Time-Course Transcriptomic Analysis Reveals Molecular Insights into the Inflorescence and Flower Development of Cardiocrinum giganteum
Abstract
:1. Introduction
2. Results
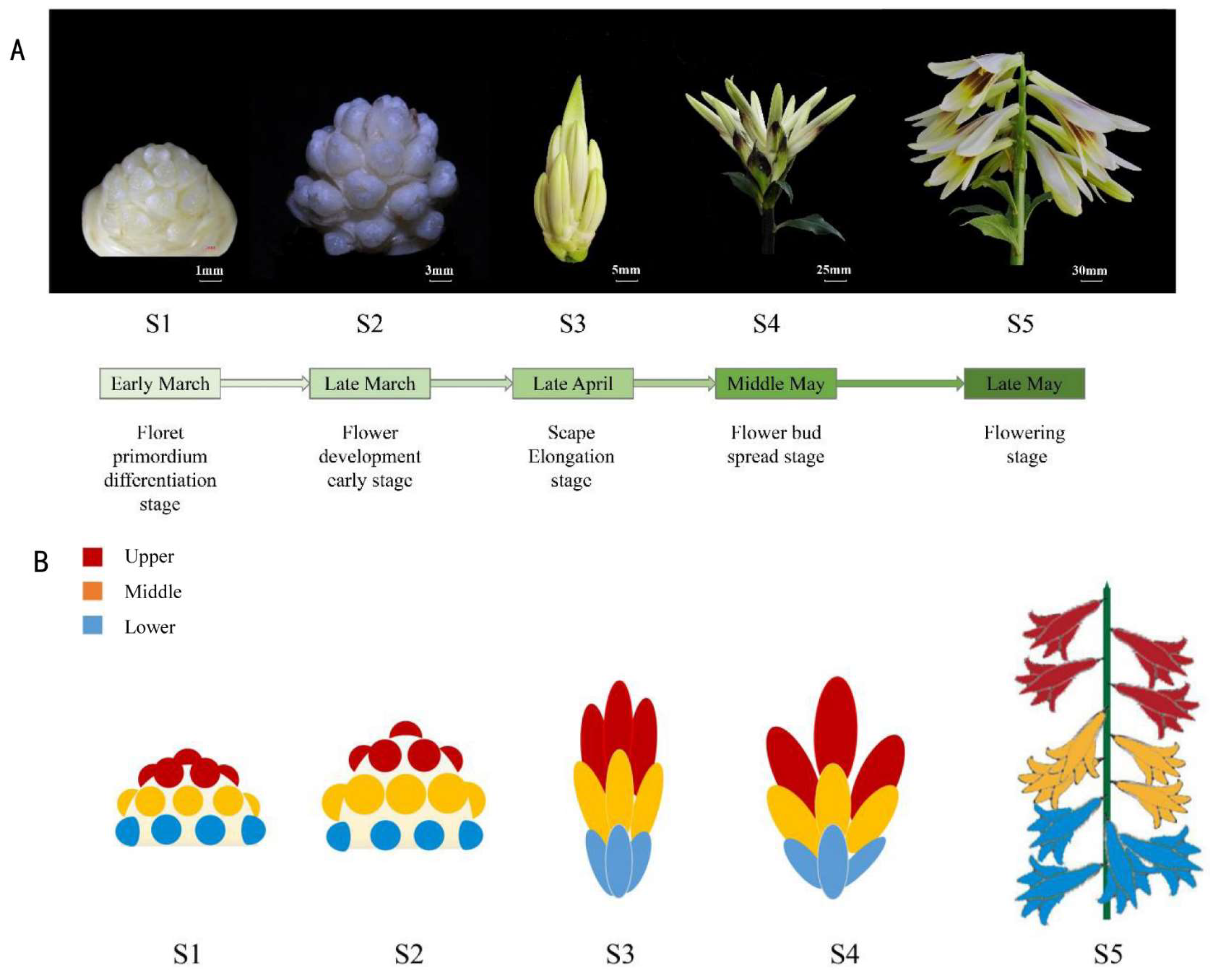
2.1. Phenology of Inflorescence Development of C. giganteum
2.2. Transcriptomic and Correlation Analysis of the Five Developmental Stages and Three Inflorescence Parts of C. giganteum
2.3. Co-Expression Modules and Expression Trends at Different Developmental Stages of the Inflorescence
2.4. Genes Specifically Expressed during Inflorescence and Flower Development
2.5. Representative Key Genes Involved in the Main Regulatory Programmes of Flower Development
2.6. Representative Candidate Genes Involved in the Synchronization of Flower Growth in Different Parts of the Inflorescence
3. Discussion
3.1. Morphological Data and GO Analysis Demonstrated the Inflorescence Development Process of C. giganteum
3.2. Autonomous Promotion and GA Promotion Together with Vernalization Promotion Might Constitute the Flower Formation Pathway of C. giganteum
3.3. The Varied ABCDE Flowering Model in C. giganteum
3.4. Preliminary Discussion on Flowering Synchronization at Different Parts of Inflorescence in C. giganteum
4. Materials and Methods
4.1. Plant Material, Inflorescence Development Observation and Sample Collection
4.2. De Novo Transcriptome Assembly, Differential Gene Expression Analysis, and Functional Annotation
4.3. Correlation Analysis between Samples
4.4. Weighted Gene Co-Expression Network Analysis and Co-Expression Module Identification
4.5. Identification of Specifically Expressed Genes and GO Enrichment Analysis
4.6. Expression Analysis of Genes Involved in Inflorescence and Flower Development
4.7. RT-qPCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.S.; Li, P.; Qiu, Y.X. The complete chloroplast genomes of three Cardiocrinum (Liliaceae) species: Comparative genomic and phylogenetic analyses. Front. Plant Sci. 2017, 7, 2054. [Google Scholar] [CrossRef] [PubMed]
- Bull-Hereñu, K.; Claßen-Bockhoff, R. Open and closed inflorescences: More than simple opposites. J. Exp. Bot. 2011, 62, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Cheng, J.; Zhang, R.; Yao, X.; Kong, H. Developmental mechanisms involved in the diversification of flowers. Nat. Plants 2019, 5, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Frédéric, B.; Guillaume, L.; Pierre, T.; Claire, P. FLOR-ID: An interactive database of flowering-time gene networks in Arabidopsis thaliana. Nucleic Acids Res. 2015, 44, D1167–D1171. [Google Scholar]
- Heisler, M.G.; Ohno, C.; Das, P.; Sieber, P.; Reddy, G.V.; Long, J.A.; Meyerowitz, E.M. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 2005, 15, 1899–1911. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Wu, M.F.; Winter, C.M.; Berns, M.C.; Nole-Wilson, S.; Yamaguchi, A.; Coupland, G.; Krizek, B.A.; Wagner, D. A molecular framework for auxin-mediated initiation of flower primordia. Dev. Cell 2013, 24, 271–282. [Google Scholar] [CrossRef]
- Krizek, B. AINTEGUMENTA and AINTEGUMENTA-LIKE6 act redundantly to regulate Arabidopsis floral growth and patterning. Plant Phys. 2009, 150, 1916–1929. [Google Scholar] [CrossRef]
- Besnard, F.; Refahi, Y.; Morin, V.; Marteaux, B.; Brunoud, G.; Chambrier, P.; Rozier, F.; Mirabet, V.; Legrand, J.; Lainé, S. Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 2014, 505, 417–421. [Google Scholar] [CrossRef]
- Song, Y.H.; Shim, J.S.; Kinmonth-Schultz, H.A.; Imaizumi, T. Photoperiodic flowering: Time measurement mechanisms in leaves. Ann. Rev. Plant Biol. 2015, 66, 441–464. [Google Scholar] [CrossRef]
- Imaizumi, T. Arabidopsis circadian clock and photoperiodism: Time to think about location. Curr. Opin. Plant Biol. 2010, 13, 83–89. [Google Scholar] [CrossRef]
- Prunet, N.; Jack, T.P. Flower development in Arabidopsis: There is more to it than learning your ABCs. Methods Mol. Biol. 2014, 1110, 3–33. [Google Scholar]
- Schnablová, R.; Herben, T.; Klimešová, J. Shoot apical meristem and plant body organization: A cross-species comparative study. Ann. Bot. 2017, 120, 833–843. [Google Scholar] [CrossRef]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.; Jürgens, G.; Laux, T. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef]
- Bradley, D.; Carpenter, R.; Copsey, L.; Vincent, C.; Rothstein, S.; Coen, E. Control of inflorescence architecture in Antirrhinum. Nature 1996, 379, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.; Ratcliffe, O.; Vincent, C.; Carpenter, R.; Coen, E. Inflorescence commitment and architecture in Arabidopsis. Science 1997, 275, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Balanzà, V.; Martínez-Fernández, I.; Sato, S.; Yanofsky, M.F.; Ferrándiz, C. Inflorescence meristem fate is dependent on seed development and FRUITFULL in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 1622. [Google Scholar] [CrossRef] [PubMed]
- Benlloch, R.; Berbel, A.; Serrano-Mislata, A.; Madueño, F. Floral initiation and inflorescence architecture: A comparative view. Ann. Bot. 2007, 100, 1609. [Google Scholar] [CrossRef]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef]
- Bowman, J.L.; Smyth, D.R.; Meyerowitz, E.M. Genetic interactions among floral homeotic genes of Arabidopsis. Development 1991, 112, 1–20. [Google Scholar] [CrossRef]
- Alejandra Mandel, M.; Gustafson-Brown, C.; Savidge, B.; Yanofsky, M.F. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 1992, 360, 273–277. [Google Scholar] [CrossRef]
- Jofuku, K.D.; Den Boer, B.; Van Montagu, M.; Okamuro, J.K. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 1994, 6, 1211–1225. [Google Scholar]
- Jack, T.; Brockman, L.L.; Meyerowitz, E.M. The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell 1992, 68, 683–697. [Google Scholar] [CrossRef]
- Goto, K.; Meyerowitz, E.M. Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev. 1994, 8, 1548–1560. [Google Scholar] [CrossRef] [PubMed]
- Yanofsky, M.F.; Ma, H.; Bowman, J.L.; Drews, G.N.; Feldmann, K.A.; Meyerowitz, E.M. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 1990, 346, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, S.; Ditta, G.S.; Baumann, E.; Wisman, E.; Yanofsky, M.F. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 2000, 405, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Theissen, G.; Saedler, H. Floral quartets. Nature 2001, 409, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Pinyopich, A.; Ditta, G.S.; Savidge, B.; Liljegren, S.J.; Baumann, E.; Wisman, E.; Yanofsky, M.F. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 2003, 424, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Borghi, M.; Fernie, A.R. Floral metabolism of sugars and amino acids: Implications for pollinators’ preferences and seed and fruit set. Plant Phys. 2017, 175, 1510–1524. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Yao, X.; Fu, X.; Cheng, J.; Shan, H.; Yin, X.; Kong, H. Mechanisms underlying the formation of complex color patterns on Nigella orientalis (Ranunculaceae) petals. New Phyt. 2023, 237, 2450–2466. [Google Scholar] [CrossRef]
- Sobel, J.M.; Streisfeld, M.A. Flower color as a model system for studies of plant evo-devo. Front. Plant Sci. 2013, 4, 321. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; McIntire, K.; Hsu, Y.C.; Lee, P.Y.; Truong, M.T.; Beals, T.; Goldberg, R. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Ma, H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu. Rev. Plant Biol. 2005, 56, 393–434. [Google Scholar] [CrossRef]
- Wilson, Z.A.; Song, J.; Taylor, B.; Yang, C. The final split: The regulation of anther dehiscence. J. Exp. Bot. 2011, 62, 1633–1649. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, R.; Wang, X.; Liang, W.; Liao, J.; Huang, X.; Cai, Z.; Liu, D.; Huang, L.; Wei, X. The starch-sugar interconversion mechanism during bulb development of Cardiocrinum giganteum (Wall.) Makino revealed by transcriptome and metabolite analysis. Ind. Crops Prod. 2022, 187, 115318. [Google Scholar] [CrossRef]
- Phartyal, S.S.; Kondo, T.; Baskin, C.C.; Baskin, J.M. Seed dormancy and germination in the giant Himalayan lily (Cardiocrinum giganteum var. giganteum): An assessment of its potential for naturalization in northern Japan. Ecol. Res. 2012, 27, 677–690. [Google Scholar] [CrossRef]
- Yang, L.Q.; Hu, H.Y.; Xie, C.; Lai, S.P.; Yang, M.; He, X.J.; Zhou, S.D. Molecular phylogeny, biogeography and ecological niche modelling of Cardiocrinum (Liliaceae): Insights into the evolutionary history of endemic genera distributed across the Sino-Japanese floristic region. Ann. Bot. 2017, 119, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Klasfeld, S.; Zhu, Y.; Fernandez Garcia, M.; Xiao, J.; Han, S.K.; Konkol, A.; Wagner, D. LEAFY is a pioneer transcription factor and licenses cell reprogramming to floral fate. Nature Commun. 2021, 12, 626. [Google Scholar] [CrossRef]
- Wang, L.; Yu, P.; Lyu, J.; Hu, Y.; Han, C.; Bai, M.Y.; Fan, M. BZR1 physically interacts with SPL9 to regulate the vegetative phase change and cell elongation in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 10415. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Jeong, C.W.; Nole-Wilson, S.; Krizek, B.A.; Wagner, D. AINTEGUMENTA and AINTEGUMENTA-LIKE6/PLETHORA3 induce LEAFY expression in response to auxin to promote the onset of flower formation in Arabidopsis. Plant Phys. 2016, 170, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Belles-Boix, E.; Hamant, O.; Witiak, S.M.; Morin, H.; Traas, J.; Pautot, V.R. KNAT6, An Arabidopsis homeobox gene involved in meristem activity and organ separation. Plant Cell 2006, 18, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Scofield, S.; Murison, A.; Jones, A.; Fozard, J.; Aida, M.; Band, L.R.; Bennett, M.; Murray, J.A. Coordination of meristem and boundary functions by transcription factors in the SHOOT MERISTEMLESS regulatory network. Development 2018, 145, 157081. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid biosynthesis in Arabidopsis: A colorful pathway. Arabidopsis Book 2012, 10, e0158. [Google Scholar] [CrossRef]
- Gu, J.N.; Zhu, J.; Yu, Y.; Teng, X.D.; Lou, Y.; Xu, X.F.; Liu, J.L.; Yang, Z.N. DYT 1 directly regulates the expression of TDF 1 for tapetum development and pollen wall formation in Arabidopsis. Plant J. 2014, 80, 1005–1013. [Google Scholar] [CrossRef]
- Verma, N.; Burma, P.K. Regulation of tapetum-specific A9 promoter by transcription factors AtMYB 80, AtMYB 1 and AtMYB 4 in Arabidopsis thaliana and Nicotiana tabacum. Plant J. 2017, 92, 481–494. [Google Scholar] [CrossRef]
- Muñoz, A.; Mangano, S.; Toribio, R.; Fernández-Calvino, L.; Del Pozo, J.C.; Castellano, M.M. The co-chaperone HOP participates in TIR1 stabilisation and in auxin response in plants. Plant Cell Environ. 2022, 45, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Yeh, T.J.; Huang, K.Y.; Li, J.Y.; Chen, H.Y.; Yang, C.H. AGAMOUS-LIKE 13, a putative ancestor for the E functional genes, specifies male and female gametophyte morphogenesis. Plant J. 2014, 77, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, P.; Browse, J. Male sterility in Arabidopsis induced by overexpression of a MYC 5-SRDX chimeric repressor. Plant J. 2015, 81, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.X.; Malitsky, S.; De Oliveira, S.; Branigan, C.; Franke, R.B.; Schreiber, L.; Aharoni, A. SHINE transcription factors act redundantly to pattern the archetypal surface of Arabidopsis flower organs. PLoS Genet. 2011, 7, e1001388. [Google Scholar] [CrossRef] [PubMed]
- Muntha, S.T.; Zhang, L.; Zhou, Y.; Zhao, X.; Hu, Z.; Yang, J.; Zhang, M. Phytochrome A signal transduction 1 and CONSTANS-LIKE 13 coordinately orchestrate shoot branching and flowering in leafy Brassica juncea. Plant Biotechnol. J. 2019, 17, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Ichim, G.; Lopez, J.; Ahmed, S.U.; Muthalagu, N.; Giampazolias, E.; Delgado, M.E.; Haller, M.; Riley, J.S.; Mason, S.M.; Athineos, D. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol. Cell 2015, 57, 860–872. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, L.; Xu, H.; Yang, P.; He, G.; Tang, Y.; Qi, X.; Song, M.; Ming, J. LhGST is an anthocyanin-related glutathione S-transferase gene in Asiatic hybrid lilies (Lilium spp.). Plant Cell Rep. 2021, 40, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Peeters, A.J.; Aarts, M.G.; Pereira, A.; Koornneef, M. ANTHOCYANINLESS2, a homeobox gene affecting anthocyanin distribution and root development in Arabidopsis. Plant Cell 1999, 11, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Chen, S.; Lian, Y.; Wang, S. Tropaeolum majus R2R3 MYB transcription factor TmPAP2 functions as a positive regulator of anthocyanin biosynthesis. Int. J. Mol. Sci. 2022, 23, 12395. [Google Scholar] [CrossRef]
- Chen, J.J.; Wang, W.; Qin, W.Q.; Men, S.Z.; Li, H.L.; Mitsuda, N.; Ohme-Takagi, M.; Wu, A.M. Transcription factors KNAT3 and KNAT4 are essential for integument and ovule formation in Arabidopsis. Plant Phys. 2023, 191, 463–478. [Google Scholar] [CrossRef]
- Xue, X.; Sun, K.; Zhu, Z. CIRCADIAN CLOCK ASSOCIATED 1 gates morning phased auxin response in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2020, 527, 935–940. [Google Scholar] [CrossRef]
- Liu, X.; Kim, Y.J.; Müller, R.; Yumul, R.E.; Liu, C.; Pan, Y.; Cao, X.; Goodrich, J.; Chen, X. AGAMOUS terminates floral stem cell maintenance in Arabidopsis by directly repressing WUSCHEL through recruitment of Polycomb Group proteins. Plant Cell 2011, 23, 3654–3670. [Google Scholar] [CrossRef]
- Dinh, T.T.; Girke, T.; Liu, X.; Yant, L.; Schmid, M.; Chen, X. The floral homeotic protein APETALA2 recognizes and acts through an AT-rich sequence element. Development 2012, 139, 1978–1986. [Google Scholar] [CrossRef]
- Sridhar, V.V.; Surendrarao, A.; Gonzalez, D.; Conlan, R.S.; Liu, Z. Transcriptional repression of target genes by LEUNIG and SEUSS, two interacting regulatory proteins for Arabidopsis flower development. Proc. Natl. Acad. Sci. USA 2004, 101, 11494–11499. [Google Scholar] [CrossRef]
- Cheng, Y.; Kato, N.; Wang, W.; Li, J.; Chen, X. Two RNA binding proteins, HEN4 and HUA1, act in the processing of AGAMOUS pre-mRNA in Arabidopsis thaliana. Dev. Cell 2003, 4, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Shang, E.; Wang, X.; Li, T.; Guo, F.; Ito, T.; Sun, B. Robust control of floral meristem determinacy by position-specific multifunctions of KNUCKLES. Proc. Natl. Acad. Sci. USA 2021, 118, e2102826118. [Google Scholar] [CrossRef]
- Pelayo, M.A.; Morishita, F.; Sawada, H.; Matsushita, K.; Iimura, H.; He, Z.; Looi, L.S.; Katagiri, N.; Nagamori, A.; Suzuki, T. AGAMOUS regulates various target genes via cell cycle–coupled H3K27me3 dilution in floral meristems and stamens. Plant Cell 2023, 35, koad123. [Google Scholar] [CrossRef]
- Jetha, K.; Theißen, G.; Melzer, R. Arabidopsis SEPALLATA proteins differ in cooperative DNA-binding during the formation of floral quartet-like complexes. Nucleic Acids Res. 2014, 42, 10927–10942. [Google Scholar] [CrossRef]
- Johnson, S.D.; Anderson, B. Coevolution between food-rewarding flowers and their pollinators. Evol. Educ. Outreach 2010, 3, 32–39. [Google Scholar] [CrossRef]
- Liao, H.; Fu, X.; Zhao, H.; Cheng, J.; Zhang, R.; Yao, X.; Duan, X.; Shan, H.; Kong, H. The morphology, molecular development and ecological function of pseudonectaries on Nigella damascena (Ranunculaceae) petals. Nat. Commun. 2020, 11, 1777. [Google Scholar] [CrossRef] [PubMed]
- McKim, S.M.; Stenvik, G.E.; Butenko, M.A.; Kristiansen, W.; Cho, S.K.; Hepworth, S.R.; Aalen, R.B.; Haughn, G.W. The BLADE-ON-PETIOLE genes are essential for abscission zone formation in Arabidopsis. Development 2008, 35, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Tae Song, J.; Soo Seo, H.; Ik Song, S.; Seob Lee, J.; Do Choi, Y. NTR1 encodes a floral nectary-specific gene in Brassica campestris L. ssp. pekinensis. Plant Mol. Biol. 2000, 42, 647–655. [Google Scholar] [CrossRef]
- Carter, C.; Thornburg, R.W. Is the nectar redox cycle a floral defense against microbial attack? Trends Plant Sci. 2004, 9, 320–324. [Google Scholar] [CrossRef]
- Compagnon, V.; Diehl, P.; Benveniste, I.; Meyer, D.; Schaller, H.; Schreiber, L.; Franke, R.; Pinot, F. CYP86B1 is required for very long chain ω-hydroxyacid and α, ω-dicarboxylic acid synthesis in root and seed suberin polyester. Plant Phys. 2009, 150, 1831–1843. [Google Scholar] [CrossRef]
- Ma, Y.; Miotk, A.; Šutiković, Z.; Ermakova, O.; Wenzl, C.; Medzihradszky, A.; Gaillochet, C.; Forner, J.; Utan, G.; Brackmann, K. WUSCHEL acts as an auxin response rheostat to maintain apical stem cells in Arabidopsis. Nat. Commun. 2019, 10, 5093. [Google Scholar] [CrossRef] [PubMed]
- Running, M.P.; Meyerowitz, E.M. Mutations in the PERIANTHIA gene of Arabidopsis specifically alter floral organ number and initiation pattern. Development 1996, 122, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Brewer, P.B.; Howles, P.A.; Dorian, K.; Griffith, M.E.; Ishida, T.; Kaplan-Levy, R.N.; Kilinc, A.; Smyth, D.R. PETAL LOSS, a trihelix transcription factor gene, regulates perianth architecture in the Arabidopsis flower. Development 2004, 131, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.M.; Broadhvest, J.; Hauser, B.A.; Meister, R.J.; Schneitz, K.; Gasser, C.S. INNER NO OUTER regulates abaxial–adaxial patterning in Arabidopsis ovules. Genes Dev. 1999, 13, 3160–3169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, F.; Su, X.; Lu, Z.; Zhou, Y. Morphological and histological observation of flower bud differentation in the bulbs of Lilium pumilum. Pratacultural Sci. 2014, 31, 878–883. [Google Scholar]
- Zhang, Y.; Miao, L.; Lin, L.; Ren, C.Y.; Liu, J.X.; Cui, Y.M. Repeated administration of Sailuotong, a fixed combination of Panax ginseng, Ginkgo biloba, and Crocus sativus extracts for vascular dementia, alters CYP450 activities in rats. Phytomedicine 2018, 38, 125–134. [Google Scholar] [CrossRef]
- Fukai, S.; Goi, M. Floral initiation and development in Lilium longiflorum Thunb. Tech. Bull. Fac. Agric. Kagawa Univ. (Jpn.) 2001, 53, 31–34. [Google Scholar]
- Ning, Y.F.; Long, M.H.; Tao, J.; Yang, M.C.; Wei, P.X. Morphological observation on flower bud differentiation of Lilium formolongi Bulb. Acta Horticult. Sin. 2008, 35, 1368. [Google Scholar]
- Hornyik, C.; Duc, C.; Rataj, K.; Terzi, L.C.; Simpson, G.G. Alternative polyadenylation of antisense RNAs and flowering time control. Biochem. Soc. Trans. 2010, 38, 1077–1081. [Google Scholar] [CrossRef]
- Michaels, S.D.; Amasino, R.M. Loss of Flowering locus C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 2001, 13, 935–941. [Google Scholar] [CrossRef]
- Feng, W.; Michaels, S.D. Dual roles for FY in the regulation of FLC. Plant Signal. Behav. 2011, 6, 703–705. [Google Scholar] [CrossRef]
- Feng, W.; Jacob, Y.; Veley, K.M.; Ding, L.; Yu, X.; Choe, G.; Michaels, S.D. Hypomorphic alleles reveal FCA-independent roles for FY in the regulation of Flowering locus C. Plant Phys. 2011, 155, 1425–1434. [Google Scholar] [CrossRef]
- Guo, X.; Yu, C.; Luo, L.; Wan, H.; Zhen, N.; Xu, T.; Tan, J.; Pan, H.; Zhang, Q. Transcriptome of the floral transition in Rosa chinensis ‘Old Blush’. BMC Genom. 2017, 18, 199. [Google Scholar] [CrossRef]
- Corbesier, L.; Coupland, G. Photoperiodic flowering of Arabidopsis: Integrating genetic and physiological approaches to characterization of the floral stimulus. Plant Cell Environ. 2005, 28, 54–66. [Google Scholar] [CrossRef]
- Schmitz, R.J.; Sung, S.; Amasino, R.M. Histone arginine methylation is required for vernalization-induced epigenetic silencing of FLC in winter-annual Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 411–416. [Google Scholar] [CrossRef]
- Hu, X.; Kong, X.; Wang, C.; Ma, L.; Zhao, J.; Wei, J.; Zhang, X.; Loake, G.J.; Zhang, T.; Huang, J. Proteasome-mediated degradation of FRIGIDA modulates flowering time in Arabidopsis during vernalization. Plant Cell 2014, 26, 4763–4781. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Suzuki, H.; Kim, Y.C.; Iuchi, A.; Kuromori, T.; Ueguchi-Tanaka, M.; Asami, T.; Yamaguchi, I.; Matsuoka, M.; Kobayashi, M. Multiple loss-of-function of Arabidopsis gibberellin receptor AtGID1s completely shuts down a gibberellin signal. Plant J. 2007, 50, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Silverstone, A.L.; Sun, C.T.P. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 1998, 10, 155–169. [Google Scholar] [CrossRef]
- Saini, P.; Yadav, R.K. C-terminal domain of APETALA1 is essential for its functional divergence from CAULIFLOWER in Arabidopsis. J. Plant Biochem. Biotechnol. 2020, 29, 824–831. [Google Scholar] [CrossRef]
- Chen, M.K.; Lin, I.C.; Yang, C.H. Functional analysis of three lily (Lilium longiflorum) APETALA1-like MADS box genes in regulating floral transition and formation. Plant Cell Phys. 2008, 49, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ng, K.H.; Lim, T.S.; Yu, H.; Meyerowitz, E.M. The homeotic protein AGAMOUS controls late stamen development by regulating a jasmonate biosynthetic gene in Arabidopsis. Plant Cell 2007, 19, 3516–3529. [Google Scholar] [CrossRef] [PubMed]
- Kramer, E.M.; Jaramillo, M.A.; Di Stilio, V.S. Patterns of gene duplication and functional evolution during the diversification of the AGAMOUS subfamily of MADS box genes in angiosperms. Genetics 2004, 166, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.F.; Talle, B.; Wilson, Z.A. Anther and pollen development: A conserved developmental pathway. J. Integr. Plant Biol. 2015, 57, 876–891. [Google Scholar] [CrossRef]
- Mizukami, Y.; Ma, H. Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell 1992, 71, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ditta, G.; Pinyopich, A.; Robles, P.; Pelaz, S.; Yanofsky, M.F. The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr. Biol. 2004, 14, 1935–1940. [Google Scholar] [CrossRef]
- Krizek, B.A.; Fletcher, J.C. Molecular mechanisms of flower development: An armchair guide. Nat. Rev. Genet. 2005, 6, 688–698. [Google Scholar] [CrossRef]
- Sommer, H.; Beltran, J.P.; Huijser, P.; Pape, H.; Lönnig, W.E.; Saedler, H.; Schwarz-Sommer, Z. Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus: The protein shows homology to transcription factors. EMBO J. 1990, 9, 605–613. [Google Scholar] [CrossRef]
- Benedito, V.A.; Visser, P.B.; Van Tuyl, J.M.; Angenent, G.C.; De Vries, S.C.; Krens, F.A. Ectopic expression of LLAG1, an AGAMOUS homologue from lily (Lilium longiflorum Thunb.) causes floral homeotic modifications in Arabidopsis. J. Exp. Bot. 2004, 55, 1391–1399. [Google Scholar] [CrossRef]
- Barrett, S.C.H.; Harder, L.D.; Worley, A.C. The comparative biology of pollination and mating in flowering plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 1271–1280. [Google Scholar]
- Zhang, D.; Wang, S. Theoretical ecology in the 21st century. Biodivers. Sci. 2020, 28, 1301–1303. [Google Scholar] [CrossRef]
- Bin, Z.; Steve, H. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4, 1–45. [Google Scholar]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. AgriGO v2.0, a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Meth. Enzymol. 2001, 25, 402–408. [Google Scholar] [CrossRef]






| Annotated Database | Isoform Amount |
|---|---|
| COG | 17,760 |
| GO | 28,050 |
| KEGG | 21,425 |
| KOG | 28,425 |
| Pfam | 35,356 |
| Swiss-Prot | 32,322 |
| eggNOG | 43,232 |
| NR | 44,172 |
| All | 44,454 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Li, A.; Zhao, Y.; Li, W.; Dong, Z.; Zhang, L.; Zhu, Y.; Zhang, H.; Gao, Y.; Zhang, Q. Time-Course Transcriptomic Analysis Reveals Molecular Insights into the Inflorescence and Flower Development of Cardiocrinum giganteum. Plants 2024, 13, 649. https://doi.org/10.3390/plants13050649
Wei Y, Li A, Zhao Y, Li W, Dong Z, Zhang L, Zhu Y, Zhang H, Gao Y, Zhang Q. Time-Course Transcriptomic Analysis Reveals Molecular Insights into the Inflorescence and Flower Development of Cardiocrinum giganteum. Plants. 2024; 13(5):649. https://doi.org/10.3390/plants13050649
Chicago/Turabian StyleWei, Yu, Aihua Li, Yiran Zhao, Wenqi Li, Zhiyang Dong, Lei Zhang, Yuntao Zhu, Hui Zhang, Yike Gao, and Qixiang Zhang. 2024. "Time-Course Transcriptomic Analysis Reveals Molecular Insights into the Inflorescence and Flower Development of Cardiocrinum giganteum" Plants 13, no. 5: 649. https://doi.org/10.3390/plants13050649
APA StyleWei, Y., Li, A., Zhao, Y., Li, W., Dong, Z., Zhang, L., Zhu, Y., Zhang, H., Gao, Y., & Zhang, Q. (2024). Time-Course Transcriptomic Analysis Reveals Molecular Insights into the Inflorescence and Flower Development of Cardiocrinum giganteum. Plants, 13(5), 649. https://doi.org/10.3390/plants13050649





