement conventional therapies since they can target several molecular pathways other than those targeted by conventional therapy, and therefore may overcome the refractoriness that develops against cancer therapeutics [7,12,13,14,15,44,45]. When combined with chemotherapy, plant-based therapies can enhance the response to chemotherapy, help overcome resistance to therapy, and decrease the severity of the side effects of chemotherapy, including cancer-related fatigue [7,12,13,14,15]. Chemotherapeutic agents, including cisplatin, can become refractory in lung cancer patients, and as result the cancer may relapse with poor prognosis [3,4]. The combination of cisplatin with a multitargeted herbal remedy may circumvent this problem. In this study, the cytotoxic effect of cisplatin alone on A549 cells was confirmed. The IC50 values of cisplatin with A549 that we obtained are similar to those reported in the literature [37]. In order to determine the impact of a combination treatment on the cisplatin-induced death of A549 cells, we applied a co-treatment of cisplatin and the ethanolic extract of M. orbicularis fruits. The results show that the combination treatment significantly augmented the cisplatin-induced decrease in the cell viability of A549 cells, when compared to either the extract alone or cisplatin alone. These results show that M. orbicularis may be a source of agents for a complementary therapy for lung cancer, in combination with chemotherapy. The combination treatment showed a similar result when tested on an aggregation of A549 cells. The co-treatment of M. orbicularis’ fruits’ extracts and cisplatin proved to significantly augment the increase in aggregation of A549 cells, when compared to either the extract alone or cisplatin alone. Taken together, these results support M. orbicularis as a source for the development of anti-lung cancer drug candidates that can complement chemotherapy.
M. orbicularis’ phytochemical composition has not been defined yet. But other Medicago species have been reported to be rich in phytochemicals [21,22,23], which have anti-cancer activities [24,25,26,27,28]. Natural polyphenols are plant secondary metabolites which have two or more phenol rings. Polyphenols’ health benefits include being antioxidant, antidiabetic, cardioprotective, and neuroprotective. Relatedly, phenolics have been reported to have strong anti-cancer effects through various mechanisms including removal of cancer cells, inhibition of cell cycle, induction of apoptosis, and inhibition of metastasis, among others [42]. Flavonoids are natural polyphenols with documented anti-cancer properties. Flavonoids have been reported to inhibit carcinogenesis by suppressing oxidative stress through their antioxidant activities [26,41]. Our results indicate that M. orbicularis’ fruits’ ethanolic extracts have cytotoxic effects against A549 cells. These effects could be mediated by polyphenols and flavonoids of M. orbicularis’ fruits’ extracts among other phytochemicals. In this study, Medicago orbicularis fruits were shown to be rich in phenolics and flavonoids, which may explain the anti-oxidant and anti-cancer properties of Medicago orbicularis fruits against A549 cells.
To further evaluate the phytochemical composition and establish a more comprehensive profile of the phytochemical constituents of M. orbicularis fruits, GC/MS analysis was conducted. We identified 20 prominent peaks that correspond to bioactive compounds of M. orbicularis fruits. The M. orbicularis’ fruits’ ethanol extract was found to be a complex mixture of various classes of phytochemicals, including flavonoids, alkaloids, diterpenes, triterpenes, sesquiterpenes, sterols, alcohols, aldehydes, and fatty acids. Among the diverse array of phytochemicals present in the extract, several compounds were correlated with therapeutic properties, including anti-cancerous activities. For example, lupeol and its derivatives, which were the most frequently identified compounds, have been recently demonstrated to possess a diverse array of pharmacological activities [65]. These activities included anticancer, antimicrobial, and antidiabetic effects, with certain lupeol derivatives exhibiting greater potency than lupeol [66]. Furthermore, the phytochemical composition of the stems and leaves of M. orbicularis was also identified by GC/MS. This revealed that M. orbicularis fruits have more phytochemical types than either the leaves or the stems. Moreover, a comparison of the abundance of the phytochemicals commonly present in fruits, leaves, and stems may provide hints about the phytochemicals responsible for the antiproliferative activities of M. orbicularis’ fruits’ extracts. For example, lupeol and its derivatives have anti-cancerous activities [65,66], and are more enriched in the fruits’ extracts. Future investigation should focus on a more comprehensive assessment of the plant extracts of M. orbicularis, using more advanced techniques such as preparative HPLC followed by LC-MS/MS or NMR, to identify the bioactives responsible for the therapeutic effects of crude extracts from this plant.
In conclusion, this study has determined, for the first time, the anti-oxidant, anti-hemolytic, and cytotoxic properties of the hydroalcoholic extracts of M. orbicularis against A549 lung cancer cells. The decrease in cell viability may be, at least partly, mediated by the intrinsic pathway of apoptosis, pending confirmation by future studies. M. orbicularis was also able to enhance the cell aggregation of A549 cells. These activities could be attributed to the phytochemicals present in M. orbicularis such as polyphenols and flavonoids, and the other compounds that were identified by GC/MS. These properties place M. orbicularis as a potential new candidate to offer effective natural agents for the treatment of lung cancer.
Author Contributions
Conceptualization: S.N.; Experimental design: S.N., F.K. and A.A.S.; Formal analysis: A.A.S., F.K. and S.N. who conducted the analysis and interpretation of data; Resources: S.N., F.K. and A.A.S.; Performed experiments: I.O., N.A.-T. and F.S.; Writing––original draft preparation: A.A.S., I.O. and F.S.; Writing––review and editing: all authors; Supervision: S.N., F.K. and A.A.S.; Funding acquisition: S.N., F.K. and A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Lebanese University (grant no. 4/6132) to SN and student grants number QUST-1-BRC-2022-315; QUST-1-BRC-2022-316, QUST-1-BRC-2023-836; and QUST-1-BRC-2023-846 to AS.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors acknowledge the help they received to perform the spectrophotometric assays and GC/MS analysis, which were accomplished in the Central Laboratories Unit, Qatar University.
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Lung and Bronchus Cancer; National Cancer Institute: Bethesda, MD, USA, 2023. [Google Scholar]
- Rossi, A.; Sacco, P.C.; Sgambato, A.; Casaluce, F.; Santabarbara, G.; Palazzolo, G.; Maione, P.; Gridelli, C. Optimal drugs for second-line treatment of patients with small-cell lung cancer. Expert Opin. Pharmacother. 2016, 17, 969–976. [Google Scholar] [CrossRef]
- Rudin, C.M.; Ismaila, N.; Hann, C.L.; Malhotra, N.; Movsas, B.; Norris, K.; Pietanza, M.C.; Ramalingam, S.S.; Turrisi, A.T., 3rd; Giaccone, G. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J. Clin. Oncol. 2015, 33, 4106–4111. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Almquist, D.; Mosalpuria, K.; Ganti, A.K. Multimodality Therapy for Limited-Stage Small-Cell Lung Cancer. J. Oncol. Pract. 2016, 12, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.Y.; Lee, B.; Kim, K.I.; Lee, B.J. Herbal medicine on cancer-related fatigue of lung cancer survivors: Protocol for a systematic review. Medicine 2020, 99, e18968. [Google Scholar] [CrossRef]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J.F. Plants as sources of natural and recombinant anti-cancer agents. Biotechnol. Adv. 2018, 36, 506–520. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J Ethnopharmacol 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Shaik, B.B.; Katari, N.K.; Jonnalagadda, S.B. Role of Natural Products in Developing Novel Anticancer Agents: A Perspective. Chem. Biodivers. 2022, 19, e202200535. [Google Scholar] [CrossRef]
- Konkimalla, V.B.; Efferth, T. Evidence-based Chinese medicine for cancer therapy. J. Ethnopharmacol. 2008, 116, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.J.; Meng, M.; Liu, Y.; Su, T.; Kwan, H.Y. Medicinal herbs and bioactive compounds overcome the drug resistance to epidermal growth factor receptor inhibitors in non-small cell lung cancer. Oncol. Lett. 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, S.; Li, C.; Li, T.; Huang, Y. Remodeling tumor microenvironment with natural products to overcome drug resistance. Front. Immunol. 2022, 13, 1051998. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Masoodi, T.; Prabhu, K.S.; Kuttikrishnan, S.; Zarif, L.; Khatoon, S.; Ali, S.; Uddin, S.; Akil, A.A.; Singh, M.; et al. Natural products as chemo-radiation therapy sensitizers in cancers. Biomed. Pharmacother. 2022, 154, 113610. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeong, D.; Song, M.; Kim, B. Recent Advances in Anti-Metastatic Approaches of Herbal Medicines in 5 Major Cancers: From Traditional Medicine to Modern Drug Discovery. Antioxidants 2021, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Malik, J.A.; Ahmed, S.; Kameshwar, V.A.; Alanazi, J.; Alamri, A.; Ahemad, N. Can Natural Products Targeting EMT Serve as the Future Anticancer Therapeutics? Molecules 2022, 27, 7668. [Google Scholar] [CrossRef] [PubMed]
- Zitouna, N.; Marghali, S.; Gharbi, M.; Haddioui, A.; Trifi-Farah, N. Sequence divergence of microsatellites for phylogeographic assessment of Moroccan Medicago species. Genet. Mol. Res. 2014, 13, 1548–1562. [Google Scholar] [CrossRef]
- Morshedi, Z.; Assadi, M.; Small, E.; Dehshiri, M.M.; Mehregan, I. Systematic Studies on Populations of Medicago orbicularis (L.) Bartal: Molecular, Morphological and Ecological Characterizations. J. Genet. Resour. 2022, 8, 178–187. [Google Scholar] [CrossRef]
- Karam, N.; Choueiry, Z.; Al-Beyrouthy, J.; Shehadeh, A.; Chalak, L.; Yazbek, M. Phenotypic diversity of Medicago crop wild relatives growing in Lebanon. Genet. Resour. Crop Evol. 2023, 70, 1487–1499. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. Phytochemical and pharmacological potential of Medicago sativa: A review. Pharm. Biol. 2011, 49, 211–220. [Google Scholar] [CrossRef]
- Khan, M.I.; Asad, S.; Zaman, G.; Rehman, H.; Rehman, S.; Iqbal, A.; Ullah, A.; Ullah, I.; Ali, S. Antioxidant And Cytotoxic Activities Of Crude Methanolic Extract Of Medicago Polymorpha. IOSR J. Pharm. 2013, 3, 32–37. [Google Scholar]
- Usman, M.; Khan, W.R.; Yousaf, N.; Akram, S.; Murtaza, G.; Kudus, K.A.; Ditta, A.; Rosli, Z.; Rajpar, M.N.; Nazre, M. Exploring the Phytochemicals and Anti-Cancer Potential of the Members of Fabaceae Family: A Comprehensive Review. Molecules 2022, 27, 3863. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Anifowose, S.O.; Alqahtani, W.S.N.; Al-Dahmash, B.A.; Sasse, F.; Jalouli, M.; Aboul-Soud, M.A.M.; Badjah-Hadj-Ahmed, A.Y.; Elnakady, Y.A. Efforts in Bioprospecting Research: A Survey of Novel Anticancer Phytochemicals Reported in the Last Decade. Molecules 2022, 27, 8307. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Mesmar, J.; Abdallah, R.; Hamade, K.; Baydoun, S.; Al-Thani, N.; Shaito, A.; Maresca, M.; Badran, A.; Baydoun, E. Ethanolic extract of Origanum syriacum L. leaves exhibits potent anti-breast cancer potential and robust antioxidant properties. Front. Pharmacol. 2022, 13, 994025. [Google Scholar] [CrossRef] [PubMed]
- AlKahlout, A.; Fardoun, M.; Mesmar, J.; Abdallah, R.; Badran, A.; Nasser, S.A.; Baydoun, S.; Kobeissy, F.; Shaito, A.; Iratni, R.; et al. Origanum syriacum L. Attenuates the Malignant Phenotype of MDA-MB231 Breast Cancer Cells. Front. Oncol. 2022, 12, 922196. [Google Scholar] [CrossRef] [PubMed]
- Güleç, M.; Erarslan, Z.B.; Kültür, Ş. The Medicinal Plants Traditionally Used Against Cardiovascular Diseases in Türkiye. Int. J. Tradit. Complement. Med. Res. 2023, 4, 81–96. [Google Scholar] [CrossRef]
- Nasreddine, S.; Mcheik, M.; Khalil, M.; El-Rashed, Z.; Daher, A.; Khalife, A. The antioxidant, antibacterial, antihemolytic and epithelial ovarian cancer antiproliferative activities of the lebanese plant salvia libanotica. Asian J. Sci. Technol. 2018, 09, 8695–8703. [Google Scholar]
- Malagoli, D. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Invertebr. Surviv. J. 2007, 4, 92–94. [Google Scholar]
- James, O.; Alewo, I.M. In vitro Antihemolytic Activity of Gymnema Sylvestre Extracts Against Hydrogen Peroxide (H2O2) Induced Haemolysis in Human Erythrocytes. Am. J. Phytomedicine Clin. Ther. 2014, 2, 861–869. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Katarina, Š.; Živković, J.; Zdunić, G.; Gođevac, D.; Đorđević, B.; Dojčinović, B.; Đorđević, N. Phenolic and mineral profiles of four Balkan indigenous apple cultivars monitored at two different maturity stages. J. Food Compos. Anal. 2014, 35, 101–111. [Google Scholar] [CrossRef]
- Rolim, P.M.; Fidelis, G.P.; Padilha, C.E.A.; Santos, E.S.; Rocha, H.A.O.; Macedo, G.R. Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumis melo L. var. reticulatus) and their antiproliferative effect in cancer cells. Braz. J. Med. Biol. Res. 2018, 51, e6069. [Google Scholar] [CrossRef]
- Tseng, C.Y.; Lin, C.H.; Wu, L.Y.; Wang, J.S.; Chung, M.C.; Chang, J.F.; Chao, M.W. Potential Combinational Anti-Cancer Therapy in Non-Small Cell Lung Cancer with Traditional Chinese Medicine Sun-Bai-Pi Extract and Cisplatin. PLoS ONE 2016, 11, e0155469. [Google Scholar] [CrossRef] [PubMed]
- Omairi, I.; Kobeissy, F.; Nasreddine, S. Anti-Oxidant, Anti-Hemolytic Effects of Crataegus aronia Leaves and Its Anti- Proliferative Effect Enhance Cisplatin Cytotoxicity in A549 Human Lung Cancer Cell Line. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 2993–3003. [Google Scholar] [CrossRef]
- De Giorgi, U.; Casadei, C.; Bergamini, A.; Attademo, L.; Cormio, G.; Lorusso, D.; Pignata, S.; Mangili, G. Therapeutic challenges for cisplatin-resistant ovarian germ cell tumors. Cancers 2019, 11, 1584. [Google Scholar] [CrossRef]
- Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef]
- Seglab, F.; Hamia, C.; Khacheba, I.; Djeridane, A.; Yousfi, M. High in vitro antioxidant capacities of Algerian Cleome arabica leaves’ extracts. Phytothérapie 2021, 19, 16–24. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Winslow, L.C.; Kroll, D.J. Herbs as medicines. Arch. Intern. Med. 1998, 158, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.W.; Leung, Y.; Chan, C. Herbal medicine in the treatment of cancer. Curr. Med. Chem. -Anti-Cancer Agents 2002, 2, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef]
- Alsamri, H.; Athamneh, K.; Pintus, G.; Eid, A.H.; Iratni, R. Pharmacological and Antioxidant Activities of Rhus coriaria L. (Sumac). Antioxidants 2021, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Visnevschi-Necrasov, T.; Barreira, J.C.; Cunha, S.C.; Pereira, G.; Nunes, E.; Oliveira, M.B. Advances in isoflavone profile characterisation using matrix solid-phase dispersion coupled to HPLC/DAD in Medicago species. Phytochem. Anal. 2015, 26, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Al Dhaheri, Y.; Attoub, S.; Arafat, K.; Abuqamar, S.; Viallet, J.; Saleh, A.; Al Agha, H.; Eid, A.; Iratni, R. Anti-metastatic and anti-tumor growth effects of Origanum majorana on highly metastatic human breast cancer cells: Inhibition of NFκB signaling and reduction of nitric oxide production. PLoS ONE 2013, 8, e68808. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, K.; Hasasna, H.E.; Samri, H.A.; Attoub, S.; Arafat, K.; Benhalilou, N.; Rashedi, A.A.; Dhaheri, Y.A.; AbuQamar, S.; Eid, A.; et al. Rhus coriaria increases protein ubiquitination, proteasomal degradation and triggers non-canonical Beclin-1-independent autophagy and apoptotic cell death in colon cancer cells. Sci. Rep. 2017, 7, 11633. [Google Scholar] [CrossRef]
- Suffness, M.P. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1991; Volume 6, pp. 71–133. [Google Scholar]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef]
- Wolf, B.B.; Green, D.R. Suicidal tendencies: Apoptotic cell death by caspase family proteinases. J. Biol. Chem. 1999, 274, 20049–20052. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Alnemri, T.; Litwack, G.; Alnemri, E.S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J. Biol. Chem. 1994, 269, 30761–30764. [Google Scholar] [CrossRef] [PubMed]
- Widłak, P. The DFF40/CAD endonuclease and its role in apoptosis. Acta Biochim. Pol. 2000, 47, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Mayer, B.; Bartolmäs, T.; Yürek, S.; Salama, A. Variability of Findings in Drug-Induced Immune Haemolytic Anaemia: Experience over 20 Years in a Single Centre. Transfus. Med. Hemother. 2015, 42, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- Thiery, J.P.; Lim, C.T. Tumor dissemination: An EMT affair. Cancer Cell 2013, 23, 272–273. [Google Scholar] [CrossRef]
- El-Hajjar, L.; Jalaleddine, N.; Shaito, A.; Zibara, K.; Kazan, J.M.; El-Saghir, J.; El-Sabban, M. Bevacizumab induces inflammation in MDA-MB-231 breast cancer cell line and in a mouse model. Cell Signal 2019, 53, 400–412. [Google Scholar] [CrossRef]
- Abduljauwad, S.N.; Ahmed, H.U. Enhancing cancer cell adhesion with clay nanoparticles for countering metastasis. Sci. Rep. 2019, 9, 5935. [Google Scholar] [CrossRef]
- Jalaleddine, N.; El-Hajjar, L.; Dakik, H.; Shaito, A.; Saliba, J.; Safi, R.; Zibara, K.; El-Sabban, M. Pannexin1 Is Associated with Enhanced Epithelial-To-Mesenchymal Transition in Human Patient Breast Cancer Tissues and in Breast Cancer Cell Lines. Cancers 2019, 11, 1967. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef]
- Sharma, N.; Palia, P.; Chaudhary, A.; Verma, K.; Kumar, I. A review on pharmacological activities of lupeol and its triterpene derivatives. J. Drug Deliv. Ther. 2020, 10, 325–332. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).