No- or Low-Content Copper Compounds for Controlling Venturia oleaginea, the Causal Agent of Olive Leaf Spot Disease
Abstract
:1. Introduction
2. Results
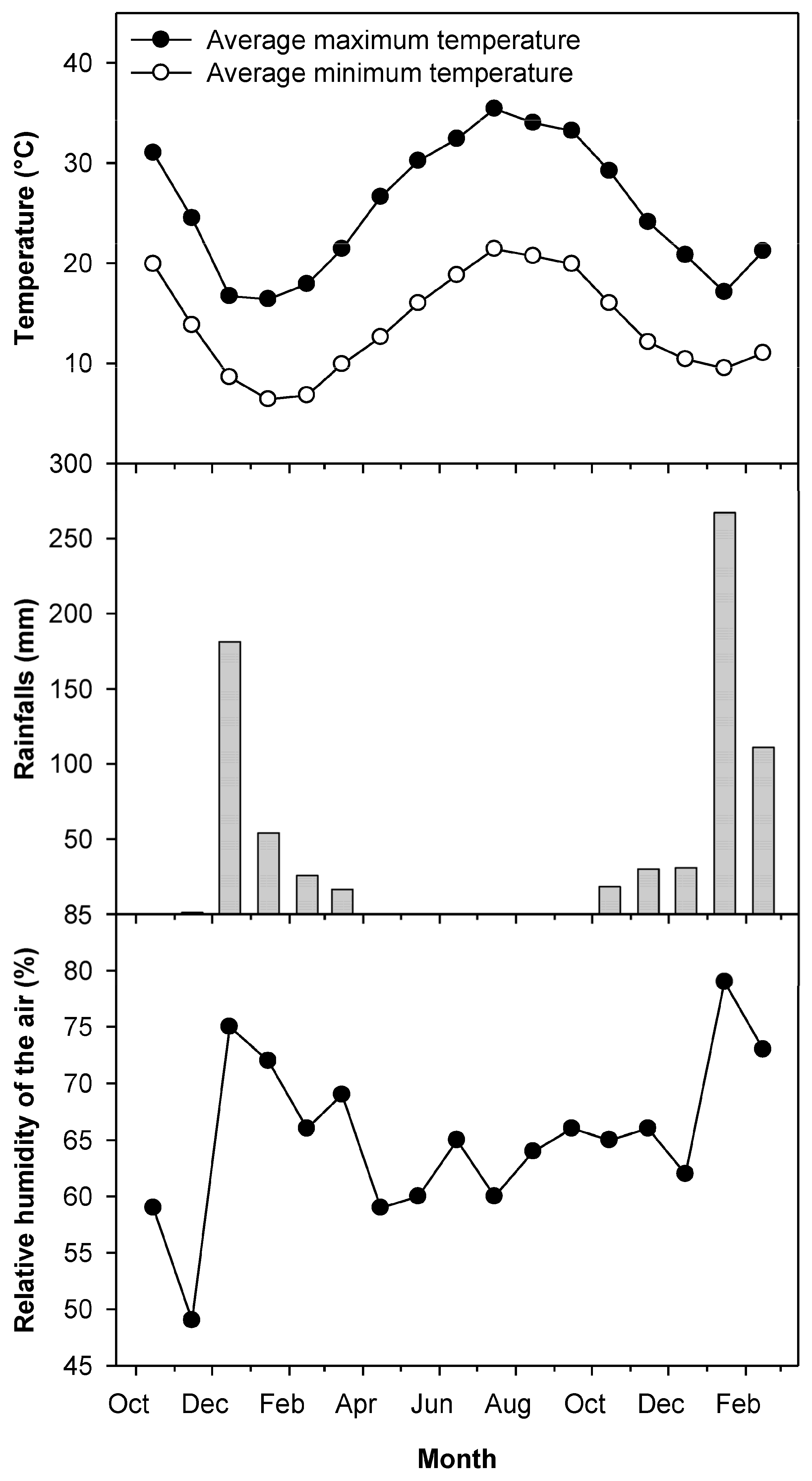
2.1. Climatic Data
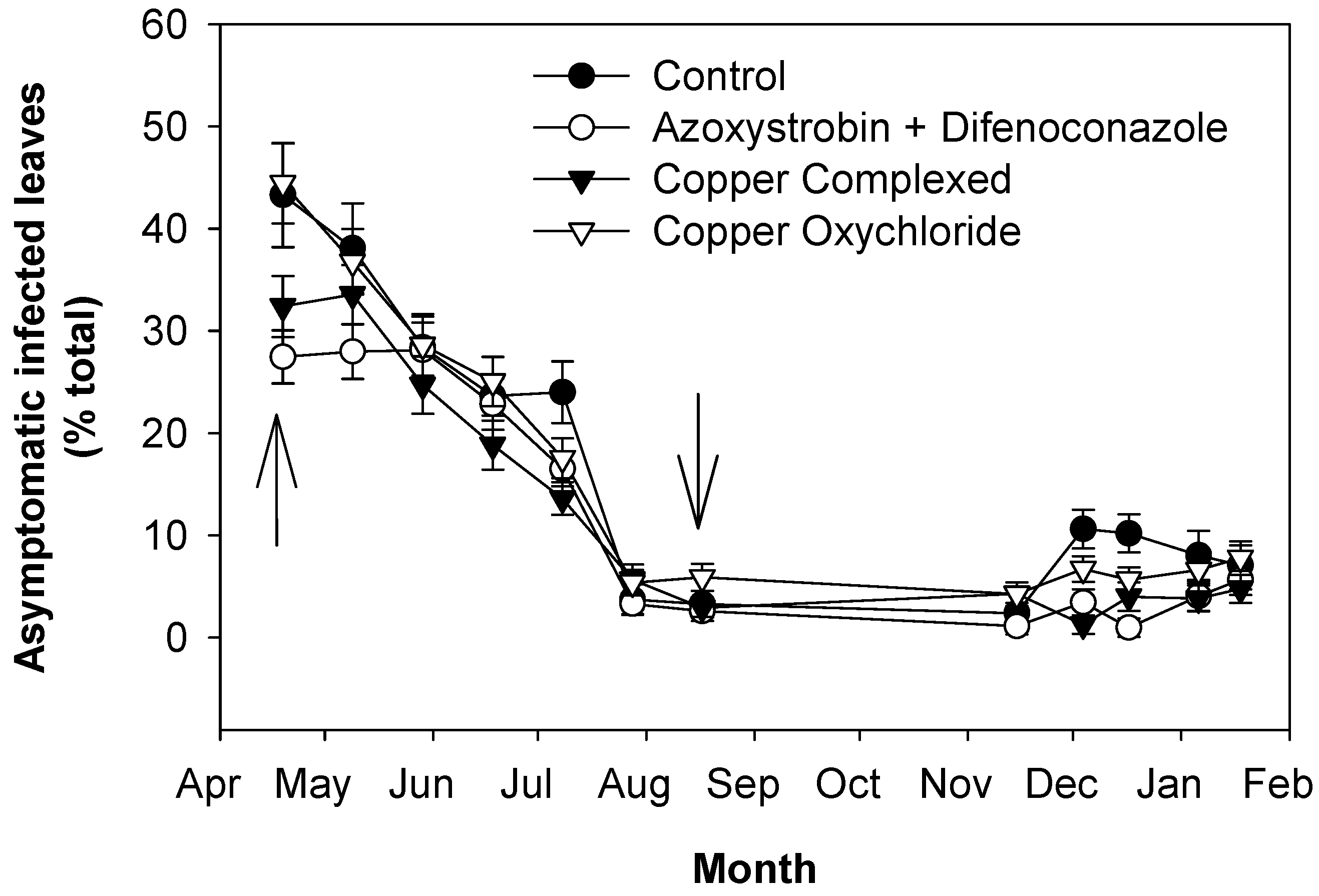
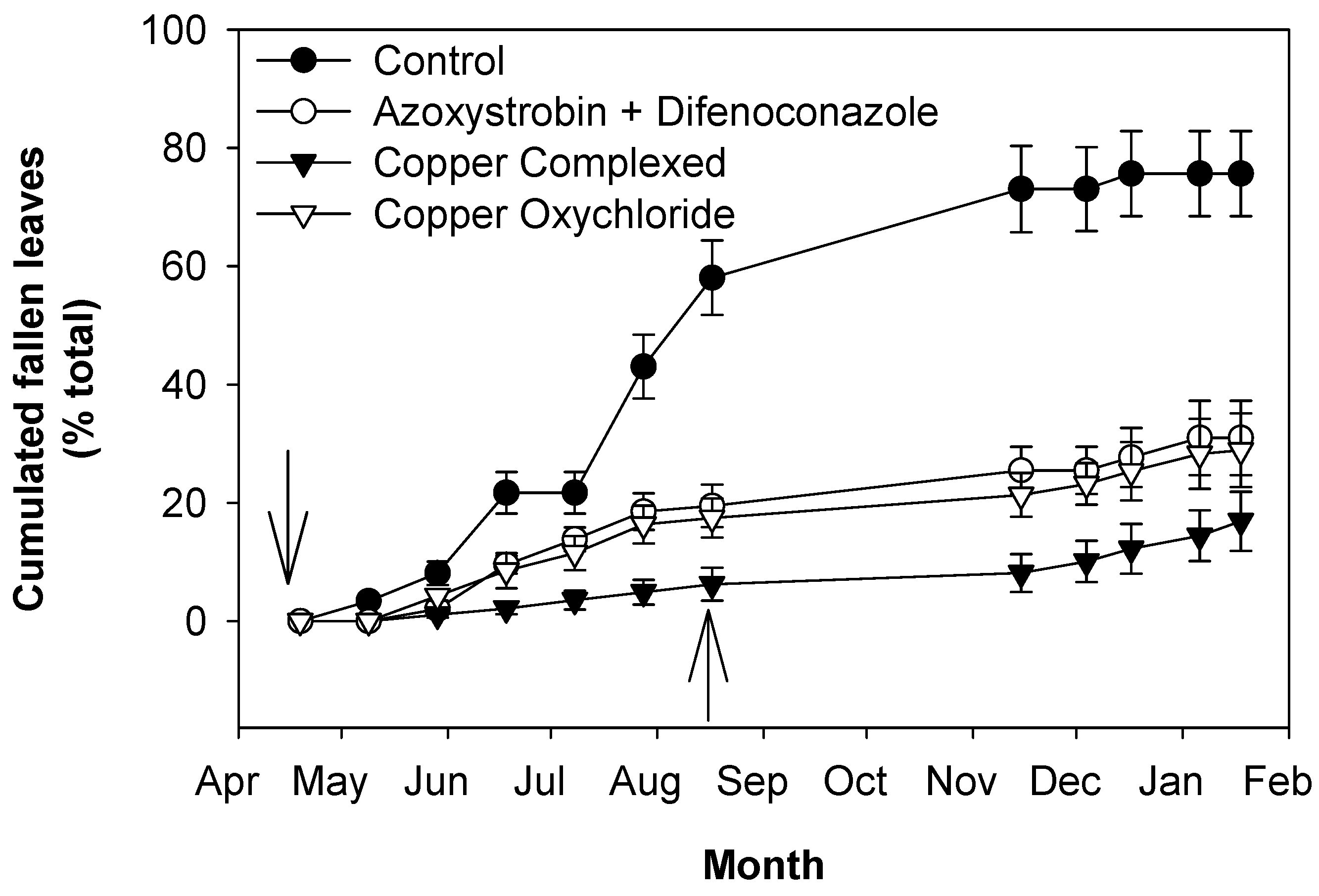
2.2. Effects of OLS Disease on Leaves Grown in 2016 and Still Present at the Beginning of 2017
2.3. Effects of OLS Disease on Leaves Grown in 2017
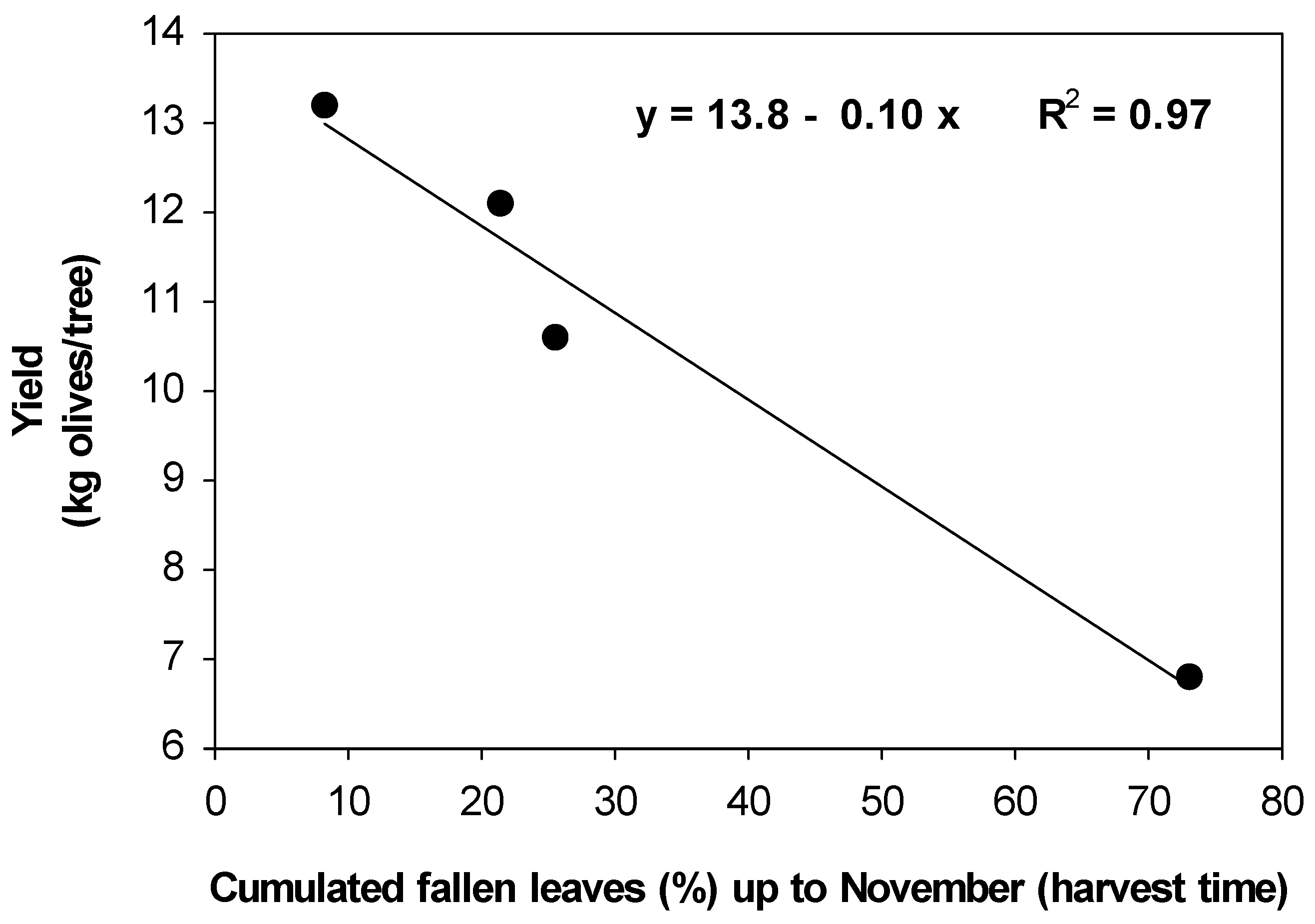
2.4. Effects of OLS Disease on Inflorescence Development and Olive Yield
3. Discussion
4. Materials and Methods
4.1. Location and Characteristics of the Experimental Field
4.2. Fungicides and Time of Application
4.3. Experimental Design
4.4. Field Observations
4.5. Laboratory Observations
4.6. Leaves Grown in 2017: Percentages of Defoliation and Infected Leaves
4.7. Climatic Data
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azeri, T. Research on olive leaf spot, olive knot and verticillium wilt of olive in Turkey. EPPO Bull. 1993, 23, 437–440. [Google Scholar] [CrossRef]
- Vossen, P. Timing Sprays for Control Peacock Spot and Olive Knot Disease. Olive News 2004, 7, 11. [Google Scholar]
- Nascimento-Silva, K.; Roca-Castillo, L.; Benlloch-González, M.; Fernández-Escobar, R. Silicon Reduces the Incidence of Venturia oleaginea (Castagne) Rossman & Crous in Potted Olive Plants. HortScience 2019, 54, 1962–1966. [Google Scholar]
- Hladnik, M.; Unković, N.; Janakiev, T.; Grbić, M.L.; Arbeiter, A.B.; Stanković, S.; Janaćković, P.; Gavrilović, M.; Rančić, D.; Bandelj, D.; et al. An insight into an olive scab on the “Istrska Belica” variety: Host-pathogen interactions and phyllosphere mycobiome. Microb. Ecol. 2023, 86, 1343–1363. [Google Scholar] [CrossRef] [PubMed]
- González-Lamothe, R.; Segura, R.; Trapero, A.; Baldoni, L.; Botella, M.A.; Valpuesta, V. Phylogeny of the fungus Spilocaea oleagina, the causal agent of peacock leaf spot in olive. FEMS Microbiol. Lett. 2002, 210, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, V.; Braun, U.; Spooner-Hart, R.; Nair, N. Observations on spot caused by Fusicladium oleagineum on olives (Olea europaea) in New South Wales, Australia. Australas. Plant Dis. Notes 2009, 4, 26–28. [Google Scholar] [CrossRef]
- Viruega, J.R.; Roca, L.F.; Moral, J.; Trapero, A. Factors affecting infection and disease development on olive leaves inoculated with Fusicladium oleagineum. Plant Dis. 2011, 95, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Issa, T.; Almadi, L.; Jarrar, S.; Tucci, M.; Buonaurio, R.; Famiani, F. Factors affecting Venturia oleaginea infections on olive and effects of the disease on floral biology. Phytopathol. Mediterr. 2019, 58, 221–229. [Google Scholar]
- Hajjeh, H.; Salman, M.; Jawabreh, M.; Rumaileh, B.A. The effect of local fungicides on conidial germination of Spilocaea oleagina in Palestine. PTUUK 2014, 2, 26–28. [Google Scholar]
- Salman, M.; Hawamda, A.A.; Al-Ashqar Amarni, A.; Rahil, M.; Hajjeh, H.; Natsheh, B. Evaluation of the incidence and severity of olive leaf spot caused by Spilocaea oleagina on olive trees in Palestine. Am. J. Plant. Sci. 2011, 2, 457–460. [Google Scholar] [CrossRef]
- Buonaurio, R.; Almadi, L.; Famiani, F.; Moretti, C.; Agosteo, G.E.; Schena, L. Olive leaf spot caused by Venturia oleaginea: An updated review. Front. Plant Sci. 2023, 13, 1061136. [Google Scholar] [CrossRef] [PubMed]
- Trapero, A.; Blanco, M.A. Enfermedades. In El Cultivo de Olivo; Coedición Junta de Andalucía/Mundi-Prensa: Madrid, Spain, 2008; pp. 595–656. [Google Scholar]
- Lanza, B.; Ragnelli, A.M.; Priore, M.; Aimola, P. Morphological and histochemical investigation of the response of Olea europaea leaves to fungal attack by Spilocaea oleagina. Plant Pathol. 2017, 66, 1239–1247. [Google Scholar] [CrossRef]
- Khalil, H.A.; El-Ansary, D.O. Morphological, physiological and anatomical responses of two olive cultivars to deficit irrigation and mycorrhizal inoculation. Eur. J. Hortic. 2020, 85, 51–62. [Google Scholar] [CrossRef]
- Graniti, A. Olive scab: A review1. EPPO Bull. 1993, 23, 377–384. [Google Scholar] [CrossRef]
- Larbi, A.; Vázquez, S.; El-Jendoubi, H.; Msallem, M.; Abadía, J.; Abadía, A.; Morales, F. Canopy light heterogeneity drives leaf anatomical, eco-physiological, and photosynthetic changes in olive trees grown in a high-density plantation. Photosynth. Res. 2015, 123, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.T.; Ferreira, H.; Moutinho-Pereira, J.; Correia, C. The role of nighttime water balance on Olea europaea plants subjected to contrasting water regimes. J. Plant Physiol. 2018, 226, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Thomidis, T.; Michos, K.; Chatzipapadopoulos, F.; Tampaki, A. Evaluation of two predictive models for forecasting olive leaf spot in northern Greece. Plants 2021, 10, 1200. [Google Scholar] [CrossRef]
- Iannotta, N.; Monardo, D.; Perri, E. Effects of different treatments against Spilocaea oleagina (Cast.) Hugh. Acta Hortic. 2002, 586, 741–744. [Google Scholar] [CrossRef]
- Obanor, F.O.; Jasper, M.V.; Jones, E.E.; Walter, M. Greenhouse and field evaluation for control of olive leaf spot in New Zeland. Crop Prot. 2008, 27, 1335–1342. [Google Scholar] [CrossRef]
- Sistani, F.; Ramezanpour, S.; Nasrollanejad, S. Field evaluation of different fungicides application to control olive leaf spot. Aust. J. Basic Appl. Sci. 2009, 3, 3341–3345. [Google Scholar]
- Obanor, F.O.; Walter, M.; Jones, E.E.; Jaspers, M.V. Efficacy of systemic acquired resistance inducers in olive leaf spot management. Australas. Plant Pathol. 2013, 42, 163–168. [Google Scholar] [CrossRef]
- Belfiore, T.; Perri, E.; Scalercio, S.; Iannotta, N.; Tocci, C.A. Systemic fungicide (Tetraconazole) against the olive spot, Spilocaea oleagina, in Italy. Acta Hortic. 2014, 1057, 185–189. [Google Scholar] [CrossRef]
- Pennisi, A.M.; Agosteo, G.E. Efficacy of natural and chemical active ingredients in the control of bird’s eye spots disease of olive. In Proceedings of the Atti Giornate Fitopatologiche, Baselga di Piné, Trento, Italy, 7–11 April 2002; Volume 2, pp. 249–254. [Google Scholar]
- Zuffa, M.; Ricci, V. Azoxystrobin + difenoconazole: Experiences on the control of peacock on olive. In Proceedings of the Atti Giornate Fitopatologiche, Siena, Italy, 6–9 March 2018; Volume 2, pp. 297–301. [Google Scholar]
- D’Ascenzo, D.; Crivelli, L.; Camillo, L.D. New defence strategies against olive leaf spot in central Italy. In Proceedings of the Atti Giornate Fitopatologiche, Siena, Italy, 18–21 March 2014; Volume 2, pp. 139–146. [Google Scholar]
- Rongai, D.; Basti, C.; Di Marco, C. A natural product for the control of olive leaf spot caused by Fusicadium oleaginum. Phytopathol. Mediterr. 2012, 51, 276–282. [Google Scholar]
- Adawi, A.; Jarrar, S.; Almadi, L.; Alkowni, R.; Gallo, M.; D’onghia, A.M.; Buonaurio, R.; Famiani, F. Effectiveness of low copper-containing chemicals against Olive Leaf Spot Disease caused by Venturia oleaginea. Agriculture 2022, 2, 326. [Google Scholar] [CrossRef]
- Abuamsha, R.; Abueid, M.; Hajjeh, H.; Salman, M. Evaluation of the incidence and severity of olive leaf spot caused by Spilocaea oleagina in different olive cultivars in Palestine. J. Agric. Environ. Int. Dev. 2013, 107, 201–212. [Google Scholar]
- Obanor, F.O.; Walter, M.; Jones, E.E.; Jaspers, M.V. Effects of temperatures, relative umidity, leaf wetness and leaf age on Spilocaea oleagina conidium germination on olive leaves. Eur. J. Plant Pathol. 2008, 3, 211–222. [Google Scholar] [CrossRef]
- Roubal, C.; Regis, S.; Nicot, P. Field models for the prediction of leaf infection and latent period of Fusicladium oleagineum on olive based on rain, temperature and relative humidity. Plant Pathol. 2013, 62, 657–666. [Google Scholar] [CrossRef]
- Agosteo, G.; Schena, L. Olive leaf spot. In Olive Diseases and Disorders; Schena, L., Agosteo, G.E., Cacciola, S.O., di San Lio, G.M., Eds.; Research Signpost: Thiruvananthapuram, India, 2011; pp. 143–176. [Google Scholar]
- Obanor, F.O.; Walter, M.; Jones, E.E.; Jaspers, M.V. Effects of temperature, inoculum concentration, leaf age, and continuous and interrupted wetness on infection of olive plants by Spilocaea oleagina. Plant Pathol. 2011, 60, 190–199. [Google Scholar] [CrossRef]
- Proietti, P.; Nasini, L.; Famiani, F. Effect of different leaf-to-fruit ratios on photosynthesis and fruit growth in olive (Olea europaea L.). Photosynthetica 2006, 44, 275–285. [Google Scholar] [CrossRef]



| Treatment | Symptomatic Leaves (%Total) | Asymptomatic Infected Leaves (%Total) | Symptomatic + Asymptomatic Infected (%Total) |
|---|---|---|---|
| Control | 68.1 a | 24.6 a | 92.7 a |
| Azoxystrobin + difenoconazole | 66.3 a | 25.6 a | 91.9 a |
| Copper oxychloride | 58.8 a | 29.1 a | 87.9 a |
| Copper complexed with gluconate and lignosulphonate | 59.4 a | 31.3 a | 90.7 a |
| Treatment | Dropped Leaves (%Total) | Symptomatic Leaves (%Total) | Asymptomatic Infected Leaves (%Total) | Non-Dropped Leaves (%Total) |
|---|---|---|---|---|
| Control | 75.7 a | 14.7 a | 7.1 a | 26.3 c |
| Azoxystrobin + difenoconazole | 31.0 b | 12.0 a | 5.7 a | 69.0 a |
| Copper oxychloride | 28.9 bc | 12.5 a | 7.8 a | 71.1 a |
| Copper complexed with gluconate and lignosulphonate | 16.9 c | 11.2 a | 4.8 a | 83.1 a |
| Treatment | Symptomatic Leaves (%Total) | Asymptomatic Infected Leaves (%Total) | Symptomatic + Asymptomatic but Infected (%) | Asymptomatic Non-Infected Leaves (%) |
|---|---|---|---|---|
| Control | 55.9 a | 30.0 a | 85.9 a | 14.1 b |
| Azoxystrobin + difenoconazole | 17.4 b | 8.3 b | 25.7 b | 74.3 a |
| Copper oxychloride | 17.6 b | 11.0 b | 28.6 b | 71.4 a |
| Copper complexed with gluconate and lignosulphonate | 13.5 b | 13.5 b | 27.0 b | 73.0 a |
| Treatment | Inflorescence Dry Weight (mg/Inflorescence) | Fruit Weight (g/Fruit) | Yield (kg/Tree) |
|---|---|---|---|
| Control | 19.8 c | 2.40 a | 6.8 a |
| Azoxystrobin + difenoconazole | 26.0 a | 2.55 a | 10.6 b |
| Copper oxychloride | 23.0 b | 2.60 a | 12.1 b |
| Copper complexed with gluconate and lignosulphonate | 24.0 b | 2.55 a | 13.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almadi, L.; Jarrar, S.; Sbaihat, L.; Issa, T.; Tucci, M.; Moretti, C.; Buonaurio, R.; Famiani, F. No- or Low-Content Copper Compounds for Controlling Venturia oleaginea, the Causal Agent of Olive Leaf Spot Disease. Plants 2024, 13, 600. https://doi.org/10.3390/plants13050600
Almadi L, Jarrar S, Sbaihat L, Issa T, Tucci M, Moretti C, Buonaurio R, Famiani F. No- or Low-Content Copper Compounds for Controlling Venturia oleaginea, the Causal Agent of Olive Leaf Spot Disease. Plants. 2024; 13(5):600. https://doi.org/10.3390/plants13050600
Chicago/Turabian StyleAlmadi, Leen, Samer Jarrar, Layth Sbaihat, Tahreer Issa, Michele Tucci, Chiaraluce Moretti, Roberto Buonaurio, and Franco Famiani. 2024. "No- or Low-Content Copper Compounds for Controlling Venturia oleaginea, the Causal Agent of Olive Leaf Spot Disease" Plants 13, no. 5: 600. https://doi.org/10.3390/plants13050600







