Methods for Improving the Germination of Rhodotypos scandens (Thunb.) Makino Seeds through Endocarp Removal
Abstract
:1. Introduction
2. Results
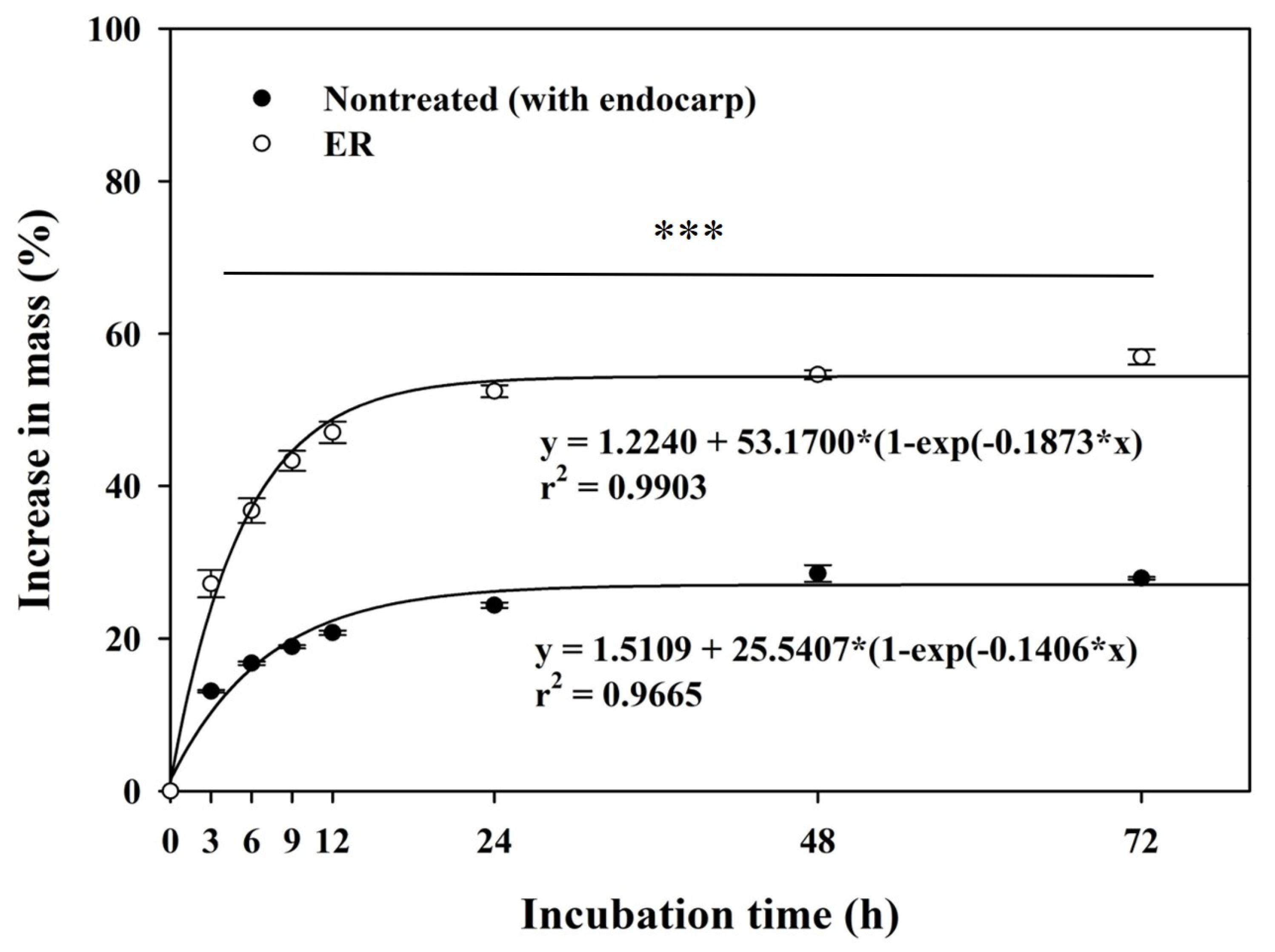
2.1. Seed Characteristics and Water Imbibition Test
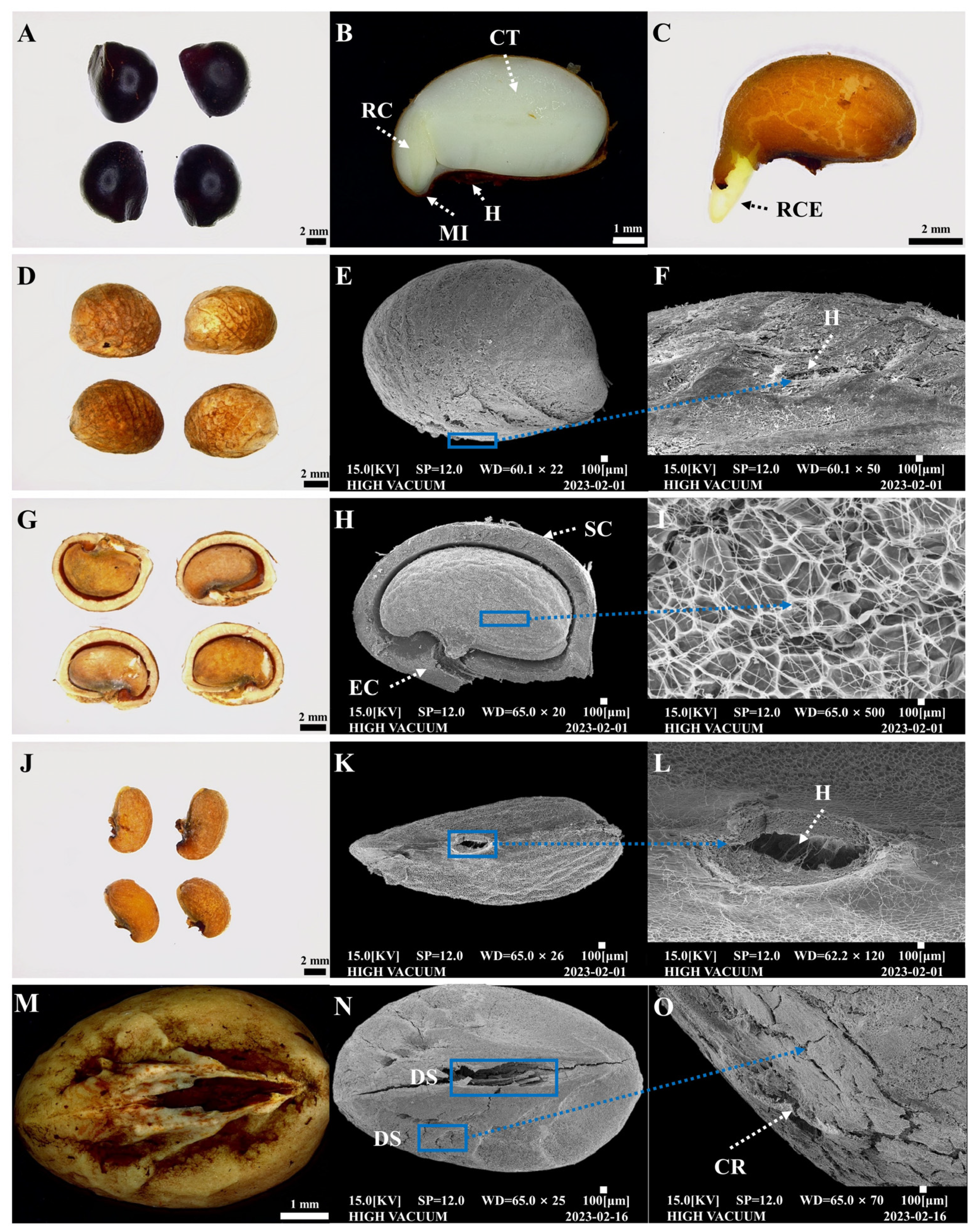
2.2. Structural Analysis of Internal and External Seed Morphoanatomy
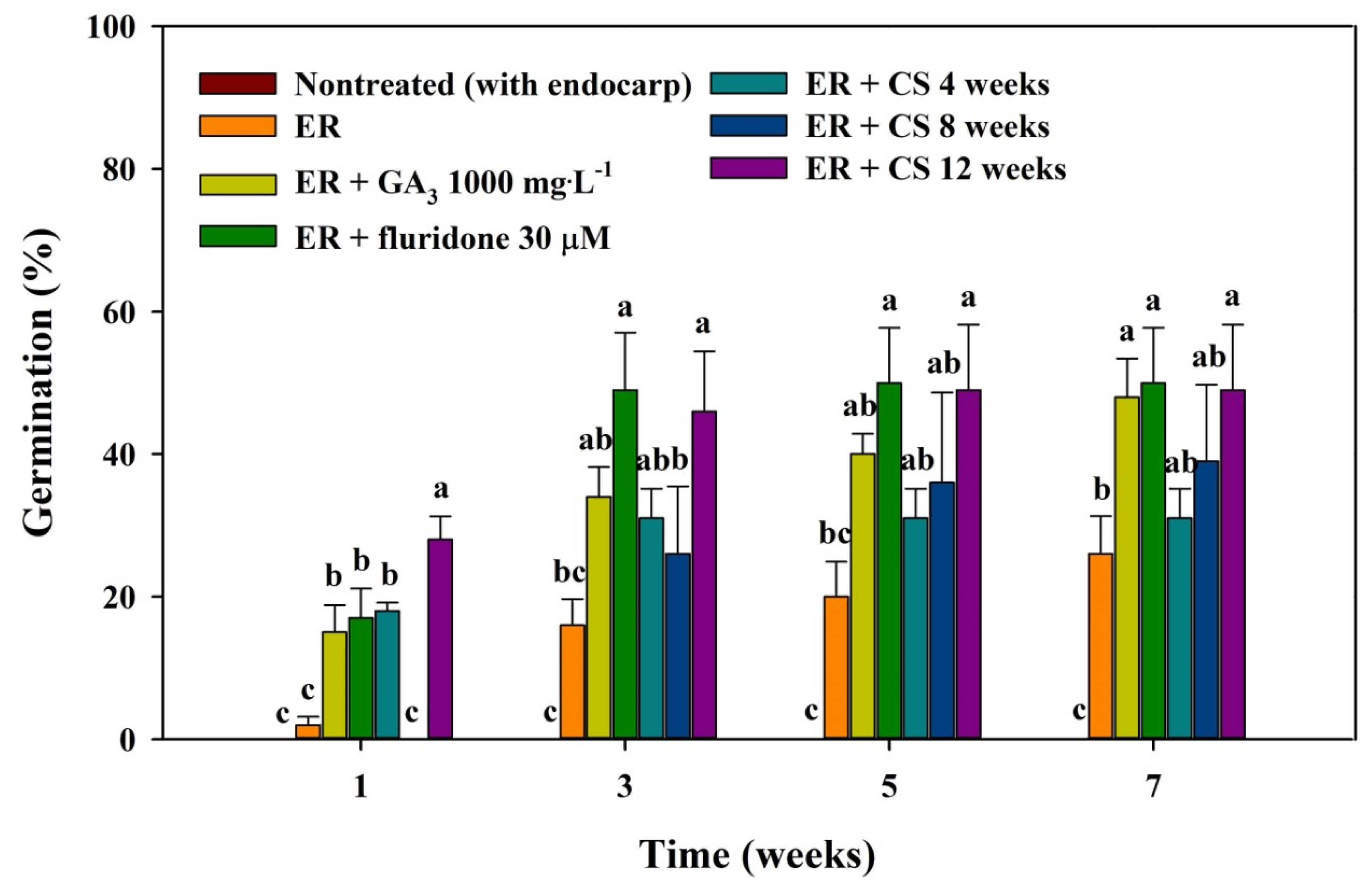
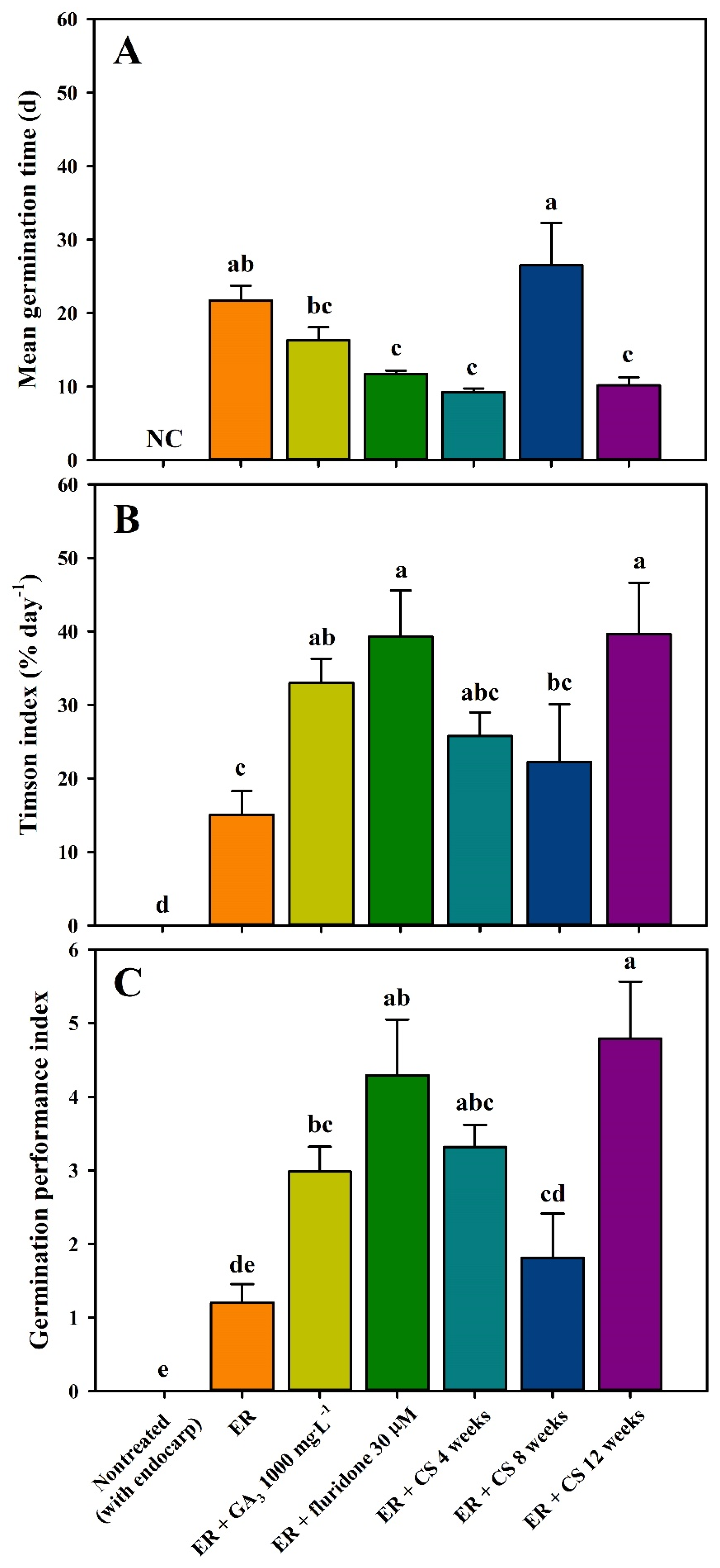
2.3. Seed Germination following Scarification, Phytohormone Treatment, and CS
3. Discussion
3.1. Seed Characteristics and Water Imbibition Test
3.2. Structural Analysis of Internal and External Seed Morphoanatomy
3.3. Seed Germination by Scarification, Phytohormone Treatment, and CS
4. Materials and Methods
4.1. Seed Material
4.2. Water Imbibition Test
4.3. Structural Analysis of Internal and External Seed Morphoanatomy
4.4. Seed Germination following Scarification, Phytohormone Treatment, and CS
4.5. Data Collection and Germination Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sharrock, S. The contribution of botanic gardens to ex situ conservation through seed banking. Plant Divers. 2017, 39, 373–378. [Google Scholar] [CrossRef]
- Fahey, M.; Yenson, A.M.; Offord, C. Historic seed collections germinated for the Australian PlantBank opening. Australas. Plant Conserv. 2014, 22, 7–8. [Google Scholar] [CrossRef]
- Lupton, D.; Al Moqbalie, H.; Al Rahaili, B.; Al Qassabi, Z.; Al Hajri, B.; Anderson, A.; Patzelt, A. The Oman Botanic Garden: A review of progress 2010–2016 with emphasis on herbarium and seed bank collections, propagation challenges and garden design principles. Sibbaldia 2017, 14, 119–132. [Google Scholar] [CrossRef]
- Society for Ecological Restoration; International Network for Seed Based Restoration; Royal Botanic Gardens, Kew. Seed Information Database (SID). 2023. Available online: https://ser-sid.org/ (accessed on 23 February 2023).
- Herranz, J.M.; Ferrandis, P.; Martínez-Duro, E. Seed germination ecology of the threatened endemic Iberian Delphinium fissum subsp. sordidum (Ranunculaceae). Plant Ecol. 2010, 211, 89–106. [Google Scholar] [CrossRef]
- Shen, S.K.; Wu, F.Q.; Yang, G.S.; Wang, Y.H.; Sun, W.B. Seed germination and seedling emergence in the extremely endangered species Rhododendron protistum var. giganteum-the world’s largest Rhododendron flora. Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 216, 65–70. [Google Scholar] [CrossRef]
- Chahtane, H.; Kim, W.; Lopez-Molina, L. Primary seed dormancy: A temporally multilayered riddle waiting to be unlocked. J. Exp. Bot. 2017, 68, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Gremer, J.R.; Wilcox, C.J.; Chiono, A.; Suglia, E.; Schmitt, J. Germination timing and chilling exposure create contingency in life history and influence fitness in the native wildflower Streptanthus tortuosus. J. Ecol. 2020, 108, 239–255. [Google Scholar] [CrossRef]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Lee, S.Y.; Rhie, Y.H.; Kim, K.S. Non-deep simple morphophysiological dormancy in seeds of Thalictrum rochebrunianum, an endemic perennial herb in the Korean Peninsula. Hortic. Environ. Biotechnol. 2015, 56, 366–375. [Google Scholar] [CrossRef]
- Née, G.; Xiang, Y.; Soppe, W.J. The release of dormancy, a wake-up call for seeds to germinate. Curr. Opin. Plant Biol. 2017, 35, 8–14. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Walck, J.L.; Tao, J. Non-deep simple morphophysiological dormancy in seeds of Angelica keiskei (Apiaceae). Sci. Hortic. 2019, 255, 202–208. [Google Scholar] [CrossRef]
- Saffari, P.; Majd, A.; Jonoubi, P.; Najafi, F. Effect of treatments on seed dormancy breaking, seedling growth, and seedling antioxidant potential of Agrimonia eupatoria L. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100282. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Tang, Y.; Zhang, K.; Zhang, Y.; Tao, J. Dormancy-breaking and germination requirements for seeds of Sorbus alnifolia (Siebold & Zucc.) K.Koch (Rosaceae), a mesic forest tree with high ornamental potential. Forests 2019, 10, 319. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.G.; Lee, H.; Na, C.S.; Lee, D.H. The dormancy types and germination characteristics of the seeds of Berberis koreana Palibin, an endemic species of Korea. Horticulturae 2023, 9, 547. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chien, C.T.; Chung, J.D.; Yang, Y.S.; Kuo, S.R. Dormancy-break and germination in seeds of Prunus campanulata (Rosaceae): Role of covering layers and changes in concentration of abscisic acid and gibberellins. Seed Sci. Res. 2007, 17, 21–32. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, K.; Jang, B.K.; Ji, B.; Lee, H.; Baskin, C.C.; Cho, J.S. Exogenous gibberellin can effectively and rapidly break intermediate physiological dormancy of Amsonia elliptica seeds. Front. Plant Sci. 2022, 13, 1043897. [Google Scholar] [CrossRef]
- Cho, J.S.; Jeong, J.H.; Lee, C.H. The effects of environmental conditions and chemical treatments on seed germination in Astilboides tabularis (Hemsl.) Engl. Korean J. Hortic. Sci. Technol. 2016, 34, 363–371. [Google Scholar] [CrossRef]
- Yang, L.E.; Peng, D.L.; Li, Z.M.; Huang, L.; Yang, J.; Sun, H. Cold stratification, temperature, light, GA3, and KNO3 effects on seed germination of Primula beesiana from Yunnan, China. Plant Divers. 2020, 42, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ghayyad, M.; Kurbysa, M.; Napolsy, G. Effect of endocarp removal, gibberellin, stratification and sulfuric acid on germination of Mahaleb (Prunus mahaleb L.) seeds. Am. Eurasian J. Agric. Environ. Sci. 2010, 9, 163–168. [Google Scholar]
- Choi, G.E.; Ghimire, B.; Lee, H.; Jeong, M.J.; Kim, H.J.; Ku, J.J.; Lee, K.M.; Son, S.W.; Lee, C.H.; Park, J.I.; et al. Scarification and stratification protocols for breaking dormancy of Rubus (Rosaceae) species in Korea. Seed Sci. Technol. 2016, 44, 239–252. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Dicenta, F. Mechanisms of dormancy in seeds of peach (Prunus persica (L.) Batsch) cv. GF305. Sci. Hortic. 2001, 91, 51–58. [Google Scholar] [CrossRef]
- García-Gusano, M.; Martínez-Gomez, P.; Dicenta, F. Breaking seed dormancy in almond (Prunus dulcis (Mill.) D.A. Webb). Sci. Hortic. 2004, 99, 363–370. [Google Scholar] [CrossRef]
- Essl, F. An overview of the first occurrences of Rhodotypos scandens in Austria. Bioinvasions Rec. 2019, 8, 736–741. [Google Scholar] [CrossRef]
- Korea National Arboretum. Available online: http://www.nature.go.kr (accessed on 1 August 2023).
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 1 August 2023).
- Baskin, C.C.; Baskin, J.M. When breaking seed dormancy is a problem: Try a move-along experiment. Nat. Plants J. 2003, 4, 17–21. [Google Scholar] [CrossRef]
- Sarkar, B.K.; Yang, W.Y.; Wu, Z.; Tang, H.; Ding, S. Variations of water uptake, lipid consumption, and dynamics during the germination of Sesamum indicum seed: A nuclear magnetic resonance spectroscopic investigation. J. Agric. Food Chem. 2009, 57, 8213–8219. [Google Scholar] [CrossRef]
- Louf, J.F.; Zheng, Y.; Kumar, A.; Bohr, T.; Gundlach, C.; Harholt, J.; Poulsen, H.F.; Jensen, K.H. Imbibition in plant seeds. Phys. Rev. E 2018, 98, 042403. [Google Scholar] [CrossRef]
- Dai, L.; Chen, Y.; Wei, X. Hard seed characteristics and seed vigor of Ormosia hosiei. Agriculture 2023, 13, 1077. [Google Scholar] [CrossRef]
- De Paula, A.; Delgado, C.; Paulilo, M.; Santos, M. Breaking physical dormancy of Cassia leptophylla and Senna macranthera (Fabaceae: Caesalpinioideae) seeds: Water absorption and alternating temperatures. Seed Sci. Res. 2012, 22, 259–267. [Google Scholar] [CrossRef]
- Zhou, Z.; Bao, W. Levels of physiological dormancy and methods for improving seed germination of four rose species. Sci. Hortic. 2011, 129, 818–824. [Google Scholar] [CrossRef]
- Jackson, M.B. Root-to-shoot communication in flooded plants: Involvement of abscisic acid, ethylene, and 1-aminocyclopropane-1-carboxylic acid. Agron. J. 1994, 86, 775–782. [Google Scholar] [CrossRef]
- Bo, J.; Huiru, D.; Xiaohan, Y. Shortening hybridization breeding cycle of rose-a study on mechanisms controlling achene dormancy. Acta Hortic. 1995, 404, 40–47. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Wu, N.; Bao, W.K.; Qiu, P.Q. Post-dispersal factors regulating dormancy and germination of Rosa soulieana seeds. Belg. J. Bot. 2008, 141, 103–111. [Google Scholar]
- Pipinis, E.; Milios, E.; Mavrokordopoulou, O.; Gkanatsiou, C.; Aslanidou, M.; Smiris, P. Effect of pretreatments on seed germination of Prunus mahaleb L. Not. Bot. Horti Agrobot. 2012, 40, 183–189. [Google Scholar] [CrossRef]
- Mira, S.; Arnal, A.; Pérez-García, F. Seed germination of Phillyrea angustifolia L., a species of difficult propagation. For. Syst. 2017, 26, e002. [Google Scholar] [CrossRef]
- Jang, B.K.; Park, K.T.; Lee, S.Y.; Lee, H.Y.; Song, S.K.; Kim, J.K.; Lee, C.H.; Cho, J.S. Comparison of the seed dormancy and germination characteristics of six Clematis species from South Korea. Sci. Hortic. 2023, 307, 111488. [Google Scholar] [CrossRef]
- Sun, J.; Jia, H.; Wang, P.; Zhou, T.; Wu, Y.; Liu, Z. Exogenous gibberellin weakens lipid breakdown by increasing soluble sugars levels in early germination of zanthoxylum seeds. Plant Sci. 2019, 280, 155–163. [Google Scholar] [CrossRef]
- Dekkers, B.J.W.; Bentsink, L. Regulation of seed dormancy by abscisic acid and DELAY OF GERMINATION 1. Seed Sci. Res. 2015, 25, 82–98. [Google Scholar] [CrossRef]
- Baek, S.G.; Im, J.H.; Kwak, M.J.; Park, C.H.; Lee, M.H.; Na, C.S.; Woo, S.Y. Non-deep physiological dormancy in seed and germination requirements of Lysimachia coreana Nakai. Horticulturae 2021, 7, 490. [Google Scholar] [CrossRef]
- Zhang, K.; Ji, Y.; Fu, G.; Yao, L.; Liu, H.; Tao, J. Dormancy-breaking and germination requirements of Thalictrum squarrosum Stephan ex Willd. seeds with underdeveloped embryos. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100311. [Google Scholar] [CrossRef]
- Quatrano, R.S.; Bartels, D.; Ho, T.D.; Pages, M. New insights into ABA-mediated processes. Plant Cell 1997, 9, 470–475. [Google Scholar] [CrossRef]
- Grappin, P.; Bouinot, D.; Sotta, B.; Miginiac, E.; Jullien, M. Control of seed dormancy in Nicotiana plumbaginifolia: Post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 2000, 210, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Worarad, K.; Xie, X.; Martha Rumainum, I.M.; Burana, C.; Yamane, K. Effects of fluridone treatment on seed germination and dormancy-associated gene expression in an ornamental Peach (Prunus persica (L.) Batsch). Hortic. J. 2017, 86, 317–326. [Google Scholar] [CrossRef]
- Qi, S.H.; Yao, G.Q.; Hasan, M.M.; Jiang, H.; Liu, X.D.; Nie, Z.F.; Zhang, X.Y.; Du, Y.X.; Tian, X.Q.; Li, F.P.; et al. The mechanisms of rapid seed germination in Caragana species adapted to low mean annual precipitation. Trees 2023, 37, 933–945. [Google Scholar] [CrossRef]
- Chae, S.H.; Yoneyama, K.; Takeuchi, Y.; Joel, D.M. Fluridone and norflurazon, carotenoid-biosynthesis inhibitors, promote seed conditioning and germination of the holoparasite Orobanche minor. Physiol. Plant. 2004, 120, 328–337. [Google Scholar] [CrossRef]
- Rudolf, P.O.; Owston, P.W. Rhodotypos scandens (Thunb.) Makino. In The Woody Plant Seed Manual; USDA FS Agriculture HandBook; US Department of Agriculture: Washington, DC, USA, 2008; Volume 727, pp. 925–953. Available online: https://www.fs.usda.gov/research/treesearch/32626 (accessed on 12 February 2024).
- Zhang, L.; Xu, C.; Liu, H.; Wu, Q.; Tao, J.; Zhang, K. Intermediate complex morphophysiological dormancy in seeds of Aconitum barbatum (Ranunculaceae). BMC Plant Biol. 2023, 23, 350. [Google Scholar] [CrossRef]
- Šerá, B. Methodological contribution on seed germination and seedling initial growth tests in wild plants. Not. Bot. Horti Agrobot. Cluj Napoca 2023, 51, 13164. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved equations for the prediction of seed longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Sundstrom, F.J.; Reader, R.B.; Edwards, R.L. Effect of seed treatment and planting method on Tabasco pepper. J. Am. Soc. Hortic. Sci. 1987, 112, 641–644. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B. High salt tolerance in germinating dimorphic seeds of Arthrocnemum indicum. Int. J. Plant Sci. 1998, 159, 826–832. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Kim, J.H.; Lee, M.H.; Kim, G.M.; Park, C.Y.; Lee, D.H.; Na, C.S. Methods for Improving the Germination of Rhodotypos scandens (Thunb.) Makino Seeds through Endocarp Removal. Plants 2024, 13, 648. https://doi.org/10.3390/plants13050648
Kim HM, Kim JH, Lee MH, Kim GM, Park CY, Lee DH, Na CS. Methods for Improving the Germination of Rhodotypos scandens (Thunb.) Makino Seeds through Endocarp Removal. Plants. 2024; 13(5):648. https://doi.org/10.3390/plants13050648
Chicago/Turabian StyleKim, Hyeon Min, Jun Hyeok Kim, Mi Hyun Lee, Gun Mo Kim, Chung Youl Park, Da Hyun Lee, and Chae Sun Na. 2024. "Methods for Improving the Germination of Rhodotypos scandens (Thunb.) Makino Seeds through Endocarp Removal" Plants 13, no. 5: 648. https://doi.org/10.3390/plants13050648
APA StyleKim, H. M., Kim, J. H., Lee, M. H., Kim, G. M., Park, C. Y., Lee, D. H., & Na, C. S. (2024). Methods for Improving the Germination of Rhodotypos scandens (Thunb.) Makino Seeds through Endocarp Removal. Plants, 13(5), 648. https://doi.org/10.3390/plants13050648





