Assessment of Chemical Composition and In Vitro Antioxidant, Antidiabetic, Anticholinesterase and Microbial Virulence-Quenching Effects of Salad Burnet (Sanguisorba minor L.) Harvested from Algeria
Abstract
1. Introduction
2. Results
2.1. Yields of Obtained Extracts
2.2. Total Polyphenols, Flavonoids, Tannins, and Anthocyanin Contents
2.3. Identification and Quantification of Phenolic Compounds by HPLC-DAD
2.4. Antioxidant Activity
2.5. Anticholinesterase and Antidiabetic Activities of the S. minor
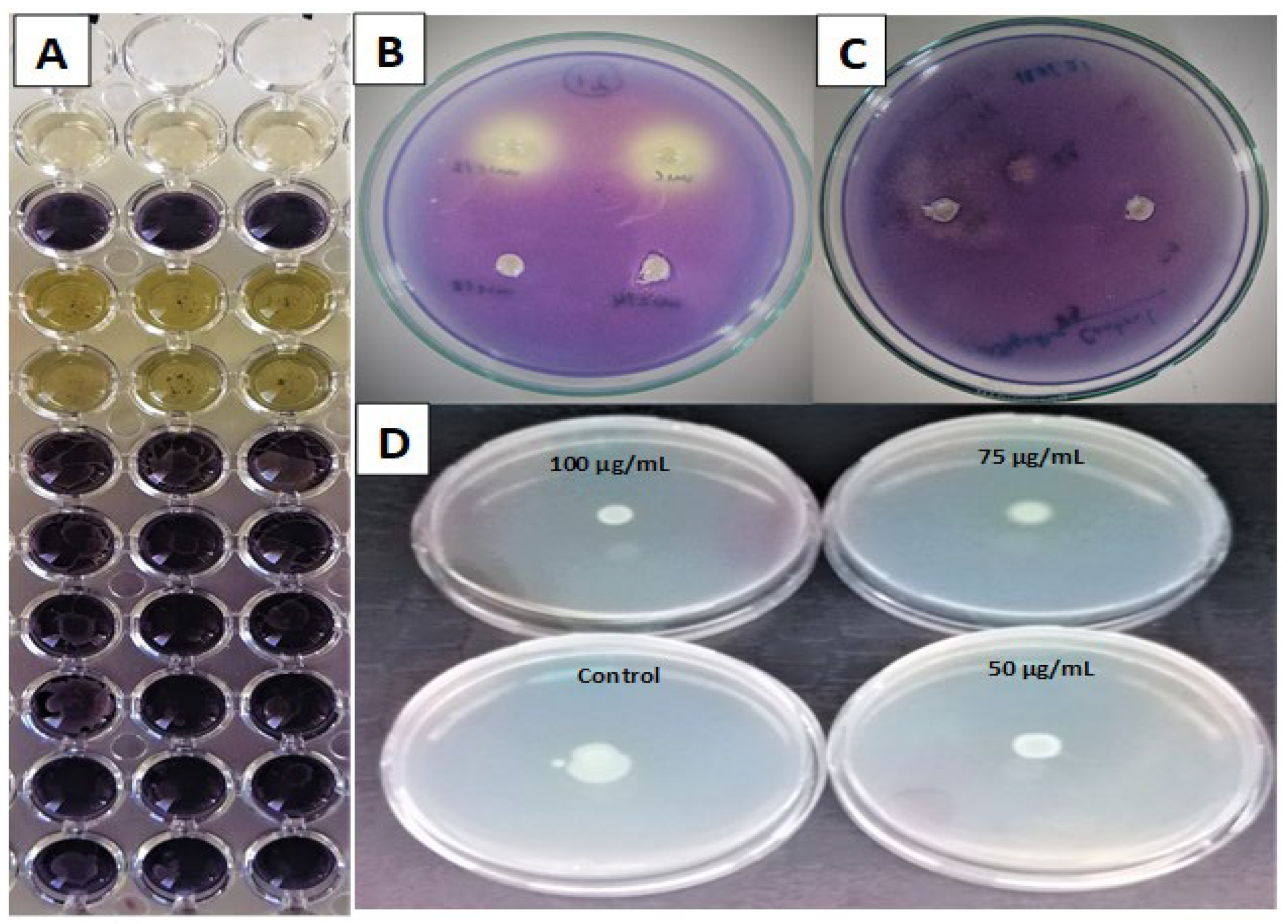
2.6. Violacein and Quorum-Sensing (QS) Inhibitions
2.7. Swarming Motility Inhibition on P. aeruginosa PA01
2.8. Antimicrobial and Antibiofilm Properties
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Total Phenolic and Total Flavonoid Content
4.3. Determination of Total Condensed Tannins
4.4. Determination of Total Anthocyanin by Absorption Spectroscopy
4.5. HPLC–DAD Phenolic Profiles of Plant Extracts
4.6. Evaluation of Antioxidant Activity
4.7. Anticholinesterase Activity
4.8. In Vitro Antidiabetic (α-Amylase and α-Glucosidase Inhibition) Assay
4.9. Microbial Strains
4.10. Determination of Minimum Inhibitory Concentrations
4.11. Effect of Extracts on Bacterial Biofilm Formation
4.12. Bioassay for Quorum-Sensing Inhibition (QSI) Activity Using C. violaceum CV026
4.13. Violacein Inhibition Assay Using C. violaceum CV12472
4.14. Swarming Motility Inhibition on Pseudomonas aeruginosa PA01
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamfu, A.N.; Kucukaydin, S.; Ceylan, O.; Sarac, N.; Duru, M.E. Phenolic composition, enzyme inhibitory and anti-quorum sensing activities of cinnamon (Cinnamomum zeylanicum Blume) and Basil (Ocimum basilicum Linn). Chem. Afr. 2021, 4, 759–767. [Google Scholar] [CrossRef]
- Ngenge, T.A.; Ceylan, O.; Fru, G.; Arab, Y.; Duru, M.E.; Ozturk, M. Antimicrobial, antibiofilm, anti-quorum sensing and motility inhibition activities of essential oil from seeds of food spice Xylopia aethiopica (Dunal) A. Rich. on some pathogenic bacteria. Res. J. Biotechnol. 2021, 16, 68–76. [Google Scholar]
- Tamfu, A.N.; Ceylan, O.; Kucukaydin, S.; Ozturk, M.; Duru, M.E.; Dinica, R.M. Antibiofilm and enzyme inhibitory potentials of two annonaceous food spices, African pepper (Xylopia aethiopica) and African nutmeg (Monodora myristica). Foods 2020, 9, 1768. [Google Scholar] [CrossRef] [PubMed]
- Catuogno, S.; Esposito, C.L.; De Franciscis, V. Aptamer-mediated targeted delivery of therapeutics: An update. Pharmaceuticals 2016, 9, 69. [Google Scholar] [CrossRef]
- Tamma, P.D.; Edina, A.; David, X.L.; Kathryn, D.; Sara, E.C. Association of adverse events with antibiotic use in hospitalized patients. JAMA Int. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yue, Z.; Yongze, G.; Xuan, Z.; Jianyi, P. Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, V.; Dietrich, R.; Klingl, A.; Martlbauer, E.; Jessberger, N. Characterization of strain-specific Bacillus cereus swimming motility and flagella by means of specific antibodies. PLoS ONE 2022, 17, 0265425. [Google Scholar] [CrossRef]
- García-Contreras, R.; Wood, T.K.; Tomás, M. Quorum Network (Sensing/Quenching) in Multidrug-Resistant Pathogens. Front. Cell Infect. Microbiol. 2019, 9, 80. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Ceylan, O.; Kucukaydin, S.; Duru, M.E. HPLC-DAD phenolic profiles, antibiofilm, anti-quorum sensing and enzyme inhibitory potentials of Camellia sinensis (L.) O. Kuntze and Curcuma longa L. LWT-Food Sci. Technol. 2020, 133, 110150. [Google Scholar] [CrossRef]
- Tocai, A.C.; Kokeric, T.; Tripon, S.; Barbu-Tudoran, L.; Barjaktarevic, A.; Cupara, S.; Vicas, S.I. Sanguisorba minor Scop.: An Overview of Its Phytochemistry and Biological Effects. Plants 2023, 12, 2128. [Google Scholar] [CrossRef]
- Alain, K.Y.; Tamfu, A.N.; Kucukaydin, S.; Ceylan, O.; Pascal, A.D.; Félicien, A.; Dominique, S.C.; Duru, M.E.; Dinica, R.M. Phenolic profiles, antioxidant, antiquorum sensing, antibiofilm and enzyme inhibitory activities of selected Acacia species collected from Benin. LWT-Food Sci. Technol. 2022, 171, 114162. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Roland, N.; Mfifen, A.M.; Kucukaydin, S.; Gaye, M.; Botezatu, A.V.; Emin Duru, M.; Dinica, R.M. Phenolic composition, antioxidant and enzyme inhibitory activities of Parkia biglobosa (Jacq.) Benth., Tithonia diversifolia (Hemsl) A. Gray, and Crossopteryx febrifuga (Afzel.) Benth. Arab. J. Chem. 2022, 15, 103675. [Google Scholar] [CrossRef]
- Geronikaki, A. Recent Trends in Enzyme Inhibition and Activation in Drug Design. Molecules 2022, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Küçükaydın, S.; Çayan, F.; Tel-Çayan, G.; Duru, M.E. HPLC-DAD phytochemical profiles of Thymus cariensis and T. cilicicus with antioxidant, cytotoxic, anticholinesterase, anti-urease, anti-tyrosinase, and antidiabetic activities. S. Afr. J. Bot. 2021, 143, 155–163. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Kucukaydin, S.; Yeskaliyeva, B.; Ozturk, M.; Dinica, R.M. Non-Alkaloid Cholinesterase Inhibitory Compounds from Natural Sources. Molecules 2021, 26, 5582. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Hsu, C.Y.; Chou, W.L.; Fang, J.Y.; Chuang, S.Y. Bioactive Agent Discovery from the Natural Compounds for the Treatment of Type 2 Diabetes Rat Model. Molecules 2020, 25, 5713. [Google Scholar] [CrossRef]
- Khan, S.N.; Shaheen, F.; Aleem, U.; Sheikh, S.; Tamfu, A.N.; Ashraf, S.; Ul-Haq, Z.; Ullah, S.; Choudhary, M.I.; Jahan, H. Peptide conjugates of 18β-glycyrrhetinic acid as potent inhibitors of α-glucosidase and AGEs-induced oxidation. Eur. J. Pharm. Sci. 2022, 168, 106045. [Google Scholar] [CrossRef]
- Hosseini, Z.; Mansouritorghabeh, F.; Kakhki, F.S.H.; Hosseini, M.; Rakhshandeh, H.; Hosseini, A.; Hasanpour, M.; Iranshahi, M.; Rajabian, A. Effect of Sanguisorba Minor on Scopolamine-Induced Memory Loss in Rat: Involvement of Oxidative Stress and Acetylcholinesterase. Metab. Brain Dis. 2022, 37, 473–488. [Google Scholar] [CrossRef]
- Cuccioloni, M.; Bonfili, L.; Mozzicafreddo, M.; Cecarini, V.; Eleuteri, A.M.; Angeletti, M. Sanguisorba Minor Extract Suppresses Plasmin-Mediated Mechanisms of Cancer Cell Migration. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 1027–1034. [Google Scholar] [CrossRef]
- Sanchez-Bel, P.; Romojaro, A.; Egea, I.; Pretel, M.T. Wild Edible Plants as Potential Antioxidant or Nutritional Supplements for Beverages Minimally Processed. LWT-Food Sci. Technol. 2015, 62, 830–837. [Google Scholar] [CrossRef]
- Sabbatini, A.; Jurnatan, Y.; Fraatz, M.A.; Govori, S.; Haziri, A.; Millaku, F.; Zorn, H.; Zhang, Y. Aroma Characterization of a Wild Plant (Sanguisorba albanica) from Kosovo Using Multiple Headspace Solid Phase Microextraction Combined with Gas Chromatography-Mass Spectrometry-Olfactometry. Food Res. Int. 2019, 120, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, J.; Chen, Q.; Wang, L.; Yang, J.; Wu, A.; Jiang, N.; Liu, Y.; Chen, J.; Zou, W.; et al. A Comprehensive Review of Genus Sanguisorba: Traditional Uses, Chemical Constituents and Medical Applications. Front. Pharmacol. 2021, 12, 750165. [Google Scholar] [CrossRef]
- Kaufmann, D.; Herrmann, F.; Wink, M. Extracts from traditional Chinese medical plants inhibit glycogen synthase kinase 3β activity, a potential Alzheimer target. Z. Für Phytother. 2009, 30, 16. [Google Scholar] [CrossRef]
- Akbari, S.; Soodi, M.; Hajimehdipoor, M.; Ataei, N. Protective effects of Sanguisorba minor and Ferulago angulata total extracts against beta-amyloid induced cytotoxicity and oxidative stress in cultured cerebellar granule neurons. J. Herbmed Pharmacol. 2019, 8, 248–255. [Google Scholar] [CrossRef]
- Ennerfelt, H.E.; Lukens, J.R. The role of innate immunity in Alzheimer’s disease. Immunol. Rev. 2020, 297, 225–246. [Google Scholar] [CrossRef]
- Kawanishi, N.; Sugimoto, T.; Shibata, J.; Nakamura, K.; Masutani, K.; Ikuta, M.; Hirai, H. Structure-based drug design of a highly potent CDK1, 2, 4, 6 inhibitor with novel macrocyclic quinoxalin-2-one structure. Bioorg. Med. Chem. Lett. 2006, 16, 5122–5126. [Google Scholar] [CrossRef]
- Romojaro, A.; Sanchez-Bel, P.; Serrano, M.; Teresa, P.M. Wild edible plants as potential antioxidants in vegetables oils. J. Chem. 2013, 2013, 457902. [Google Scholar] [CrossRef]
- Bedoya, L.M.; Sanchez-Palomino, S.; Abad, L.J.; Bermejo, P.; Alcami, J. Anti-HIV activity of medicinal plant extracts. J. Ethnopharmacol. 2001, 77, 113–116. [Google Scholar] [CrossRef]
- Viano, J.; Masotti, V.; Gaydou, E.M. Nutritional value of Mediterranean sheep’s burnet (Sanguisorba minor ssp. muricata). J. Agric. Food Chem. 1999, 47, 4645–4648. [Google Scholar] [CrossRef]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.M.; Araújo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; He, X.; Zhang, Q.; Wei, X.; Huang, L.; Cheng Fang, J.; Wang, X.; Zhao, M.; Bai, Y.; Zheng, X. Traditional uses, chemical constituents and biological activities of plants from the genus Sanguisorba L. Am. J. Chin. Med. 2017, 45, 199–224. [Google Scholar] [CrossRef] [PubMed]
- Cecarini, V.; Bonfili, L.; Cuccioloni, M.; Mozzicafreddo, M.; Rossi, G.; Buizza, L.; Uberti, D.; Angeletti, M.; Eleuteri, A.M. Crosstalk between the ubiquitin-proteasome system and autophagy in a human cellular model of Alzheimer’s disease. Biochim. Biophys. Acta 2012, 1822, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Finimundy, T.C.; Karkanis, A.; Fernandes, Â.; Petropoulos, S.A.; Calhelha, R.; Petrović, J.; Soković, M.; Rosa, E.; Barros, L.; Ferreira, I.C.F.R. Bioactive properties of Sanguisorba minor L. cultivated in central Greece under different fertilization regimes. Food Chem. 2020, 327, 127043. [Google Scholar] [CrossRef] [PubMed]
- Karkanis, A.C.; Fernandes, Â.; Vaz, J.; Petropoulos, S.; Georgiou, E.; Ciric, A.; Sokovic, M.; Oludemi, T.; Barros, L.; Ferreira, I.C.F.R. Chemical Composition and Bioactive Properties of Sanguisorba Minor Scop. Under Mediterranean Growing Conditions. Food Funct. 2019, 10, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Tocai (Mo¸toc), A.-C.; Ranga, F.; Teodorescu, A.G.; Pallag, A.; Vlad, A.M.; Bandici, L.; Vicas, S.I. Evaluation of Polyphenolic Composition and Antimicrobial Properties of Sanguisorba Officinalis L. and Sanguisorba Minor Scop. Plants 2022, 11, 3561. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, T.; Barjaktarevic, A.; Ninkovic, M.; Bauer, R.; Nikles, S.; Brankovic, S.; Markovic, M.; Stankov Jovanovic, V.; Ilic, M.; Milovanovic, O.; et al. Biological Activities of Sanguisorba minor L. Extracts—In vitro and In vivo Evaluations. Acta Pol. Pharm. Drug Res. 2020, 77, 745–758. [Google Scholar]
- Ceccanti, C.; Finimundy, T.C.; Heleno, S.A.; Pires, T.C.S.P.; Calhelha, R.C.; Guidi, L.; Ferreira, I.C.F.R.; Barros, L. Differences in the Phenolic Composition and Nutraceutical Properties of Freeze Dried and Oven-Dried Wild and Domesticated Samples of Sanguisorba Minor Scop. LWT 2021, 145, 111335. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains--a review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–919. [Google Scholar] [CrossRef] [PubMed]
- Mizzi, L.; Chatzitzika, C.; Gatt, R.; Valdramidis, V. HPLC Analysis of Phenolic Compounds and Flavonoids with Overlapping Peaks. FTB Food Technol. Biotechnol. 2020, 58, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Beddiar, H.; Boudiba, S.; Benahmed, M.; Tamfu, A.N.; Ceylan, Ö.; Hanini, K.; Kucukaydin, S.; Elomri, A.; Bensouici, C.; Laouer, H.; et al. Chemical composition, anti-quorum sensing, enzyme inhibitory, and antioxidant properties of phenolic extracts of Clinopodium nepeta L. Kuntze. Plants 2021, 10, 1955. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M. Phenolic Compounds in Food: Characterization and Health Benefits. Molecules 2022, 27, 783. [Google Scholar] [CrossRef]
- Arab, Y.; Sahin, B.; Ceylan, O.; Zellagui, A.; Olmez, O.T.; Kucukaydin, S.; Tamfu, A.N.; Ozturk, M.; Gherraf, N. Assessment of in vitro activities and chemical profiling of Senecio hoggariensis growing in Algerian Sahara. Biodiversitas 2022, 23, 3498–3506. [Google Scholar] [CrossRef]
- Gharaati Jahromi, S. Extraction Techniques of Phenolic Compounds from Plants. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar] [CrossRef]
- Gatto, M.A.; Sergio, L.; Ippolito, A.; Di Venere, D. Phenolic extracts from wild edible plants to control postharvest diseases of sweet cherry fruit. Postharvest Biol. Technol. 2016, 120, 180–187. [Google Scholar] [CrossRef]
- Karkanis, A.; Vellios, E.; Thomaidis, T.; Bilalis, D.; Efthimiadou, A.; Travlos, I. Phytochemistry and Biological Properties of Burnet Weed (Sanguisorba spp.): A Review. Not. Sci. Biol. 2014, 6, 395–398. [Google Scholar] [CrossRef]
- Tocai, A.C.; Memete, A.R.; Vica¸s, S.; Burescu, P. Antioxidant capacity of sanguisorba officinalis l. And sanguisorba minor scop. Nat. Resour. Sustain. Dev. 2021, 11, 121–133. [Google Scholar] [CrossRef]
- Cirovic, T.; Barjaktarevic, A.; Cupara, S.; Mitic, V.; Nikolic, J.; Jovanovic, V.S. Antioxidant and Antimicrobial Activity of Sanguisorba Minor L. Extracts. Serb. J. Exp. Clin. Res. 2022, 23, 51–57. [Google Scholar] [CrossRef][Green Version]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Sarengaowa; Guan, Y.; Feng, K. Biosynthesis of Phenolic Compounds and Antioxidant Activity in Fresh-Cut Fruits and Vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, C.; Landi, M.; Rocchetti, G.; Miras Moreno, M.B.; Lucini, L.; Incrocci, L.; Pardossi, A.; Guidi, L. Hydroponically Grown Sanguisorba minor Scop.: Effects of Cut and Storage on Fresh-Cut Produce. Antioxidants 2019, 8, 631. [Google Scholar] [CrossRef] [PubMed]
- Tamfu, A.N.; Tagatsing, F.M.; Talla, E.; Ozturk, M.; Mbafor, J.T.; Duru, M.E.; Farzana, S. Chemical composition and evaluation of anticholinesterase activity of essential oil from Cameroonian propolis. Issues Biol. Sci. Pharma Res. 2019, 7, 58–63. [Google Scholar]
- Colizzi, C. The protective effects of polyphenols on Alzheimer’s disease: A systematic review. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-beta aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Ali Asgar, M. Anti-Diabetic Potential of Phenolic Compounds: A Review. Int. J. Food Prop. 2012, 16, 91–103. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Dogaç, Y.I.; Ceylan, O.; Dediu, A.B.; Bozkurt, S.; Dinica, R.M. Antibiofilm and Anti-Quorum Sensing Potential of Safely-Synthesized Hydrated Zirconium Oxide-Coated Alginate Beads against Some Pathogenic Bacteria. J. Chem. 2023, 2023, 9924845. [Google Scholar] [CrossRef]
- Krzyżek, P. Challenges and Limitations of Anti-quorum Sensing Therapies. Front. Microbiol. 2019, 10, 2473. [Google Scholar] [CrossRef]
- Kocak, G.; Tamfu, A.N.; Bütün, V.; Ceylan, O. Synthesis of quaternary piperazine methacrylate homopolymers and their antibiofilm and anti-quorum sensing effects on pathogenic bacteria. J. Appl. Polym. Sci. 2021, 138, 50466. [Google Scholar] [CrossRef]
- Raju, D.V.; Nagarajan, A.; Pandit, S.; Nag, M.; Lahiri, D.; Upadhye, V. Effect of bacterial quorum sensing and mechanism of antimicrobial resistance. Biocatal. Agri Biotechnol. 2022, 43, 102409. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Boukhedena, W.; Boudiba, S.; Deghboudj, S.; Ceylan, O. Synthesis and evaluation of inhibitory potentials of microbial biofilms and quorum-sensing by 3-(1, 3-dithian-2-ylidene) pentane-2, 4-dione and ethyl-2-cyano-2-(1, 3-dithian-2-ylidene) acetate. Pharmacia 2022, 69, 973–980. [Google Scholar] [CrossRef]
- Dong, Y.-H.; Zhang, L.-H. Quorum sensing and quorum-quenching enzymes. J. Microbiol. 2005, 43, 101–109. [Google Scholar] [PubMed]
- Tamfu, A.N.; Ceylan, O.; Cârâc, G.; Talla, E.; Dinica, R.M. Antibiofilm and anti-quorum sensing potential of cycloartane-type triterpene acids from Cameroonian grassland propolis: Phenolic profile and antioxidant activity of crude extract. Molecules 2022, 27, 4872. [Google Scholar] [CrossRef] [PubMed]
- Tamfu, A.N.; Kocak, G.; Ceylan, O.; Citak, F.; Bütün, V.; Çiçek, H. Synthesis of cross-linked diazaborine-based polymeric microparticles with antiquorum sensing, anti-swarming, antimicrobial, and antibiofilm properties. J. Appl. Polym. Sci. 2023, 140, e53631. [Google Scholar] [CrossRef]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-Sensing Regulation of Antimicrobial Resistance in Bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Gerginova, D.; Trusheva, B.; Simova, S.; Tamfu, A.N.; Ceylan, O.; Clark, K.; Bankova, V. A preliminary study of chemical profles of honey, cerumen, and propolis of the African stingless bee Meliponula ferruginea. Foods 2021, 10, 997. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Talla, E.; Tamfu, A.N.; Biyanzi, P.; Sakava, P.; Asobo, F.P.; Mbafor, J.T.; Tchuenguem, F.F.N.; Ndjouenkeu, R. Phytochemical screening, antioxidant activity, total polyphenols and flavonoids content of different extracts of propolis from Tekel (Ngaoundal, Adamawa region, Cameroon). J. Phytopharmacol. 2014, 3, 321–329. [Google Scholar] [CrossRef]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; Elsohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid. -Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Quantification of Tannins in Tree Foliage; Working document; FAO/IAEA: Vienna, Austria, 2000. [Google Scholar]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. “Analysis of condensed tannins” a review. Anim. Feed. Sci. Technol. 2001, 91, 21. [Google Scholar] [CrossRef]
- Sun, B.; Richardo-da-Silvia, J.M.; Spranger, I. Critical factors of vanillin assay forcatechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Unit F1.2.1-13. Anthocyanins. In Characterization and Measurement with UV Visible Spectroscopy; Wrolstad, R.E., Ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Barros, L.; Dueñas, M.; Ferreira, I.C.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC-DAD-ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Mikwangock, H.D.; Tamfu, A.N.; Amang, A.P.; Siwe, G.T.; Mezui, C.; Kucukaydin, S.; Enow-Orock, E.G.; Tan, P.V. Chronic Gastric Ulcer Healing Actions of the Aqueous Extracts of Staple Plant Foods of the North-West, Adamawa, and West Regions of Cameroon. BioMed Res Int. 2023, 2023, 2657278. [Google Scholar] [CrossRef] [PubMed]
- Çayan, F.; Tel-Çayan, G.; Deveci, E.; Duru, M.E. HPLC–DAD characterization of phenolic profile and in vitro antioxidant, anticholinesterase, and antidiabetic activities of five mushroom species from Turkey. 3 Biotech. 2021, 11, 273. [Google Scholar] [CrossRef]
- Öztürk, M.; Duru, M.E.; Kivrak, Ş.; Mercan-Doğan, N.; Türkoglu, A.; Özler, M.A. In vitro antioxidant, anticholinesterase and antimicrobial activity studies on three Agaricus species with fatty acid compositions and iron contents: A comparative study on the three most edible mushrooms. Food Chem. Toxicol. 2011, 49, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Sawalda, M.; Tamfu, A.N.; Fadimatou; Essongue, C.F.; Talla, E.; Céline, H.; Sophie, L.; Shaheen, F.; Mbafor, J.T. Evaluation of Radical Scavenging and Metal Chelating Potential of Cameroonian Propolis and Isolation of Some Chemical Constituents. Rec. Agric. Food Chem. 2022, 2, 84–93. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Alfred, T.N.; Ceylan, O.; Kucukaydin, S.; Olmez, O.T.; Godloves, C.F.; Sylvain, S.K.; Yeskaliyeva, B.; Duru, M.E.; Ozturk, M. HPLC-DAD and GC-MS characterization of Cameroonian honey samples and evaluation of their antibioflm, anti-quorum sensing and antioxidant activities. Bull. Environ. Pharmacol. Life Sci. 2020, 9, 132–142. [Google Scholar]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Chelliah, R.; Banan-MwineDaliri, E.; Oh, D.H. Screening for Antioxidant Activity: Hydrogen Peroxide Scavenging Assay. In Methods in Actinobacteriology, Springer Protocols Handbooks; Dharumadurai, D., Ed.; Humana: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Tamfu, A.N.; Kucukaydin, S.; Quradha, M.M.; Ceylan, O.; Ugur, A.; Duru, M.E. Ultrasound-Assisted Extraction of Syringa vulgaris Mill., Citrus sinensis L. and Hypericum perforatum L.: Phenolic Composition, Enzyme Inhibition and Anti-quorum Sensing Activities. Chem. Afr. 2022, 5, 237–249. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, C.S.; Son, K.H. Inhibition of α-glucosidase and amylaze by luteolin, a flavonoid. Biosci Biotechnol. Biochem. 2010, 64, 2458–2461. [Google Scholar] [CrossRef]
- Ceylan, O.; Tamfu, A.N.; Doğaç, Y.İ.; Teke, M. Antibiofilm and anti-quorum sensing activities of polyethylene imine coated magnetite and nickel ferrite nanoparticles. 3 Biotech. 2020, 10, 513. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Munvera, A.M.; Botezatu, A.V.D.; Talla, E.; Ceylan, O.; Fotsing, M.T.; Mbafor, J.T.; Shaheen, F.; Dinica, R.M. Synthesis of benzoyl esters of β-amyrin and lupeol and evaluation of their antibiofilm and antidiabetic activities. Results Chem. 2022, 4, 100322. [Google Scholar] [CrossRef]
- Koh, K.M.; Tham, F.Y. Screening of traditional Chinese medicinal plants for quorum-sensing inhibitors activity. J. Microbiol. Immunol. Infect. 2011, 44, 144–148. [Google Scholar] [CrossRef]
- Alfred Ngenge, T.; Kucukaydin, S.; Ceylan, O.; Duru, M.E. Evaluation of enzyme inhibition and anti-quorum sensing potentials of Melaleuca alternifolia and Citrus sinensis essential oils. Nat. Prod. Commun. 2021, 16, 1934578X211044565. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Ceylan, O.; Fru, G.C.; Ozturk, M.; Duru, M.E.; Shaheen, F. Antibiofilm, antiquorum sensing and antioxidant activity of secondary metabolites from seeds of Annona senegalensis, Persoon. Microb. Pathog. 2020, 144, 104191. [Google Scholar] [CrossRef]
- Packiavathy, I.A.S.V.; Agilandeswari, P.; Musthafa, K.S.; Pandian, S.K.; Ravi, A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int. 2012, 45, 85–92. [Google Scholar] [CrossRef]
- Boudiba, S.; Tamfu, A.N.; Berka, B.; Hanini, K.; Hioun, S.; Allaf, K.; Boudiba, L.; Ceylan, O. Anti-quorum sensing and antioxidant activity of essential oils extracted from juniperus species, growing spontaneously in tebessa region (East of Algeria). Nat. Prod. Commun. 2021, 16, 1934578X211024039. [Google Scholar] [CrossRef]


| Sample | TPC mg GAE/g DW (a) | FC mg QE/g DW (b) | TC mg CE/g DW (c) | AC mg CGE/g DW (d) |
|---|---|---|---|---|
| DCME | 240.0 ± 4.9 | 155.7 ± 1.1 | 113.5 ± 3.0 | 6.5 ± 0.7 |
| EAE | 811.0 ± 4.7 | 254.9 ± 3.5 | 129.7 ± 2.1 | 2.7 ± 0.1 |
| BE | 461.0 ± 4.8 | 101.6 ± 2.0 | 102.2 ± 4.3 | 8.6 ± 0.2 |
| AQE | 232.0 ± 4.4 | 78.4 ± 2.2 | 58.6 ± 1.6 | 1.5 ± 0.0 |
| No. | Phenolic Compounds | RT (min) | DCME | EAE | BE | AQE | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 5.70 | - | - | - | - | |
| 2 | Protocatechuic acid | 8.85 | 0.85 ± 0.06 | 10.20 ± 0.17 | - | - | [1,11,12,23] |
| 3 | Catechin | 10.68 | - | - | - | - | |
| 4 | Pyrocatechol | 11.04 | 0.52 ± 0.04 | 13.44 ± 0.23 | - | - | [1,12] |
| 5 | Chlorogenic acid | 12.35 | - | - | - | - | |
| 6 | p-Hydroxy benzoic acid | 12.77 | - | 22.63 ± 0.28 | 2.52 ± 0.15 | 8.15 ± 0.08 | [1,11] |
| 7 | 6.7-Dihydroxy coumarin | 14.10 | - | - | - | - | |
| 8 | Caffeic acid | 15.09 | 5.05 ± 0.12 | 70.49 ± 0.35 | - | 1.86 ± 0.05 | [12,23] |
| 9 | 3-Hydroxy benzoic acid | 15.98 | - | - | - | - | |
| 10 | Syringic acid | 16.56 | 0.41 ± 0.02 | 75.82 ± 0.48 | - | 2.97 ± 0.10 | [1,11,23] |
| 11 | Vanillin | 17.78 | 5.68 ± 0.10 | 2.85 ± 0.08 | - | 2.70 ± 0.08 | [1,23] |
| 12 | p-Coumaric acid | 20.56 | - | 120.1 ± 0.75 | - | 10.44 ± 0.12 | [1,12] |
| 13 | Taxifolin | 21.26 | - | - | - | - | |
| 14 | Ferulic acid | 22.14 | - | 25.34 ± 0.25 | 0.85 ± 0.04 | 0.57 ± 0.05 | [1,11,12] |
| 15 | Coumarin | 24.49 | - | - | - | - | |
| 16 | Rutin | 25.30 | - | 85.47 ± 0.44 | 278.4 ± 1.20 | 32.87 ± 0.23 | [1,11,12] |
| 17 | Ellagic acid | 26.11 | - | - | - | - | |
| 18 | Rosmarinic acid | 26.57 | 10.22 ± 0.17 | 124.5 ± 0.80 | 0.98 ± 0.06 | - | [1,12,14] |
| 19 | Myricetin | 27.35 | - | - | - | - | |
| 20 | Quercetin | 30.83 | 7.19 ± 0.21 | 9.73 ± 0.35 | - | - | [1,23] |
| 21 | trans-Cinnamic acid | 31.33 | - | - | - | - | |
| 22 | Luteolin | 31.70 | 3.42 ± 0.25 | 133.6 ± 0.70 | - | - | [1,11,14] |
| 23 | Hesperetin | 32.14 | - | - | - | - | |
| 24 | Kaempferol | 33.21 | - | - | - | - | |
| 25 | Apigenin | 33.77 | 84.75 ± 0.60 | 156.8 ± 0.95 | - | - | [1,12,23] |
| 26 | Chrysin | 38.40 | - | - | - | - |
| Sample | β-Carotene Assay | DPPH• Assay | ABTS•+ Assay | CUPRAC Assay | Metal Chelating Assay | H2O2 Assay |
|---|---|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | A0.50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | |
| DCME | 51.30 ± 0.98 | 7.16 ± 0.80 | 62.50 ± 0.94 | 8.36 ± 0.25 | 5.83 ± 1.20 | 53.60 ± 0.36 |
| EAE | 7.44 ± 0.28 | 17.37 ± 0.45 | 9.27 ± 0.33 | 21.70 ± 0.51 | 19.48 ± 0.88 | 49.20 ± 0.21 |
| BE | 20.86 ± 0.84 | 39.51 ± 0.96 | 28.19 ± 0.57 | 34.81 ± 0.65 | 51.65 ± 1.14 | 66.60 ± 0.39 |
| AQE | 92.48 ± 0.76 | 128.30 ± 1.30 | 87.40 ± 1.12 | 83.53 ± 0.55 | 102.90 ± 0.98 | 71.00 ± 0.82 |
| α-Tocopherol | 2.10 ± 0.05 | 38.20 ± 0.50 | 35.50 ± 0.55 | 60.20 ± 0.45 | NT | NT |
| BHA | 1.50 ± 0.03 | 19.50 ± 0.30 | 12.70 ± 0.10 | 25.40 ± 0.38 | NT | NT |
| EDTA | NT | NT | NT | NT | 5.50 ± 0.35 | NT |
| Ascorbic acid | NT | NT | NT | NT | NT | 28.70 ± 0.36 |
| Cholinesterase Inhibitory Activity | Antidiabetic Activity | |||
|---|---|---|---|---|
| AChE | BChE | α-Glucosidase | α-Amylase | |
| Sample | Inhibition (%) (at 200 µg/mL) | |||
| DCME | 32.75 ± 0.48 | 41.25 ± 0.69 | 55.18 ± 0.26 | 32.45 ± 0.43 |
| EAE | 36.11 ± 0.51 | 54.50 ± 0.74 | 41.09 ± 0.85 | 29.87 ± 0.67 |
| BE | 18.71 ± 0.45 | 24.80 ± 0.61 | 32.73 ± 0.90 | 25.51 ± 0.82 |
| AQE | 10.42 ± 0.33 | 21.60 ± 0.78 | 21.44 ± 0.72 | 14.08 ± 0.26 |
| Galantamine | 88.70 ± 0.50 | 80.20 ± 0.30 | NT | NT |
| Acarbose | NT | NT | 86.51 ± 0.45 | 81.33 ± 0.90 |
| Sample | MIC (mg/mL) | Violacein Inhibition (%) | |||||
|---|---|---|---|---|---|---|---|
| MIC | MIC/2 | MIC/4 | MIC/8 | MIC/16 | MIC/32 | ||
| DCME | 0.625 | 100 ± 0.0 | 78.5 ± 1.9 | 49.4 ± 1.5 | 31.0 ± 0.7 | 16.1 ± 0.2 | - |
| EAE | 0.625 | 100 ± 0.0 | 61.9 ± 0.6 | 29.9 ± 1.0 | 13.0 ± 0.5 | - | - |
| BE | 2.5 | 100 ± 0.0 | 40.7 ± 0.8 | 19.8 ± 0.4 | - | - | - |
| AQE | 1.25 | 100 ± 0.0 | 61.9 ± 1.3 | 61.9 ± 1.1 | 24.9 ± 0.5 | 11.7 ± 0.3 | - |
| Antiquorum-Sensing Inhibition Zones (mm) | |||||
|---|---|---|---|---|---|
| Sample | MIC (mg/mL) | MIC | MIC/2 | MIC/4 | MIC/8 |
| DCME | 1.25 | 14.0 ± 1.5 | 10.0 ± 1.0 | - | - |
| EAE | 1.25 | 13.0 ± 0.8 | 09.5 ± 0.2 | - | - |
| BE | 0.625 | 14.5 ± 0.6 | 10.0 ± 0.1 | - | - |
| AQE | 0.625 | 17.0 ± 1.2 | 15.0 ± 0.9 | 11.5 ± 0.4 | 09.0 ± 0.5 |
| Sample | 100 µg/mL | 75 µg/mL | 50 µg/mL |
|---|---|---|---|
| DCME | 18.4 ± 0.6 | 05.5 ± 0.2 | - |
| EAE | 34.9 ± 1.5 | 16.3 ± 0.7 | 06.2 ± 0.1 |
| BE | 39.5 ± 0.3 | 23.0 ± 0.6 | 10.5 ± 0.3 |
| AQE | 20.1 ± 0.5 | 03.8 ± 0.1 | - |
| Microorganisms | Sample Codes | ||||
|---|---|---|---|---|---|
| DCME | EAE | BE | AQE | ||
| MIC (mg/mL) | |||||
| S. aureus | 1.25 | 1.25 | 0.625 | 0.312 | |
| E. coli | 1.25 | 2.5 | 2.5 | 1.25 | |
| C. albicans | 0.625 | 1.25 | 0.625 | 0.625 | |
| Biofilm inhibition (%) | |||||
| S. aureus | MIC | 71.25 ± 1.78 | 55.65 ± 0.59 | 76.14 ± 1.95 | 26.36 ± 0.27 |
| MIC/2 | 42.38 ± 0.96 | 23.82 ± 0.35 | 45.24 ± 0.85 | 8.29 ± 0.06 | |
| MIC/4 | 16.92 ± 0.56 | 5.87 ± 0.18 | 27.32 ± 0.66 | - | |
| MIC/8 | - | - | 10.22 ± 0.15 | - | |
| E. coli | MIC | 60.16 ± 1.24 | 56.65 ± 1.05 | 46.13 ± 0.78 | 51.11 ± 0.64 |
| MIC/2 | 26.63 ± 0.76 | 30.14 ± 0.65 | 21.34 ± 0.25 | 29.21 ± 0.44 | |
| MIC/4 | 13.15 ± 0.28 | 18.62 ± 0.44 | 6.18 ± 0.10 | 17.33 ± 0.15 | |
| MIC/8 | - | 06.21 ± 0.32 | - | 05.27 ± 0.1 | |
| C. albicans | MIC | - | - | - | 11.26 ± 0.13 |
| MIC/2 | - | - | - | 02.44 ± 0.05 | |
| MIC/4 | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haouam, C.; Boudiba, S.; Tamfu, A.N.; Kucukaydin, S.; Hanini, K.; Zohra, H.F.; Hioun, S.; Botezatu, A.D.; Ceylan, Ö.; Boudiba, L.; et al. Assessment of Chemical Composition and In Vitro Antioxidant, Antidiabetic, Anticholinesterase and Microbial Virulence-Quenching Effects of Salad Burnet (Sanguisorba minor L.) Harvested from Algeria. Plants 2023, 12, 4134. https://doi.org/10.3390/plants12244134
Haouam C, Boudiba S, Tamfu AN, Kucukaydin S, Hanini K, Zohra HF, Hioun S, Botezatu AD, Ceylan Ö, Boudiba L, et al. Assessment of Chemical Composition and In Vitro Antioxidant, Antidiabetic, Anticholinesterase and Microbial Virulence-Quenching Effects of Salad Burnet (Sanguisorba minor L.) Harvested from Algeria. Plants. 2023; 12(24):4134. https://doi.org/10.3390/plants12244134
Chicago/Turabian StyleHaouam, Chahrazed, Sameh Boudiba, Alfred Ngenge Tamfu, Selcuk Kucukaydin, Karima Hanini, Haouaouchi Fatma Zohra, Soraya Hioun, Andreea Dediu Botezatu, Özgür Ceylan, Louiza Boudiba, and et al. 2023. "Assessment of Chemical Composition and In Vitro Antioxidant, Antidiabetic, Anticholinesterase and Microbial Virulence-Quenching Effects of Salad Burnet (Sanguisorba minor L.) Harvested from Algeria" Plants 12, no. 24: 4134. https://doi.org/10.3390/plants12244134
APA StyleHaouam, C., Boudiba, S., Tamfu, A. N., Kucukaydin, S., Hanini, K., Zohra, H. F., Hioun, S., Botezatu, A. D., Ceylan, Ö., Boudiba, L., Duru, M. E., & Dinica, R. M. (2023). Assessment of Chemical Composition and In Vitro Antioxidant, Antidiabetic, Anticholinesterase and Microbial Virulence-Quenching Effects of Salad Burnet (Sanguisorba minor L.) Harvested from Algeria. Plants, 12(24), 4134. https://doi.org/10.3390/plants12244134









