An Experimental Investigation of the Precipitation Utilization of Plants in Arid Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Field

2.2. Sap Flow Observation
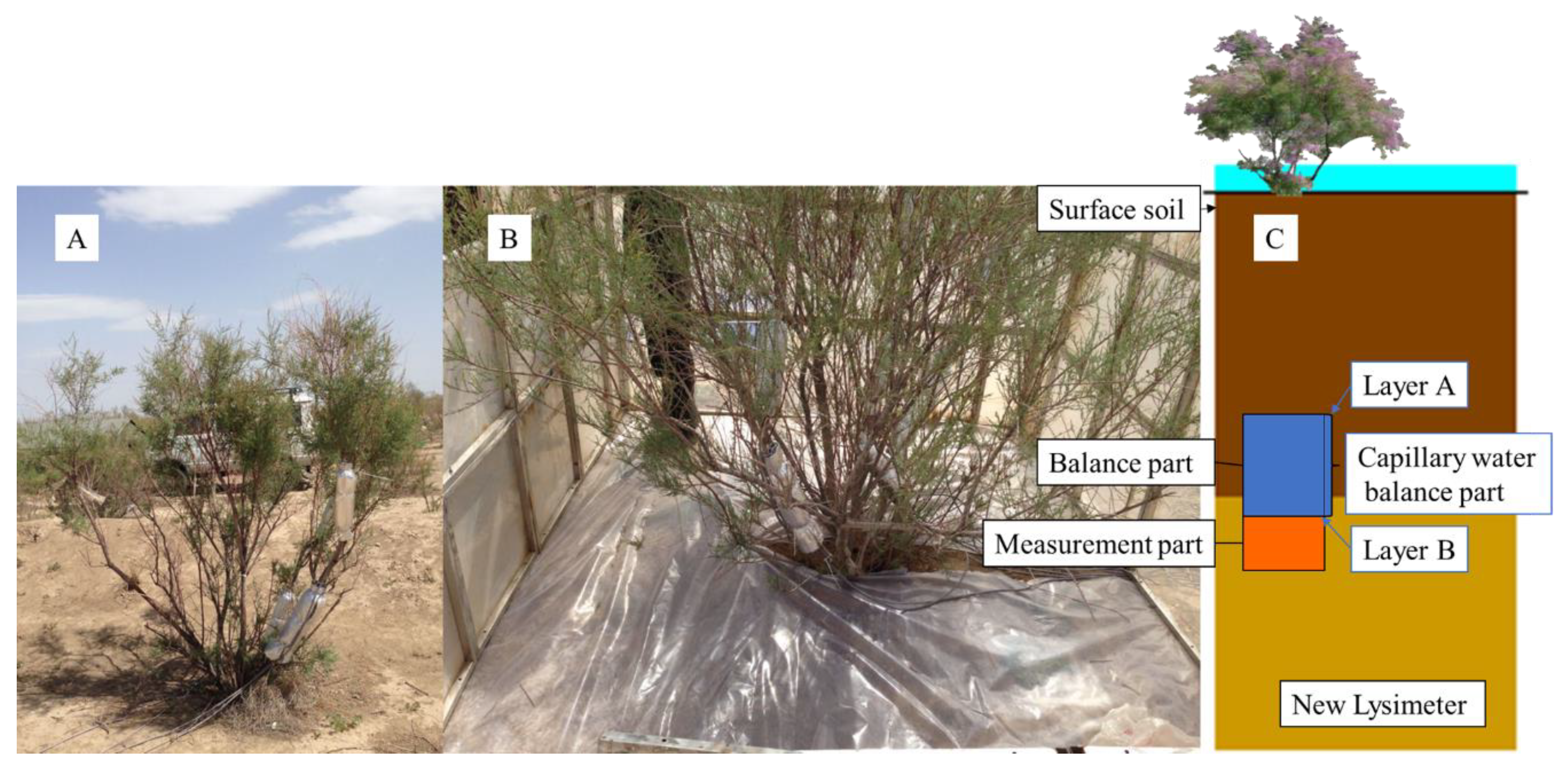
2.2.1. In Situ Observation Site
2.2.2. Determination of Leaf Absorption of Atmospheric Water
2.3. Deep-Soil Recharge Observation
2.4. Calculation of Atmospheric Water Absorption
2.4.1. Reverse Flow Measurement
2.4.2. Calculation of Leaf Water Absorption
2.5. Observation of Air Relative Humidity
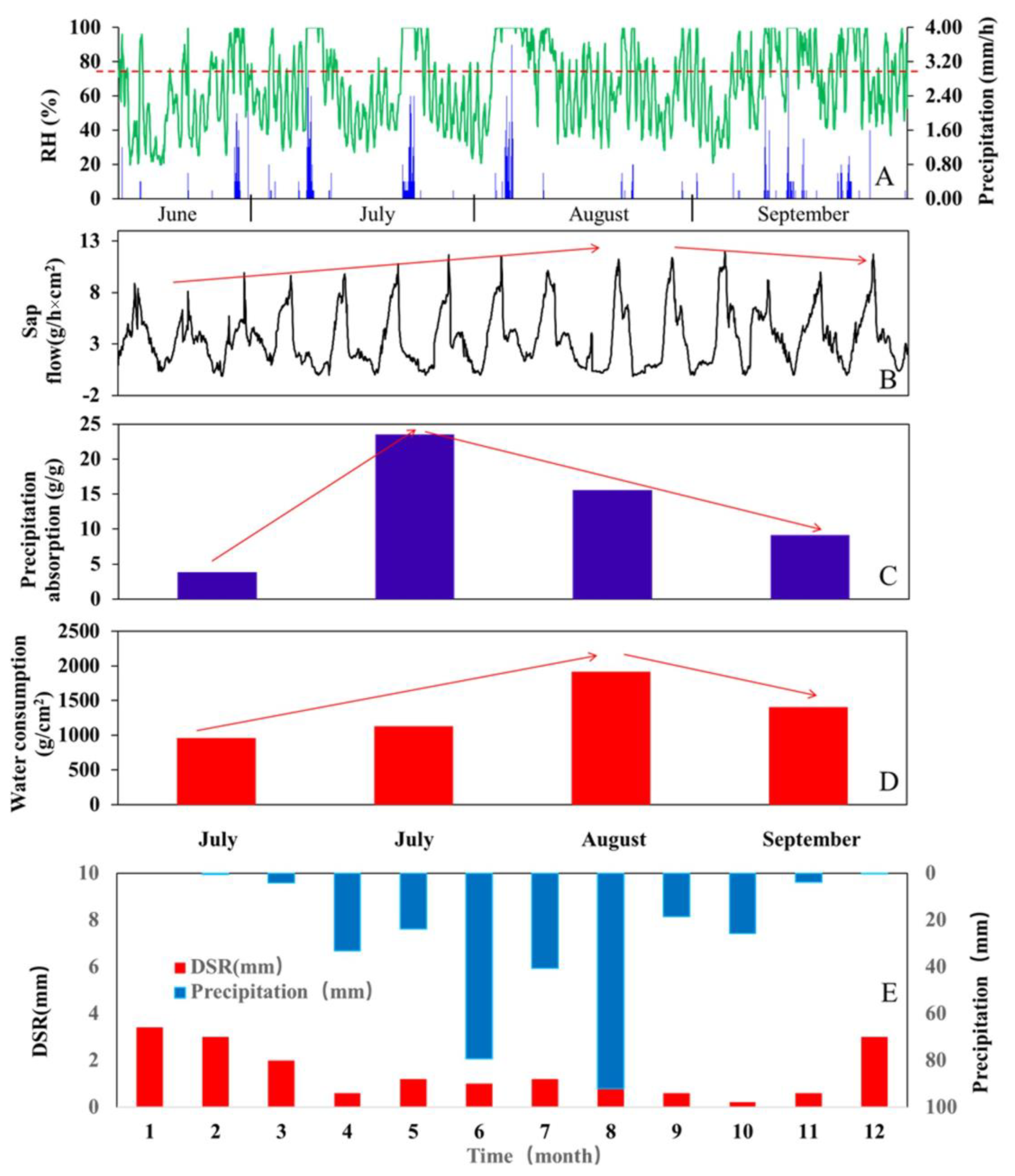
3. Results
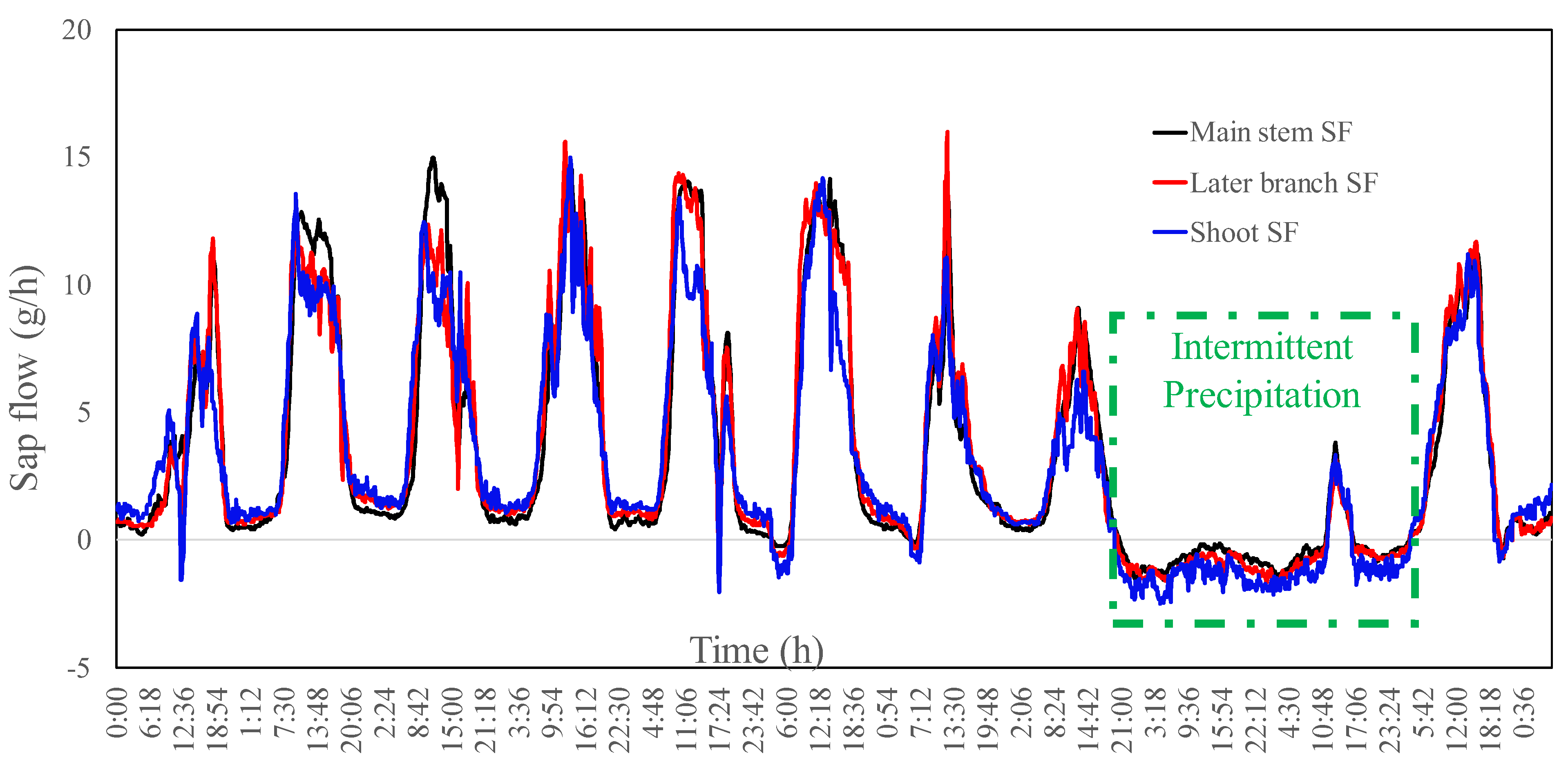
3.1. Time for Vegetation to Absorb Atmospheric Water
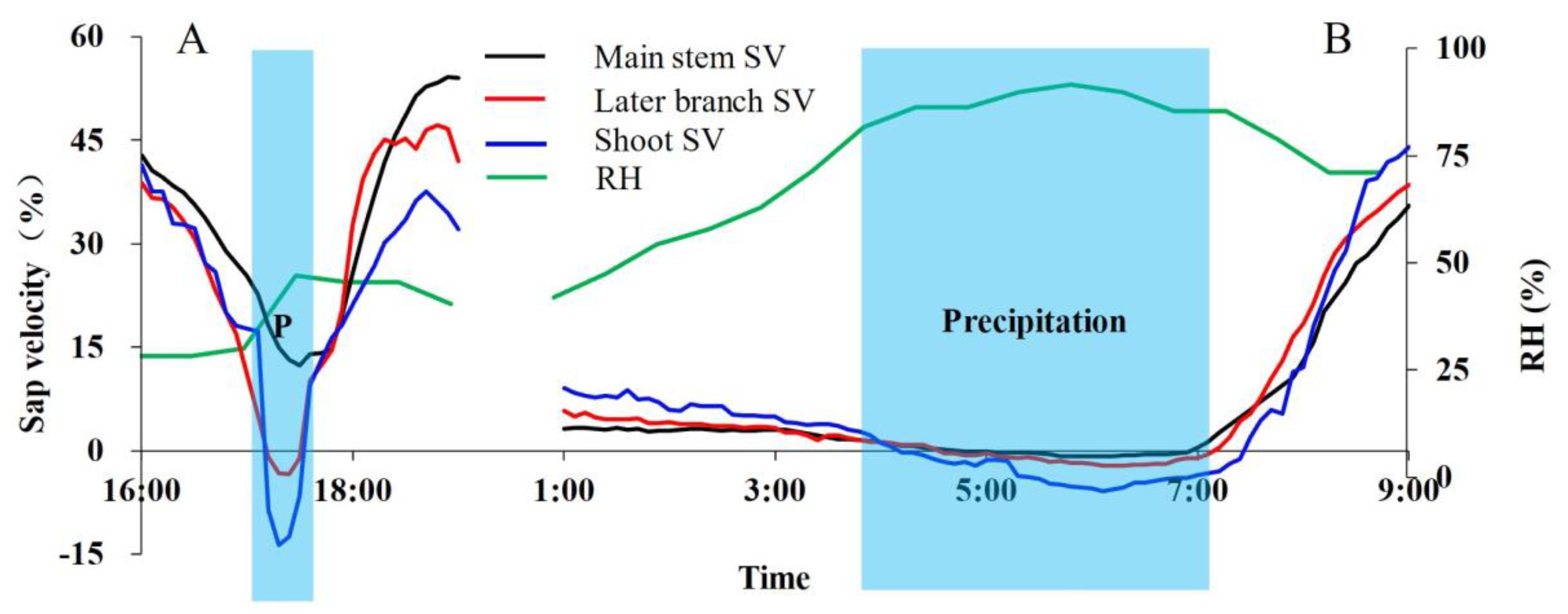
3.2. Precipitation Events That Can Cause Water Absorption in Leaves
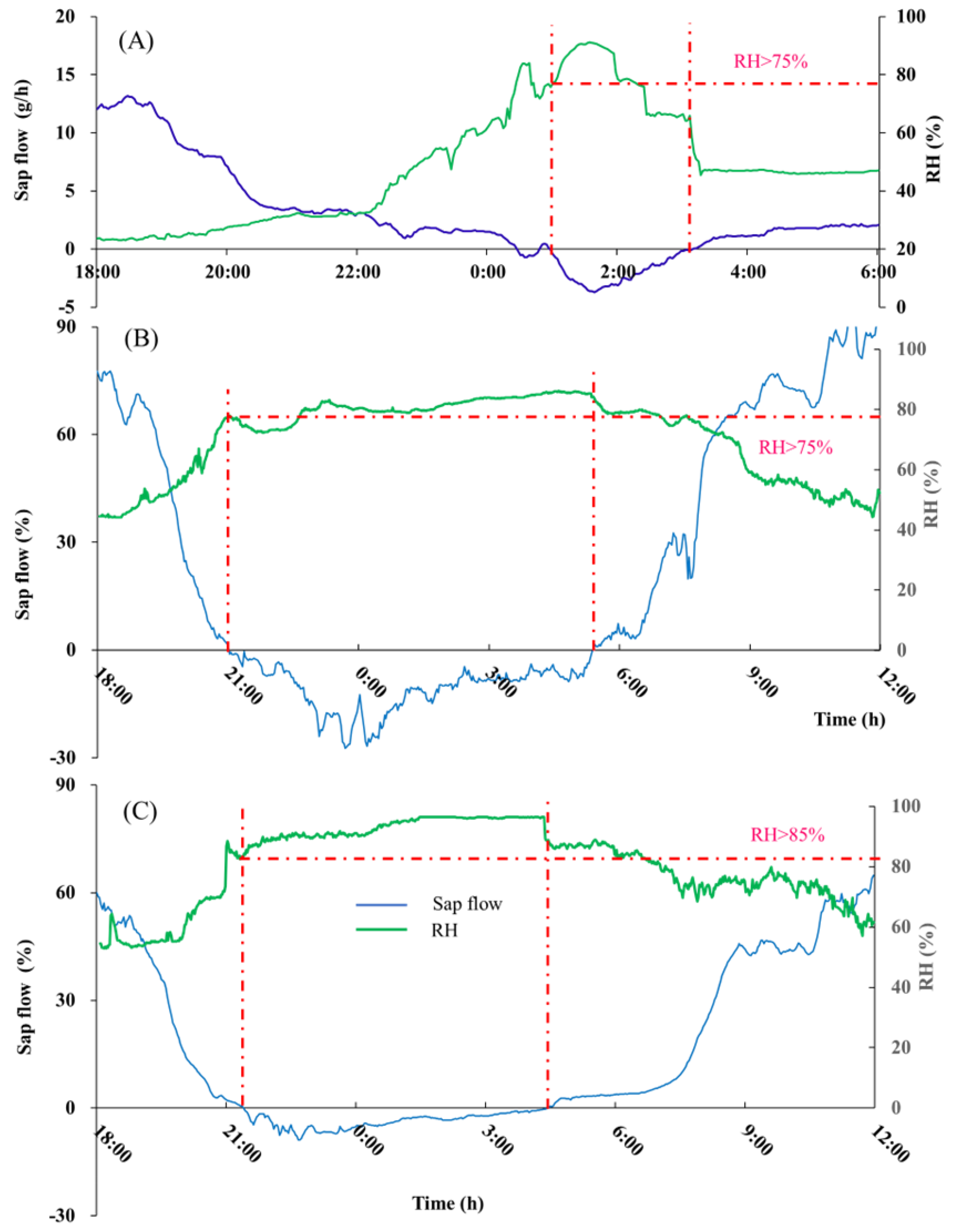
3.3. Environmental Factors Affect Vapor Absorption
3.4. Calculation of Precipitation Absorption and Utilization
3.5. Water Absorption and Consumption Characteristics of Tamarisk
4. Discussion
4.1. The Timing of Absorbing Atmospheric Moisture
4.2. Characteristics of Atmospheric Water Absorption by Leaves
4.3. The Fate of the Absorbed Water
4.4. The Significance of Absorbed Atmospheric Water for Plants
5. Conclusions
- The precipitation in the arid region was dominated by light precipitation events (with an intensity below 0.5 mm/d). Our results show that Tamarisk leaves could absorb unsaturated water vapor and precipitation directly. During the experiment, a single nighttime light precipitation was, surprisingly, absorbed by the Tamarisk leaves at a rate of 42.6%. The Tamarisk leaves can absorb precipitation moisture, even when the precipitation intensity is less than 0.2 mm/d, especially if the precipitation event occurs late at night or early in the morning.
- The reverse sap flow usually appears in the shoots soon after precipitation, and then in the branch and stem in turn. The rate of reverse sap flow was not only related to the amount of precipitation, but also related to the timing and duration of precipitation. Continuous precipitation results in the escalated reversed sap flow.
- The reversed sap flows at the stem and branch accounted for 13.6% and 21.5% of the precipitation amount, respectively, during an observation year.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berry, Z.C.; Emery, N.C.; Gotsch, S.G.; Goldsmith, G.R. Foliar water uptake: Processes, pathways, and integration into plant water budgets. Plant Cell Environ. 2019, 42, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Fricke, W. Night-time transpiration–favouring growth? Trends Plant Sci. 2019, 24, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Philip, J.R. Plant water relations: Some physical aspects. Annu. Rev. Plant Physiol. 1966, 17, 245–268. [Google Scholar] [CrossRef]
- Aung, K.; Jiang, Y.; He, S.Y. The role of water in plant–microbe interactions. Plant J. 2018, 93, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Holanda AE, R.; Souza, B.C.; Carvalho EC, D.; Oliveira, R.S.; Martins, F.R.; Muniz, C.R.; Costa, R.C.; Soares, A.A. How do leaf wetting events affect gas exchange and leaf lifespan of plants from seasonally dry tropical vegetation? Plant Biol. 2019, 21, 1097–1109. [Google Scholar] [CrossRef]
- Delf, E.M. Transpiration in succulent plants. Ann. Bot. 1912, 26, 409–442. [Google Scholar] [CrossRef]
- Hietz, P.; Wanek, W.; Popp, M. Stable isotopic composition of carbon and nitrogen and nitrogen content in vascular epiphytes along an altitudinal transect. Plant Cell Environ. 1999, 22, 1435–1443. [Google Scholar] [CrossRef]
- Dacey, J.W. Internal winds in water lilies: An adaptation for life in anaerobic sediments. Science 1980, 210, 1017–1019. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.; Hemmingsen, E.; Bradstreet, E. Hydrostatic pressure and osmotic potential in leaves of mangroves and some other plants. Proc. Natl. Acad. Sci. USA 1964, 52, 119. [Google Scholar] [CrossRef]
- Gotsch, S.G.; Asbjornsen, H.; Holwerda, F.; Goldsmith, G.R.; Weintraub, A.E.; Dawson, T.E. Foggy days and dry nights determine crown-level water balance in a seasonal tropical montane cloud forest. Plant Cell Environ. 2014, 37, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Riveros-Iregui, D.A. Life in the clouds: Are tropical montane cloud forests responding to changes in climate? Oecologia 2016, 180, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.M.; Szyska, B.; Kessler, M. Microhabitat partitioning promotes plant diversity in a tropical montane forest. Glob. Ecol. Biogeogr. 2011, 20, 558–569. [Google Scholar] [CrossRef]
- Los, S.O.; Street-Perrott, F.A.; Loader, N.J.; Froyd, C.A. Detection of signals linked to climate change, land-cover change and climate oscillators in Tropical Montane Cloud Forests. Remote Sens. Environ. 2021, 260, 112431. [Google Scholar] [CrossRef]
- Schreel, J.D.; Steppe, K. Foliar water uptake in trees: Negligible or necessary? Trends Plant Sci. 2020, 25, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.L.; De Boer, H.J.; Schymanski, S.J.; Veneklaas, E.J. Two sides to every leaf: Water and CO2 transport in hypostomatous and amphistomatous leaves. New Phytol. 2019, 222, 1179–1187. [Google Scholar] [CrossRef]
- Sheil, D. Forests, atmospheric water and an uncertain future: The new biology of the global water cycle. For. Ecosyst. 2018, 5, 19. [Google Scholar] [CrossRef]
- Ganthaler, A.; Bär, A.; Dämon, B.; Losso, A.; Nardini, A.; Dullin, C.; Tromba, G.; von Arx, G.; Mayr, S. Alpine dwarf shrubs show high proportions of nonfunctional xylem: Visualization and quantification of species-specific patterns. Plant Cell Environ. 2022, 45, 55–68. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zhang, S.; Cai, J.; Tyree, M.T. Water relations of Robinia pseudoacacia L.: Do vessels cavitate and refill diurnally or are R-shaped curves invalid in Robinia? Plant Cell Environ. 2014, 37, 2667–2678. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Dawson, T.E.; Richards, J.H. Hydraulic lift: Consequences of water efflux from the roots of plants. Oecologia 1998, 113, 151–161. [Google Scholar] [CrossRef]
- Sprenger, M.; Stumpp, C.; Weiler, M.; Aeschbach, W.; Allen, S.T.; Benettin, P.; Dubbert, M.; Hartmann, A.; Hrachowitz, M.; Kirchner, J.W. The demographics of water: A review of water ages in the critical zone. Rev. Geophys. 2019, 57, 800–834. [Google Scholar] [CrossRef]
- Dubbert, M.; Werner, C. Water fluxes mediated by vegetation: Emerging isotopic insights at the soil and atmosphere interfaces. New Phytol. 2019, 221, 1754–1763. [Google Scholar] [CrossRef]
- Beyer, M.; Kühnhammer, K.; Dubbert, M. In situ measurements of soil and plant water isotopes: A review of approaches, practical considerations and a vision for the future. Hydrol. Earth Syst. Sci. 2020, 24, 4413–4440. [Google Scholar] [CrossRef]
- Domínguez-Niño, J.M.; Oliver-Manera, J.; Girona, J.; Casadesús, J. Differential irrigation scheduling by an automated algorithm of water balance tuned by capacitance-type soil moisture sensors. Agric. Water Manag. 2020, 228, 105880. [Google Scholar] [CrossRef]
- Coopman, R.E.; Nguyen, H.T.; Mencuccini, M.; Oliveira, R.S.; Sack, L.; Lovelock, C.E.; Ball, M.C. Harvesting water from unsaturated atmospheres: Deliquescence of salt secreted onto leaf surfaces drives reverse sap flow in a dominant arid climate mangrove, Avicennia marina. New Phytol. 2021, 231, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Assouline, S.; Kamai, T. Liquid and vapor water in vadose zone profiles above deep aquifers in hyper-arid environments. Water Resour. Res. 2019, 55, 3619–3631. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, W.; Zhan, H.; Jiang, Q.; Shi, M.; Wang, Y. On the origin of deep soil water infiltration in the arid sandy region of China. Water 2020, 12, 2409. [Google Scholar] [CrossRef]
- Mackay, D.S.; Savoy, P.R.; Grossiord, C.; Tai, X.; Pleban, J.R.; Wang, D.R.; McDowell, N.G.; Adams, H.D.; Sperry, J.S. Conifers depend on established roots during drought: Results from a coupled model of carbon allocation and hydraulics. New Phytol. 2020, 225, 679–692. [Google Scholar] [CrossRef]
- Heffelfinger, L.J.; Stewart, K.M.; Bush, A.P.; Sedinger, J.S.; Darby, N.W.; Bleich, V.C. Timing of precipitation in an arid environment: Effects on population performance of a large herbivore. Ecol. Evol. 2018, 8, 3354–3366. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.-Y.; Wang, Y.; Wu, H.; Wang, P.; Li, W.; Bai, Y.; Li, E.; Wang, S.; Miao, C. Responses of two desert shrubs to simulated rainfall pulses in an arid environment, northwestern China. Plant Soil 2019, 435, 239–255. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, W. Adventitious roots are key to the development of nebkhas in extremely arid regions. Plant Soil 2019, 442, 471–482. [Google Scholar] [CrossRef]
- Ouyang, L.; Gao, J.; Zhao, P.; Rao, X. Species-specific transpiration and water use patterns of two pioneer dominant tree species under manipulated rainfall in a low-subtropical secondary evergreen forest. Ecohydrology 2020, 13, e2234. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhan, H.; Yang, W.; Jiang, Q.; Wang, Y.; Guo, F. An ecohydrological perspective of reconstructed vegetation in the semi-arid region in drought seasons. Agric. Water Manag. 2021, 243, 106488. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhan, H.; Yang, W.; Dang, H.; Li, W. Is annual recharge coefficient a valid concept in arid and semi-arid regions? Hydrol. Earth Syst. Sci. 2017, 21, 5031–5042. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, W.; Zhan, H.; Jiang, Q.; Shi, M.; Wang, Y.; Li, X.; Xin, Z. On Change of Soil Moisture Distribution with Vegetation Reconstruction in Mu Us Sandy Land of China, with Newly Designed Lysimeter. Front. Plant Sci. 2021, 12, 182. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, C.; Yan, W.; Zhao, X. Influences of 1.5 °C and 2.0 °C global warming scenarios on water use efficiency dynamics in the sandy areas of northern China. Sci. Total Environ. 2019, 664, 161–174. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhan, H.; Yang, W.; Feng, W.; Lu, Q.; Wang, Y.; Jiang, Q.; Wang, B.; Shi, M.; Wang, T. Redistribution process of precipitation in ecological restoration activity of Pinus sylvestris var. mongolica in Mu Us Sandy Land, China. Int. Soil Water Conserv. Res. 2023, 11, 572–585. [Google Scholar] [CrossRef]
- Burgess, S.S.; Adams, M.A.; Turner, N.C.; Beverly, C.R.; Ong, C.K.; Khan, A.A.; Bleby, T.M. An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol. 2001, 21, 589–598. [Google Scholar] [CrossRef]
- Campbell, G.; Calissendorff, C.; Williams, J. Probe for measuring soil specific heat using a heat-pulse method. Soil Sci. Soc. Am. J. 1991, 55, 291–293. [Google Scholar] [CrossRef]
- Mennekes, D.; Rinderer, M.; Seeger, S.; Orlowski, N. Ecohydrological travel times derived from in situ stable water isotope measurements in trees during a semi-controlled pot experiment. Hydrol. Earth Syst. Sci. 2021, 25, 4513–4530. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, X.; Wang, Y.; Zhan, H.; Yang, W.; Jiang, Q. New measures of deep soil water recharge during the vegetation restoration process in semi-arid regions of northern China. Hydrol. Earth Syst. Sci. 2020, 24, 5875–5890. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Zhan, H.; Liang, H.; Yang, W.; Zhao, Y.; Li, T. New comparative experiments of different soil types for farmland water conservation in arid regions. Water 2018, 10, 298. [Google Scholar] [CrossRef]
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics; Springer Science & Business Media: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Henschel, J.R.; Seely, M.K. Ecophysiology of atmospheric moisture in the Namib Desert. Atmos. Res. 2008, 87, 362–368. [Google Scholar] [CrossRef]
- Marks, W.; Dobelle, W.; MacNichol, E.F., Jr. Visual pigments of single primate cones. Science 1964, 143, 1181–1183. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.; Henschel, J.R.; Hetem, R.S.; Wassenaar, T.D.; Strauss, W.M.; Hanrahan, S.A.; Seely, M.K. Fog and fauna of the Namib Desert: Past and future. Ecosphere 2020, 11, e02996. [Google Scholar] [CrossRef]
- Stone, E.C. Dew as an ecological factor: I. A review of the literature. Ecology 1957, 38, 407–413. [Google Scholar] [CrossRef]
- Stone, E.C. The ecological importance of dew. Q. Rev. Biol. 1963, 38, 328–341. [Google Scholar] [CrossRef]
- Hill, A.J.; Dawson, T.E.; Shelef, O.; Rachmilevitch, S. The role of dew in Negev Desert plants. Oecologia 2015, 178, 317–327. [Google Scholar] [CrossRef]
- Alvarado-Barrientos, M.; Holwerda, F.; Asbjornsen, H.; Dawson, T.; Bruijnzeel, L. Suppression of transpiration due to cloud immersion in a seasonally dry Mexican weeping pine plantation. Agric. For. Meteorol. 2014, 186, 12–25. [Google Scholar] [CrossRef]
- Smith, D.; Jackson, N.; Roberts, J.; Ong, C. Reverse flow of sap in tree roots and downward siphoning of water by Grevillea robusta. Funct. Ecol. 1999, 13, 256–264. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C. Hydraulic redistribution of soil water by neotropical savanna trees. Tree Physiol. 2002, 22, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Schreel, J.D.; Van de Wal, B.A.; Hervé-Fernandez, P.; Boeckx, P.; Steppe, K. Hydraulic redistribution of foliar absorbed water causes turgor-driven growth in mangrove seedlings. Plant Cell Environ. 2019, 42, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Vandegehuchte, M.W.; Van de Wal, B.A.; Hoste, P.; Guyot, A.; Lovelock, C.E.; Lockington, D.A. Direct uptake of canopy rainwater causes turgor-driven growth spurts in the mangrove Avicennia marina. Tree Physiol. 2018, 38, 979–991. [Google Scholar] [CrossRef]
- He, Q.-Y.; Yan, M.-J.; Miyazawa, Y.; Chen, Q.-W.; Cheng, R.-R.; Otsuki, K.; Yamanaka, N.; Du, S. Sap flow changes and climatic responses over multiple-year treatment of rainfall exclusion in a sub-humid black locust plantation. For. Ecol. Manag. 2020, 457, 117730. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sakikawa, N.; Miyata, T. Thermo-responsive gels that absorb moisture and ooze water. Nat. Commun. 2018, 9, 2315. [Google Scholar] [CrossRef]



| Date | Time | R2 | |
|---|---|---|---|
| July 2019 | daytime | SF = −0.044 VPD2 + 1.951 VPD + 0.794 | 0.501 |
| nighttime | SF = −0.033 VPD 2 + 0.893 VPD − 2.013 | 0.718 | |
| August 2019 | daytime | SF = −0.068 VPD 2 + 2.868 VPD − 0.758 | 0.658 |
| nighttime | SF = −0.024 VPD 2 + 0.759 VPD − 1.981 | 0.703 |
| Date | Time | Duration (h) | Precipitation (mm) | Sap Flux (mm) | Reversed Sap Flux on Precipitation% | ||
|---|---|---|---|---|---|---|---|
| Stem | Branch | Stem | Branch | ||||
| 6 July | Midday | 0.33 | Under 0.2 | 0 | 0.004 | 0.000 | 0.020 |
| 18 July | Midday | 0.5 | 0.2 | 0 | 0.006 | 0.000 | 0.030 |
| 20 July | Late afternoon | 0.17 | Under 0.2 | 0 | 0 | 0.000 | 0.000 |
| 22 July | Late afternoon | 0.5 | 0.2 | 0 | 0.004 | 0.000 | 0.020 |
| 23 July | Afternoon | 0.17 | Under 0.2 | 0 | 0 | 0.000 | 0.000 |
| 24 July | Dawn | 3 | Under 0.2 | 0.002 | 0.01 | 0.010 | 0.050 |
| 25 July | Late afternoon | 0.08 | Under 0.2 | 0 | 0.006 | 0.000 | 0.030 |
| 27 July | Dawn and night | >12 h | 23 (5.6; 10.4; 7.0) | 5.93 | 9.802 | 0.258 | 0.426 |
| 2 August | Late afternoon | 1.5 | 5.2 | 0.028 | 0.055 | 0.005 | 0.011 |
| 6 August | Afternoon | 0.08 | Under 0.2 | 0 | 0.004 | 0.000 | 0.020 |
| 12 August | Midnight to early morning | 8 | 2 | 0.135 | 0.222 | 0.068 | 0.111 |
| 18 August | Afternoon | 0.17 | Under 0.2 | 0 | 0 | 0.000 | 0.000 |
| 25 August | Midmorning | 1 | 0.4 | 0.002 | 0.006 | 0.005 | 0.015 |
| 27 August | Late afternoon | 0.17 | Under 0.2 | 0 | 0.004 | 0.000 | 0.020 |
| 30 August~1 September | Day and night | >12 h | 22 (6; 7; 9) | 1.312 | 1.608 | 0.060 | 0.073 |
| Sum | 7.409 | 11.731 | 0.136 | 0.215 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Ma, X.; Yuan, Z.; Li, W.; Yan, Y.; Yang, W. An Experimental Investigation of the Precipitation Utilization of Plants in Arid Regions. Plants 2024, 13, 594. https://doi.org/10.3390/plants13050594
Feng W, Ma X, Yuan Z, Li W, Yan Y, Yang W. An Experimental Investigation of the Precipitation Utilization of Plants in Arid Regions. Plants. 2024; 13(5):594. https://doi.org/10.3390/plants13050594
Chicago/Turabian StyleFeng, Wei, Xiaoxu Ma, Zixuan Yuan, Wei Li, Yujie Yan, and Wenbin Yang. 2024. "An Experimental Investigation of the Precipitation Utilization of Plants in Arid Regions" Plants 13, no. 5: 594. https://doi.org/10.3390/plants13050594
APA StyleFeng, W., Ma, X., Yuan, Z., Li, W., Yan, Y., & Yang, W. (2024). An Experimental Investigation of the Precipitation Utilization of Plants in Arid Regions. Plants, 13(5), 594. https://doi.org/10.3390/plants13050594





