Nano-Priming for Inducing Salinity Tolerance, Disease Resistance, Yield Attributes, and Alleviating Heavy Metal Toxicity in Plants
Abstract
:1. Introduction
2. Nanomaterials Used in Seed Priming
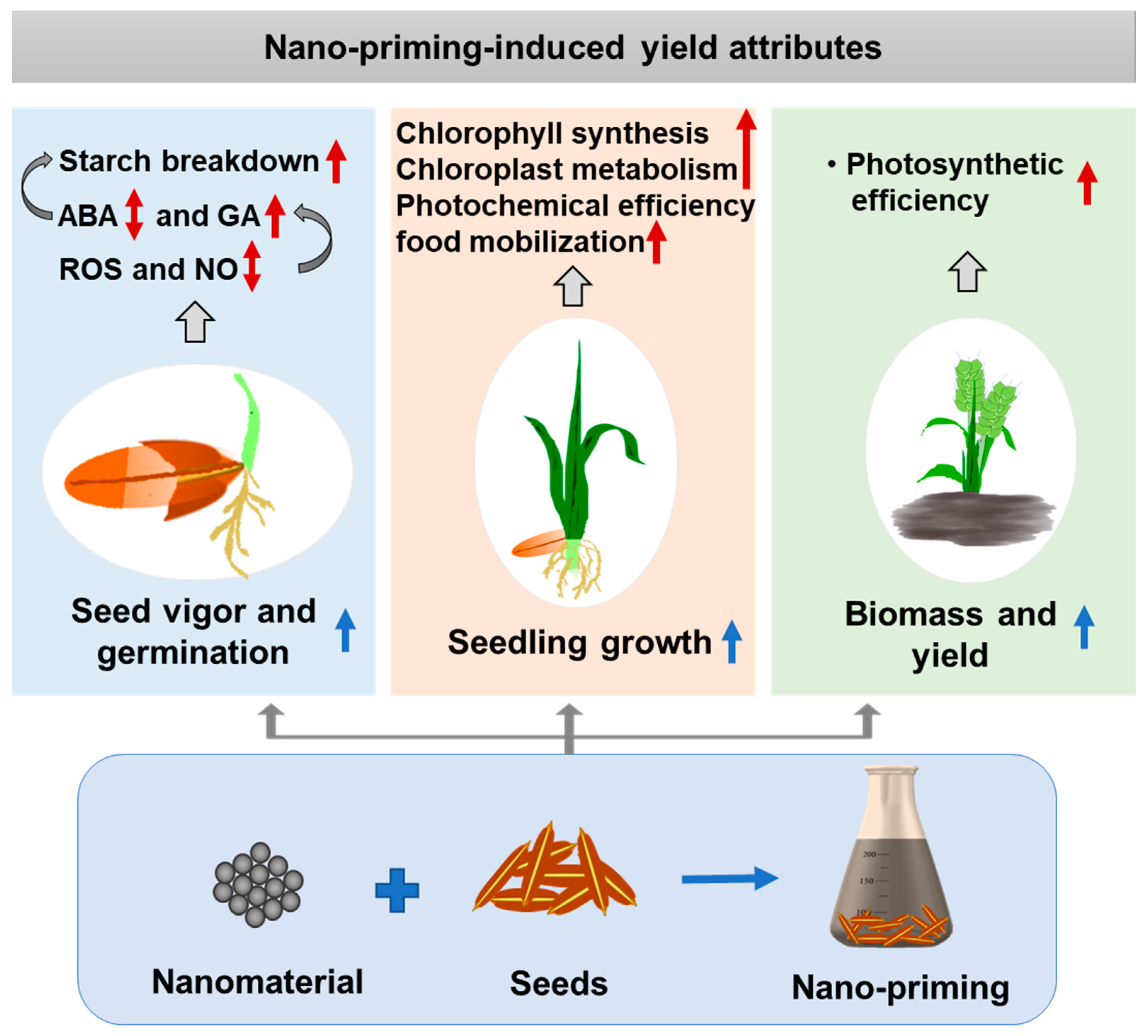
3. Nano-Priming to Induce Yield Attributes
3.1. Nano-Priming to Boost Seed Vigor, Germination, and Early Growth
3.2. Nano-Priming to Induce Photosynthetic Output, Biomass, and Yield
4. Nano-Priming to Mount Physiological Responses
4.1. Nano-Priming to Modulate Hormonal Responses
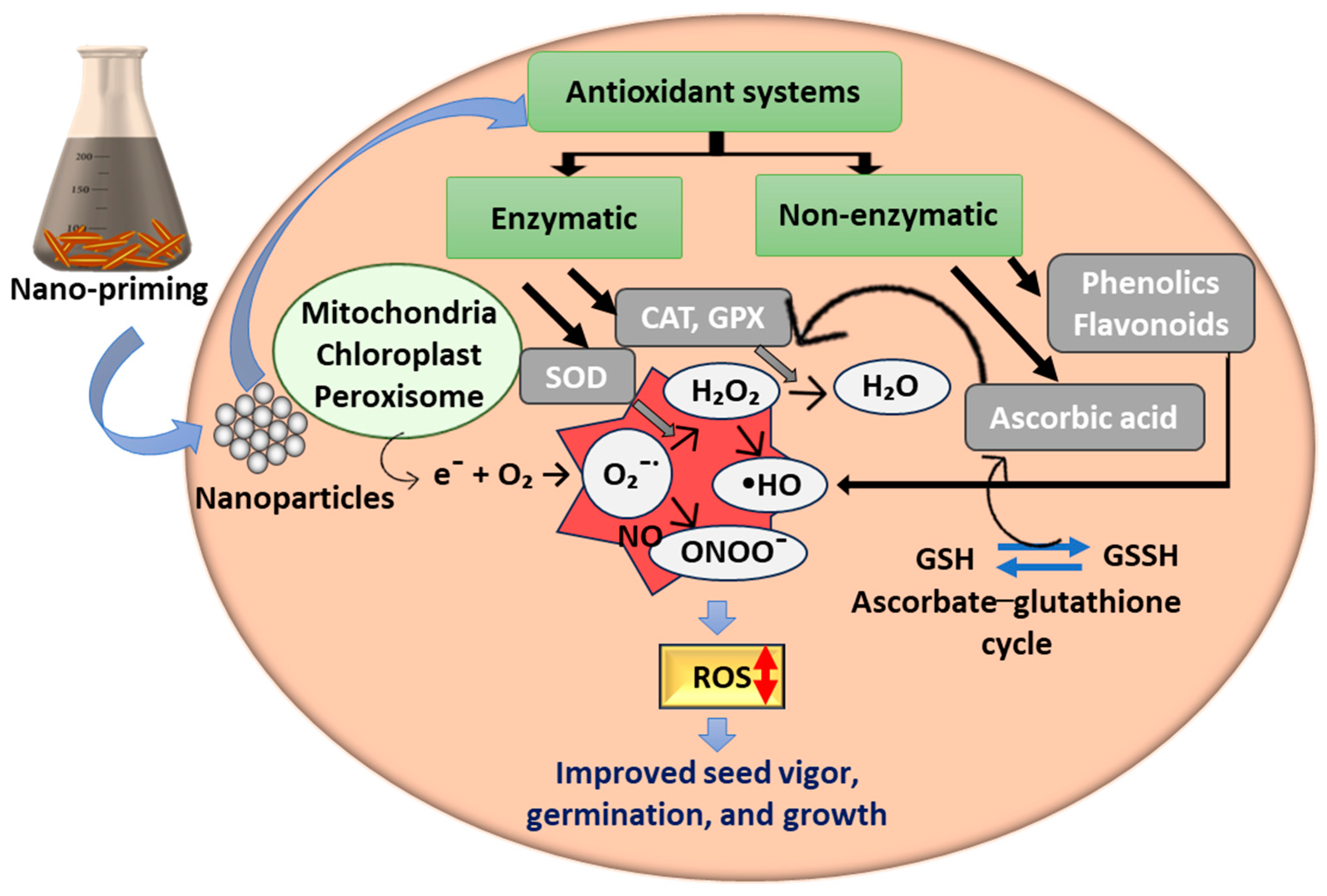
4.2. Nano-Priming to Modulate Plant Antioxidant Defense
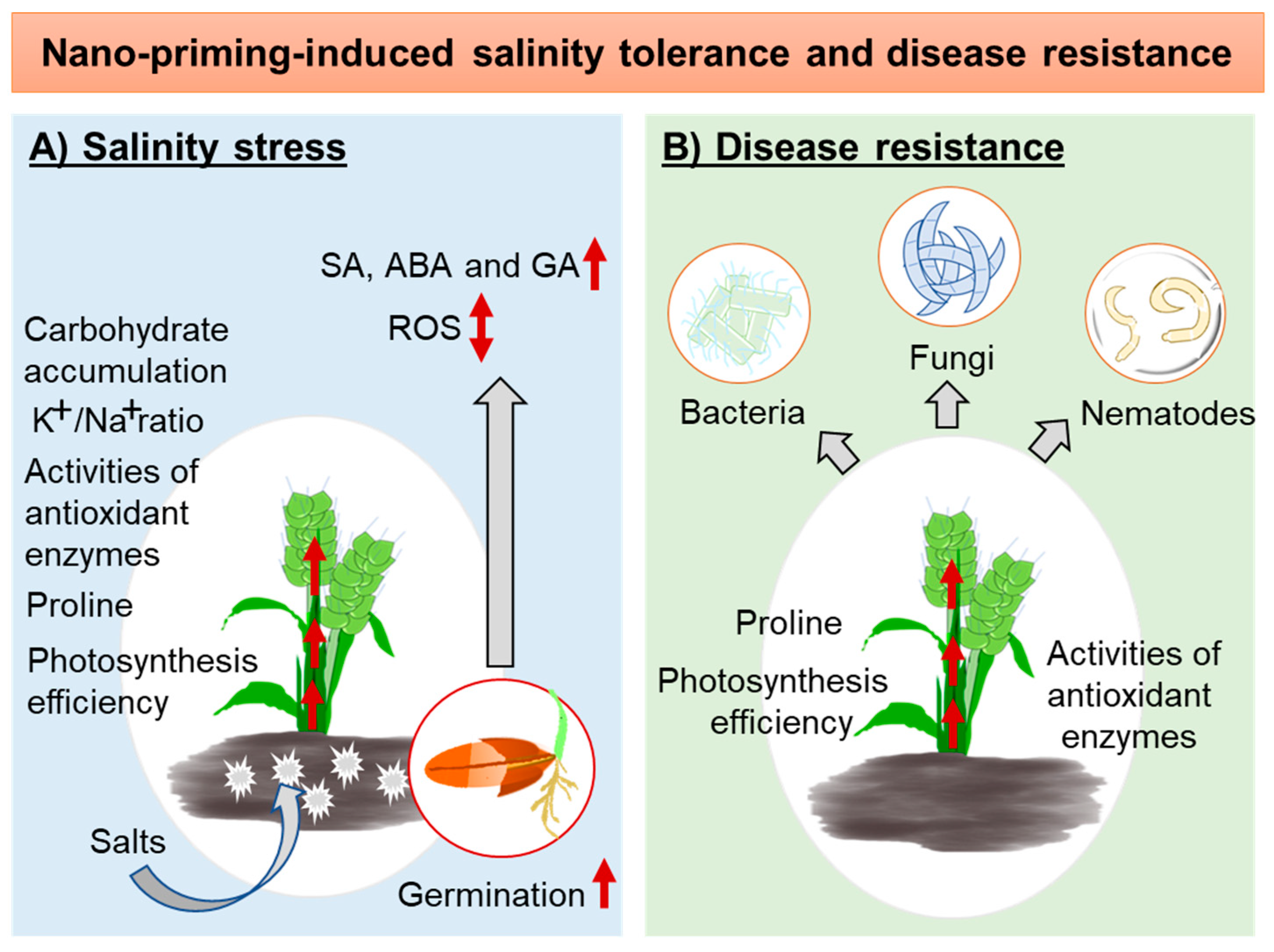
4.3. Nano-Priming to Develop a Tolerance against Salinity Stress
4.4. Nano-Priming to Mount Disease Resistance
5. Nano-Priming to Alleviate the Heavy Metals Associated with Soil Pollution
6. Nano-Priming for Micronutrients Biofortification
7. Nano-Priming in the Context of Phytotoxicity and Environment Safety
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K. Climate-smart agriculture for food security. Nat. Clim. Chang. 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- Haris, M.; Hussain, T.; Mohamed, H.I.; Khan, A.; Ansari, M.S.; Tauseef, A.; Khan, A.A.; Akhtar, N. Nanotechnology–A new frontier of nano-farming in agricultural and food production and its development. Sci. Total Environ. 2023, 857, 159639. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K. Nanotechnology in agri-food sector. Crit. Rev. Food Sci. Nutr. 2014, 54, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 20. [Google Scholar] [CrossRef]
- Trujillo-Reyes, J.; Peralta-Videa, J.; Gardea-Torresdey, J. Supported and unsupported nanomaterials for water and soil remediation: Are they a useful solution for worldwide pollution? J. Hazard. Mater. 2014, 280, 487–503. [Google Scholar] [CrossRef]
- Wen, L.X.; Li, Z.Z.; Zou, H.K.; Liu, A.Q.; Chen, J.F. Controlled release of avermectin from porous hollow silica nanoparticles. Pest Manag. Sci. Former. Pestic. Sci. 2005, 61, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, Z.; Prud’homme, R.K. Stabilized polymeric nanoparticles for controlled and efficient release of bifenthrin. Pest Manag. Sci. Former. Pestic. Sci. 2008, 64, 808–812. [Google Scholar] [CrossRef]
- Rexlin, J.; Vijayakumar, S.; Nilavukkarasi, M.; Vidhya, E.; Alharthi, N.S.; Sajjad, M.; Punitha, V.; Praseetha, P. Bioengineered ZnO nanoparticles as a nano priming agent in Cyamopsis tetragonoloba (L). Taub. to improve yield and disease resistance. Appl. Nanosci. 2022, 13, 5993–6001. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-mediated seed priming improves germination, growth, yield, and quality of watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef]
- Kasote, D.M.; Lee, J.H.; Jayaprakasha, G.; Patil, B.S. Seed priming with iron oxide nanoparticles modulate antioxidant potential and defense-linked hormones in watermelon seedlings. ACS Sustain. Chem. Eng. 2019, 7, 5142–5151. [Google Scholar] [CrossRef]
- Raj, A.B.; Raj, S.K. Seed priming: An approach towards agricultural sustainability. J. Appl. Nat. Sci. 2019, 11, 227–234. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Kasote, D.M.; Lee, J.H.; Jayaprakasha, G.K.; Patil, B.S. Manganese oxide nanoparticles as safer seed priming agent to improve chlorophyll and antioxidant profiles in watermelon seedlings. Nanomaterials 2021, 11, 1016. [Google Scholar] [CrossRef]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Del Buono, D.; Luzi, F.; Puglia, D. Lignin nanoparticles: A promising tool to improve maize physiological, biochemical, and chemical traits. Nanomaterials 2021, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanoparticle Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Esper Neto, M.; Britt, D.W.; Jackson, K.A.; Coneglian, C.F.; Cordioli, V.R.; Braccini, A.L.; Inoue, T.T.; Batista, M.A. Assessments in early growth of corn seedlings after hausmanite (Mn3O4) nanoscale seed priming. J. Plant Nutr. 2021, 44, 1611–1620. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; GonzáLez-GarcíA, Y.; Cadenas-Pliego, G.; Olivares-SáEnz, E.; Trejo-TéLlez, L.I.; Benavides-Mendoza, A. Seed priming with ZnO nanoparticles promotes early growth and bioactive compounds of Moringa oleifera. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12546. [Google Scholar] [CrossRef]
- Sharma, P.; Urfan, M.; Anand, R.; Sangral, M.; Hakla, H.R.; Sharma, S.; Das, R.; Pal, S.; Bhagat, M. Green synthesis of zinc oxide nanoparticles using Eucalyptus lanceolata leaf litter: Characterization, antimicrobial and agricultural efficacy in maize. Physiol. Mol. Biol. Plants 2022, 28, 363–381. [Google Scholar] [CrossRef]
- Adhikary, S.; Biswas, B.; Chakraborty, D.; Timsina, J.; Pal, S.; Chandra Tarafdar, J.; Banerjee, S.; Hossain, A.; Roy, S. Seed priming with selenium and zinc nanoparticles modifies germination, growth, and yield of direct-seeded rice (Oryza sativa L.). Sci. Rep. 2022, 12, 7103. [Google Scholar] [CrossRef]
- Afzal, S.; Sharma, D.; Singh, N.K. Eco-friendly synthesis of phytochemical-capped iron oxide nanoparticles as nano-priming agent for boosting seed germination in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2021, 28, 40275–40287. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Green-synthesized nanoparticles enhanced seedling growth, yield, and quality of onion (Allium cepa L.). ACS Sustain. Chem. Eng. 2019, 7, 14580–14590. [Google Scholar] [CrossRef]
- Pawar, V.; Ambekar, J.; Kale, B.; Apte, S.; Laware, S. Response in chickpea (Cicer arietinum L.) seedling growth to seed priming with iron oxide nanoparticles. Int. J. Biosci. 2019, 14, 82–91. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, X.; Zhang, Z.; Chen, K.; Wang, L.; Chen, H.; Yang, Z.; Li, C.; Zhao, L. AgNPs seed priming accelerated germination speed and altered nutritional profile of Chinese cabbage. Sci. Total Environ. 2022, 808, 151896. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Qu, G.; Li, S.; Song, K.; Zhao, D.; Li, X.; Yang, P.; He, X.; Hu, T. Iron nanoparticles induced the growth and physio-chemical changes in Kobresia capillifolia seedlings. Plant Physiol. Biochem. 2023, 194, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Li, Y.; Fu, C.; Hu, J.; Chen, L.; Yan, J.; Khan, Z.; Wu, H.; Li, Z. CeO2 nanoparticles seed priming increases salicylic acid level and ROS scavenging ability to improve rapeseed salt tolerance. Glob. Chall. 2022, 6, 2200025. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.G.; Pelegrino, M.T.; Ferreira, A.S.; Bazzo, J.H.; Zucareli, C.; Seabra, A.B.; Oliveira, H.C. Seed priming with copper-loaded chitosan nanoparticles promotes early growth and enzymatic antioxidant defense of maize (Zea mays L.) seedlings. J. Chem. Technol. Biotechnol. 2021, 96, 2176–2184. [Google Scholar] [CrossRef]
- Guha, T.; Ravikumar, K.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Thuesombat, P.; Hannongbua, S.; Ekgasit, S.; Chadchawan, S. Effects of silver nanoparticles on hydrogen peroxide generation and antioxidant enzyme responses in rice. J. Nanosci. Nanotechnol. 2016, 16, 8030–8043. [Google Scholar] [CrossRef]
- Sharma, A.B.; Sidhu, A.; Manchanda, P.; Ahuja, R. 1, 2, 4-triazolyldithiocarbamate silver nano conjugate: Potent seed priming agent against bakanae disease of rice (Oryzae sativa). Eur. J. Plant Pathol. 2022, 162, 825–841. [Google Scholar] [CrossRef]
- Joshi, S.M.; De Britto, S.; Jogaiah, S. Myco-engineered selenium nanoparticles elicit resistance against tomato late blight disease by regulating differential expression of cellular, biochemical and defense responsive genes. J. Biotechnol. 2021, 325, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Siddiqui, Z.A. Use of silicon dioxide nanoparticles for the management of Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solani disease complex of beetroot (Beta vulgaris L.). Sci. Hortic. 2020, 265, 109211. [Google Scholar] [CrossRef]
- Farooq, T.; Nisa, Z.U.; Hameed, A.; Ahmed, T.; Hameed, A. Priming with copper-chitosan nanoparticles elicit tolerance against PEG-induced hyperosmotic stress and salinity in wheat. BMC Chem. 2022, 16, 23. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Raza, M.A.; Awan, S.A.; Shah, G.A.; Rizwan, M.; Ali, B.; Tariq, R.; Hassan, M.J.; Alyemeni, M.N.; Brestic, M. Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): The oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem. 2020, 156, 221–232. [Google Scholar] [CrossRef]
- Mosavikia, A.A.; Mosavi, S.G.; Seghatoleslami, M.; Baradaran, R. Chitosan nanoparticle and pyridoxine seed priming improves tolerance to salinity in milk thistle seedling. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 221–233. [Google Scholar] [CrossRef]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.; Kumar, A. Seed priming with iron oxide nanoparticles triggers iron acquisition and biofortification in wheat (Triticum aestivum L.) grains. J. Plant Growth Regul. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- Hatami, M.; Khanizadeh, P.; Bovand, F.; Aghaee, A. Silicon nanoparticle-mediated seed priming and Pseudomonas spp. inoculation augment growth, physiology and antioxidant metabolic status in Melissa officinalis L. plants. Ind. Crop. Prod. 2021, 162, 113238. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Li, W.; Ashraf, U.; Ma, L.; Tang, X.; Pan, S.; Tian, H.; Mo, Z. ZnO nanoparticle-based seed priming modulates early growth and enhances physio-biochemical and metabolic profiles of fragrant rice against cadmium toxicity. J. Nanobiotechnol. 2021, 19, 75. [Google Scholar] [CrossRef]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef] [PubMed]
- Ventura, L.; Donà, M.; Macovei, A.; Carbonera, D.; Buttafava, A.; Mondoni, A.; Rossi, G.; Balestrazzi, A. Understanding the molecular pathways associated with seed vigor. Plant Physiol. Biochem. 2012, 60, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.; Garcia, Q. Reactive oxygen species and seed germination. Biologia 2013, 68, 351–357. [Google Scholar] [CrossRef]
- Guha, T.; Gopal, G.; Das, H.; Mukherjee, A.; Kundu, R. Nanopriming with zero-valent iron synthesized using pomegranate peel waste: A “green” approach for yield enhancement in Oryza sativa L. cv. Gonindobhog. Plant Physiol. Biochem. 2021, 163, 261–275. [Google Scholar] [CrossRef] [PubMed]
- de Ribou, S.d.B.; Douam, F.; Hamant, O.; Frohlich, M.W.; Negrutiu, I. Plant science and agricultural productivity: Why are we hitting the yield ceiling? Plant Sci. 2013, 210, 159–176. [Google Scholar] [CrossRef]
- Del Buono, D.; Luzi, F.; Tolisano, C.; Puglia, D.; Di Michele, A. Synthesis of a lignin/zinc oxide hybrid nanoparticles system and its application by nano-priming in maize. Nanomaterials 2022, 12, 568. [Google Scholar] [CrossRef]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y.-G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Isayenkov, S. Physiological and molecular aspects of salt stress in plants. Cytol. Genet. 2012, 46, 302–318. [Google Scholar] [CrossRef]
- El-Serafy, R.S.; El-Sheshtawy, A.-N.A.; Atteya, A.K.; Al-Hashimi, A.; Abbasi, A.M.; Al-Ashkar, I. Seed priming with silicon as a potential to increase salt stress tolerance in Lathyrus odoratus. Plants 2021, 10, 2140. [Google Scholar] [CrossRef] [PubMed]
- Graeber, K.; Nakabayashi, K.; Miatton, E.; Leubner-Metzger, G.; Soppe, W.J. Molecular mechanisms of seed dormancy. Plant Cell Environ. 2012, 35, 1769–1786. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major biological control strategies for plant pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Worrall, E.A.; Hamid, A.; Mody, K.T.; Mitter, N.; Pappu, H.R. Nanotechnology for plant disease management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Thummar, K.A.; Trivedi, S.K.; Gajera, H.; Savaliya, D. Antioxidant defence system induced by seed priming with nanoparticles to restrain Fusarium wilt in cumin (Cuminum cyminum L.). Indian J. Agric. Biochem. 2022, 35, 27–34. [Google Scholar] [CrossRef]
- Parveen, A.; Siddiqui, Z.A. Foliar spray and seed priming of titanium dioxide nanoparticles and their impact on the growth of tomato, defense enzymes and some bacterial and fungal diseases. Arch. Phytopathol. Plant Prot. 2022, 55, 527–548. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, S.-H.; Lee, D.-G.; Lee, H.; Lee, S.W.; Bahk, J.D.; Lee, B.-H. Physiological and protein profiles alternation of germinating rice seedlings exposed to acute cadmium toxicity. Comptes Rendus Biol. 2007, 330, 735–746. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Hamurcu, M.; Gezgin, S.; Athar, T.; Rajput, V.D.; Gupta, O.P.; Minkina, T. Insight into the prospects for nanotechnology in wheat biofortification. Biology 2021, 10, 1123. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-Roche, R.; Cantu, J.; Tamez, C.; Zuverza-Mena, N.; Hamdi, H.; Adisa, I.O.; Elmer, W.; Gardea-Torresdey, J.; White, J.C. Seed biofortification by engineered nanomaterials: A pathway to alleviate malnutrition? J. Agric. Food Chem. 2020, 68, 12189–12202. [Google Scholar] [CrossRef] [PubMed]
| Sr. No. | Nanomaterials | Average Size of Nanomaterials (nm) | Seeds | Physiological/Productivity-Linked Response(s) | Effective Seed Priming Concentration and Time | Ref. |
|---|---|---|---|---|---|---|
| 1. | Mn3O4 NPs | 20 | Corn | Increased germination, vigor, dry biomass, and length | 20 mg L−1 (h) | [19] |
| 2. | ZnO NPs | 16.49 | Moringa oleifera L. | Increased early growth and bioactive compounds | 10 mg L−1 (1 h) | [20] |
| 3. | ZnO NPs | 100 | Maize | Improved seed vigor index, germinationpercentage, shoot and root length, and fresh biomass | 200 mg L−1 (24 h) | [21] |
| 4. | ZnO NPs combined with sodium selenite and sodium selenate | <10 | Direct-seeded rice | Enhanced seed vigor, metabolic profiles, nutrient uptake, growth, and yield | 10 µmoL (24 h) | [22] |
| 5. | FeO NPs | 20–50 | Rice | Improved seed germination and growth | 20 mg L−1 (24 h) | [23] |
| 6. | Ag NPs | 36.5–171.3 | Watermelon | Increased seed germination, growth, and yield | 31.3 mg L−1 (12 h) | [11] |
| 7. | Au NPs | 30–113 | Onion | Enhanced germination, growth, and yield | 5.4 mg L−1 (12 h) | [24] |
| 8. | Fe2O3 NPs | 8–10 | Chickpea | Increased seedling growth | <12 μg mL−1 (4–5 min.) | [25] |
| 9. | Ag NPs | 19.9–36.9 | Cabbage | Accelerated seed germination speed, seedling development, yield, and nutritional quality | 20 and 40 mg L−1 (15 h) | [26] |
| 10. | Fe2O3 NPs | 12–50 | Kobresia capillifolia (Decne.) C.B.Clarke | Increased rubisco activity and photosynthetic rate | 10–100 mg L−1 (12 h) | [27] |
| 11. | Mn2O3 NPs | 22–39 | Watermelon | Modulated chlorophyll and antioxidant profiles | 20 mg L−1 (14 h) | [15] |
| 12. | Fe2O3 NPs | 19–30 | Watermelon | Modified the jasmonic acid and 12-oxo phytodienoic acid levels | 20–160 mg L−1 (14 h) | [12] |
| 13. | Polyacrylic acid-coated CeO2 NPs | 9.2 | Rapeseed | Increased salicylic acid level and ROS scavenging ability | 0.1 mM (8 h) | [28] |
| 14. | Chitosan nanoparticles containing Cu | 174.2 | Maize | Promoted early growth and enzymatic antioxidant defense | 0.0625 mmol L−1 | [29] |
| 15. | Nanoscale zerovalent Fe | 33.8 | Rice | Regulated intracellular ROS levels, increased activity of hydrolytic, dehydrogenase, and antioxidant enzymes | 20 mg L−1 (30 min.) | [30] |
| 16. | Ag NPs | 150 | Rice | Enhanced hydrogen peroxide generation and antioxidant enzymes | 10 mg L−1 (24 h) | [31] |
| 17. | 1,2,4-triazolyldithiocarbamate conjugated Ag NPs | 45.48 | Rice | Showed activity against Fusarium fujikuroi | 100 mg L−1 (8 h) | [32] |
| 18. | Se NPs | 60.48–123.16 | Tomato | Elicited resistance against tomato late blight disease | 100 mg L−1 (4 h) | [33] |
| 19. | SiO2 NPs | 5–15 | Beta vulgaris L. (beetroot) | Control Meloidogyne incognita, Pectobacterium betavasculorum, and Rhizoctonia solani disease complex of beetroot | 200 mg L−1 (12 h) | [34] |
| 20. | Cu–chitosan NPs | 19–21 | Wheat | Mitigated hyperosmotic stress and salinity | 0.12% and 0.16% (8 h) | [35] |
| 21. | Se and ZnO NPs | 10–55 and ~20 | Brassica napus L. (rapeseed) | Modulated the expression of ABA and GA genes during the germination stage; induced salinity tolerance by reducing the oxidative damage | 150 µmol L−1 of Se-NPs and 100 mg L−1 of ZnO-NPs (8 h) | [36] |
| 22. | Ag NPs | 50–100 | Pennisetum glaucum L. (pearl millet) | Enhanced salinity tolerance by reducing oxidative damage, reducing Na+ uptake, and maintaining the Na+/K+ ratio | 20 mM (20 h) | [37] |
| 23. | Chitosan nanoparticle | - | Silybum marianum (L.) Gaertn. (milk thistle) | Enhanced salt stress by increasing photosynthetic pigment synthesis, antioxidant enzyme activity, and free proline content | 0.25% (6 h) | [38] |
| 24. | Fe2O3 NPs | 80 | Wheat | Biofortified iron | 25 mg L−1 (12 h) | [39] |
| 25. | Si NPs and Pseudomonas putida | 20–30 | Melissa officinalis L. | Increased primary and secondary metabolites | 100 mg L−1 (24 h) | [40] |
| 26 | ZnO NPs | 30 | Rice | Promoted early growth and resilience against cadmium toxicity | 50 and 100 mg L−1 (20 h) | [41] |
| 27. | ZnO NPs | 20 | Maize | Alleviated cobalt’s toxic effect by decreasing its uptake and improved photosynthetic apparatus | 500 mg L−1 (24 h) | [42] |
| 28. | Si NPs | - | Wheat | Increased growth chlorophyll contents, activity of enzymatic antioxidants, diminished oxidative stress, and reduced Cd contents | 1200 mg L−1 (20 h) | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.J.; Kasote, D.M. Nano-Priming for Inducing Salinity Tolerance, Disease Resistance, Yield Attributes, and Alleviating Heavy Metal Toxicity in Plants. Plants 2024, 13, 446. https://doi.org/10.3390/plants13030446
Lee JHJ, Kasote DM. Nano-Priming for Inducing Salinity Tolerance, Disease Resistance, Yield Attributes, and Alleviating Heavy Metal Toxicity in Plants. Plants. 2024; 13(3):446. https://doi.org/10.3390/plants13030446
Chicago/Turabian StyleLee, Jisun H. J., and Deepak M. Kasote. 2024. "Nano-Priming for Inducing Salinity Tolerance, Disease Resistance, Yield Attributes, and Alleviating Heavy Metal Toxicity in Plants" Plants 13, no. 3: 446. https://doi.org/10.3390/plants13030446
APA StyleLee, J. H. J., & Kasote, D. M. (2024). Nano-Priming for Inducing Salinity Tolerance, Disease Resistance, Yield Attributes, and Alleviating Heavy Metal Toxicity in Plants. Plants, 13(3), 446. https://doi.org/10.3390/plants13030446








