Reddening of the Unicellular Green Alga Euglena gracilis by Dried Bonito Stock and Intense Red Light Irradiation
Abstract
1. Introduction
2. Results
2.1. Exploration of Conditions for Cell Reddening
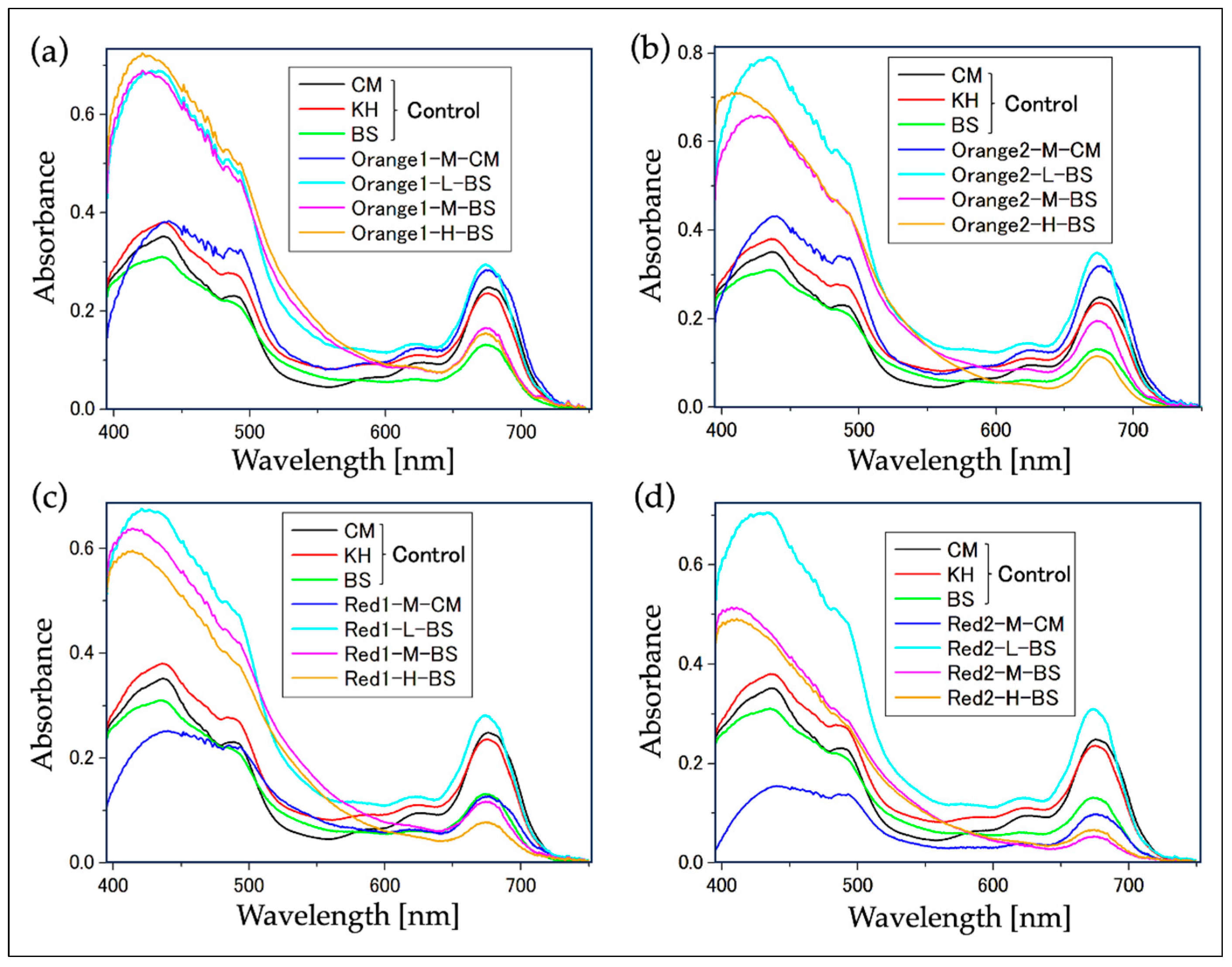
2.2. Confirmation of Reddening through Absorption Spectra
2.2.1. Absorption Spectrum of Cell Suspensions
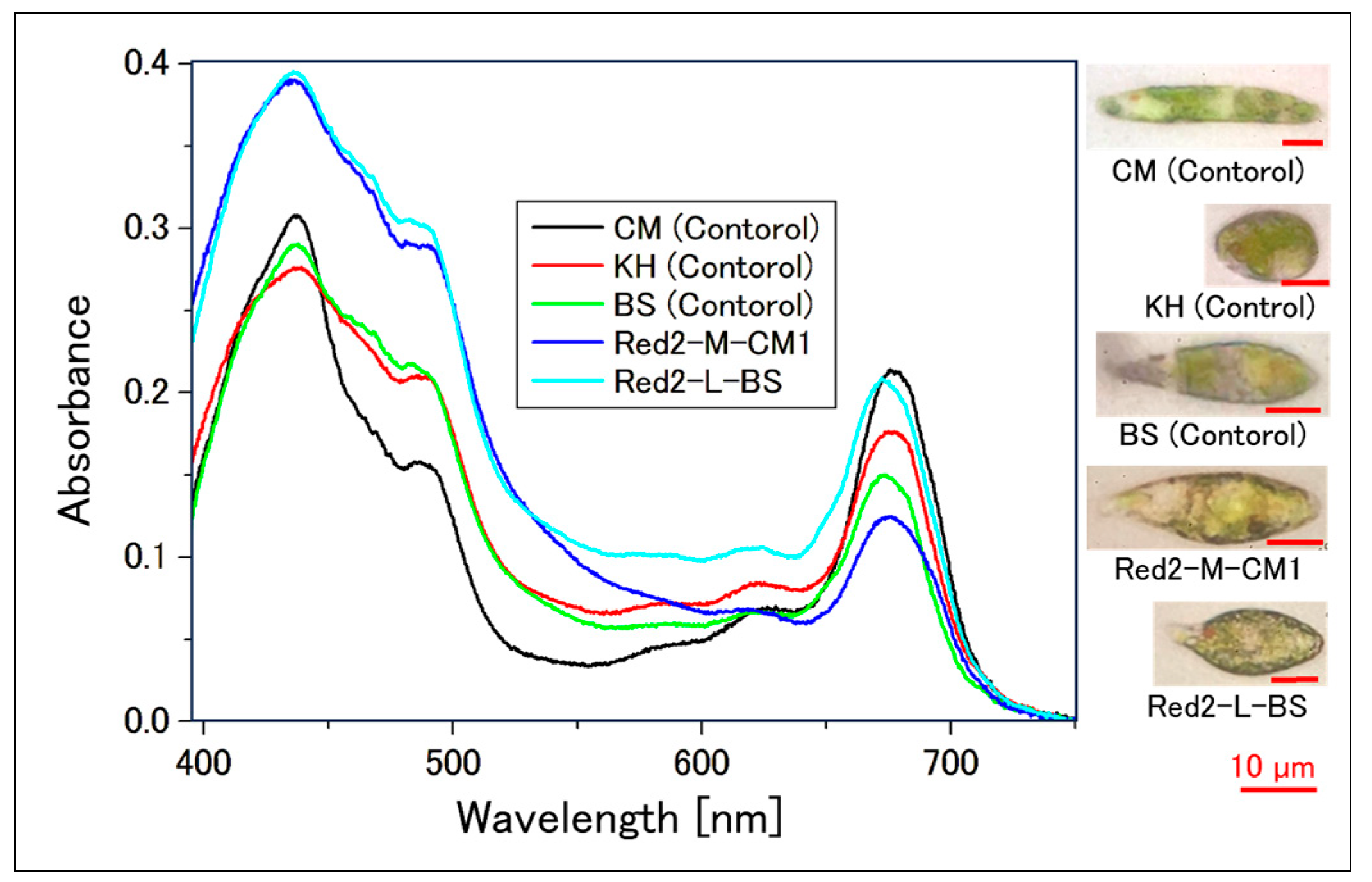
2.2.2. Absorption Spectrum of a Single Cell
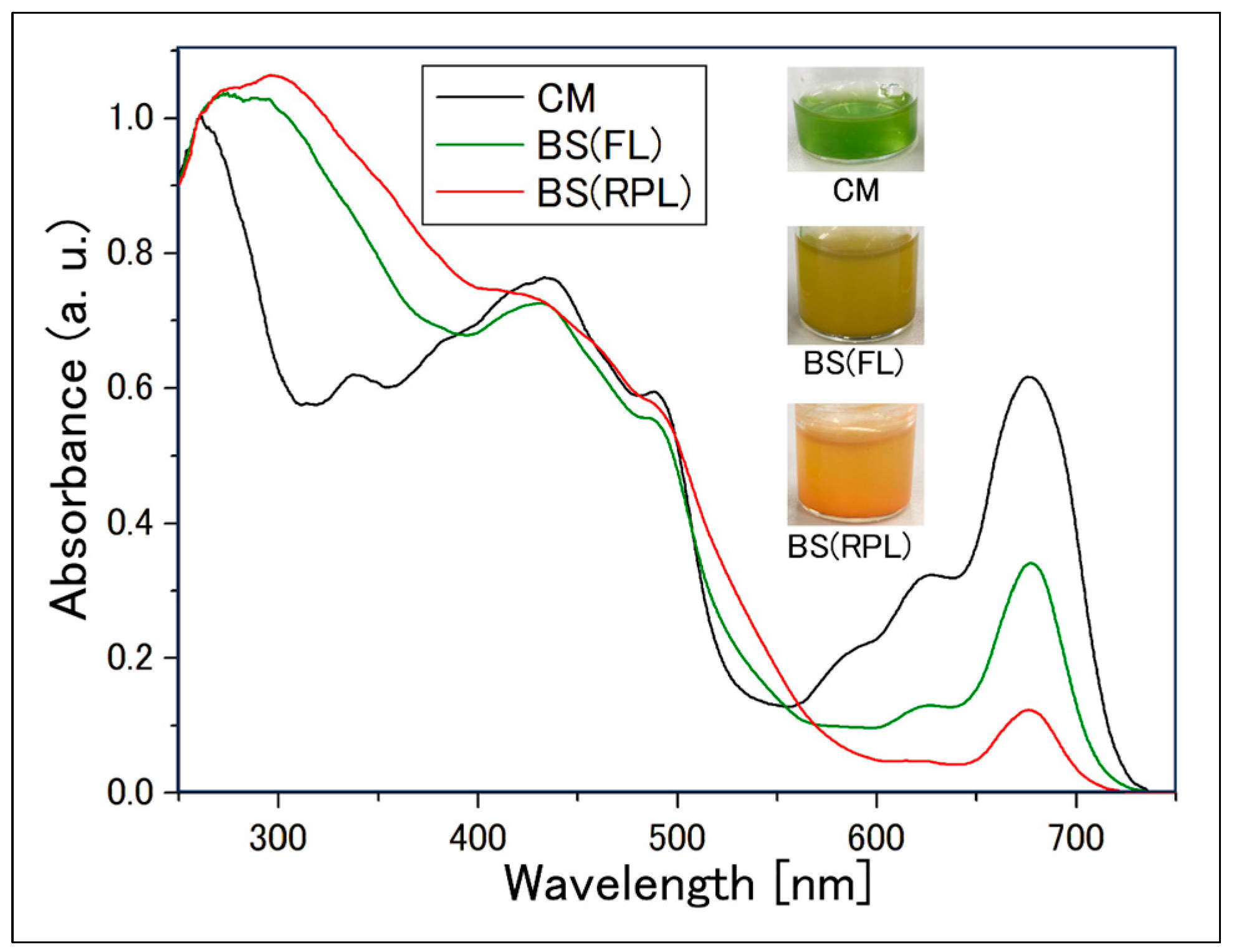
2.2.3. Measurement of Absorption Spectrum of Cell Suspensions Using an Integrating Sphere
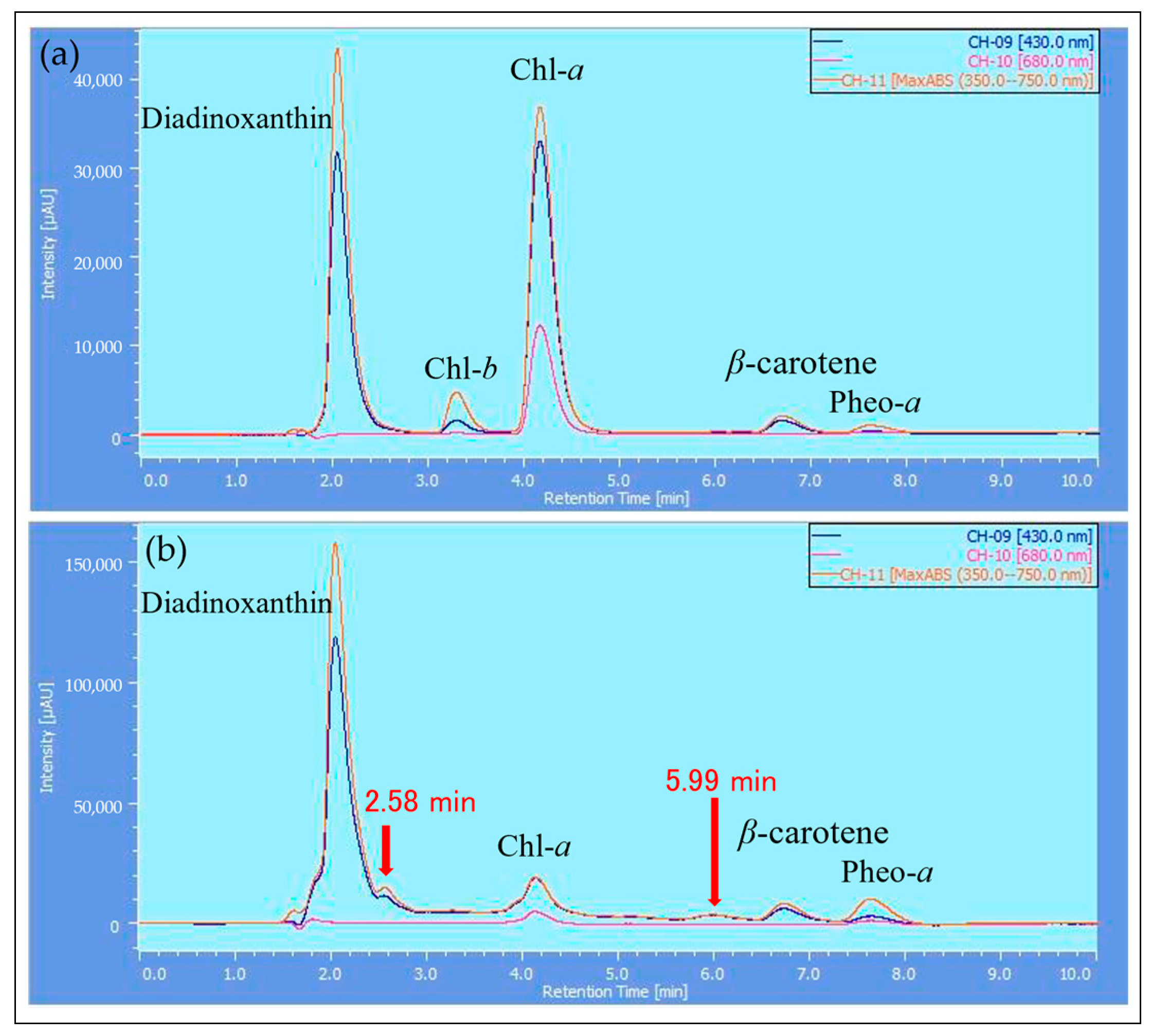
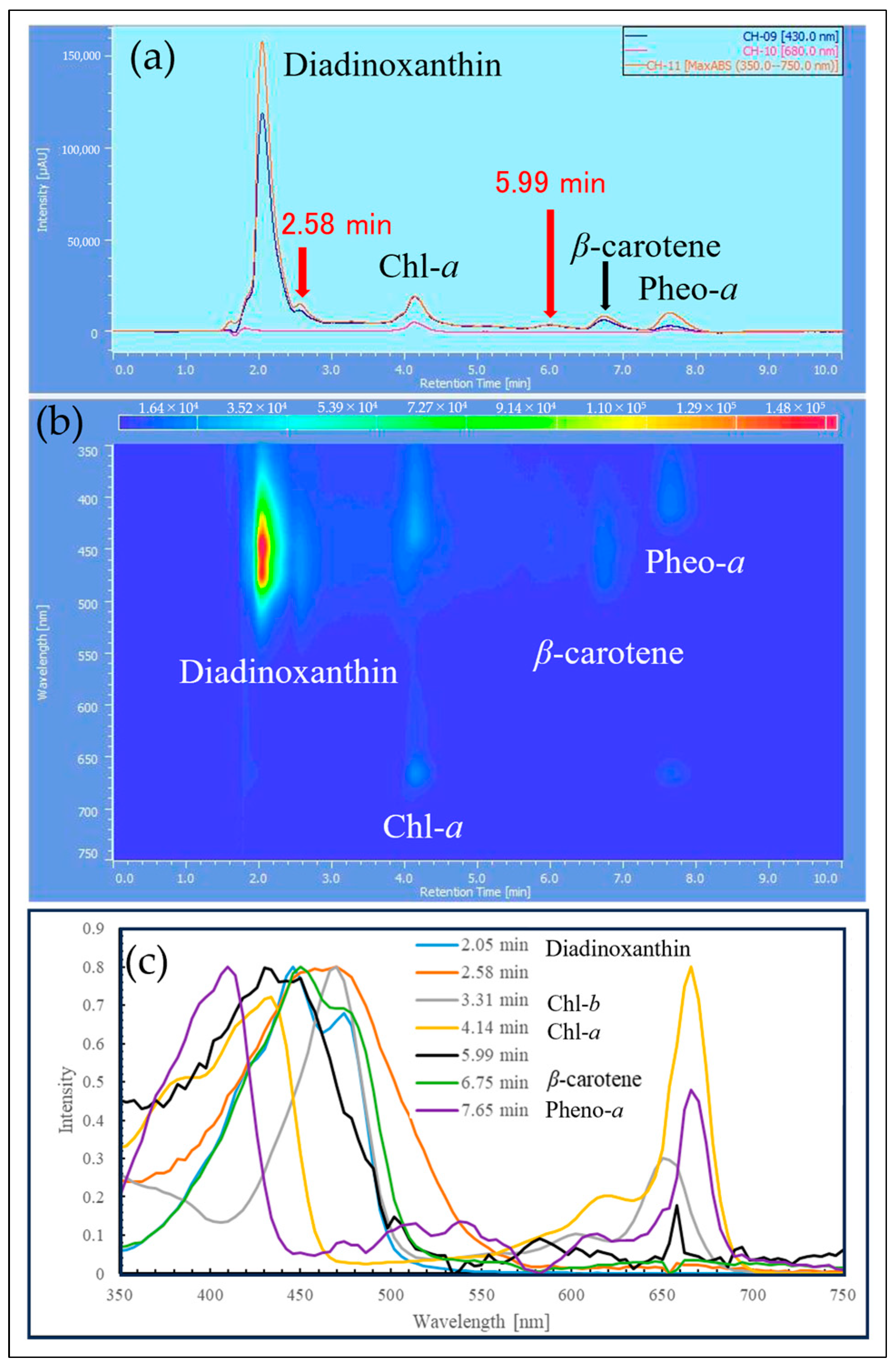
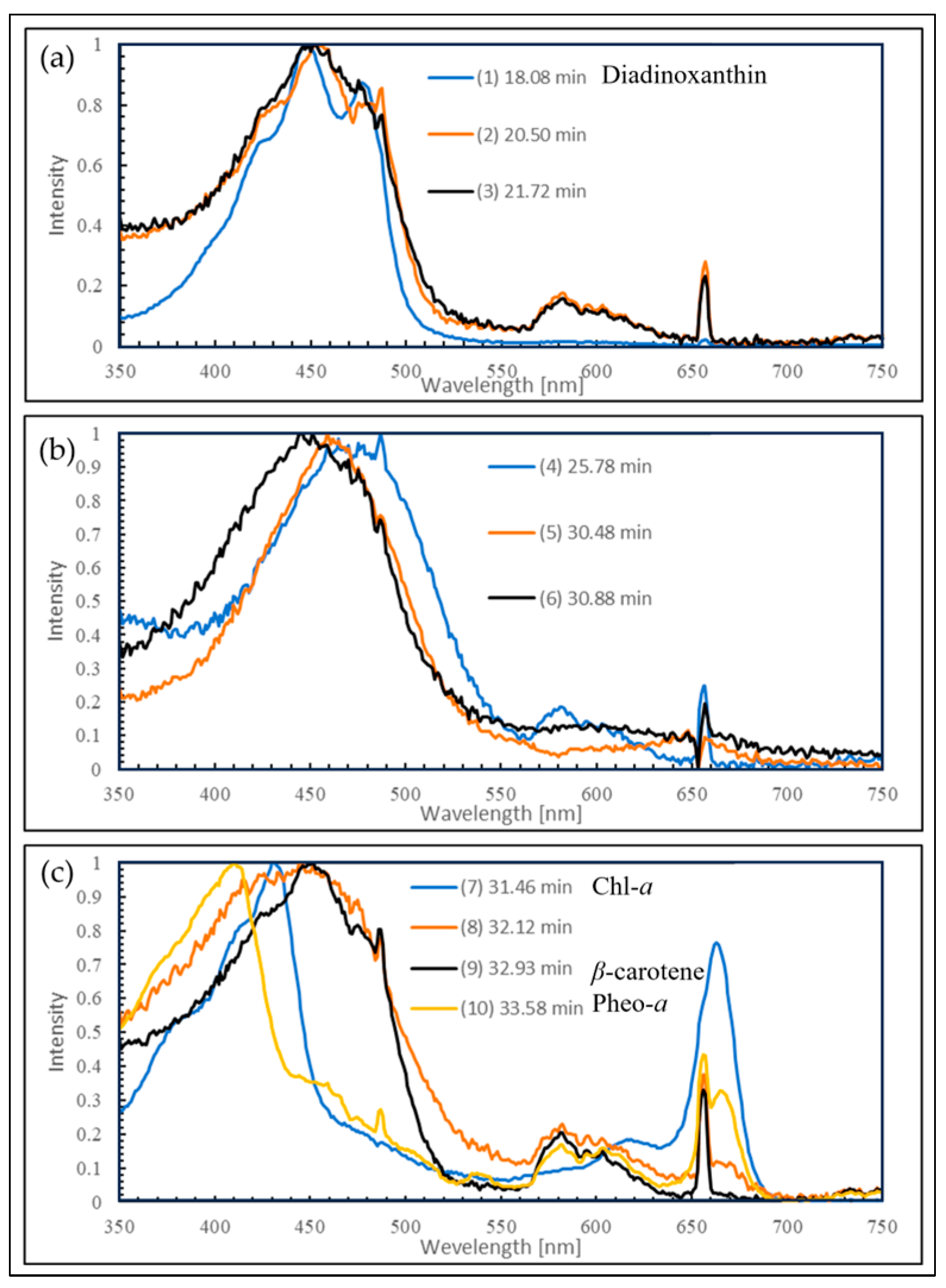
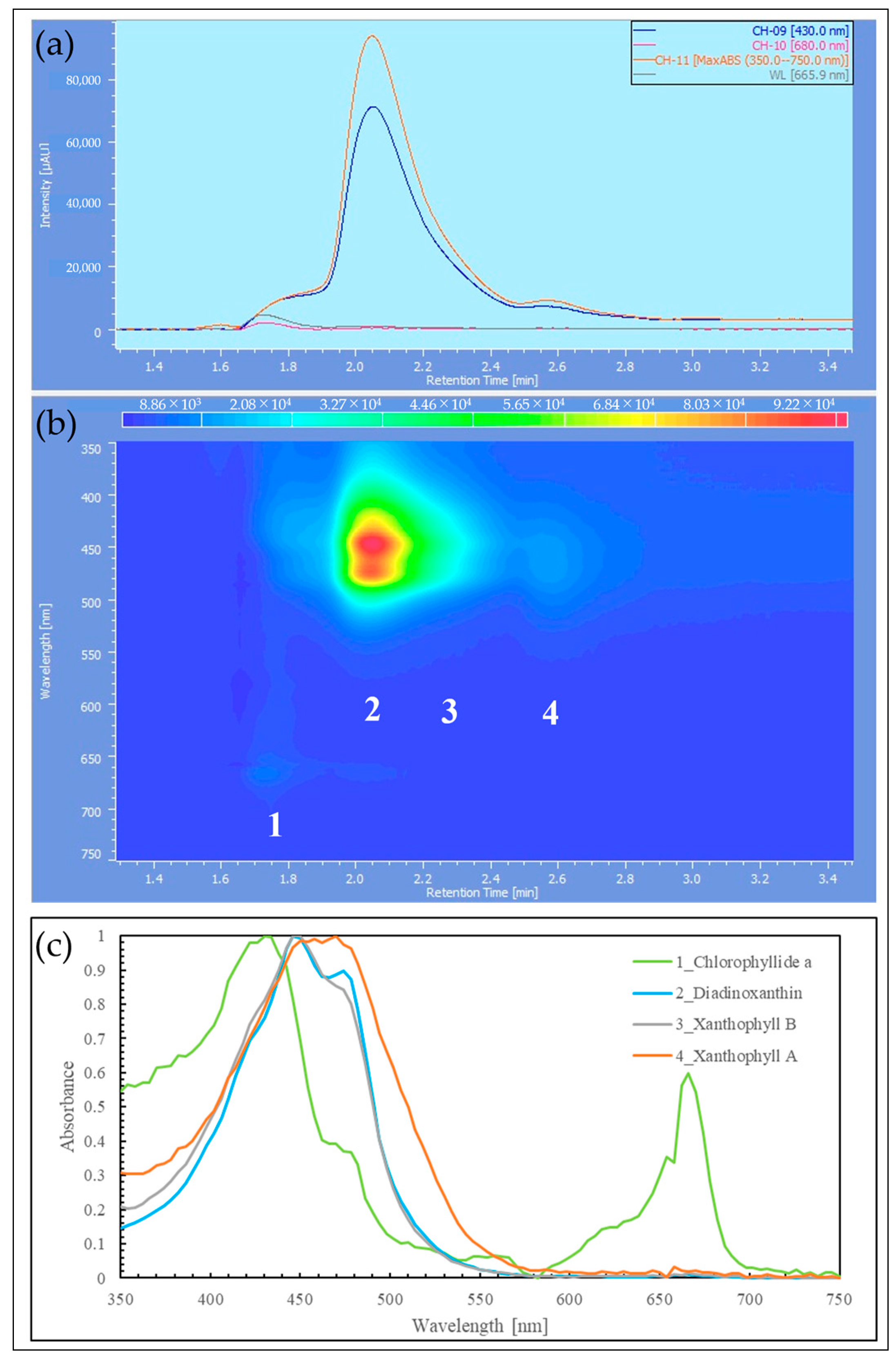
2.3. HPLC Analysis of Reddened Cells
3. Discussion
4. Materials and Methods
4.1. E. gracilis
4.2. Cell Counting
4.3. For Exploring Cell Reddening Conditions (Influence of Wavelength and Light Intensity of LEDs and Culture Medium)
4.3.1. Preparation of Bonito Stock Culture Medium
4.3.2. Growth Curve in Bonito Stock Culture Medium
4.3.3. Confirmation of the Effect of Light Source Wavelength, Light Intensity, and Culture Medium on Cell Reddening
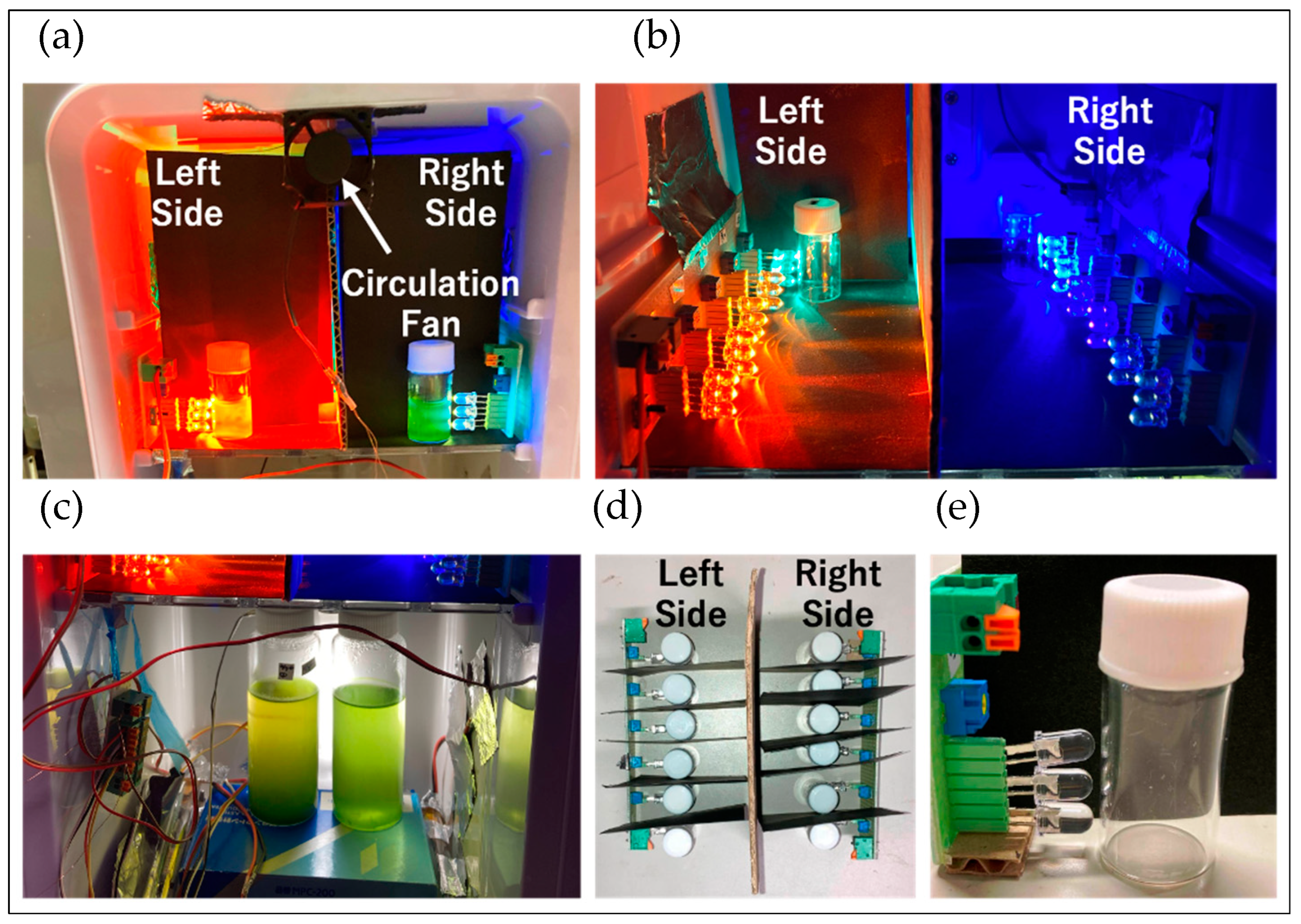
4.3.4. Confirmation of Reddening through Absorbance Measurement
Cultivation of Control Sample
Measurement of Absorption Spectra of Cell Suspensions and Individual Cells by Scan-Free Absorbance Spectral Imaging
4.4. Measurement of Absorption Spectrum of Cell Suspensions Using an Integrating Sphere
4.5. HPLC Analysis of Red Substance
4.5.1. HPLC (Condition1) Analysis of Normal E. gracilis with MeOH Extraction

4.5.2. HPLC (Condition1) Analysis of Reddened E. gracilis with MeOH Extraction
4.5.3. HPLC (Condition2) Analysis of Reddened E. gracilis by Zapata’s Method
4.5.4. Comparison of HPLC Elution Profiles of Samples Extracted with Low Polarity Solvent
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Gordon, H.T.; Bauernfeind, J.C. Carotenoids as Food Colorants. Crit. Rev. Food Sci. Nutr. 1982, 18, 59–97. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial Pigments as Natural Color Sources: Current Trends and Future Perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. The Role of Carotenoids in Human Health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Food Carotenoids: Chemistry, Biology, and Technology; Wiley Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Darvin, M.E.; Lademann, J.; von Hagen, J.; Lohan, S.B.; Kolmar, H.; Meinke, M.C.; Jung, S. Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants 2022, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Carotenoids Market Size, Share, Trends, Industry Analysis 2023–2028. Available online: https://www.imarcgroup.com/carotenoids-market (accessed on 12 December 2023).
- Syun, T.; Yuki, K.; Tomoko, S. Diverse Carotenoid Functions in Photoresponse in Euglena Gracilis. Oleoscience 2023, 23, 79–86. [Google Scholar] [CrossRef]
- Wan, M.; Hou, D.; Li, Y.; Fan, J.; Huang, J.; Liang, S.; Wang, W.; Pan, R.; Wang, J.; Li, S. The Effective Photoinduction of Haematococcus Pluvialis for Accumulating Astaxanthin with Attached Cultivation. Bioresour. Technol. 2014, 163, 26–32. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.-M.; Sun, Z.; Liu, S.-F.; Qin, Z.-H.; Mou, J.-H.; Zhou, Z.-G.; Lin, C.S.K. Sustainable Lipid and Lutein Production from Chlorella Mixotrophic Fermentation by Food Waste Hydrolysate. J. Hazard. Mater. 2020, 400, 123258. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Jiang, H.; Mao, X. Biotechnology Advances in β-Carotene Production by Microorganisms. Trends Food Sci. Technol. 2021, 111, 322–332. [Google Scholar] [CrossRef]
- Xu, Y.; Harvey, P.J. Carotenoid Production by Dunaliella Salina under Red Light. Antioxidants 2019, 8, 123. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Deng, J.; Huang, J.; Chen, F. Carotenoid Production from Microalgae: Biosynthesis, Salinity Responses and Novel Biotechnologies. Mar. Drugs 2021, 19, 713. [Google Scholar] [CrossRef]
- Wolken, J.J. Euglena: An Experimental Organism for Biochemical and Biophysical Studies, 2nd ed.; Appleton-Century-Crofts: New York, NY, USA, 1967. [Google Scholar]
- Suzuki, K.; Mitra, S.; Iwata, O.; Ishikawa, T.; Kato, S.; Yamada, K. Selection and Characterization of Euglena Anabaena Var. Minor as a New Candidate Euglena Species for Industrial Application. Biosci. Biotechnol. Biochem. 2015, 79, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Kott, Y.; Wachs, A.M. Amino Acid Composition of Bulk Protein of Euglena Grown in Waste Water. Appl. Microbiol. 1964, 12, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.R.; McLaughlin, J.J.A.; Hutner, S.H.; DeAngelis, B.; Feingold, S.; Frank, O.; Baker, H. Water-Soluble Vitamins in Cells and Spent Culture Supernatants of Poteriochromonas Stipitata, Euglena Gracilis, and Tetrahymena Thermophila. Arch. Microbiol. 1981, 129, 310–313. [Google Scholar] [CrossRef]
- Evans, M.; Falcone, P.H.; Crowley, D.C.; Sulley, A.M.; Campbell, M.; Zakaria, N.; Lasrado, J.A.; Fritz, E.P.; Herrlinger, K.A. Effect of a Euglena gracilis Fermentate on Immune Function in Healthy, Active Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 2926. [Google Scholar] [CrossRef] [PubMed]
- Feuzing, F.; Mbakidi, J.P.; Marchal, L.; Bouquillon, S.; Leroy, E. A Review of Paramylon Processing Routes from Microalga Biomass to Non-Derivatized and Chemically Modified Products. Carbohydr. Polym. 2022, 288, 119181. [Google Scholar] [CrossRef]
- Kottuparambil, S.; Thankamony, R.L.; Agusti, S. Euglena as a Potential Natural Source of Value-Added Metabolites. A Review. Algal Res. 2019, 37, 154–159. [Google Scholar] [CrossRef]
- Takeyama, H.; Kanamaru, A.; Yoshino, Y.; Kakuta, H.; Kawamura, Y.; Matsunaga, T. Production of Antioxidant Vitamins, Beta-Carotene, Vitamin C, and Vitamin E, by Two-Step Culture of Euglena gracilis Z. Biotechnol. Bioeng. 1997, 53, 185–190. [Google Scholar] [CrossRef]
- Kato, S.; Soshino, M.; Takaichi, S.; Ishikawa, T.; Nagata, N.; Asahina, M.; Shinomura, T. Suppression of the Phytoene Synthase Gene (EgcrtB) Alters Carotenoid Content and Intracellular Structure of Euglena gracilis. BMC Plant Biol. 2017, 17, 125. [Google Scholar] [CrossRef]
- Doege, M.; Ohmann, E.; Tschiersch, H. Chlorophyll Fluorescence Quenching in the Alga Euglena gracilis. Photosynth. Res. 2000, 63, 159–170. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Carotenoids and Photoprotection in Plants: A Role for the Xanthophyll Zeaxanthin. Biochim. Biophys. Acta BBA-Bioenerg. 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as Membrane Stabilizers in Chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and Photosynthesis. Subcell. Biochem. 2016, 79, 111–139. [Google Scholar] [CrossRef]
- Tamaki, S.; Ozasa, K.; Nomura, T.; Ishikawa, M.; Yamada, K.; Suzuki, K.; Mochida, K. Zeaxanthin Is Required for Eyespot Formation and Phototaxis in Euglena gracilis. Plant Physiol. 2023, 191, 2414–2426. [Google Scholar] [CrossRef]
- Tamaki, S.; Mochida, K.; Suzuki, K. Diverse Biosynthetic Pathways and Protective Functions against Environmental Stress of Antioxidants in Microalgae. Plants 2021, 10, 1250. [Google Scholar] [CrossRef]
- Yao, R.; Fu, W.; Du, M.; Chen, Z.-X.; Lei, A.-P.; Wang, J.-X. Carotenoids Biosynthesis, Accumulation, and Applications of a Model Microalga Euglena gracilis. Mar. Drugs 2022, 20, 496. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Goodwin, T.W. Carotenoid Chemistry and Biochemistry, 6th ed.; Pergamon Press: Oxford, UK, 2013. [Google Scholar]
- Yamashita, K.; Yagi, T.; Isono, T.; Nishiyama, Y.; Hashimoto, M.; Yamada, K.; Suzuki, K.; Tokunaga, E. Absorbance Spectra of the Hematochrome-like Granules and Eyespot of Euglena gracilis by Scan-Free Absorbance Spectral Imaging A(x, y, λ) within the Live Cells. J. Plant Res. 2019, 132, 431–438. [Google Scholar] [CrossRef]
- Yamashita, K.; Yamada, K.; Suzuki, K.; Tokunaga, E. Method for Growing Edible Euglena gracilis in an Inexpensive Medium with Tomato Juice to a High Cell Density Equivalent to the Density in KH Medium. Sustain. Food Technol. 2023, 1, 709–721. [Google Scholar] [CrossRef]
- Koren, L.E. High-Yield Media for Photosynthesizing Euglena gracilis Z. J. Protozool. 1967, 14, 17. [Google Scholar]
- Nakano, Y. Chloroplast Replication in Euglena Gracilis Grown in Cadmium-Ion Containing Media. Agric. Biol. Chem. 1980, 44, 2733–2734. [Google Scholar] [CrossRef]
- Cramer, M.; Myers, J. Growth and Photosynthetic Characteristics of Euglena gracilis. Arch. Mikrobiol. 1952, 17, 384–402. [Google Scholar] [CrossRef]
- Kim, S.; Wirasnita, R.; Lee, D.; Yu, J.; Lee, T. Enhancement of Growth and Paramylon Production of Euglena gracilis by Upcycling of Spent Tomato Byproduct as an Alternative Medium. Appl. Sci. 2021, 11, 8182. [Google Scholar] [CrossRef]
- Šantek, B.; Felski, M.; Friehs, K.; Lotz, M.; Flaschel, E. Production of Paramylon, a β-1,3-Glucan, by Heterotrophic Cultivation of Euglena Gracilis on Potato Liquor. Eng. Life Sci. 2010, 10, 165–170. [Google Scholar] [CrossRef]
- Ivušić, F.; Šantek, B. Optimization of Complex Medium Composition for Heterotrophic Cultivation of Euglena Gracilis and Paramylon Production. Bioprocess Biosyst. Eng. 2015, 38, 1103–1112. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.; Lim, D.; Lim, S.; Park, S.; Kang, C.; Yu, J.; Lee, T. Paramylon Production from Heterotrophic Cultivation of Euglena Gracilis in Two Different Industrial Byproducts: Corn Steep Liquor and Brewer’s Spent Grain. Algal Res. 2020, 47, 101826. [Google Scholar] [CrossRef]
- Amit; Kumar Ghosh, U. Utilization of Kinnow Peel Extract with Different Wastewaters for Cultivation of Microalgae for Potential Biodiesel Production. J. Environ. Chem. Eng. 2019, 7, 103135. [Google Scholar] [CrossRef]
- Asiandu, A.P.; Nugroho, A.P.; Naser, A.S.; Sadewo, B.R.; Koerniawan, M.D.; Budiman, A.; Siregar, U.J.; Suwanti, S.; Suyono*, E.A. The Effect of Tofu Wastewater and pH on the Growth Kinetics and Biomass Composition of Euglena sp. Curr. Appl. Sci. Technol. 2023, 23, 2. [Google Scholar] [CrossRef]
- Rubiyatno; Matsui, T.; Mori, K.; Toyama, T. Alterations of Membrane. Bioresour. Technol. Rep. 2021, 15, 100735. [Google Scholar] [CrossRef]
- Maekawa, T.; Nomura, N.; Ogushi, M.; Ebara, S.; Fukui, T.; Watanabe, T. Comparative Study on the Composition of Amino Acids Contained in Bonito Bouillon and Extracts. Trace Nutr. Res. 2005, 22, 125–129. [Google Scholar] [CrossRef]
- Funatsu, S.; Kondoh, T.; Kawase, T.; Ikeda, H.; Nagasawa, M.; Denbow, D.M.; Furuse, M. Long-Term Consumption of Dried Bonito Dashi (a Traditional Japanese Fish Stock) Reduces Anxiety and Modifies Central Amino Acid Levels in Rats. Nutr. Neurosci. 2015, 18, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Matsuda, H. Identification of Antioxidant Active Compounds in Dried Bonito Stock (Katsuo-Dashi). J. Brew. Soc. Jpn. 2009, 104, 866–873. [Google Scholar] [CrossRef][Green Version]
- Kato, S.; Tanno, Y.; Takaichi, S.; Shinomura, T. Low Temperature Stress Alters the Expression of Phytoene Desaturase Genes (crtP1 and crtP2) and the ζ-Carotene Desaturase Gene (crtQ) Together with the Cellular Carotenoid Content of Euglena gracilis. Plant Cell Physiol. 2019, 60, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Isono, T.; Yamashita, K.; Momose, D.; Kobayashi, H.; Kitamura, M.; Nishiyama, Y.; Hosoya, T.; Kanda, H.; Kudo, A.; Okada, N.; et al. Scan-Free Absorbance Spectral Imaging A(x, y, λ) of Single Live Algal Cells for Quantifying Absorbance of Cell Suspensions. PLoS ONE 2015, 10, e0128002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mori, A.; Yamashita, K.; Tabata, Y.; Seto, K.; Tokunaga, E. Absorbance Spectroscopy of Light Scattering Samples Placed inside an Integrating Sphere for Wide Dynamic Range Absorbance Measurement. Rev. Sci. Instrum. 2021, 92, 123103. [Google Scholar] [CrossRef] [PubMed]
- Laza-Martínez, A.; Fernández-Marín, B.; García-Plazaola, J.I. Rapid Colour Changes in Euglena sanguinea (Euglenophyceae) Caused by Internal Lipid Globule Migration. Eur. J. Phycol. 2019, 54, 91–101. [Google Scholar] [CrossRef]
- Kulczycka, A.; Łukomska-Kowalczyk, M.; Zakryś, B.; Milanowski, R. PCR Identification of Toxic Euglenid Species Euglena Sanguinea. J. Appl. Phycol. 2018, 30, 1759–1763. [Google Scholar] [CrossRef]
- Cohoe, D.; Hanczarek, I.; Wallace, J.K.; Nadeau, J. Multiwavelength Digital Holographic Imaging and Phase Unwrapping of Protozoa Using Custom Fiji Plug-Ins. Front. Phys. 2019, 7, 94. [Google Scholar] [CrossRef]
- Pancaldi, S.; Bonora, A.; Dall’Olio, G.; Bruni, A.; Fasulo, M.P. Ageing of Euglena Chloroplasts in Vitro I. Variations in Pigment Pattern and in Morphology. J. Exp. Bot. 1996, 47, 49–60. [Google Scholar] [CrossRef]
- Cunningham, F.X.; Schiff, J.A. Chlorophyll-Protein Complexes from Euglena gracilis and Mutants Deficient in Chlorophyll b. Plant Physiol. 1986, 80, 223–230. [Google Scholar] [CrossRef]
- Matsumoto, T.; Inui, H.; Miyatake, K.; Nakano, Y.; Murakami, K. Effect of Light Quality and CO2 Concentrationon Antioxidant Vitamin Contents in Autotrophycally Cultured Euglena gracilis. Eco-Engineering 2007, 19, 33–38. [Google Scholar] [CrossRef]
- Heelis, D.V.; Kernick, W.; Phillips, G.O.; Davies, K. Separation and Identification of the Carotenoid Pigments of Stigmata Isolated from Light-Grown Cells of Euglena gracilis Strain Z. Arch. Microbiol. 1979, 121, 207–211. [Google Scholar] [CrossRef]
- Grung, M.; Kreimer, G.; Calenberg, M.; Melkonian, M.; Liaaen-Jensen, S. Carotenoids in the Eyespot Apparatus of the Flagellate Green Alga Spermatozopsis similis: Adaptation to the Retinal-Based Photoreceptor. Planta 1994, 193, 38–43. [Google Scholar] [CrossRef]
- Abomohra, A.E.-F.; Shang, H.; El-Sheekh, M.; Eladel, H.; Ebaid, R.; Wang, S.; Wang, Q. Night Illumination Using Monochromatic Light-Emitting Diodes for Enhanced Microalgal Growth and Biodiesel Production. Bioresour. Technol. 2019, 288, 121514. [Google Scholar] [CrossRef] [PubMed]
- Seasonings and Spices (Japanese Food Standard Composition Table. 2015 Edition (7th Edition): Chapter 3 Materials (1. Points to Note by Food Group)). Available online: https://www.mext.go.jp/a_menu/syokuhinseibun/1365449.htm (accessed on 13 December 2023).
- Yamashita, K.; Yamada, K.; Suzuki, K.; Tokunaga, E. Noninvasive and Safe Cell Viability Assay for Euglena Gracilis Using Natural Food Pigment. PeerJ 2019, 7, e6636. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Tokunaga, E. Noninvasive and Safe Cell Viability Assay for Paramecium Using Natural Pigment Extracted from Food. Sci. Rep. 2020, 10, 10996. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of Chlorophylls and Carotenoids from Marine Phytoplankton: A New HPLC Method Using a Reversed Phase C8 Column and Pyridine-Containing Mobile Phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]








| Sample | Incubation Period [Day] | Intensity [µmol Photons/m2/s] | Light Diameter [mm] | Exposure Time [s] |
|---|---|---|---|---|
| CM, KH, BS | 14 | 7400 | 3.0 | 0.3 |
| Orange 1, 2 | 16 | 7100 | 3.5 | 0.4 |
| Red 1, 2 | 15 | 7700 | 3.5 | 0.4 |
| LED Color | Wavelength [nm] | Intensity (Symbol) | Intensity [µmol photons/m2/s] Average (Each LED) | Culture Medium |
|---|---|---|---|---|
| Dark | - | - | 0 (-) | CM |
| - | 0 (-) | BS | ||
| Ultraviolet | 365 | - | 10 (9, 11, 10) | BS |
| 375 | - | 21 (19, 25, 20) | BS | |
| 395 | - | 290 (270, 276, 325) | BS | |
| 405 | L | 496 (470, 542, 476) | BS | |
| Blue | 475 | M | 1029 (978, 1140, 969) | CM |
| M | 1008 (912, 1074, 1037) | BS | ||
| Green | 505 | M | 1029 (1040, 1044, 1002) | CM |
| M | 1033 (1004, 1062, 1033) | BS | ||
| Yellow | 590 | M | 508 (482, 538, 503) | CM |
| M | 495 (477, 530, 478) | BS | ||
| Orange1 | 605 | M | 1027 (963, 1108, 1010) | CM |
| L | 495 (486, 510, 490) | BS | ||
| M | 1031 (987, 1054, 1051) | BS | ||
| H | 1282 (1256, 1315, 1275) | BS | ||
| Orange2 | 611 | M | 980 (964, 1036, 940) | CM |
| L | 496 (486, 511, 491) | BS | ||
| M | 1004 (980, 1043, 990) | BS | ||
| H | 1282 (1262, 1367, 1218) | BS | ||
| Red1 | 625 | M | 1012 (1036, 1035, 965) | CM |
| L | 494 (456, 510, 517) | BS | ||
| M | 1015 (999, 1020, 1025) | BS | ||
| H | 1337 (1297, 1336, 1378) | BS | ||
| Red2 | 660 | M | 1012 (1023, 1023, 990) | CM |
| L | 490 (504, 514, 451) | BS | ||
| M | 1002 (975, 1005, 1025) | BS | ||
| H | 1299 (1214, 1368, 1314) | BS | ||
| White | - | L | 514 (494, 536, 513) | CM |
| L | 502 (485, 536, 484) | BS |
| Sample | Culture Medium | Incubation Period [Day] | Light Source | Intensity [µmol Photons/m2/s] | Temperature [℃] |
|---|---|---|---|---|---|
| CM | CM | 18 | White fluorescent light | 90 | 25 |
| BS (FL) | BS* | 33 | White fluorescent light | 90 | 25 |
| BS (Red-PLED) | BS* | 33 | Red Power LED (625 nm) | 1300 | 25 |
| Min | B Solvent [%] |
|---|---|
| 0 | 0 |
| 22 | 40 |
| 28 | 95 |
| 38 | 95 |
| 40 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, K.; Hanaki, R.; Mori, A.; Suzuki, K.; Tomo, T.; Tokunaga, E. Reddening of the Unicellular Green Alga Euglena gracilis by Dried Bonito Stock and Intense Red Light Irradiation. Plants 2024, 13, 510. https://doi.org/10.3390/plants13040510
Yamashita K, Hanaki R, Mori A, Suzuki K, Tomo T, Tokunaga E. Reddening of the Unicellular Green Alga Euglena gracilis by Dried Bonito Stock and Intense Red Light Irradiation. Plants. 2024; 13(4):510. https://doi.org/10.3390/plants13040510
Chicago/Turabian StyleYamashita, Kyohei, Ryusei Hanaki, Ayaka Mori, Kengo Suzuki, Tatsuya Tomo, and Eiji Tokunaga. 2024. "Reddening of the Unicellular Green Alga Euglena gracilis by Dried Bonito Stock and Intense Red Light Irradiation" Plants 13, no. 4: 510. https://doi.org/10.3390/plants13040510
APA StyleYamashita, K., Hanaki, R., Mori, A., Suzuki, K., Tomo, T., & Tokunaga, E. (2024). Reddening of the Unicellular Green Alga Euglena gracilis by Dried Bonito Stock and Intense Red Light Irradiation. Plants, 13(4), 510. https://doi.org/10.3390/plants13040510








